Abstract
Fertile plants undergoing male gametogenesis can be treated with nitrous oxide (N2O) gas to obtain 2n male gametes. N2O treatment is also expected to restore the fertility of interspecific hybrids through meiotic restitution or mitotic amphidiploidization. However, this technique has few applications to date, and it is un-known how N2O treatment restores fertility in sterile hybrids. To establish optimal N2O treatment conditions and determine its cytological mechanism of action, we treated various sized floral buds with N2O gas at different anther developmental stages from fertile and sterile hybrid lilies. N2O treatment using the optimal 1–4 mm floral buds induced mitotic polyploidization of male archesporial cells to produce 2n pollen in fertile hybrid lilies. In sterile hybrid lilies, N2O treatment doubled the chromosome number in male archesporial cells followed by homologous chromosome pairing and normal meiosis in pollen mother cells (PMC), resulting in restoration of pollen fertility. Backcrossing the resultant fertile pollen to Lilium × formolongi produced many triploid BC1 plants. Thus N2O treatment at the archesporial cell proliferating stage effectively overcame pollen sterility in hybrid lilies, resulting in fertile, 2n pollen grains that could produce progeny. The procedure presented here will promote interspecific or interploidy hybridization of lilies.
Keywords: chromosome doubling, diploid male gamete, hybrid sterility, interspecific hybridization, Lilium, nitrous oxide, polyploid
Introduction
Interspecific hybridization is useful for increasing genetic diversity. In ornamental flower breeding, interspecific hybridization is an essential technique that produces novel cultivars, as driven by consumer demand. Many such cultivars are used as ornamental crops (Fukai and Tsuji 2004, Okazaki et al. 1992, 1994, 1995, Saruwatri et al. 2008), whereas hybrids obtained from remote interspecific crosses are mostly sterile and cannot be used in cross breeding (Choudhary et al. 2000, Gangadevi et al. 1985, Poysa 1990). The traditional method of overcoming hybrid sterility is to double chromosome number using polyploidizing agents such as colchicine to produce allopolyploids that may be fertile (Asano 1982, Nimura et al. 2006).
Nitrous oxide (N2O) has been applied to zygotes and seedlings in many crops as a polyploidizing agent in lieu of colchicine treatment (Berdahl and Barker 1991, Dvorak et al. 1973, Kato 2002, Kato and Birchler 2006, Nygren 1955, Östergren 1954, 1957, Taylor et al. 1976, Zeilinga and Schouten 1968). Meiotic metaphase stage anthers of male-fertile cultivars in tulips and lilies are optimal for N2O treatment to produce fertile 2n pollen grains (Akutsu et al. 2007, Okazaki et al. 2001, 2005). Compared to other chemicals that mostly induce mitotic polyploidization by arresting cell division, N2O is not damaging and leads to first division restitution (FDR) or second division restitution (SDR) in meiosis in male-fertile plants, producing 2n gametes that are functional and available for crossing. Some polyploid plants have been produced using 2n gametes obtained by N2O treatment (Akutsu et al. 2007, Okazaki et al. 2005).
Lilies (Lilium spp., 2n = 2x = 24) are one of the most important horticultural crops, with about 100 species widespread in the northern hemisphere (Nishikawa et al. 1999). The most important modern commercial lilies mainly belong to the Asiatic, Oriental, and Longiflorum hybrid groups, and over 10,000 cultivars, including many sterile interspecific hybrids, are registered (Matthews 2007). Barba-Gonzalez et al. (2006) obtained fertile 2n pollen from sterile lily hybrids by using N2O treatment. Postulating that N2O treatment could bring about meiotic restitution in sterile as well as fertile lily cultivars, they concluded from cytological analyses of progeny that N2O had induced FDR gametes in most cases. However, the particular FDR mechanism by which hybrid sterility can be overcome does not explain the finding of Kitamura et al. (2009). In their histological study on pollen meiosis in fertile plants treated with N2O gas for 24 h, microtubules were effectively depolymerized, which prevented chromosomes from moving to the poles and resulted in chromosome retention in the center of N2O-treated cells. Cell plate formation took place without delay, however, yielding one daughter cell with a diploid genome and another daughter cell without chromosomes. As a result, a 2n male gamete was produced during meiotic metaphase. In sterile interspecific hybrids where chromosomes are scattered in the cytoplasm owing to non-homologous parental genomes, the cell plate divides the chromosomes unequally to make aneuploid daughter cells with or without N2O treatment (Kitamura et al. 2009). This suggests that N2O treatment during meiotic division does not lead to chromosome doubling in a daughter cell through the FDR mechanism in sterile hybrids. The cytological mechanism of action of N2O treatment for overcoming hybrid sterility remains to be determined.
Therefore, this study investigated how N2O gas leads to the development of fertile gametes in sterile interspecific hybrid lilies and determined the optimal PMC developmental stage for overcoming hybrid sterility. In addition, to verify whether the resulting fertile pollen could produce progeny, we attempted to produce BC1 progeny using the fertile pollen obtained from completely sterile interspecific hybrids.
Materials and Methods
Plant materials
The cultivars used in this study were collected from Yamaki Noen Co., Ltd. (Niigata, Japan). The cultivars used are listed in Table 1: four fertile cultivars belonging to Lilium spp. Asiatic hybrid lilies, a fertile Oriental hybrid lily, two LA hybrids, an OT hybrid, and an LO hybrid. Two cultivars of L. × formolongi were crossed with pollen from N2O-treated hybrids. Single bulbs of each cultivar were planted in 10-cm pots in October or November and grown in an unheated greenhouse at Niigata University, Niigata, Japan.
Table 1.
Ploidy level, pollen fertility and origin of cultivars used in the study
| Cultivars | Horticultural groups | Ploidy level | Chromosome number | Pollen fertility | Origin |
|---|---|---|---|---|---|
| Regata | Asiatic hybrids | 2× | 24 | fertile | Intra-sectional hybrid (Sinomartagon) |
| Mona | Asiatic hybrids | 2× | 24 | fertile | Intra-sectional hybrid (Sinomartagon) |
| Gran Paradiso | Asiatic hybrids | 4× | 48 | fertile | Intra-sectional hybrid (Sinomartagon) |
| Loreto | Asiatic hybrids | 4× | 48 | fertile | Intra-sectional hybrid (Sinomartagon) |
| Willeke Alberti | Oriental hybrids | 2× | 24 | fertile | Intra-sectional hybrid (Archelirion) |
| Serrada | LA hybrids | 3× | 36 | almost sterile | Inter-sectional hybrid; L. longiflorum (Leucolirion) × Asiatic hybrids (Sinomartagon) |
| Royal Trinity | LA hybrids | 3× | 36 | low fertile | Inter-sectional hybrid; L. longiflorum (Leucolirion) × Asiatic hybrids (Sinomartagon) |
| Yelloween | OT hybrids | 2× | 24 | completely sterile | Unknown |
| Otome no Sugata | LO hybrids | 2× | 24 | completely sterile | Inter-sectional hybrid; L. × formolongi (Leucolirion) × L. rubellum (Archelirion) |
| Katsuki | L. × formolongi | 2× | 24 | fertile | Intra-sectional hybrid (Leucolirion); L. formosanum × L. longiflorum |
| Original Ougo | L. × formolongi | 2× | 24 | fertile | Intra-sectional hybrid (Leucolirion); L. formosanum × L. longiflorum |
N2O treatment
N2O treatment (modified from Akutsu et al. 2007) was performed at room temperature for 48 h in a pressure-tolerant cylinder, with N2O gas applied at 6 atm without oxygen. Prophase I and meiotic metaphase I in Asiatic hybrid lilies occur in 15- or 20-mm floral buds, respectively (Akutsu et al. 2007). In L. longiflorum, premeiotic mitosis occurs in 10-mm floral buds (Taylor and McMaster 1954), and N2O treatment during prophase I does not produce 2n pollen grains at flowering (Akutsu et al. 2007). Based on these results, we treated hybrid lilies with attached 1–10 mm floral buds, which presumably contained the archesporial cell proliferating stage. Buds were small at the time of treatment, making direct length measurements difficult. Hence, longitudinal lengths of detached floral buds from plants grown under similar conditions were measured with a caliper or ruler to estimate the floral bud sizes of treated plants at the time of N2O treatment. The treated plants were grown in a greenhouse. N2O treatments were conducted for Asiatic hybrid lilies, Oriental hybrid lilies and LA hybrids in 2007, ‘Yelloween’ in 2007 and 2008 and ‘Otome no Sugata’ in 2008.
Pollen assessments and statistical analysis
At flowering time, a small amount of pollen was collected from six anthers of each bud, mixed, and then stained with 1% (w/v) acetocarmine solution. Lengths of short axes of pollen grains (100–400) were microscopically measured using Image-Pro PLUS (Media Cybernetics Co., Ltd., Bethesda, MD, USA). In the control and N2O-treated plants of ‘Yelloween’, microspore tetrad sizes were measured from young anthers of 30–35 mm floral buds. Pollen fertility was determined using the acetocarmine method, whereby acetocarmine-stained pollen was regarded as fertile pollen. Before pollination, the germinability of the pollen obtained by N2O treatment was assessed after culture for 24 h at 25°C in agar medium containing 10% (w/v) sucrose, 0.7% (w/v) agar, 0.3 g/L calcium nitrate and 10 mg/L boric acid.
For data analysis, a non-parametric multiple comparison method (Steel-Dwass test) was performed using Microsoft Excel 2003 and statistical add-in software (Excel Toukei 2010 for Windows, Social Survey Research Information Co., Ltd., Tokyo, Japan).
Crossing and hybrid embryo rescue
For intraspecific hybridization of Asiatic hybrid lilies, control crosses were made as follows: 2x × 2x; 4x × 2x; and 4x × 4x. Tetraploid ‘Gran Paradiso’ was pollinated with pollen masses including putative 2n grains. Before flowering, flowers were emasculated in each cross. Capsules were harvested 2 months after pollination, and the seed number was counted for each cross.
For interspecific hybridization using fertile pollen restored from the sterile hybrid lilies, the capsules were collected about 40 d after pollination and embryo culture was performed as described by Okazaki et al. (1994).
Histological observation
PMC developmental stages of the Asiatic hybrid lily ‘Regata’ were observed. Floral buds of different sizes were collected and fixed for 24 h in Carnoy’s fluid, dehydrated in an alcohol gradient series, and embedded in paraffin. Paraffin-embedded samples were cut into 6-μm sections, stained with 0.05% (w/v) toluidine blue, mounted in balsam jelly, and observed by light microscopy (BX-60; Olympus, Tokyo, Japan).
Determination of ploidy and hybridity
Ploidy of seedlings and pollen grains was measured using flow cytometry as described previously (Akutsu et al. 2007, Okazaki et al. 2005). To verify the hybridity of the progeny from the cross between L. × formolongi and the N2O-treated ‘Otome no Sugata’, genomic DNA was extracted from the leaves using the CTAB method (Murray and Thompson 1980), and the internal transcribed spacer (ITS) region of rDNA was amplified according to the methods of Nishikawa et al. (1999, 2001). The amplified fragments were digested by MspI and analyzed on polyacrylamide gels (Nagaoka et al. 2010).
Genomic in situ hybridization (GISH)
For mitotic metaphase chromosome analysis, the root tips were collected early in the morning. The root tips were incubated in 0.05% (w/v) colchicine solution for 2–3 h and then fixed in ethanol-acetic acid (3 : 1) solution for 24 h and stored at 4°C until use. The root tips were washed in distilled water and incubated in a pectolytic enzyme mixture containing 2% (w/v) cellulose Onozuka-RS (Yakult Pharmaceutical Industry Co. Ltd., Tokyo, Japan) and 1% (w/v) pectolyase Y-23 (Kanto Chemical Co., Inc.) in 10 mM citrate buffer (pH 4.5) at 37°C for 1–2 h. Squash preparations were made in a drop of 45% (v/v) acetic acid and frozen using liquid nitrogen. The cover slips were removed using a razor blade.
Genomic DNA of L. × formolongi was used as a probe and labeled with digoxigenin-11-dUTP by a standard nick translation protocol (Roche, Basel, Switzerland). GISH was performed as described by Marasek et al. (2006). The probe was detected with fluorescein-conjugated anti-digoxigenin. The preparations were analyzed with a microscope (BX60, Olympus) and photographed with a digital camera (DP70, Olympus). For each plant, the total number of chromosomes and the number of crossover points were determined.
Results
Mitotic polyploidization of archesporial cells in fertile hybrid lilies
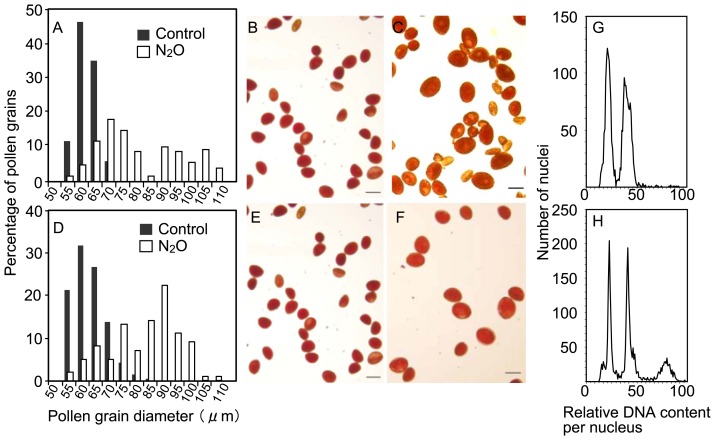
Control pollen grain diameters ranged 51–71 μm (59 ± 4 μm, mean ± SD) in diploid Asiatic hybrid lily ‘Regata’ (Fig. 1A, 1B) and 50–84 μm (60 ± 6 μm) in diploid Oriental hybrid lily ‘Willeke Alberti’ (Fig. 1D, 1E). Plants with small (1–10 mm) floral buds treated with N2O produced giant pollen in ‘Regata’ (Fig. 1C) and in ‘Willeke Alberti’ (Fig. 1F), whereas treated pollen shape was the same as the oval-shaped control pollen. For example, a 3-mm floral bud of ‘Regata’ and a 4-mm ‘Willeke Alberti’ bud treated with N2O gas resulted in a pollen size distribution of 59–119 μm (79 ± 15 μm) and 53–110 μm (81 ± 12 μm), respectively (Fig. 1A, 1D).
Fig. 1.
Size distribution, appearance, and flow cytometry histograms of pollen grains in diploid cultivars. Pollen size distributions were obtained from control pollen (shaded bars) and pollen obtained from plants treated with N2O gas for 48 h (open bars) in ‘Regata’ (A) and ‘Willeke Alberti’ (D). The appearance of untreated (B, E) and N2O-induced (C, F) pollen grains are shown for ‘Regata’ (B, C) and ‘Willeke Alberti’ (E, F). The histograms show pollen samples for untreated (G) and N2O-induced (H) pollen grains in ‘Regata’. Bars = 50 μm (B, C, E, F).
Flow cytometric analysis of untreated pollen of ‘Regata’ revealed two peaks corresponding to 1C and 2C DNA content because the binucleate-type lily pollen contains one 2C generative and one 1C vegetative cell (Fig. 1G). In contrast, the N2O-induced pollen gave rise to peaks at 1C, 2C and additional 4C levels, indicating both n and 2n pollen (Fig. 1H). These results indicated that N2O treatment was able to induce chromosome doubling in male gametes. Based on the difference in pollen size distribution between the control and N2O-induced pollen, we considered grains with a diameter of >75 or 85 μm diameter as putative 2n pollen grains in ‘Regata’ or ‘Willeke Alberti’, respectively.
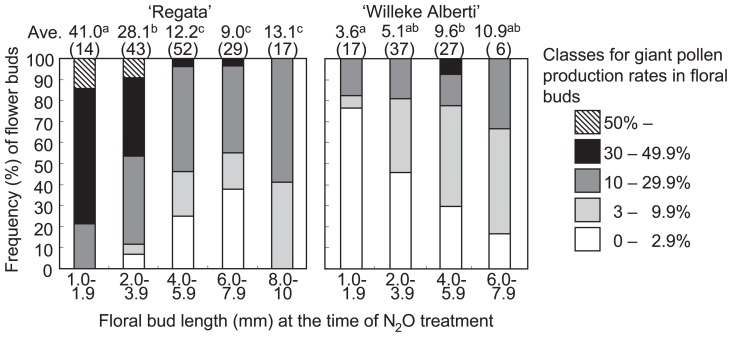
To determine the most suitable floral bud stage for mitotic chromosome doubling of male archesporial cells, 1–10 mm floral buds were treated with N2O. Mean production rates of giant pollen in a treated flower were classified into five categories (Fig. 2). The efficacy of giant pollen formation fluctuated among treated flowers of ‘Regata’, with up to 83% (n = 128) of flowers having more than 3% giant pollen among the 155 treated flowers (Fig. 2). Flowers producing giant pollen grains with a frequency of higher than 50% occurred in 1.0–1.9 mm and 2.0–3.9 mm floral buds. The average rate of giant pollen production in the 1.0–1.9 mm category was significantly higher than in the other categories. N2O treatment using smaller floral buds tended to produce more giant pollen grains.
Fig. 2.
Frequency (%) of giant pollen production classes in floral buds of fertile hybrid lilies after 48 h of N2O treatment of floral buds of different lengths. Ave., mean percentage of giant pollen grains produced in each floral bud size, with number of N2O-treated floral buds in parentheses. Different letters between cultivars represent significant differences at P = 0.05, as determined by the Steel-Dwass test.
Similarly, in Oriental hybrid lily ‘Willeke Alberti’, N2O treatment induced giant pollen in flower buds ranging from 1–7.9 mm (Fig. 2). The average rate of giant pollen production in the 4.0–5.9 mm category was significantly higher than in the 1.0–1.9 mm category. Although there were no significant differences on giant pollen production rate among the 2.0–3.9 mm, 4.0–5.9 mm and 6.0–7.9 mm categories, we found flowers producing giant pollen with a frequency of higher than 30% in the 4.0–5.9 mm category. Therefore, floral buds ca. 5 mm long at the time of N2O treatment were likely to be suitable for induction of giant pollen. Thus, the optimal bud size of ‘Willeke Alberti’ was larger than that of ‘Regata. The efficacy of giant pollen formation fluctuated among the treated flowers, as well as among the anthers within a given flower, in ‘Willeke Alberti’ and ‘Regata’ (data not shown).
Restoration of fertility in sterile and partially sterile hybrid lilies
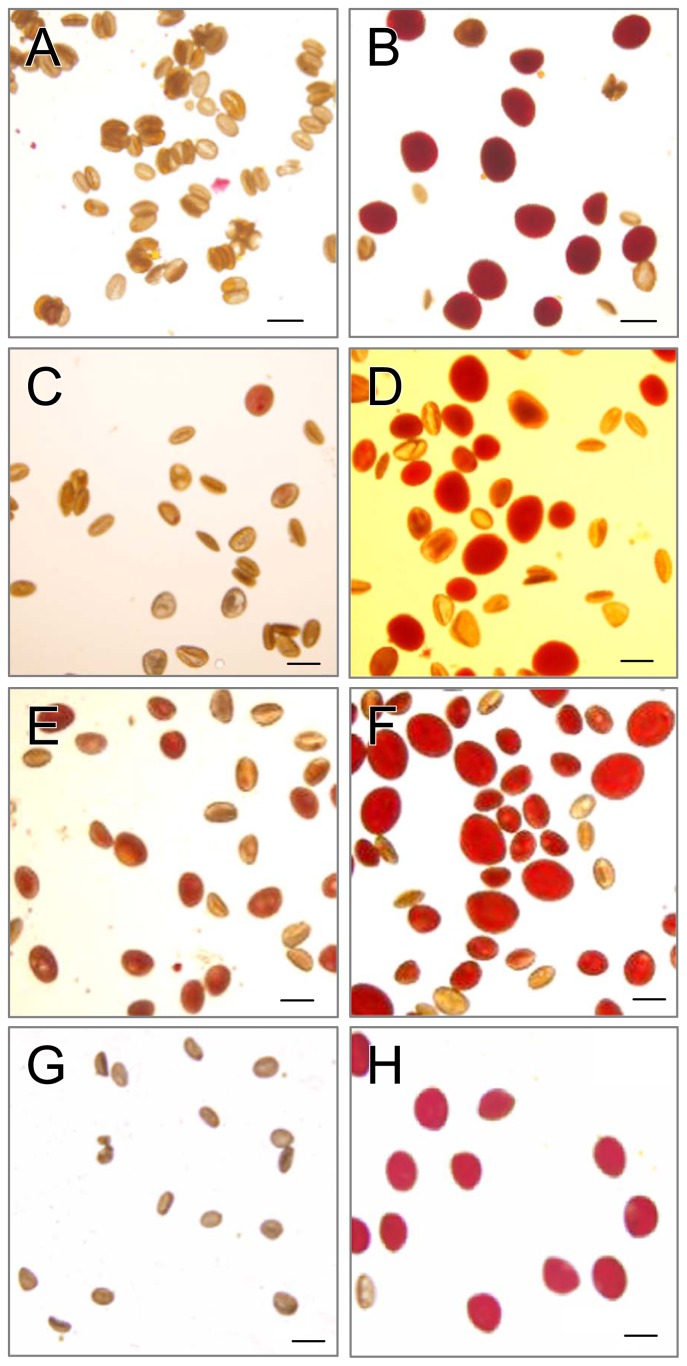
OT hybrid ‘Yelloween’ was completely pollen sterile (Fig. 3A). LA hybrid ‘Serrada’ displayed low pollen fertility ranging from 0–20% (10 ± 9.7%) (Fig. 3C). To examine whether N2O treatment could restore pollen fertility in sterile interspecific hybrid lilies, ‘Yelloween’ and ‘Serrada’ plants were treated with N2O in 2007. A partially sterile LA hybrid ‘Royal Torinity’ also was included. N2O treatment restored pollen fertility in both sterile hybrid lilies (Fig. 3B, 3D). Although acetocarmine-stained pollen is not always fertile, 76% of pollen on average was stained in the untreated plants of LA hybrid ‘Royal Trinity’ (Fig. 3E), whereas N2O-treated plants produced larger, positively stained pollen (Fig. 3F). The smaller-sized, positively stained pollen likely came from normal meiosis.
Fig. 3.
Overcoming hybrid sterility by treating sterile or partially sterile hybrid lilies with N2O for 48 h. Appearance of control pollen (left side) and N2O-induced pollen (right side) in ‘Yelloween’ (A, B), ‘Serrada’ (C, D), ‘Royal Trinity’ (E, F) and ‘Otome no Sugata’ (G, H) is shown. Bars = 100 μm.
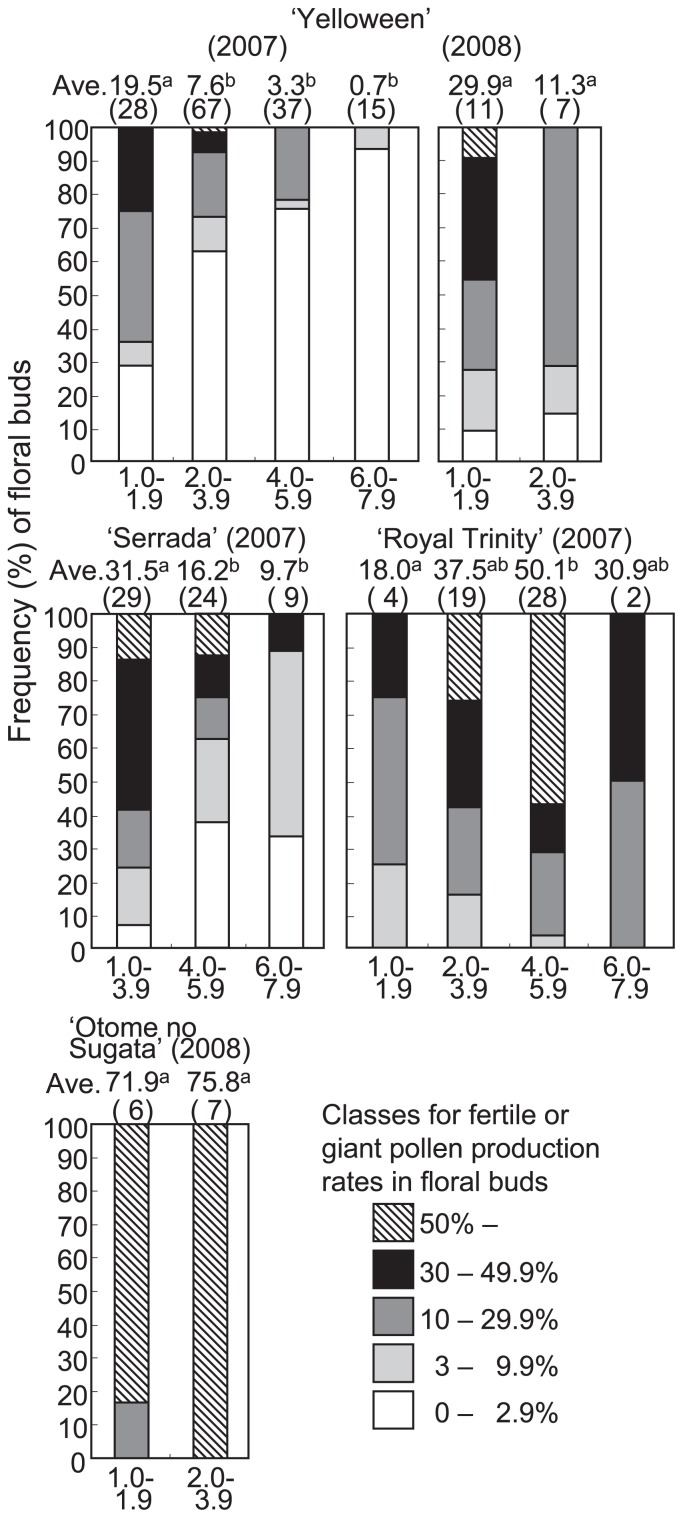
To determine the optimal growth stage for overcoming pollen sterility, the aforementioned sterile and partially sterile cultivars were treated at various stages (1–7.9 mm) of floral buds in 2007. N2O treatment of 1–3.9 mm floral buds of ‘Yelloween’ produced more fertile pollen grains, with the highest pollen fertility (50%) achieved from 2-mm floral buds (Fig. 4). In ‘Serrada’, fertile pollen grains were most effectively produced using 1.0–3.9 mm floral buds, with the most fertile pollen (71%) obtained from 4-mm floral buds (Fig. 4). Untreated plants of LA hybrid ‘Royal Trinity’ showed normal meiosis, with the diameter of pollen grain ranging between 57–97 μm (83 ± 10 μm). Based on this control data for pollen grain diameter in ‘Royal Trinity’, to examine the effectiveness of N2O treatment, we scored the frequency of giant pollen grains as that of pollen grains with a diameter of >100 μm in the N2O-treated plants. Although there were no significant differences among the 2.0–3.9 mm, 4.0–5.9 mm and 6.0–7.9 mm categories, the 2.0–5.9 mm floral buds produced more giant pollen (>100 μm) in ‘Royal Trinity’ (Fig. 4).
Fig. 4.
Frequency (%) of floral buds in each fertile or giant pollen production class in the sterile or partially sterile hybrid lily floral buds treated with N2O for 48 h. Ave., mean percentage of fertile or giant pollen grains in each floral bud size, with number of floral buds treated with N2O in parentheses. The fertile pollen production rate at flowering (‘Yelloween’, ‘Serrada’ and ‘Otome no Sugata’) and the giant pollen production rate (‘Royal Trinity’) were measured. Different letters between cultivars represent significant differences at P = 0.05, as determined by the Steel-Dwass test.
To confirm the reproducibility and reliability of these results, we repeated the experiments to overcome hybrid sterility using ‘Yelloween’ and a completely sterile LO hybrid ‘Otome no Sugata’ in 2008. ‘Otome no Sugata’ produced highly fertile pollen upon N2O treatment (Fig. 3G, 3H). In ‘Yelloween’, the efficacy of recovery of fertile pollen varied among the treated flowers (Fig. 4) and ranged 1–67% (23% average in the 1.0–1.9 mm floral buds; 11% average in the 2.0–3.9 mm floral buds). The efficacy of fertile pollen formation fluctuated among the six anthers within a flower. For example, four of six anthers of ‘Yelloween’ had <10% recovery of fertile pollen, whereas the remaining two anthers had 55% and 50% recovery. ‘Otome no Sugata’ also had variable efficacy of fertile pollen recovery among the N2O-treated flowers (21–90% range; 72% average in 1.0–1.9 mm floral buds; 76% average in 2.0–3.9 mm floral buds).
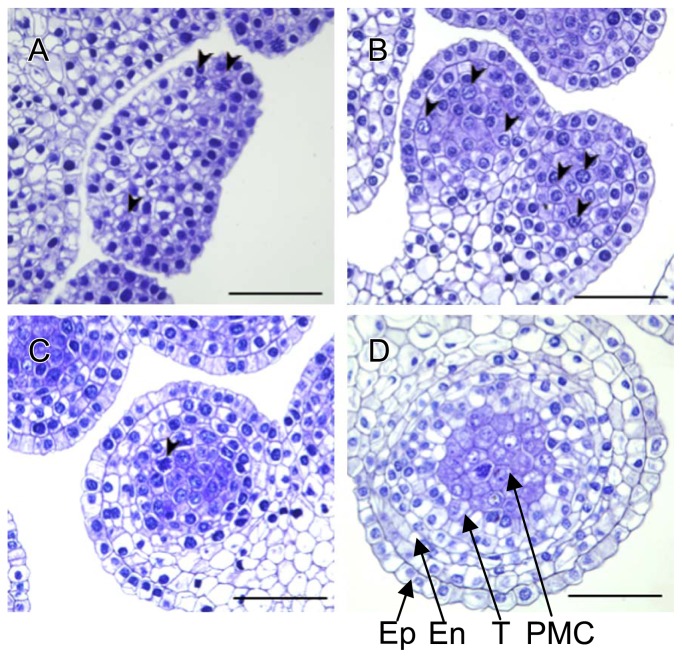
Histological observations of archesporial cells in anther primordia
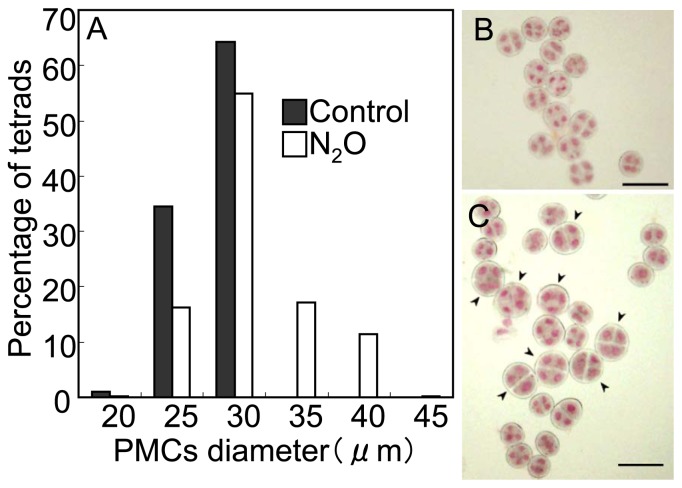
We observed cell development in anther primordia at the optimal floral bud developmental stage (1–4 mm) after N2O treatment in ‘Regata’. Transverse sections of the 1–3 mm floral buds showed actively dividing archesporial cells inside the peripheral layers of the stamen (Fig. 5A, 5B). As a result of the cell proliferation along the longitudinal axis of anther locules, the oblong locules were filled with archesporial cells in 3–5 mm floral buds (Fig. 5C). Cell division in anther primordia was almost finished at the 7-mm floral bud stage, with 0.6% mitotic index of the archesporial cells (Fig. 5D). In 10-mm floral buds, tapetum cells surrounded PMCs, and no mitotic divisions were observed (data not shown). In the 32-mm floral buds of ‘Yelloween’ undergoing meiotic division, untreated control microspore tetrads had 20–29 μm (25 ± 1.8 μm) diameters, whereas floral buds treated with N2O had some larger microspore tetrads with 19–41 μm (29 ± 4.2 μm) diameters (Fig. 6). Among them, 29% were >30 μm in diameter, which was similar to the recovery percentage of fertile pollen at anthesis. These results suggest that N2O treatment overcame hybrid sterility in the interspecific hybrid lilies owing to chromosome doubling of archesporial cells of PMCs.
Fig. 5.
Transverse sections of young anthers of ‘Regata’. The floral buds were sampled at lengths of 1 mm (A), 3 mm (B), 5 mm (C) and 7 mm (D). Ep, epidermis; En, endothecium; T, tapetum; PMC, pollen mother cell. The archesporial cell undergoing mitotic division is marked with arrowheads. Bars = 50 μm.
Fig. 6.
Size distribution and appearance of microspore tetrads in control and N2O-treated plants of ‘Yelloween’. The plants were untreated (black bar) or treated (open bar) with N2O for 48 h (A). The appearance of microspore tetrads of untreated (B) and N2O-treated (C) plants is indicated. Large microspore tetrads derived from chromosome doubling by N2O treatment are marked with arrowheads. Bars = 50 μm.
Crosses with putative 2n pollen obtained from the N2O-treated plants
In the 2x-level control cross (between 2x Asiatic hybrid lilies ‘Mona’ and ’Regata’), all capsules set normally with about 120 normal seeds per capsule (Table 1). In the 4x-level control cross (between 4x Asiatic hybrid lilies ‘Gran Paradiso’ and ‘Loreto’), normal seeds were obtained with 34 seeds per capsule. The ploidy levels of embryos excised from the resulting seeds were determined using flow cytometry (Table 2). All seeds obtained from the 2x × 2x and 4x × 4x crosses were diploid and tetraploid, respectively. These data indicate that the diploids and tetraploids used have normal fertility in male and female gametes. Nevertheless, no seeds were obtained in the interploidy cross between the tetraploid ‘Gran Paradiso’ and the diploid ‘Regata’, owing to triploid block. This phenomenon can act as a selective barrier against triploids in crosses of tetraploid cultivars with mixed n and 2n pollen (Akutsu et al. 2007). In such crosses, the resulting progeny seeds will be tetraploids. In fact, when the tetraploid cultivar ‘Gran Paradiso’ was crossed with 2n grain including pollen from N2O-treated plants of the diploid cultivar ‘Regata’, 59% of the flowers pollinated set capsule and the resulting embryos were tetraploid. In the crosses using the 2n grain including pollen from N2O-treated 3x LA hybrid ‘Royal Torinity’, the examined embryos were penta-ploid. When L. × formolongi was pollinated with pollen obtained from the N2O-treated ‘Yelloween’ (OT hybrid) and ‘Otome no Sugata’ (LO hybrid), some hybrid embryos were obtained. These were diploid, triploid or tetraploid (Table 3). However, no embryos were obtained using the control pollen that contained no viable grains.
Table 2.
Results of interploidy crosses between diploid and tetraploid lily varieties and crosses between tetraploids and N2O-treated plants
| Female parent | Male parent | No. of flowers pollinated | No. of ovaries developed | No. of seeds obtained per capsule (mean ± SD) | Ploidy level of seeds (%) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2×a | 4× | 5× | |||||
| Diploid × diploid | |||||||
| Mona (2×) | Regata (2×) | 5 | 5 | 125.8 ± 18.2 | 100 n = 20 |
0 | 0 |
| Tetraploid × tetraploid | |||||||
| Gran Paradiso (4×) | Loreto (4×) | 9 | 9 | 33.7 ± 16.7 | 0 | 100 n = 45 |
0 |
| Tetraploid × diploid | |||||||
| Gran Paradiso (4×) | Regata (2×) | 10 | 0 | – | – | – | – |
| Tetraploid × N2O-treated plants | |||||||
| Gran Paradiso (4×) | Regata (2×) | 71 | 42 | 3.1 ± 2.7 | 0 | 100 n = 41 |
0 |
| Gran Paradiso (4×) | Royal Torinity (3×) | 11 | 8 | 5.5 | 0 | 0 | 100 n = 41 |
Ploidy of mature seeds was determined by flow cytometry (n = the number of seeds examined).
Table 3.
Results of interspecific crosses between L. × formolongi and interspecific hybrids
| Male parent | No. of flowers pollinated | No. of capsules set | No. of plantlets obtained | Ploidy level of progeny | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 2×a | 3× | 4× | ||||
| Untreated control | ||||||
| ‘Otome no Sugata’ (2×) | 14 | 5 | 0 | – | – | – |
| ‘Yelloween’ (2×) | 37 | 7 | 0 | – | – | – |
| Pollinated with N2O-treated plants | ||||||
| ‘Otome no Sugata’ (2×) | 32 | 21 | 24 | 9 | 14 | 1 |
| ‘Yelloween’ (2×) | 39 | 22 | 17 | 15 | 2 | 0 |
Ploidy of progeny was determined by flow cytometry.
Verification of hybridity
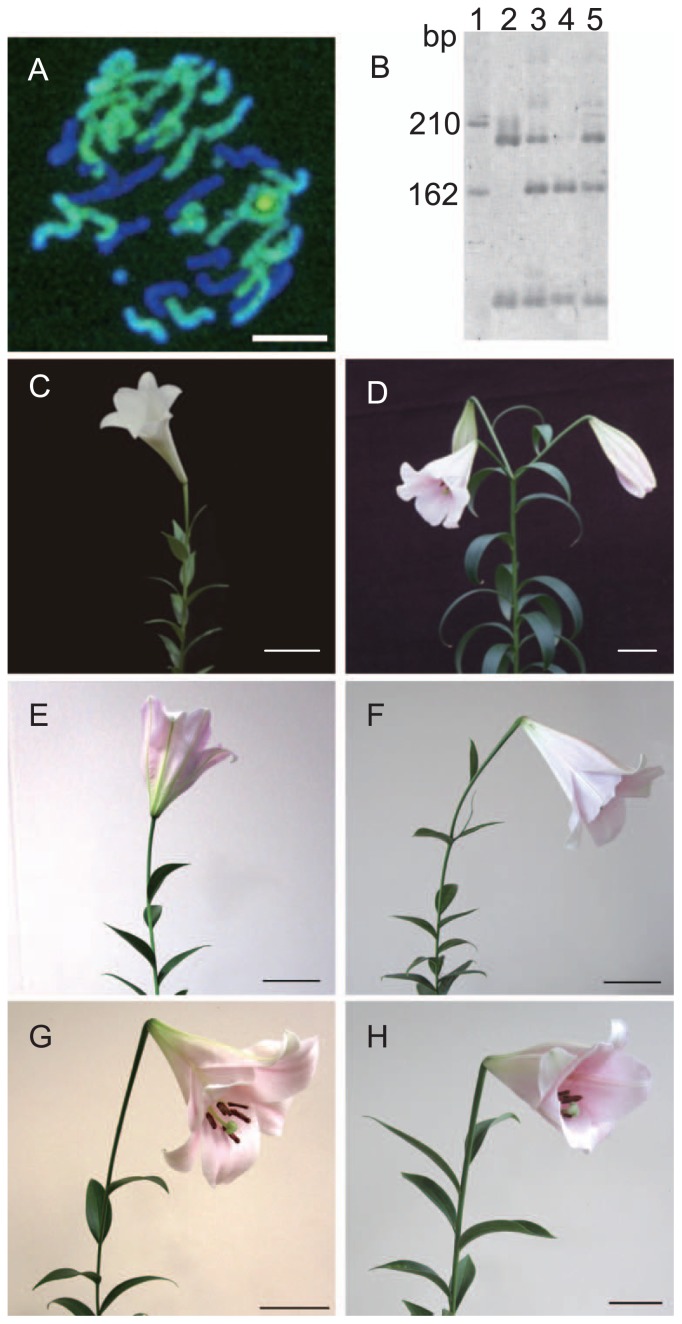
‘Otome no Sugata’ was the diploid hybrid developed by crossing L. × formolongi with L. rubellum (Okazaki et al. 1992). Crossing L. × formolongi with 2n grain including pollen of ‘Otome no Sugata’ produced in this study was regarded as a backcross, which would lead to production of triploids having the diploid genomes of L. × formolongi and a haploid genome of L. rubellum. When genomic DNA of L. × formolongi was used as a probe, 24 chromosomes were detected in the triploid progeny (Fig. 7A), indicating that these 24 chromosomes came from L. × formolongi and the remaining 12 from L. rubellum. The GISH analysis revealed that no chromosome recombination occurred between the genomes of L. × formolongi and L. rubellum in either of the three hybrid plants examined.
Fig. 7.
Hybridity and appearance of the parents and their hybrids (BC1 plants). GISH analysis of a triploid progeny with 24 L. × formolongi (green) and 12 L. rubellum (dark blue) chromosomes (A). CAPS analysis of MspI-digestion of the rDNA ITS region (B): 1, HincII-digested ϕX174 markers; 2, L. × formolongi; 3, ‘Otome no Sugata’; 4, L. rubellum; and 5, a hybrid. The appearance of a female parent (L. × formolongi) (C), male parent (‘Otome no Sugata’) (D) and BC1 shoots (E–H). Bars = 10 μm (A) or 10 cm (C–H).
The ITS regions of rDNA in L. × formolongi, L. rubellum, ‘Otome no Sugata’, and the backcrossed progeny were amplified by PCR, and the resulting PCR products were <700 bp in size, and were cleaved by MspI (Fig. 7B). The ca. 160 bp restriction fragment, specific to L. rubellum genome, was transmitted to the backcrossed triploid and tetraploid progeny, indicating that the triploid and tetraploid plants derived from crossing L. × formolongi with N2O-treated ‘Otome no Sugata’ were true hybrids, whereas the diploids were false hybrids (data not shown). The two triploid plants derived from the cross of L. × formolongi and ‘Yelloween’ died after transplant. Appearance of diploids and tetraploids was an unexpected result; the tetraploid BC1 plants were arisen from the fusion of spontaneous 2n female gametes and the N2O-induced 2n pollen. The diploids might be developed by spontaneous 2n female gamete formation, followed by pseudogamy.
Characteristics of hybrids
Four plants from the cross of L. × formolongi with N2O-treated ‘Otome no Sugata’ flowered in the 2 years after pollination. L. × formolongi has white, trumpet-shaped, upright flowers with yellow pollen (Fig. 7C). ‘Otome no Sugata’ has pink, trumpet-shaped flowers that are slightly drooping with brownish anthers (Fig. 7D). The hybrid floral sizes were intermediate between both parents, whereas flower color and anther color were similar to ‘Otome no Sugata’ (Fig. 7E–7H). The appearance of flowers varied among hybrids, with upright positioned (Fig. 7E), slightly drooping flower positions and trumpet-shaped (Fig. 7F, 7H) or bowl-shaped (Fig. 7G) pink flowers. The appearance of the trumpet-shaped flowers of the hybrids was more like L. × formolongi than ‘Otome no Sugata’.
Discussion
The mechanism of action of N2O for overcoming hybrid sterility
Barba-Gonzalez et al. (2006) reported that N2O treatment overcame pollen sterility through the FDR mechanism in 5–10 mm floral buds. At this developmental stage, however, the archesporial cells in anthers are thought to undergo premeiotic mitosis. In general, microspore developmental stages correlate with floral bud lengths (Goldberg et al. 1993). In L. longiflorum, floral buds <10 mm or 10–22 mm have the proliferating stage of archesporial cells or meiotic prophase I PMCs, respectively (Taylor and McMaster 1954). Our histological study confirmed a similar correlation between microspore developmental stage and floral bud length in Asiatic hybrids, although these lilies have smaller floral buds than L. longiflorum. Therefore, in the 5–10 mm floral buds used for N2O treatment by Barba-Gonzalez et al. (2006), archesporial cells should undergo mitosis, indicating that production of 2n gametes in 5–10 mm floral buds does not occur via FDR during meiosis. Furthermore, if fertile pollen was produced in sterile hybrid lilies through the FDR mechanism, the resulting meiotic products should be dyads (unreduced microspores) having the same size as tetrads. In our study, however, larger microspore tetrads were observed in plants treated with N2O. Therefore, our results suggest that the FDR mechanism is not involved in restoration of pollen fertility, and chromosome doubling in archesporial cells followed by formation of amphidiploid PMCs (2n = 4x) is the mechanism for the recovery of hybrid fertility.
Kato (2002) obtained doubled haploid lines by treating haploid maize floral primordia with N2O, suggesting that applying N2O to the floral primordial stage might overcome hybrid sterility. Unlike the treatment of floral primordia by Kato (2002), we treated anther archesporial cells. Thus, the use of floral tissues at different developmental stages may overcome interspecific sterility through mitotic polyploidization.
Application of N2O treatment
The proportion of 2n pollen varied in each anther within a bud for cultivars treated with N2O gas. This may be due to incomplete synchronized mitotic division of archesporial cells in the different anthers within a flower. Nevertheless, the developmental stages of archesporial cells correlated to some extent with floral bud lengths. As a result, the measurement of floral bud length could be used to determine the optimal timing for N2O treatment: 1–4 mm for Asiatic hybrid lilies; 2–6 mm for Oriental hybrid lilies and LA hybrid lilies; and 1–4 mm for completely sterile OT hybrid ‘Yelloween’ and LO hybrid ‘Otome no Sugata’. This size-based criterion is not absolute because the developmental stages of floral buds and archesporial cells are affected by environmental and/or culture conditions.
Treatment using smaller floral buds tended to produce greater numbers of giant pollen grains. This indicates that if some archesporial cells undergo chromosome doubling at an early developmental stage with N2O treatment, the resulting 4x archesporial cells could predominate, with preferential cell proliferation of the archesporial cell lineage. Additionally, in contrast to the colchicine-induced chimeric somatic tissues where 2x cells proliferate better than 4x cells, the N2O-induced 4x archesporial cells effectively proliferated along the oblong anther locules (Fig. 5) because this narrow oblong locule allowed a certain space for 4x archesporial cells to outcompete 2x archesporial cells with 2n ploidy levels.
Chromosome doubling in archesporial cells with N2O can shorten the period of polyploidy breeding in lilies compared to the traditional method of soaking bulb scales in colchicine, which requires a long duration to flowering. Taken together, compared to soaking in colchicine, chromosome doubling in archesporial cells treated with N2O can save time and enhance production of polyploids.
Two methods for obtaining 2n pollen in fertile cultivars
Okazaki et al. (2005) and Akutsu et al. (2007) reported that N2O treatment at metaphase I produces 2n pollen in Tulipa and Lilium, respectively. In the present study, N2O treatment at the archesporial cell proliferating stage allowed mitotic diploidization of male gametes of fertile cultivars. Thus, to obtain 2n pollen in fertile cultivars, chromosome doubling by N2O treatment has two optimal stages—the meiotic division stage and the archesporial cell proliferating stage. N2O treatment during the short duration of meiotic metaphase I stage requires skillful techniques for optimizing timing during meiotic divisions. However, it can produce more 2n pollen than in the archesporial cell proliferating stage because PMCs at meiotic metaphase I have more synchronized cell divisions at the time of N2O treatment than archesporial cells. On the other hand, treatment is easier at the archesporial cell proliferating stage because plants in premeiotic stage are smaller and easier to handle. Moreover, the duration applicable for N2O treatment at the archesporial cell proliferating stage is longer than for the meiotic stage.
Characteristics of the BC1 plants
In the backcrosses using the resultant fertile pollen, production of progeny is evidence of functional pollen obtained by overcoming pollen sterility. Although the resultant pollen is functional, only a limited number of the BC1 plants were obtained due to interspecific incongruity in the BC1 progeny. We did not find any chromosome recombination between the genomes of L. × formolongi and L. rubellum, probably because of the preferential homologous chromosome pairing in the amphidiploid PMCs formed by N2O treatment. This result is consistent with previous studies by Barba-Gonzalez et al. (2004) and Lim et al. (2000), who reported that fewer chromosomal recombinations occur in progenies obtained from crosses using fertile pollen of amphidiploids restored by somatic chromosome doubling compared to crosses using fertile pollen obtained from meiotic polyploidization. In this study, we found some phenotypic variations in the BC1 progeny, such as in the flower direction, an important trait for lily breeding (Fig. 7E–7H). This probably resulted from genetic variation between the parental plants of L. × formolongi because chromosome recombinations which would be a potential source of phenotypic variation in the progeny did not occur between the genomes of L. × formolongi and L. rubellum.
Overcoming hybrid sterility
Hybrid sterility derived from remote hybridization between two different species is a common phenomenon in plants and is a key problem in interspecific breeding. The selection of 2n gamete producers has contributed to overcoming hybrid sterility in crop breeding (Hanneman and Peloquin 1968, Khan et al. 2009, Marasek et al. 2006, Ramanna et al. 2003). The diploid LA hybrid lilies derived from the cross of L. longiflorum and Asiatic hybrid lilies showed intermediate morphology between the parental species. This intermediate morphology is esthetically undesirable. To improve this undesirable trait, 2n gamete producers were selected for further backcrossing of diploid LA hybrids with Asiatic hybrid lilies because diploid LA hybrids were basically pollen sterile. As a result, triploid LA hybrids have been developed for commercial distribution. Similarly, other hybrid lilies such as OT, OA and LO have been derived from more remote interspecific hybridization than LA hybrids, and hence, they have serious hybrid sterility problems that require breeding involving selection of 2n gamete producers. However, 2n gametes may not be widely applicable in breeding because their occurrence is not always predictable (Asano 1984, Barba-Gonzalez et al. 2004, Lim et al. 2001). Thus, techniques that can overcome hybrid sterility of lilies and perhaps other crops are eagerly awaited. N2O treatment would be a useful technique in this regard, and the procedure presented here will promote interspecific or interploidy hybridization of lilies and other crops.
Acknowledgements
We thank Dr. K. Shinoda (National Agricultural Research Center for Hokkaido Region, Japan) for his invaluable comments on this study and two anonymous reviewers for valuable comments. This work was supported in part by grants from the Ministry of Agriculture, Forestry and Fisheries, Japan, and a grant from Japan Society for the Promotion of Science (Grant in Aid for Scientific Research No. 19580024).
Literature Cited
- Akutsu M, Kitamura S, Toda R, Miyajima I, Okazaki K. Production of 2n pollen of Asiatic hybrid lilies by nitrous oxide treatment. Euphytica. 2007;155:143–152. [Google Scholar]
- Asano Y. Overcoming interspecific hybrid sterility in Lilium. J Jpn Soc Hort Sci. 1982;51:75–81. [Google Scholar]
- Asano Y. Fertility of a hybrid between distantly related species in Lilium. Cytologia. 1984;49:447–456. [Google Scholar]
- Barba-Gonzalez R, Lokker AC, Lim K-B, Ramanna MS, Van Tuyl JM. Use of 2n gametes for the production of sexual polyploids from sterile Oriental × Asiatic hybrids of lilies (Lilium) Theor Appl Genet. 2004;109:1125–1132. doi: 10.1007/s00122-004-1739-0. [DOI] [PubMed] [Google Scholar]
- Barba-Gonzalez R, Miller CT, Ramanna MS, Van Tuyl JM. Nitrous oxide (N2O) induces 2n gametes in sterile F1 hybrids between Oriental × Asiatic lily (Lilium) hybrids and leads to intergenomic recombination. Euphytica. 2006;148:308–309. [Google Scholar]
- Berdahl JD, Barker RE. Characterization of autotetraploid Russian wildrye produced with nitrous oxide. Crop Sci. 1991;31:1153–1155. [Google Scholar]
- Choudhary BR, Joshi P, Ramarao S. Interspecific hybridization between Brassica carinata and Brassica rapa. Plant Breed. 2000;119:417–420. [Google Scholar]
- Dvorak J, Harevery BL, Coulman BE. The use of nitrous oxide for producing eupolyploids and aneuploids in wheat and barley. Can J Genet Cytol. 1973;15:205–214. [Google Scholar]
- Fukai S, Tsuji K. Interspecific hybrids between Lilium × formolongi and some Asian Trumpet species. J Jpn Soc Hort Sci. 2004;73:447–452. [Google Scholar]
- Gangadevi T, Rao PN, Rao BH, Satyanarayana KV. A study of morphology, cytology and sterility in interspecific hybrids and amphidiploids of Nicotiana knightiana × N. umbratica. Theor Appl Genet. 1985;70:330–332. doi: 10.1007/BF00304921. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM. Anther development: basic principles and practical applications. Plant Cell. 1993;5:1217–1229. doi: 10.1105/tpc.5.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanneman RE, Peloquin SJ. Ploidy levels of progeny from diploid-tetraploid crosses in the potato. Am Potato J. 1968;45:255–261. [Google Scholar]
- Kato A. Chromosome doubling of haploid maize seedling using nitrous oxide gas at the flower primordial stage. Plant Breed. 2002;121:370–377. [Google Scholar]
- Kato A, Birchler JA. Induction of tetraploid derivatives of maize inbred lines by nitrous oxide gas treatment. J Hered. 2006;97:39–44. doi: 10.1093/jhered/esj007. [DOI] [PubMed] [Google Scholar]
- Khan N, Zhou S, Ramanna MS, Arens P, Herrera J, Visser RGF, Van Tuyl JM. Potential for analytic breeding in allopolyploids: an illustration from Longiflorum × Asiatic hybrid lilies (Lilium) Euphytica. 2009;166:399–409. [Google Scholar]
- Kitamura S, Akutsu M, Okazaki K. Mechanism of action of nitrous oxide gas applied as a polyploidizing agent during meiosis in lilies. Sex Plant Reprod. 2009;22:9–14. doi: 10.1007/s00497-008-0084-x. [DOI] [PubMed] [Google Scholar]
- Lim K-B, Chung J-D, Kronenburg BCE, Ramanna MS, de Jong JH, Jacobsen E, VanTuyl JM. Introgression of Lilium rubellum Baker chromosomes into L. Longiflorum Thunb.: a genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromos Res. 2000;8:119–125. doi: 10.1023/a:1009290418889. [DOI] [PubMed] [Google Scholar]
- Lim K-B, Ramanna MS, de Jong JH, Jacobsen E, Van Tuyl JM. Indeterminate meiotic restitution (IMR): a novel type of meiotic nuclear restitution mechanism detected in interspecific lily hybrids by GISH. Theor Appl Genet. 2001;103:219–230. [Google Scholar]
- Marasek A, Mizuochi H, Okazaki K. The origin of Darwin hybrid tulips analyzed by flowcytometry, karyotype analyses and genomic in situ hybridization. Euphytica. 2006;151:279–290. [Google Scholar]
- Matthews V. Lily register and checklist 2007, the international. 4th edition. The Royal Horticultural Society; London: 2007. [Google Scholar]
- Murray M, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka T, Doullah MAU, Matsumoto S, Kawasaki S, Ishikawa T, Hori H, Okazaki K. Identification of QTLs that control clubroot resistance in Brassica oleracea and comparative analysis of clubroot resistance genes between B. rapa and B. oleracea. Theor Appl Genet. 2010;120:1335–1346. doi: 10.1007/s00122-010-1259-z. [DOI] [PubMed] [Google Scholar]
- Nimura M, Kato J, Horaguchi H, Mii M, Sakai K, Katoh T. Induction of fertile amphidiploids by artificial chromosome-doubling in intespecific hybrid between Dianthus caryophyllus L. and D. japonicus Thunb. Breed Sci. 2006;56:303–310. [Google Scholar]
- Nishikawa T, Okazaki K, Uchio T, Arakawa K, Nagamine T. Molecular phylogeny of Lilium in the internal transcribed spacer region of nuclear ribosomal DNA. J Mol Evol. 1999;49:238–249. doi: 10.1007/pl00006546. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Okazaki K, Arakawa K, Nagamine T. Phylogenetic analysis of section Sinomartagon in genus Lilium using sequences of the internal transcribed spacer region in nuclear ribosomal DNA. Breed Sci. 2001;51:39–46. [Google Scholar]
- Nygren A. Polyploids in Melandrium produced by nitrous oxide. Hereditas. 1955;41:287–290. [Google Scholar]
- Okazaki K, Umada Y, Urashima O, Kawada J, Kunishige M, Murakami K. Interspecific hybrids of Lilium longiflorum and L. formolongi with L. rubellum and L. japnicum through embryo culture. J Jpn Soc Hort Sci. 1992;60:997–1002. [Google Scholar]
- Okazaki K, Asano Y, Oosawa K. Interspecific hybrids between Lilium ‘Oriental’ hybrid and L. ‘Asiatic’ hybrid produced by embryo culture with revised media. Breed Sci. 1994;44:59–64. [Google Scholar]
- Okazaki K, Kawada J, Kunishige M, Murakami K. Introduction of the characteristics of Lilium concolor into L. ‘Asiatic Hybrid’ by crossing through style-cutting pollination and embryo culture. J Jpn Soc Hort Sci. 1995;63:825–833. [Google Scholar]
- Okazaki K, Kurimoto K, Ohya H, Kato A. Induction of un-reduced gamete of tulips with N2O treatment. Breed. Res (Suppl) 2001;3:66. [Google Scholar]
- Okazaki K, Kurimoto K, Miyajima I, Enami A, Mizuochi H, Matsumoto Y, Ohya H. Induction of 2n pollen in tulips by arresting meiotic process with nitrous oxide gas. Euphytica. 2005;143:101–114. [Google Scholar]
- Östergren G. Polyploids and aneuploids of Crepis capillaris produced by treatment with nitrous oxide. Genetica. 1954;24:54–64. [PubMed] [Google Scholar]
- Östergren G. Production of polyploids and aneuploids of Phalaris by means of nitrous oxide. Hereditas. 1957;43:512–516. [Google Scholar]
- Poysa V. The development of bridge lines for interspeeific gene transfer between Lycopersicon esculentum and L. peruvianum. Theor Appl Genet. 1990;79:187–192. doi: 10.1007/BF00225950. [DOI] [PubMed] [Google Scholar]
- Ramanna MS, Kuipers AGJ, Jacobsen E. Occurrence of numerically unreduced (2n) gametes in Alstroemeria interspecific hybrids and their significance for sexual polyploidisation. Euphytica. 2003;133:95–106. [Google Scholar]
- Saruwatari H, Shuto-Nakano Y, Nakano K, Hiramatsu M, Ozaki Y, Okubo H. Interspecific lily hybrids with the ability to flower precociously and to produce multiple flower stalks from Lilium formosanum. J Jpn Soc Hort Sci. 2008;77:312–317. [Google Scholar]
- Taylor JH, McMaster RD. Autoradiographic and micro-photometric studies of desoxyribose nucleic acid during micro-gametogenesis in Lilium longiflorum. Chromosoma. 1954;6:489–521. doi: 10.1007/BF01259951. [DOI] [PubMed] [Google Scholar]
- Taylor NL, Anderson MK, Quesenberry KH, Watson L. Doubling the chromosome number of Trifolium species using nitrous oxide. Crop Sci. 1976;16:516–518. [Google Scholar]
- VanTuyl JM, Meijer B, vanDiën MP. The use of oryzalin as an alternative for colchicine in in-vitro chromosome doubling of Lilium and Nerine. Acta Hortic. 1992;352:625–630. [Google Scholar]
- Zeilinga AE, Schouten HP. Polyploidy in garden tulips. II. The production of tetraploids. Euphytica. 1968;17:303–310. [Google Scholar]