Abstract
A Mexican hexaploid wild potato species, Solanum demissum (dms), was only used as a female in previous breeding programs. The resulting clones with dms cytoplasm produced abundant, but non-functional pollen. A 170 bp DNA fragment, named Band 1, was originally detected in the F1 hybrid between dms and S. tuberosum. In this study, the sequenced region was extended to 1,032 bp; nevertheless, it did not show any homology to known sequences. This extended region harboring Band 1 was, without introns, all transcribed to mRNA and was maternally inherited from dms to S. tuberosum through backcrosses. Three dms accessions, 168 accessions of 38 cultivated and closely related wild species, and 158 varieties and breeding lines were surveyed, which demonstrated that Band 1 was specific to dms and varieties and breeding lines with dms cytoplasm. Thus, Band 1 is a useful marker to distinguish dms cytoplasm, which enables us to design efficient mating combinations in breeding programs.
Keywords: backcrossing, cytoplasmic male sterility, DNA marker, Solanum demissum, transcribed region, unknown sequence, W/α cytoplasm
Introduction
Solanum demissum Lindl. (2n = 6x = 72) is a hexaploid wild potato species distributed in Mexico (Hawkes 1990); this species could be one of the oldest wild species used in the history of modern breeding. As early as 1906, Salaman recognized resistance to late blight in this species (Salaman 1941). Since then, S. demissum has been extensively used as a resistance source to late blight (Plaisted and Hoopes 1989, Ross 1986, Rudorf 1950). So far, 11 hypersensitive-type resistance genes have been identified and incorporated into cultivars (Ross 1986). S. demissum is highly self-fertile, yet it shows unilateral incompatibility with the common potato (S. tuberosum L., 2n = 4x = 48). S. demissum can be easily crossed with the pollen of S. tuberosum and produces pentaploid hybrids which, as well as backcross progenies, are only crossable as female parents (Black 1943, Dionne 1961, Irikura 1968). Thus, the S. demissum cytoplasm was preferentially transmitted to the bred varieties.
Such unilateral incompatibility, or cytoplasmic male sterility, is common in potato. The common potato shares at least seven different cytoplasmic sterility factors ([ASFs], [Fms], [Ins], [SMs], [Sps], [TAs] and [VSAs]) that condition sterility in the presence of dominant chromosomal genes (ASF, Fm, In, SM, Sp, TA and VSA) (Grun et al. 1977). The cytoplasmic genome of potato is characterized by possessing T-type chloroplast DNA (Hosaka 1986) and β-type mitochondrial DNA (Lössl et al. 1999). Although the cytoplasmic sterility factors likely reside on mitochondrial DNA (Hosaka et al. 1988, Lössl et al. 2000), β-type mitochondrial DNA so far shows complete association with T-type chloroplast DNA (hereinafter, T/β cytoplasm) (Lössl et al. 2000). The T/β cytoplasm is predominant in the common potato (Bryan et al. 1999, Hosaka and Hanneman 1988, Lössl et al. 2000, Powell et al. 1993, Provan et al. 1999, Waugh et al. 1990), so sterility problems are unavoidable when T/β cytoplasm is present. In addition to such intrinsic sterility, specific male sterility associated with cytoplasmic genome is known. Varieties carrying Rysto (a resistance gene to Potato virus Y), released mainly in Germany (Ross 1986), show male sterility caused by the association with the characteristic mitochondrial DNA derived from S. stoloniferum Schlechtd. et Bché. (W/γ cytoplasm) (Lössl et al. 2000). In these cases of T/β and W/γ cytoplasm, sterility is always characterized by visible abnormalities, such as no pollen, no or poor pollen-shedding, or various deformities of anthers (Grun 1979). In contrast, F1 and backcrossed progenies carrying S. demissum cytoplasm (W/α) produce abundant and normal-looking pollen, but this pollen is non-functional on S. tuberosum (Dionne 1961). According to Lössl et al. (2000), the W/α cytoplasm occupied 40% of German varieties.
We reconfirmed the unilateral incompatibility between S. demissum and S. tuberosum; 395 berries were obtained from 488 pollinations in S. demissum (♀) × S. tuberosum (♂), while in the reciprocal cross, 45 berries from 232 pollinations (Sanetomo et al. 2011). Further, we found that the hybrid from a cross S. tuberosum (♀) × S. demissum (♂) showed higher crossability than the reciprocal hybrid: for example, the former hybrid when crossed as a male to S. demissum resulted in a significantly higher berry setting rate (64.9%) than the latter hybrid (24.2%) (Sanetomo et al. 2011). To elucidate this differential crossability, pollen DNA samples from reciprocal hybrids were compared using methylation-sensitive amplified polymorphism (MSAP) analysis. Six distinct DNA sequences were found to be different between the reciprocal hybrids (Sanetomo and Hosaka 2011).
We noticed that one of the six bands, named Band 1 in Sanetomo and Hosaka (2011), was very interesting and worthy of further investigation. We report here that the extended DNA sequence harboring Band 1 was strictly specific to S. demissum and maternally inherited to S. tuberosum, so it was a useful indicator of S. demissum cytoplasm. Although this sequence was transcribed to mRNA without an intron, it did not show any significant homology with known sequences, and the intra-cellular origin remains unknown.
Materials and Methods
Plant materials
Saikai 35 (a S. tuberosum breeding line) and 5H109-5 (S. demissum PI 186551) (referred to as T and D, respectively) were reciprocally crossed, deriving DT (D as female) and TD (T as female) F1 populations (6H37 and 6H38 families, respectively). One TD plant (6H38-19) and one DT plant (6H37-6) were backcrossed with the pollen of Saikai 35, deriving (TD)T and (DT)T BC1 populations (7H1 and 7H2 families), respectively. Since S. demissum is highly self-pollinated in nature and homozygous, we assumed that all seedlings derived by selfing were genetically identical to each other and to the parental clone 5H109-5, and were used as D. Saikai 35 has S/ɛ cytoplasm, while 5H109-5 has W/α cytoplasm.
The presence/absence of Band 1 was examined for three S. demissum accessions and 168 accessions of 38 species (Table 1), which covered all 164 different cytoplasms previously distinguished among cultivated species and wild species closely related to cultivated species, except distantly related S. pinnatisectum Dun. and S. stoloniferum (Hosaka and Sanetomo 2010). A further survey was conducted for 158 varieties and breeding lines.
Table 1.
Wild and Andean cultivated potatoes surveyed by Band 1
| Taxonomic series and species | Accessiona |
|---|---|
| Series Pinnatisecta (Rydb.) Hawkes | |
| S. pinnatisectum Dun. | PI 184764, PI 275230 |
| Series Yungasensa Corr. | |
| S. chacoense Bitt. | PI 537025, chc 525-3 |
| S. tarijense Hawkes | PI 498399* |
| Series Megistacroloba Cárd. et Hawkes | |
| S. boliviense Dun. | PI 498215, PI 545964* |
| S. megistacrolobum Bitt. | PI 265874, PI 473356, PI 473361, PI 545999 |
| S. raphaniforium Cárd. et Hawkes | PI 473371 |
| S. sogarandinum Ochoa | PI 230510 |
| Series Conicibaccata Bitt. | |
| S. chomatophilum Bitt. | PI 266387, PI 365327 |
| S. irosinum Ochoa | PI 568985 |
| Series Piurana Hawkes | |
| S. acroglossum Juz. | PI 498204 |
| S. blanco-galdosii Ochoa | PI 442701 |
| Series Tuberosa (Rydb.) Hawkes (Wild species) | |
| S. acroscopicum Ochoa | PI 365314, PI 365315 |
| S. brevicaule Bitt. | PI 498110*, PI 498111*, PI 498112*, PI 498113*, PI 498114*, PI 498115*, PI 498218, PI 545967, PI 545968, PI 545970* |
| S. bukasovii Juz. | PI 210042, PI 210051, PI 265876, PI 275271, PI 283074, PI 310937, PI 365304, PI 365318, PI 365321, PI 365349, PI 365350, PI 365355, PI 414155, PI 442698, PI 458379, PI 473447, PI 473450, PI 473453, PI 473491, PI 473492, PI 498219, PI 498220, PI 568932, PI 568933, PI 568939, PI 568944, PI 568949, PI 568954 |
| S. canasense Hawkes | PI 246533, PI 283080, PI 310938, PI 310956, PI 473346, PI 473347, PI 473348 |
| S. candolleanum Berth. | PI 498227, PI 545972, PI 568969 |
| S. coelestipetalum Vargas | PI 473354, PI 590904 |
| S. dolichocremastrum Bitt. | PI 498234 |
| S. immite Dun. | PI 365330, PI 498245 |
| S. leptophyes Bitt. | PI 283090*, PI 320340*, PI 458378, PI 473342*, PI 473343*, PI 473344*, PI 473445, PI 473451, PI 473495*, PI 545895, PI 545896, PI 545985*, PI 545986*, PI 545987, PI 545988*, PI 545990, PI 545991*, PI 545992*, PI 545993*, PI 545995 |
| S. marinasense Vargas | PI 210040, PI 310946 |
| S. medians Bitt. | PI 210045, PI 442703, PI 473496 |
| S. multidissectum Hawkes | PI 210043, PI 210044, PI 210052, PI 210055, PI 473349, PI 473353, PI 498304 |
| S. multiinterruptum Bitt. | PI 275272, PI 498267* |
| S. oplocense Hawkes | PI 435079*, PI 442693*, PI 458390*, PI 498067, PI 545876, PI 545908*, PI 545910* |
| S. pampasense Hawkes | PI 275274, PI 442697 |
| S. sparsipilum (Bitt.) Juz. et Buk. | PI 498136*, PI 498138*, PI 498139*, PI 498140*, PI 498305* |
| S. × sucrense Hawkes | PI 473506 |
| S. vernei Bitt. et Wittm. (Cultivated species) | PI 458373, PI 458374, PI 473306, PI 473311, PI 500067, PI 545884*, PI 558148, PI 558151 |
| S. ajanhuiri Juz. et Buk. | CIP 702677 |
| S. curtilobum Juz. et Buk. | CIP 700273, CIP 702455 |
| S. juzepczukii Buk. | CIP 700895 |
| S. phureja Juz. et Buk. | CIP 703275 |
| S. stenotomum Juz. et Buk. | CIP 701165, CIP 701985, CIP 702583, CIP 703088, CIP 703710, CIP 703808, CIP 703933, CIP 707297 |
| S. tuberosum L. ssp. andigena Hawkes | PI 243363, PI 246497, PI 255508, PI 265882*, PI 281080, PI 281105, PI 292089, PI 365345, PI 473285*, PI 473391*, PI 473393*, PI 498076, PI 498294, PI 498310, PI 546017*, PI 546023, CIP 700790, CIP 703268 |
| S. tuberosum L. ssp. tuberosum | CIP 703252 |
| Series Acaulia Juz. | |
| S. acaule Bitt. | PI 210030, CIP 761143 |
| S. albicans (Ochoa) Ochoa | PI 266381, PI 365306 |
| Series Longipedicellata Buk. | |
| S. stoloniferum Schlechtd. et Bché. | PI 186544*, PI 195167* |
| Series Demissa Buk. | |
| S. demissum Lindl. | PI 175411*, PI 186551*, PI 498012* |
Accessions having W/α cytoplasm are shown by asterisks.
Extending Band 1 sequence
The sequenced region was extended from both ends of Band 1 using an LA PCR™ in vitro Cloning Kit (Takara Bio Inc., Japan) by the manufacturer’s protocol briefly described below. Total DNA of D was extracted from fresh leaves by the method of Hosaka and Hanneman (1998), digested separately with various restriction enzymes, and ligated with appropriate adapters to the end of restriction digests. Using a pair of primers (one assigned to internal sequence of Band 1 and the other to the adapter sequence), polymerase chain reaction (PCR) was performed. If a single band was obtained, it was directly sequenced.
PCR detection of Band 1
The extended Band 1 sequence was divided into three overlapped regions, Region 1, 2 and 3, and the three primer sets were designed to amplify these regions (Table 2). PCR reaction was performed using a volume of 5 μl consisting of 1 μl template DNA (approximately 5 ng/μl), 2.5 μl Ampdirect Plus (Shimadzu Co., Japan), 0.125 units of Taq DNA polymerase (BIOTAQ HS DNA Polymerase; Bioline Ltd., UK) and 0.3 μM primers (Table 2). Thermal cycling was performed using a Veriti 96-well thermal cycler (Applied Biosystems) (one cycle of 10 min at 95°C, followed by 35 cycles of 30 sec at 94°C, 30 sec at 60°C and 1 min at 72°C, and then terminated with one cycle of 5 min at 72°C). PCR products were separated by electrophoresis on a 1.4% aga-rose gel in 1× TAE buffer (40 mM Tris-acetate and 1 mM EDTA pH 8.0), stained in 2.5 μl Midori Green DNA Stain (Nippon Genetics Europe GmbH, Germany) per 100 ml of 1× TAE buffer for 30 min with gentle shaking, followed by de-staining using 1× TAE buffer for 30 min with gentle shaking. Photographic images were captured using a UV lamp.
Table 2.
Primers used in this study
| Target | Primer (5′-3′ sequence)a | Size (bp) |
|---|---|---|
| Band 1 | Band 1-F (GCCTATGGCTCTCATCTTCAA ) Band 1-R (GGACCAGATCCAGAAGGTAACG) |
163 |
| Region 1 | Band 1-F11 (CGGGAGGTGGTGTACTTTCT) Band 1-R6 (ACGGCTGACTGTGTGTTTGA) |
527 |
| Region 2 | Band 1-F8 (AACTTGGAAGCGAAAGCTCA) Band 1-R9 (ATTGCCGATGTCCAAGTAGG) |
434 |
| Region 3 | Band 1-F9 (CCCTTTGTTTGAGCCCTTGT) Band 1-R3 (GCTCCCGTTTCCCACTATTT) |
446 |
| GBSS | GBSS-01 (ATGGCAAGCATCACAG) GBSS-02 (CAAAACTTTAGGTGCCTC) |
981 |
| β-tubulin | Tubf (ATGGATCTAGAGCCTGGTACTATG) Tubr (CAAACAGCAAGTAACACCACTC) |
525b |
Primer sequences for amplification of GBSS and β-tubulin were obtained from Takeuchi et al. (2009) and Turra et al. (2009), respectively.
cDNA size (Turra et al. 2009).
For a wide survey of wild and cultivated potatoes, Region 2 of the extended Band 1 was amplified by the same protocol described above except that the granule-bound starch synthase I gene (GBSS) marker (Table 2) was included at 0.3 μM concentration in the reaction as a positive control to check whether the PCR was performed correctly.
Southern hybridization
Approximately 15 μg total DNA of T and D were digested with a restriction enzyme MspI, HindIII or EcoRI, and Southern-hybridized with a PCR product of Band 1 (primers shown in Table 2) as probe DNA by the method described by Hosaka and Hanneman (1998).
Transcription analysis
Fresh leaves were sampled from each of T, D, TD and DT plants, quick-frozen in liquid nitrogen and ground to powder with a mortar and a pestle. Total RNA was extracted using the RNeasy Plant Mini Kit (QIAGEN, ME, USA). To eliminate possibly contaminated genomic DNA, all RNA samples were treated for 20 min at 37°C with DNase (TURBO DNA-free; Ambion, TX, USA). RNA concentration was measured by a fluorometer (QuantiFluor-P; Promega, WI, USA) using the Quanti-iT RiboGreen RNA Assay Kit (Invitrogen). Reverse transcription was performed using 1 μg total RNA primed with an oligo primer using SuperScript III (dT)21 First-Strand Synthesis SuperMix (Invitrogen). The synthesized cDNA was diluted to 1/10 and subjected to PCR amplification for the three regions. PCR reactions were as described above. The following thermal profile was used: 10 min at 95°C, 30 cycles of 30 sec at 95°C, 30 sec at 60°C and 1 min at 72°C, followed by a final extension step of 5 min at 72°C. To confirm the absence of genomic DNA contaminations in cDNA samples, PCR was carried out under the same condition with primers Tubf and Tubr (Table 2), which amplified a 525 bp β-tubulin gene fragment from cDNA instead of an approximately 1,600 bp fragment from genomic DNA containing an intron in potato (Turra et al. 2009).
Determination of chloroplast and mitochondrial DNA types
Chloroplast DNA types were determined by restriction fragment length polymorphism (RFLP) analysis of chloroplast DNA as described earlier (Sukhotu et al. 2004). Mitochondrial DNA type was determined by PCR using primers ALM_4 and ALM_5, which amplified a 2.4 kb, 1.6 kb or no band from α-, β- or γ-type mitochondrial DNA, respectively (Lössl et al. 2000); however, if a 1.6 kb band was associated with S or A-type chloroplast DNA, the mitochondrial DNA type was deduced to be ɛ type, based on circumstantial information from Lössl et al. (1999).
Results
Extended Band 1 sequence
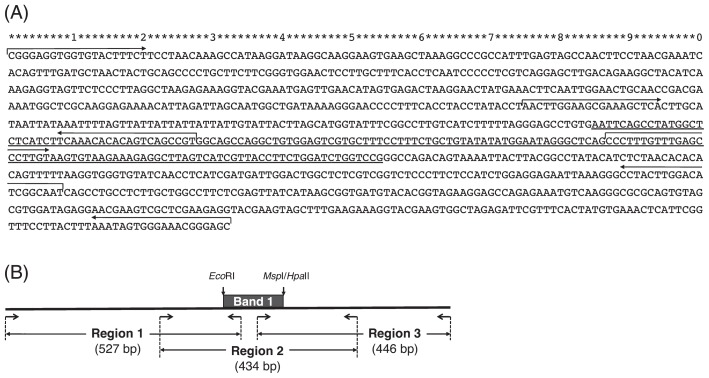
Band 1 has been detected as a 170 bp EcoRI and MspI (or HpaII) double-digested DNA fragment (NCBI Genbank Accession No. HR505437), specifically found in D and DT (Sanetomo and Hosaka 2011). This sequence was extended to 1,032 bp containing the 170 bp Band 1 (Fig. 1A). Homology search was carried out for the extended Band 1 sequence using the BLASTN program; however, it did not show high homology to any known sequences, even to those of the latest potato genome database including chloroplast and mitochondrial genome sequences (The Potato Genome Sequencing Consortium 2011).
Fig. 1.
A DNA fragment of 1,032 bp harboring Band 1 (underlined in A). Three pairs of primer sequences for amplification of Region 1, 2 and 3 are shown in (A) and the schematic representation in (B).
Inheritance and specificity of Band 1
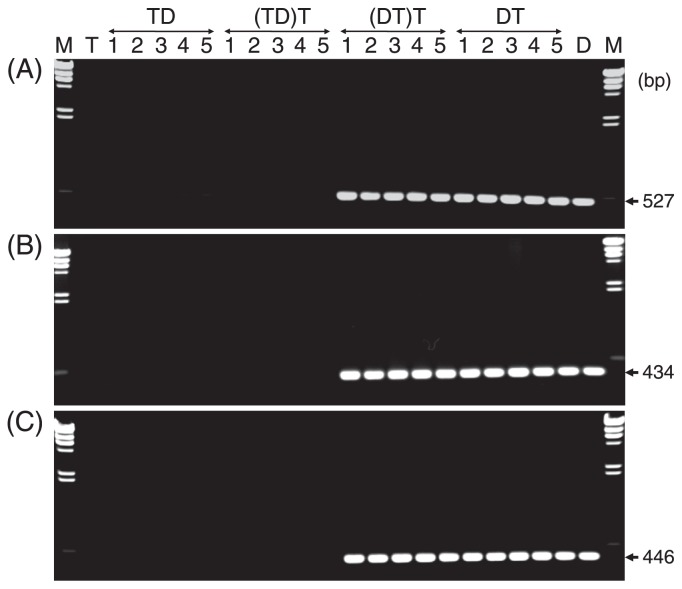
Using three primer sets for amplification of Region 1 to 3, or a primer set that specifically amplified Band 1 (Table 2), D, T, 5 DT, 5 TD, 90 (DT)T and 38 (TD)T plants were examined for the presence/absence of Band 1. As shown in Fig. 2, DNA fragments of expected sizes were present in D, all DT and (DT)T plants, but not in T, all TD and (TD)T plants. By Southern hybridization analysis, hybridization signals were obtained as single bands from all three restriction digests of D, whereas no signal was obtained from T, indicating the lack of the homologous sequence to Band 1 in T (Fig. 3).
Fig. 2.
Amplification of Region 1 (A), Region 2 (B) and Region 3 (C) from T, D, 5 reciprocal F1 plants of TD and DT, and 5 BC1 plants of (TD)T and (DT)T. M denotes λDNA HindIII digests.
Fig. 3.
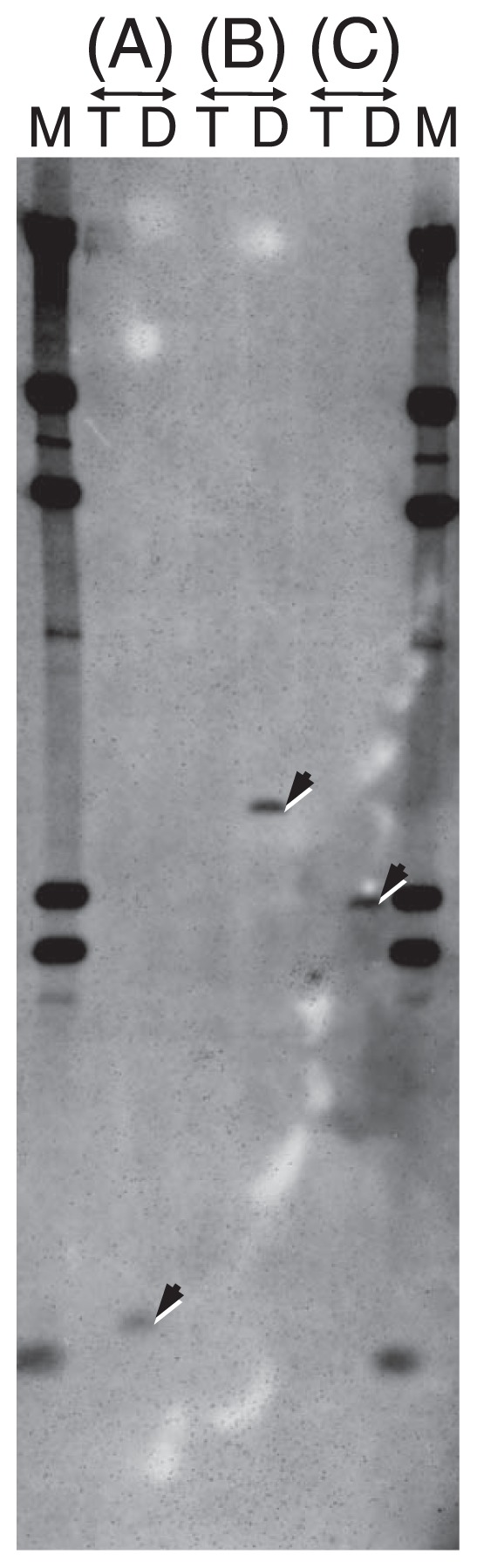
Southern hybridization of MspI (A), HindIII (B) and EcoRI (C) digests of T and D probed with Band 1. Arrows indicate hybridization signals. M denotes λDNA HindIII digests.
Transcription of Band 1
Leaf mRNA was extracted and cDNA was amplified for the Band 1 region (Fig. 4). Compared with a band of β-tubulin amplified from genomic DNA, only a smaller band (525 bp) was amplified from cDNA, indicating that an intron was removed and no genomic DNA was contaminated in cDNA samples (Fig. 4A). As shown in Fig. 4B–4D, three regions were all transcribed to mRNA in leaves of D and DT plants, but not in those of T and TD plants. The sizes of amplified bands from cDNA were similar to those from genomic DNA for all three regions. Furthermore, Region 1 PCR products from cDNA were sequenced, and showed similar sequences to those of genomic DNA. This indicates that the entire sequence of extended Band 1 is transcribed to mRNA and contains no intron.
Fig. 4.
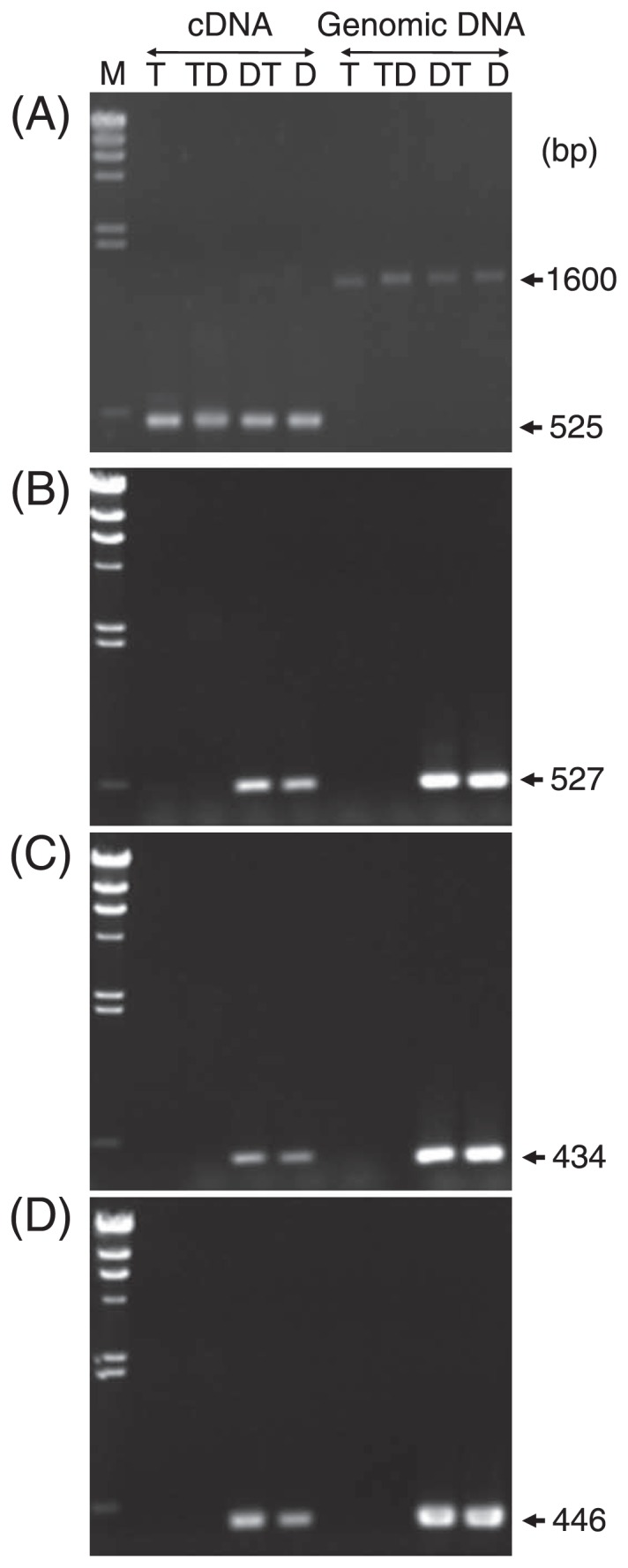
Leaf cDNA and genomic DNA of T, TD, DT and D were amplified for Region 1 (B), Region 2 (C) and Region 3 (D). A β-tubulin gene amplified from leaf cDNA samples exhibited only 525 bp band (A), demonstrating no contamination with genomic DNA. M denotes λ DNA HindIII digests.
Screening Band 1 against various cytoplasms
Band 1 was surveyed in three S. demissum accessions and 168 accessions of 38 species with various cytoplasms (Table 1). Although 43 accessions had W/α cytoplasm, none except the three accessions of S. demissum (all had W/α cytoplasm) had Band 1.
Characterization of varieties and breeding lines
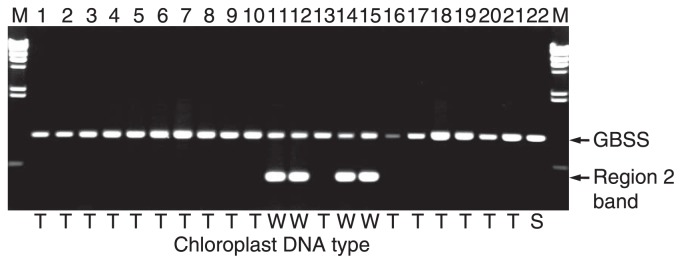
Cytoplasm types were determined for 158 varieties and breeding lines. One hundred and eight (68.4%) had T/β cytoplasm typical of S. tuberosum (Table 3). The W/α cytoplasm was found in 21 varieties and 12 breeding lines (20.9%) (Table 4). One of the landraces, Nemuromurasaki, and two others had A/ɛ cytoplasm derived possibly from S. tuberosum L. ssp. andigena Hawkes. The S/ɛ cytoplasm, derived from S. phureja Juz. et Buk., and W/γ cytoplasm, derived from S. stoloniferum, were found in 12 and 2 varieties or breeding lines, respectively (Table 3). As exemplified in Fig. 5, Band 1 was exclusively detected in varieties or breeding lines that had W/α cytoplasm (Table 4).
Table 3.
Varieties and breeding lines not possessing Band 1
| Variety or breeding linea |
|---|
|
Cytoplasm type: A = A/ɛ, S = S/ɛ, W = W/γ, no symbol = T/β.
Table 4.
Varieties and breeding lines possessing Band 1
| Clones | Year released | Pedigree (Female × male) | Cytoplasm | Cytoplasmic origin (No. of generations) |
|---|---|---|---|---|
| 1. Japanese variety | ||||
| Yoraku | 1958 | 41089-8 × Norin 1 | W/α | S. demissum T-2 (3) |
| Rishiri | 1960 | 41089-8 × Norin 1 | W/α | S. demissum T-2 (3) |
| Chijiwa | 1962 | S54042-15 × Unzen | W/α | S. demissum T-2 (5) |
| Shiretoko | 1967 | Hokkai 24 × Shimakei 291 | W/α | HLT-4 (2) |
| Setoyutaka | 1977 | Saikai 10 × Unzen | W/α | S. demissum T-2 (7) |
| Meihou | 1986 | Chijiwa × Chokei 80 | W/α | S. demissum T-2 (6) |
| Toyo-akari | 1986 | Tunika × WB61037-4 | W/α | W-race (4) |
| Ezo-akari | 1987 | Tunika × Priekulsky Ranny | W/α | W-race (4) |
| Musamaru | 1992 | Tunika × Kon-iku 20 | W/α | W-race (4) |
| Hanashibetsu | 1997 | W553-4 × R392-50 | W/α | W553-4 (1) |
| Benihisashi | 2001 | W862207-1 × WB72209-5 | W/α | W217H.2 (2) |
| Natsufubuki | 2003 | Musamaru × Shimakei 544 | W/α | W-race (5) |
| Kitamurasaki | 2004 | Shimakei 571 × Shimakei 561 | W/α | Nemuromurasaki (3) |
| Koganemaru | 2006 | Musamaru × Tokachikogane | W/α | W-race (5) |
| Northern Ruby | 2006 | Open-polination from Kitamurasaki | W/α | Nemuromurasaki (4) |
| Shadow Queen | 2006 | Open-polination from Kitamurasaki | W/α | Nemuromurasaki (4) |
| 2. Japanese breeding line | ||||
| Chokei 108 | – | Musamaru × Touya | W/α | W-race (5) |
| Hokkai 97 | – | Meihou × Tokachikogane | W/α | S. demissum T-2 (7) |
| Hoku-iku 6 | – | Kon-iku 27 × Hokkaikogane | W/α | HLT-4 (6) |
| Kitakei 29 | – | Hanashibetsu × Tokachikogane | W/α | W553-4 (2) |
| Saikai 39 | – | Aikei 125 × Saikai 35 | W/α | S. demissum T-2 (10) |
| Shimakei 284 | – | Open-pollination from Nemuromurasaki | W/α | Nemuromurasaki (1) |
| Shimakei 561 | – | Shimakei 530 × ND860-2 | W/α | W-race (5) |
| Shimakei 571 | – | Shimakei 284 × 83015-47 | W/α | Nemuromurasaki (2) |
| W553-4 | – | A possible interspecific hybrid of unknown origin | W/α | – |
| W794215-H33 | – | 2x Tunika | W/α | – |
| W794215-H34 | – | 2x Tunika | W/α | – |
| W794215-H35 | – | 2x Tunika | W/α | – |
| 3. Foreign variety | ||||
| Tunika | 1967 | Lü.56.186/21 N × Lü.51.183/2 | W/α | W-race (3) |
| Astarte | 1993 | SVP RR 62-5-43 × VTN 62-69-5 | W/α | MPI 19268 (4) |
| Eva | 1999 | Steuben × bulk pollen hybrids | W/α | B4494-3 (3) |
| Monticello | 2003 | Steuben × Kanona | W/α | B4494-3 (3) |
| Sassy | 2004 | G82TT137.1 × Promesse | W/α | ? |
Fig. 5.
Amplification of Region 2 together with GBSS as a positive control from randomly chosen varieties. Chloroplast DNA types are shown below. 1. Early Rose, 2. Irish Cobbler, 3. May Queen, 4. Desiree, 5. Russet Burbank, 6. Kennebec, 7. Atlantic, 8. Shepody, 9. Snowden, 10. Norking Russet, 11. Tunika, 12. Astarte, 13. Norin 1, 14. Rishiri, 15. Chijiwa, 16. Toyoshiro, 17. Nishiyutaka, 18. Hokkaikogane, 19. Konafubuki, 20. Kita-akari, 21. Touya, 22. Inca-no-mezame. M denotes λDNA HindIII digests.
Band 1 was detected in Tunika and its three haploid clones (Table 4) and Band 1 was inherited maternally from S. demissum even after 10 times backcrossing (see Saikai 39 in Table 4).
Homology between Band 1 sequences of different origins
PCR products amplified from Band 1 of Rishiri (the cytoplasm originally derived from S. demissum T-2), Hanashibetsu (from W553-4) and Kitamurasaki (from Nemuromurasaki), and those from Region 1 of Rishiri, Tunika (from W-race) and Astarte (from MPI 19268), were sequenced, which perfectly fit the corresponding sequences of D (Fig. 1A).
Discussion
Band 1 is a useful DNA marker distinguishing the S. demissum cytoplasm
Previously, a set of PCR primers flanking a 241 bp deletion that defined T-type chloroplast DNA (Hosaka et al. 1988, Kawagoe and Kikuta 1991) was developed (Hosaka 2002, Lössl et al. 2000), and has been used frequently worldwide for various purposes (Ames and Spooner 2008, Chimote et al. 2008, Gavrilenko et al. 2007, Spooner et al. 2007). Such a DNA marker distinguishing S. demissum cytoplasm has not been available until now. Genotypes producing abundant pollen tend to be used as pollen parents. Even if a desirable genotype produces abundant pollen, it should not be used as a pollen parent when it has S. demissum-derived cytoplasm to avoid the difficulty of obtaining hybrid seeds; thus, it is very important to identify S. demissum-derived cytoplasm in breeding programs.
According to Lössl et al. (2000), W/α cytoplasm is specific to S. demissum-derived varieties. This was confirmed, as indicated in Table 4, because the W/α cytoplasm found in varieties and breeding lines was likely all descended from S. demissum alone; however, W-type chloroplast DNA was defined by RFLP analysis of chloroplast DNA and found in many wild species (Hosaka 1986, Hosaka and Sanetomo 2010, Sukhotu et al. 2004). According to the raw data used in Hosaka and Sanetomo (2010), among 164 different cytoplasms distinguished in Andean cultivated potatoes and closely related species, 73 cytoplasms had W-type chloroplast DNA, while 49 cytoplasms had α-type mitochondrial DNA. Consequently, 40 cytoplasms were disclosed to be W/α type, which is found in many wild species including S. demissum (shown by asterisks in Table 1). Thus, W/α cytoplasm is not specific to S. demissum. Alternatively, as discussed later, Band 1 was always associated with S. demissum cytoplasm, so it is a useful DNA marker for identifying S. demissum cytoplasm. Precise identification of S. demissum-derived cytoplasm enables the design of efficient mating combinations in breeding programs.
Cytoplasmic origin of varieties and breeding lines
Varieties and breeding lines that had W/α cytoplasm had exclusively Band 1. Their cytoplasm was descended from at least eight parents: S. demissum T-2, W-race and MPI 19268 (both are famous backcross progenies from S. demissum, Ross 1986), Nemuromurasaki, HLT-4 (from USDA), W553-4, W217H.2 and B4494-3 (Table 4). The cytoplasm of W-race was incorporated into Japanese varieties via Tunika (introduced from the former East Germany in 1981), from which a potato cyst nematode resistance gene (H1) was introduced into several Japanese varieties (Mori et al. 2007).
Shimakei 571, Kitamurasaki, Northern Ruby and Shadow Queen had Band 1. According to their pedigree, they were maternally descended from Shimakei 284, which was developed from seedlings of an open-pollinated berry set on Nemuromurasaki (Table 4); however, the parent of Shimakei 284 was apparently misreported, because Nemuromurasaki is the oldest variety with A-type chloroplast DNA (Table 3), typical of S. tuberosum ssp. andigena, and was thought to be a relic of the early European potato (Hosaka 1993).
Although W553-4 was recorded as S. tuberosum ssp. andigena by Dr. Y. Irikura, who collected this clone in Colombia in 1977, now it has been recognized as an interspecific hybrid of unknown origin because of the extremely wide segregation observed in the progeny. As W553-4 shows a high level of late blight resistance, it might be a backcross progeny from S. demissum.
Furthermore, the pedigrees of other maternal parents that conferred W/α cytoplasm could not be traced. In conclusion, the W/α cytoplasm associated with Band 1 in varieties and breeding lines were all derived likely from S. demissum.
Intra-cellular origin of Band 1
We demonstrated that Band 1 is a S. demissum-specific DNA fragment maternally inherited from S. demissum to S. tuberosum through backcrossing; however, Band 1 did not show high homology to any known sequences, including complete sequences of potato chloroplast DNA (Chung et al. 2006) and mitochondrial DNA of the related genus Nicotiana (Sugiyama et al. 2005). It is generally known that plant chloroplast DNA evolves very slowly and is highly conservative in size, structure, gene content, and the linear order of genes (Palmer 1992, Palmer et al. 1988). Band 1 or Region 1 sequences, maternally inherited through many generations from different source accessions of S. demissum, were all similar to those of S. demissum used in this study, demonstrating the highly conservative nature of Band 1; however, S. demissum-specific insertion over 1 kbp was not detected in chloroplast DNA (Hosaka 1986), so that it is less likely that Band 1 is a part of chloroplast DNA.
By comparing several completely sequenced angiosperm mitochondrial DNAs, it is known that, although identified genes and introns are rather well conserved, intergenic regions are highly variable in sequence, even between two close relatives (Alverson et al. 2010, Handa 2003, Kubo and Mikami 2007); therefore, it is highly probable that Band 1 is part of the mitochondrial DNA of S. demissum.
However, mRNAs with poly(A) tails from chloroplast and mitochondrial genes are generally found only in degradation and are thus expected to occur in only a minor fraction of the steady state pool (del Campo 2009, Forner et al. 2007). Thus, alternative possibilities are that Band 1 is 1) located on one of the S. demissum chromosomes and exclusively transmitted maternally, or 2) something else, such as plasmid-like DNA, is maternally inherited. Linear or circular plasmids have been frequently reported in mitochondria in higher plants (Allen et al. 2007, Handa et al. 2002, Turpen et al. 1987). Further investigation of the intra-cellular origin of Band 1 is ongoing.
Acknowledgments
We thank the US Potato Genebank (NRSP-6), Sturgeon Bay, Wisconsin, and the CIP gene bank for providing the Solanum materials used in this study. We also thank Drs. S. Huang and Z. Zhang, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, for homology search of the extended Band 1. This study was supported by Calbee Inc.
Literature Cited
- Allen JO, Fauron CM, Minx P, Roark L, Oddiraju S, Lin GN, Meyer L, Sun H, Kim K, Wang C, et al. Comparison among two fertile and three male-sterile mitochondrial genomes of maize. Genetics. 2007;177:1173–1192. doi: 10.1534/genetics.107.073312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverson AJ, Wei X-X, Rice DW, Stern DB, Barry K, Palmer JD. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae) Mol Biol Evol. 2010;27:1436–1448. doi: 10.1093/molbev/msq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames M, Spooner DM. DNA from herbarium specimens settles a controversy about origins of the European potato. Am J Bot. 2008;95:252–257. doi: 10.3732/ajb.95.2.252. [DOI] [PubMed] [Google Scholar]
- Black W. Inheritance of resistance to two strains of blight (Phytophthora infestans de Bary) in potatoes. Trans. Roy. Soc Edinburgh. 1943;61:137–147. doi: 10.1017/s0080455x00009760. [DOI] [PubMed] [Google Scholar]
- Bryan GJ, McNicoll J, Ramsay G, Meyer RC, De Jong WS. Polymorphic simple sequence repeat markers in chloroplast genomes of Solanaceous plants. Theor Appl Genet. 1999;99:859–867. [Google Scholar]
- Chimote VP, Chakrabarti SK, Pattanayak D, Pandey SK, Naik PS. Molecular analysis of cytoplasm type in Indian potato varieties. Euphytica. 2008;162:69–80. [Google Scholar]
- Chung HJ, Jung JD, Park HW, Kim JH, Cha HW, Min SR, Jeong WJ, Liu JR. The complete chloroplast genome sequences of Solanum tuberosum and comparative analysis with Solanaceae species identified the presence of a 241-bp deletion in cultivated potato chloroplast DNA sequence. Plant Cell Rep. 2006;25:1369–1379. doi: 10.1007/s00299-006-0196-4. [DOI] [PubMed] [Google Scholar]
- del Campo EM. Post-transcriptional control of chloroplast gene expression. Gene Reg Syst Biol. 2009;3:31–47. doi: 10.4137/grsb.s2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne LA. Cytoplasmic sterility in derivatives of Solanum demissum. Am Potato J. 1961;38:117–120. [Google Scholar]
- Forner J, Weber B, Thuss S, Wildum S, Binder S. Mapping of mitochondrial mRNA termini in Arabidopsis thaliana: t-elements contribute to 5′ and 3′ end formation. Nucleic Acids Res. 2007;35:3676–3692. doi: 10.1093/nar/gkm270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilenko TA, Antonova OY, Kostina LI. Study of genetic diversity in potato cultivars using PCR analysis of organelle DNA. Russian J Genet. 2007;43:1550–1555. [PubMed] [Google Scholar]
- Grun P. Evolution of the cultivated potato: a cytoplasmic analysis. In: Hawkes JG, Lester RN, Skelding AD, editors. The biology and taxonomy of the Solanaceae. Academic Press; London: 1979. pp. 655–665. [Google Scholar]
- Grun P, Ochoa C, Capage D. Evolution of cytoplasmic factors in tetraploid cultivated potatoes (Solanaceae) Am J Bot. 1977;64:412–420. [Google Scholar]
- Handa H. The complete nucleotide sequence and RNA editing content of the mitochondrial genome of rapeseed (Brassica napus L.): comparative analysis of the mitochondrial genomes of rape-seed and Arabidopsis thaliana. Nucleic Acids Res. 2003;31:5907–5916. doi: 10.1093/nar/gkg795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H, Itani K, Sato H. Structural features and expression analysis of a linear mitochondrial plasmid in rapeseed (Brassica napus L.) Mol. Genet Genomics. 2002;267:797–805. doi: 10.1007/s00438-002-0711-4. [DOI] [PubMed] [Google Scholar]
- Hawkes JG. The potato: evolution, biodiversity and genetic resources. Belhaven Press; London: 1990. p. 259. [Google Scholar]
- Hosaka K. Who is the mother of the potato?—restriction endo-nuclease analysis of chloroplast DNA of cultivated potatoes. Theor Appl Genet. 1986;72:606–618. doi: 10.1007/BF00288998. [DOI] [PubMed] [Google Scholar]
- Hosaka K. Similar introduction and incorporation of potato chloroplast DNA in Japan and Europe. Jpn J Genet. 1993;68:55–61. [Google Scholar]
- Hosaka K. Distribution of the 241 bp deletion of chloroplast DNA in wild potato species. Am J Potato Res. 2002;79:119–123. [Google Scholar]
- Hosaka K, Hanneman RE., Jr. The origin of the cultivated tetraploid potato based on chloroplast DNA. Theor Appl Genet. 1988;76:172–176. doi: 10.1007/BF00257842. [DOI] [PubMed] [Google Scholar]
- Hosaka K, Hanneman RE., Jr Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica. 1998;103:265–271. [Google Scholar]
- Hosaka K, Sanetomo R. Comparative differentiation in mitochondrial and chloroplast DNA among cultivated potatoes and closely related wild species. Genes Genet Syst. 2010;84:371–378. doi: 10.1266/ggs.84.371. [DOI] [PubMed] [Google Scholar]
- Hosaka K, de Zoeten GA, Hanneman RE., Jr. Cultivated potato chloroplast DNA differs from the wild type by one deletion—evidence and implications. Theor Appl Genet. 1988;75:741–745. [Google Scholar]
- Irikura Y. Studies on interspecific crosses of tuber-bearing Solanums. I. Overcoming cross-incompatibility between Solanum tuberosum and other Solanum species by mean of induced poly-ploids and haploids. Hokkaido Agr. Exp. Sta Shuho. 1968;92:21–37. [Google Scholar]
- Kawagoe Y, Kikuta Y. Chloroplast DNA evolution in potato (Solanum tuberosum L.) Theor Appl Genet. 1991;81:13–20. doi: 10.1007/BF00226106. [DOI] [PubMed] [Google Scholar]
- Kubo T, Mikami T. Organization and variation of angiosperm mitochondrial genome. Physiol Plant. 2007;129:6–13. [Google Scholar]
- Lössl A, Adler N, Horn R, Frei U, Wenzel G. Chondriome-type characterization of potato: mt α, β, γ, δ, ɛ and novel plastid-mitochondrial configurations in somatic hybrids. Theor Appl Genet. 1999;99:1–10. [Google Scholar]
- Lössl A, Götz M, Braun A, Wenzel G. Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica. 2000;116:221–230. [Google Scholar]
- Mori M, Tsuda S, Mukojima N, Kobayashi A, Matsuura-Endo C, Ohara-Takada A, Zaidul ISM. Breeding of potato cyst nematode resistant varieties in Japan. In: Haverkort AJ, Anisimov BV, editors. Potato Production and Innovative Technologies. Wageningen Academic Publishers; The Netherland: 2007. pp. 328–339. [Google Scholar]
- Palmer JD. Mitochondrial DNA in plant systematics: applications and limitations. In: Soltis D, Soltis P, Doyle JJ, editors. Molecular Systematics of Plants. Chapman and Hall; New York: 1992. pp. 36–49. [Google Scholar]
- Palmer JD, Jansen RK, Michaels HJ, Chase MW, Manhart JR. Chloroplast DNA variation and plant phylogeny. Ann Missouri Bot Gard. 1988;75:1180–1206. [Google Scholar]
- Plaisted RL, Hoopes RW. The past record and future prospects for the use of exotic potato germplasm. Am Potato J. 1989;66:603–627. [Google Scholar]
- Powell W, Baird D, Duncan N, Waugh R. Chloroplast DNA variability in old and recently introduced potato cultivars. Ann Appl Biol. 1993;123:403–410. [Google Scholar]
- Provan J, Powell W, Dewar H, Bryan G, Machray GC, Waugh R. An extreme cytoplasmic bottleneck in the modern European cultivated potato (Solanum tuberosum) is not reflected in decreased levels of nuclear diversity. Proc. R. Soc. Lond B. 1999;266:633–639. [Google Scholar]
- Ross H. Potato breeding-problems and perspectives. Verlag Paul Parey; Berlin: 1986. p. 132. [Google Scholar]
- Rudorf W. Methods and results of breeding resistant strains of potatoes. Am Potato J. 1950;27:332–339. [Google Scholar]
- Salaman RN. Breeding for immunity to late blight and other diseases in the potato. Proc. 7th Int Genet Congr Edinburgh. 1941;1939:253–254. [Google Scholar]
- Sanetomo R, Hosaka K. Reciprocal differences in DNA sequence and methylation status of the pollen DNA between F1 hybrids of Solanum tuberosum × S. demissum. Euphytica. 2011;182:219–229. [Google Scholar]
- Sanetomo R, Ono S, Hosaka K. Characterization of crossability in the crosses between Solanum demissum and S. tuberosum, and the F1 and BC1 progenies. Am. J. Poato Res. 2011 doi: 10.1007/s12230-011-9217-0. [DOI] [Google Scholar]
- Spooner DM, Núñez J, Trujillo G, Herrera MDR, Guzmán F, Ghislain M. Extensive simple sequence repeat genotyping of potato landraces supports a major reevaluation of their gene pool structure and classification. Proc. Natl. Acad. Sci USA. 2007;104:19398–19403. doi: 10.1073/pnas.0709796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Watase Y, Nagase M, Makita N, Yagura S, Hirai A, Sugiura M. The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: comparative analysis of mitochondrial genomes in higher plants. Mol. Gen Genomics. 2005;272:603–615. doi: 10.1007/s00438-004-1075-8. [DOI] [PubMed] [Google Scholar]
- Sukhotu T, Kamijima O, Hosaka K. Nuclear and chloroplast DNA differentiation in Andean potatoes. Genome. 2004;47:46–56. doi: 10.1139/g03-105. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Sasaki J, Suzuki T, Horita H, Hiura S, Iketani S, Fujita R, Senda K. DNA markers for efficient selection of disease and pests resistance genes in potato. Hokkaido Nogyo-Shiken-Kaigi-Shiryo. 2009;2008:1–26. [Google Scholar]
- The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature. 2011;475:189–195. doi: 10.1038/nature10158. [DOI] [PubMed] [Google Scholar]
- Turpen T, Garger SJ, Marks MD, Grill LK. Molecular cloning and physical characterization of a Brassica linear mitochondrial plasmid. Mol Gen Genet. 1987;209:227–233. doi: 10.1007/BF00329647. [DOI] [PubMed] [Google Scholar]
- Turra D, Bellin D, Lorito M, Gebhardt C. Genotype-dependent expression of specific members of potato protease inhibitor gene families in different tissues and in response to wounding and nematode infection. J Plant Physiol. 2009;166:762–774. doi: 10.1016/j.jplph.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Waugh R, Glendinning DR, Duncan N, Powell W. Chloroplast DNA variation in European potato cultivars. Potato Res. 1990;33:505–513. [Google Scholar]