Abstract
Saponins are sterols or triterpene glycosides that are widely distributed in plants. The biosynthesis of soybean saponins is thought to involve many kinds of glycosyltransferases, which is reflected in their structural diversity. Here, we performed linkage analyses of the Sg-3 and Sg-4 loci, which may control the sugar chain composition at the C-3 sugar moieties of the soybean saponin aglycones soyasapogenols A and B. The Sg-3 locus, which controls the production of group A saponin Af, was mapped to chromosome (Chr-) 10. The Sg-4 locus, which controls the production of DDMP saponin βa, was mapped to Chr-1. To elucidate the preference of sugar chain formation at the C-3 and C-22 positions, we analyzed the F2 population derived from a cross between a mutant variety, Kinusayaka (sg-10), for the sugar chain structure at C-22 position, and Mikuriya-ao (sg-3), with respect to the segregation of the composition of the group A saponins, and found that the formation of these sugar chains was independently regulated. Furthermore, a novel saponin, predicted to be A0-γg, 3-O-[β-d-galactopyranosyl (1→2)-β-d-glucuronopyranosyl]-22-O-α-l-arabinopyranosyl-soyasapogenol A, appeared in the hypocotyl of F2 individuals with genotype sg-10/sg-10 sg-3/sg-3.
Keywords: genetic analysis, Glycine max (L.) Merrill, Glycine soja Sieb. et Zucc., DDMP saponin, group A saponin, sugar chain composition, mapping
Introduction
Soybeans are used as raw materials for foods, domestic animal feed, and soybean oil because of the abundant proteins and fats in their seeds. Recently, there has been interest in the composition of soybeans for preventing and treating chronic diseases. Some saponins show functional properties, such as antilipidemic effects (Topping et al. 1980), anti-proliferative effects on human colon cancer cells (Ellington et al. 2005, 2006), and a reduction in hepatic lipid peroxidation through the secretion of thyroid hormones (Ishii and Tanizawa 2006). Because these properties depend on the chemical structure and concentration of the saponin, the presence of different saponin components at high levels in seeds could confer different health-promoting activities (Tsukamoto and Yoshiki 2006).
Many different triterpenoid saponins have been isolated and characterized in soybean seeds (Burrows et al. 1987, Kikuchi et al. 1999, Kitagawa et al. 1982, 1988, Kudou et al. 1992, 1993, Shiraiwa et al. 1991a, 1991b, Taniyama et al. 1988, Tsukamoto et al. 1993). The soybean saponins are divided into two groups, group A saponins and DDMP (2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one) saponins, according to their aglycone components. DDMP saponins and their degradation products, groups B and E saponins, possess functional properties; group A saponins, which have an acetylated sugar, cause a bitter and astringent taste (Okubo et al. 1992).
Group A saponins, detected only in seed hypocotyls (Shimoyamada et al. 1990), are bisdesmosidic saponins with a soyasapogenol A (3β, 21β, 22β, 24-tetrahydroxyolean-12-ene) aglycone that bears two sugar chains, one on the C-3 and one on the C-22 hydroxyl group (Shiraiwa et al. 1991a). A mutant line, Mikuriya-ao, accumulates saponin Af (Fig. 1), which lacks the terminal sugar at C-3, instead of saponin Ab (Shiraiwa et al. 1991b). DDMP saponins are found in both seed hypocotyls and cotyledons. They are mono-desmosidic saponins with one carbohydrate at the C-3 of soyasapogenol B (3β, 22β, 24-trihydroxyolean-12-ene) as well as the DDMP moiety at C-22. In hypocotyls, DDMP saponins are composed of 6 components, αg, βg, γg, αa, βa and γa, by the combination of sugar in the C-3 sugar chains; however, only a few soybean varieties have αa, βa and γa, which have an arabinose in the sugar chain at the C-3 (Tsukamoto et al. 1993). In cotyledons, four saponin components, βg, γg, βa and γa are detected.
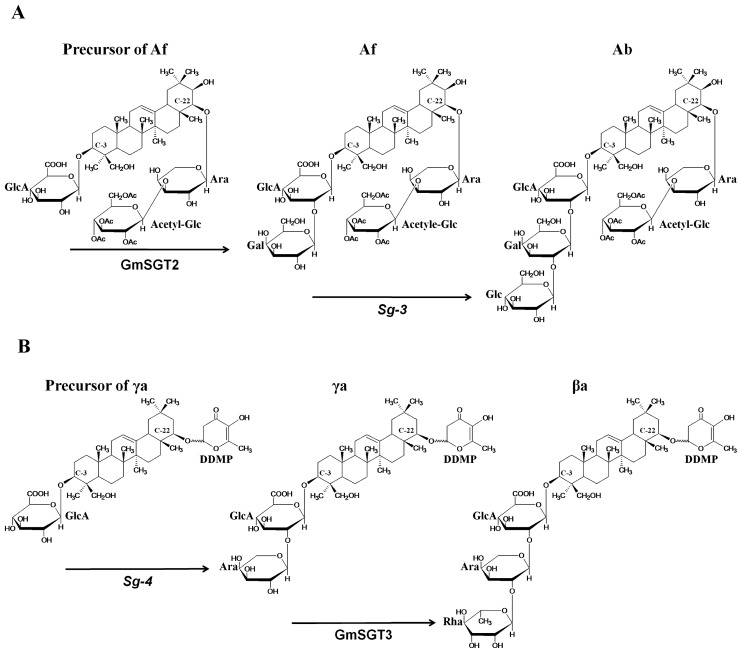
Fig. 1.
Predicted glycosylation pathway of C-3 position of group A saponin Ab (A) and DDMP saponin βa (B) in soybean seed hypocotyls. The sugar chain positions of C-3 and C-22 are indicated in each chemical structure. GmSGT2 and GmSGT3 are glycosyltransferases reported by Shibuya et al. (2010). Sg-3 and Sg-4 control glycosylation of second and third sugar moieties.
Group A saponins Aa, Ab, and A0-αg have different sugar chain sequences at the C-22 position even though they have the same sugar chain sequence at the C-3 position in all soybean varieties tested. The sugar chain sequence at the C-22 of Aa, Ab and A0-αg is acetylxylosyl-arabinose, acetylglucosyl-arabinose and arabinose, respectively. The phenotypes of saponins Aa and Ab are controlled by a co-dominant allele at a single locus named Sg-1 (Shiraiwa et al. 1990, Tsukamoto et al. 1993); saponin A0-αg is controlled by a recessive allele, named sg-1, at the same locus (Kikuchi et al. 1999). The Sg-1 locus was mapped near the simple sequence repeat marker Satt336 on chromosome (Chr-) 7 (Takada et al. 2010). The phenotype of saponin Af is controlled by a recessive allele at a single locus called Sg-3, whereas the presence of saponin βa in seed hypocotyls is controlled by a dominant allele at a single locus called Sg-4 (Tsukamoto et al. 1992, 1993). However, there is no genetic information concerning the locations and interactions of the Sg-3 and Sg-4 loci.
The aim of this study is to obtain genetic information on the accumulation of saponins Af and βa in seed hypocotyls, and to develop molecular markers linked to the Sg-3 and Sg-4 loci. This information will be valuable for breeding new cultivars through marker-assisted selection and will help clarify the mechanisms that determine the sugar chain structure at the C-3 position of soybean saponins.
Materials and Methods
Plant materials
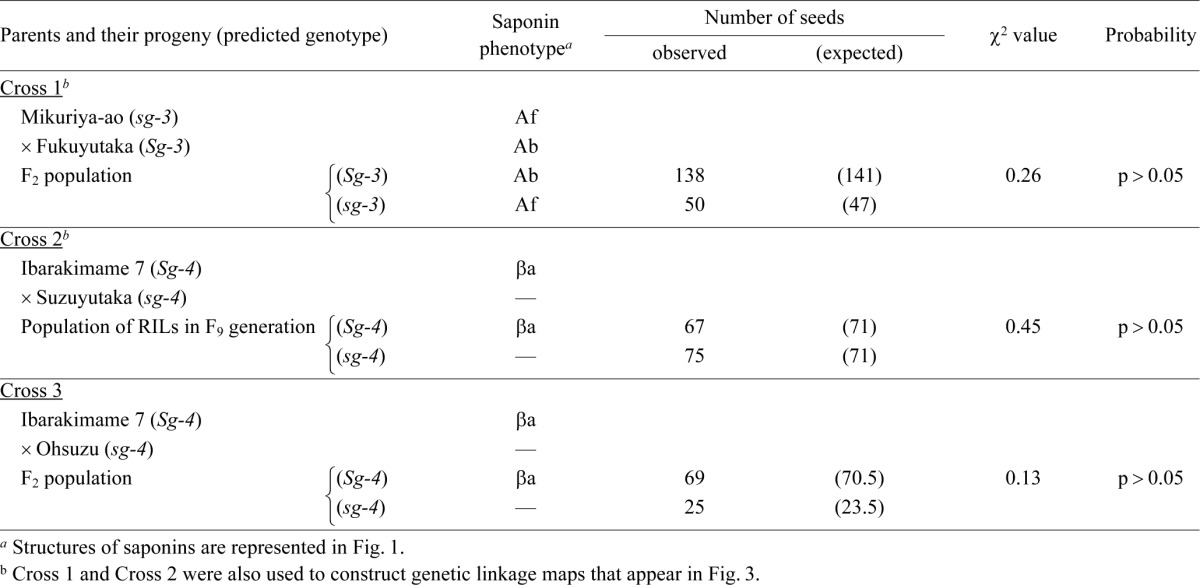
Five Japanese varieties were used to develop segregating populations for the genetic analyses. The cultivar names, together with the characteristic constituents of their group A or DDMP saponins, are listed in Table 1. Cross 1 (described below) was carried out at the NARO Western Region Agricultural Research Center, Zentsuuji, Kagawa, Japan, and segregating populations were also developed there. Crosses 2 and 3 were carried out at the NARO Tohoku Agricultural Research Center, Daisen, Akita, Japan, and a segregating population was also developed there. An F2 population (188 F2 seeds) derived from Mikuriya-ao (sg-3) × Fukuyutaka (Sg-3) (Cross 1) and a population of 142 recombinant inbred lines (RILs) in the F9 generation derived from Ibarakimame 7 (Sg-4) × Suzuyutaka (sg-4) (Cross 2) were used to construct genetic linkage maps of the loci that control saponins. Cross 3 was developed from crosses between Ibarakimame 7 (Sg-4) and Ohsuzu (sg-4) to analyze the mode of inheritance of Sg-4, as with Cross 2. We also developed an F2 population (192 F2 seeds) derived from Kinusayaka (sg-10/Sg-3) × Mikuriya-ao (Sg-1b/sg-3) (Cross 4), which was used to analyze the interaction between the sg-10 and sg-3 genes.
Table 1.
Segregation of saponin components in two F2 populations and a population of recombinant inbred lines (RILs)
|
In each cross, F2 seeds and RIL seeds were divided into hypocotyls and cotyledons. Thus, each hypocotyl was used to identify each saponin phenotype, and each cotyledon was used to analyze the genotype of each SSR locus.
Separation and detection of Group A and DDMP saponins
Each dry seed was divided into the hypocotyl and cotyledons with a small blade. Saponin extracts were prepared from each hypocotyl in a 10-fold volume (v/w) of aqueous 70% ethanol containing 0.1% acetic acid for 24 h at 25°C. The crude extract was used to analyze the composition of group A saponins by means of thin-layer chromatography (TLC) as previously described (Takada et al. 2010), and the composition of DDMP saponins by using HPLC analysis, which was performed on an ODS column (4.6 × 250 mm) (Tsukamoto et al. 1995). The mobile phase was acetonitrile : water : acetic acid (42 : 58 : 0.1). The solvent flow rate was 0.5 ml/min and the UV absorption was measured at 292 nm.
SSR marker analysis and mapping
Total genomic DNA was extracted from the seed flour of the two segregating populations, Cross 1 and Cross 2, and their parents by using a DNeasy Plant Mini Kit or a BioSprint 96 DNA Plant Kit (Qiagen, Valencia, CA, USA). PCR amplification and detection of SSR markers were performed as described previously (Hwang et al. 2009). Genotyping was carried out with polymorphic SSR markers that were used previously (Hwang et al. 2009). MAPMAKER/ EXP v. 3.0 software was used to analyze the linkage between markers. Genetic distances (cM) were calculated with Kosambi’s mapping function (Kosambi 1944). Linkage maps were drawn with MapChart software (Voorrips 2002).
Results
Variations in group A saponins and DDMP saponin βa
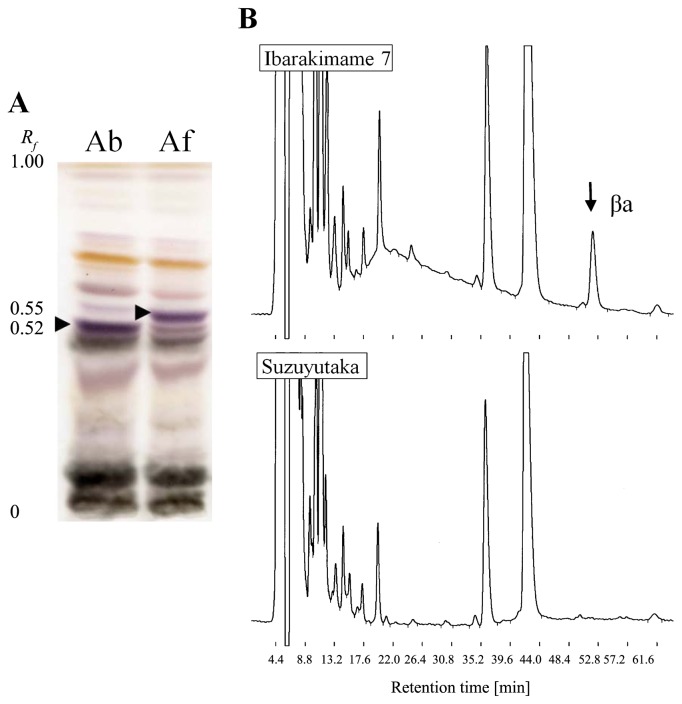
Hypocotyl extracts of Fukuyutaka gave group A saponin Ab at a relative mobility (Rf) of 0.52, whereas the extracts of Mikuriya-ao yielded group A saponin Af at Rf = 0.55 (Fig. 2A). The extracts of Ibarakimame 7 gave DDMP saponin βa at a retention time of 52.0 min, whereas the βa peak was not detected in extracts of Suzuyutaka (Fig. 2B).
Fig. 2.
Separation and detection of group A saponins, Ab and Af, by thin layer chromatography (TLC) and DDMP saponin βa by high performance liquid chromatography (HPLC). A: TLC patterns of aqueous 70% ethanol extracts of seed hypocotyls. Ab: Fukuyutaka (Ab phenotype, Sg-3), Af: Mikuriya-ao (Af phenotype, sg-3). Arrows indicate the positions of respective group A saponins. Rf: relative to front. B: HPLC chromatograms of the extracts. Upper: Ibarakimame 7 (βa presence, Sg-4), lower: Suzuyutaka (βa absence, sg-4).
Segregation patterns of saponin phenotypes
The F2 population derived from Cross 1 showed the phenotypic segregation for saponins Ab and Af (Table 1: Mikuriya-ao [Af phenotype, sg-3] × Fukuyutaka [Ab phenotype, Sg-3]). The segregation ratio (Ab : Af = 138 : 50, χ2 = 0.26, p > 0.05) fitted well with the 3 : 1 ratio, showing that the accumulation of saponin Af is controlled by a single recessive allele, as previously reported (Tsukamoto et al. 1993). The mode of inheritance of the DDMP saponin βa phenotype was examined in the RIL population derived from Cross 2 (Ibarakimame 7 [βa presence, Sg-4] × Suzuyutaka [βa absence, sg-4]) and in the F2 population derived from Cross 3 (Ibarakimame 7 [βa presence, Sg-4] × Ohsuzu [βa absence, sg-4]). The segregation ratio of Cross 2 (67 : 75, χ2 = 0.45, p > 0.05) fitted well with the 1 : 1 ratio, and that of Cross 3 (69 : 25, χ2 = 0.13, p > 0.05) fitted well with the 3 : 1 ratio (Table 1). These results show that the accumulation of DDMP saponin βa in seed hypocotyls is controlled by a single dominant allele. The gene symbols Sg-3 and Sg-4 were used to represent the genes that control the presence of the glucose residue as the third sugar at the C-3 of soyasapogenols and the addition of an arabinose residue as the second sugar at the C-3 position, respectively, following a previous report (Tsukamoto et al. 1993).
Linkage analysis and mapping of loci that control sugar chain composition at the C-3 position of soybean saponins
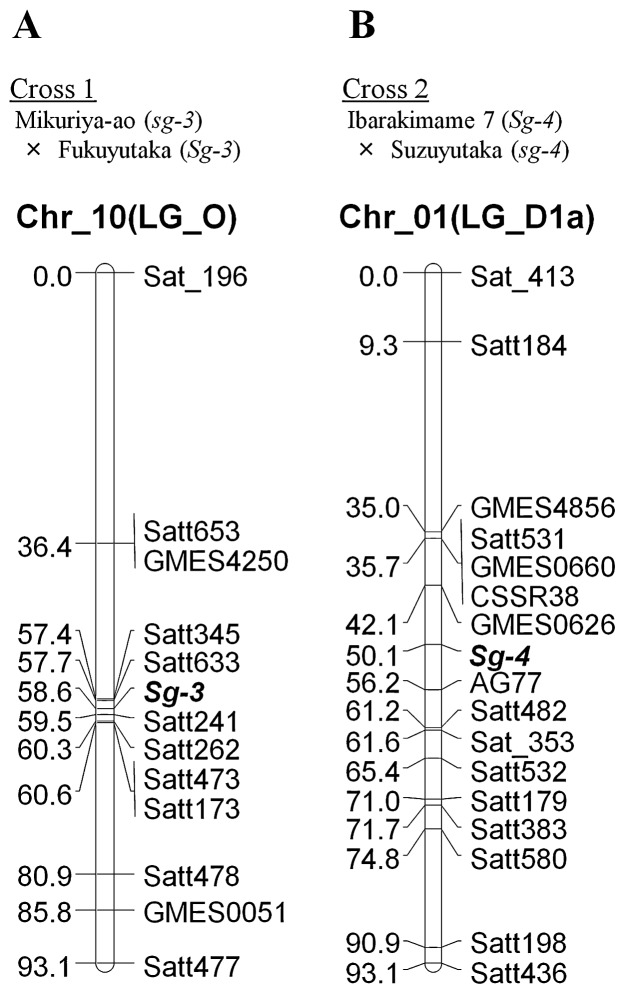
To clarify in detail the genetic relationships between the two phenotypes of sugar chain composition at the C-3 position of saponins, we performed linkage analyses with SSR markers (Fig. 3). The Sg-3 locus mapped between the SSR markers Satt633 and Satt241 on Chr-10 (linkage group [LG] O) in the segregating population of Cross 1 (Fig. 3A). Both SSR markers were located 0.9 cM from Sg-3. On the other hand, the Sg-4 locus mapped between GMES0626 and AG77 on Chr-1 (LG D1a) in the RILs derived from Cross 2 (Fig. 3B). GMES0626 and AG77 were 18.0 and 6.1 cM, respectively, from Sg-4.
Fig. 3.
Genetic linkage maps of Sg-3 locus positioned in soybean chromosome 10 (linkage group O) and Sg-4 locus in soybean chromosome 1 (linkage group D1a) along with SSR markers. A. Cross 1: an F2 population derived from crosses between Mikuriya-ao (sg-3) and Fukuyutaka (Sg-3), B. Cross 2: a population of RILs in F9 generation derived from crosses between Ibarakimame 7 (Sg-4) and Suzuyutaka (sg-4). Genetic distance of SSR markers and Sg-3 and Sg-4 loci is shown in centimorgans (cM).
Interaction between the sg-10 and sg-3 alleles
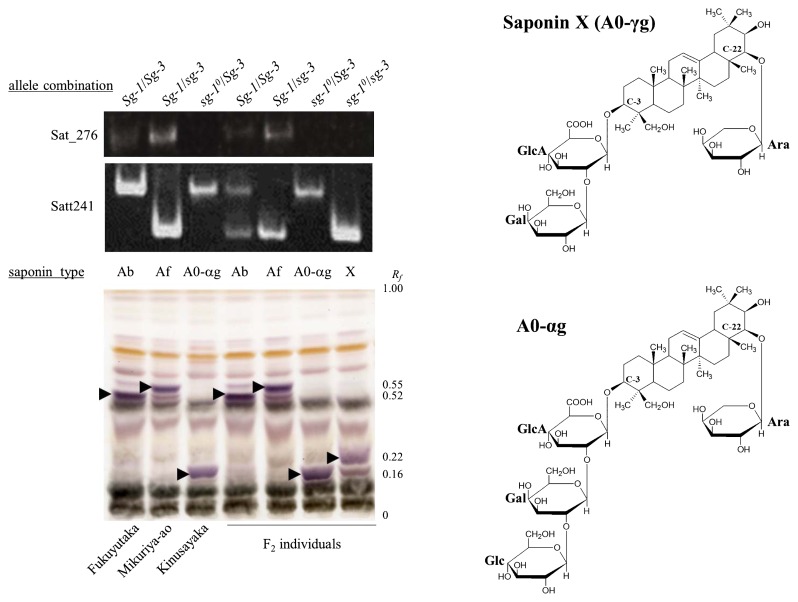
The interaction of another mutant harboring sg-10, which lacks the ability to add an acetylated sugar residue at the terminal position of the C-22 of soyasapogenol A, was examined in the F2 population derived from Cross 4 (Kinusayaka [A0-αg] × Mikuriya-ao [Af]). A novel saponin (X) at Rf = 0.22 was detected by using TLC analysis (Fig. 4). The segregation ratio of Cross 4 (Ab : Af : A0 : X = 96: 39 : 44 : 13, χ2 = 3.44, p > 0.05) fitted well with a 9 : 3 : 3 : 1 ratio. This result indicates that Kinusayaka and Mikuriya-ao harbor sg-10/Sg-3 and Sg-1b/sg-3, respectively, and that these two loci independently regulate the sugar chain structure of group A saponins. The type of SSR marker Sat_276 located near Sg-1 locus in F2 individual with saponin X was Kinusayaka [sg-10] type, and that of Satt241 located near Sg-3 locus was Mikuriya-ao [sg-3] type. Saponin X at Rf = 0.22 could be distinguished from saponin A0-αg at Rf = 0.16 (Fig. 4). The structure of the novel saponin appears to be saponin A0-γg, 3-O-[β-d-galactopyranosyl (1→2)-β-d-glucuronopyranosyl]-22-O-α-l-arabinopyranosyl-soyasapogenol A (Fig. 4), based on the integration of the mutant phenotypes of sg-10 and sg-3.
Fig. 4.
Detection a novel group A saponin in the F2 population derived from the cross between Kinusayaka (A0-αg) and Mikuriya-ao (Af) by TLC, and a predicted chemical structure of saponin X (A0-γg) and A0-αg. Allele combinations of Sg-1 and Sg-3 loci are indicated in the first line of each lane. Sat_276 and Satt241 are located near Sg-1 and Sg-3 loci, respectively. Arrows indicate the positions of group A saponins. Rf: relative to front.
Discussion
The biosynthesis of soybean saponins is complex, and many kinds of glycosyltransferases are thought to be involved in the sugar chain structural diversity of soybean saponins. Group A saponins and DDMP saponins contain six different kinds of sugar chains, which are composed of glucuronic acid as the first sugar, arabinose or galactose as the second, and glucose, rhamnose, or no sugar as the terminal third, at the C-3 position of aglycone (Tsukamoto et al. 1993). A comparison of the chemical structures of group A saponins Ab and Af shows that saponin Af lacks the third sugar residue, glucose, at the C-3 position, which is present in saponin Ab (Fig. 1A). The Sg-3 gene is thought to encode a glucosyl-transferase that catalyzes the glucosylation of a galactosyl moiety of saponin Af (Shimoyamada et al. 1991). The Sg-4 gene is thought to be an arabinosyltransferase that catalyzes the arabinosylation of a glucuronic acid residue attached at the C-3 of soyasapogenols (Tsukamoto et al. 1993). However, these glycosyltransferases have not yet been identified.
Kurosawa et al. (2002) reported that UDP-glucuronic acid : soyasapogenol glucuronosyltransferase is a specific enzyme for UDP-glucuronic acid as a donor and soyasapogenols as an acceptor, and that this enzyme is involved in the biosynthesis of sugar chains in soybean saponins. However, there is, as yet, no information about the structure or the gene of this partially purified enzyme. Recently, two glycosyltransferases from soybean, GmSGT2 and GmSGT3, were identified and characterized (Shibuya et al. 2010). GmSGT2 transfers a galactosyl group from UDP-galactose to soyasapogenol B monoglucuronide, and GmSGT3 transfers a rhamnosyl group from UDP-rhamnose to soyasaponin III, which has two sugars at the C-3 position of soyasapogenol B. Thus, GmSGT2 would transfer a galactosyl group from UDP-galactose not only to soyasapogenol B monoglucuronide but also to soyasapogenol glycosides, such as the precursor of saponin Af since there is a glucuronic acid residue at the C-3 position of soyasapogenols A and B (Fig. 1A). Similarly, GmSGT3 would transfers a rhamnosyl group from UDP-rhamnose not only to soyasaponin III but also to soyasapogenol glycosides such as saponin γa since there is an arabinose residue at the C-3 position of soyasapogenols A and B (Fig. 1B). Thus, although some glycosyltransferases of soybean has been identified recently, little is known regarding the molecular basis of the glycosylation events that are involved in the biosynthesis of soybean saponins. Therefore, spontaneous and induced mutations in saponin components in wild and cultivar germplasms and other mutant populations should be further explored. This type of genetic approach may reveal key enzymes involved in the production of the diverse sugar chain structures of soybean saponins and pave the way for agricultural uses of these mutants.
In the hypocotyl extracts of the seeds obtained from the cross between Kinusayaka (A0-αg) and Mikuriya-ao (Af), a novel saponin (saponin X) was detected by use of TLC analysis. The blue-violet color strongly suggested that saponin X was a group A saponin. However, there is no information about soybean saponins with this Rf. The genotype producing saponin X would be sg-10/sg-10 at the Sg-1 locus and sg-3/sg-3 at the Sg-3 locus based on the segregation frequency in F2 seeds. We predict that saponin X would be saponin A0-γg, containing a β-d-galactopyranosyl (1→2)-β-d-glucuronopyranosyl sugar chain at the C-3 position and an α-l-arabinopyranosyl residue at the C-22 position of soyasapogenol A, because the sg-10/sg-10 and sg-3/sg-3 genotypes could not put a terminal sugar at the C-22 and C-3 positions, respectively. To determine the chemical structure of the novel saponin component, purification and nuclear magnetic resonance analysis are required. Combining mutated genes not only changes the saponin composition but also creates a novel saponin component. Because saponin functionality depends heavily on the structure of the sugar chains (Hayashi et al. 1997, Kinjo et al. 1998), combinations of mutated genes could be used to produce profitable saponins with enhanced or novel functionalities.
Here, we excised hypocotyls from seeds to analyze saponin phenotypes. This process requires an immense amount of time and effort, and disrupts seed germination. In this study, we revealed that the Sg-3 and Sg-4 loci are located on Chr-10 and Chr-1, respectively. This positional information will enable us to screen target phenotypes by using marker-assisted selection, and to efficiently breed new varieties that might accumulate profitable saponins with health benefits and improved taste for soybean food processing. In addition, it provides useful information regarding the identities of the Sg-3 and Sg-4 genes.
Acknowledgments
This work was supported by the Research Project for Utilizing Advanced Technologies in Agriculture, Forestry and Fisheries (18063) and in part by the Research Project for Genomics for Agricultural Innovation (DD-3260) of the Ministry of Agriculture, Forestry and Fisheries of Japan.
Literature Cited
- Burrows JC, Price KR, Fenwick RG. Soyasaponin IV, and additional monodesmosidic saponin isolated from soybean. Phytochemistry. 1987;26:1214–1215. [Google Scholar]
- Ellington AA, Berhow MA, Singletary KW. Induction of macroautophagy in human colon cancer cells by soybean B-group triterpenoid saponins. Carcinogenesis. 2005;26:159–167. doi: 10.1093/carcin/bgh297. [DOI] [PubMed] [Google Scholar]
- Ellington AA, Berhow MA, Singletary KW. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis. 2006;27:298–306. doi: 10.1093/carcin/bgi214. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H, Hiraoka N, Ikeshiro Y. Inhibitory activity of soyasaponin II on virus replication in vitro. Planta Med. 1997;63:102–105. doi: 10.1055/s-2006-957622. [DOI] [PubMed] [Google Scholar]
- Hwang TY, Sayama T, Takahashi M, Takada Y, Nakamoto Y, Funatsuki H, Hisano H, Sasamoto S, Sato S, Tabata S, et al. High-density integrated linkage map based on SSR markers in soybean. DNA Res. 2009;16:213–225. doi: 10.1093/dnares/dsp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y, Tanizawa H. Effects of soyasaponins on lipid peroxidation through the secretion of thyroid hormones. Biol Pharm Bull. 2006;29:1759–1763. doi: 10.1248/bpb.29.1759. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Tsukamoto C, Tabuchi K, Adachi T, Okubo K. Inheritance and characterization of a null allele for group A acetyl saponins found in a mutant soybean (Glycine max (L.) Merrill) Breed Sci. 1999;49:167–171. [Google Scholar]
- Kinjo J, Imagire M, Udayama M, Arao T, Nohara T. Structure-hepatoprotective relationships study of soyasaponins I–IV having soyasapogenol B as aglycone. Planta Med. 1998;64:233–236. doi: 10.1055/s-2006-957416. [DOI] [PubMed] [Google Scholar]
- Kitagawa I, Yoshikawa M, Wang KH, Saito M, Tosirisuk V, Fujiwara T, Tomita T. Revised structures of soyasapogenols A, B and E, oleanene-sapogenols from soybean. Structures of soyasaponins I, II and III. Chem Pharm Bull. 1982;30:2294–2297. [Google Scholar]
- Kitagawa I, Taniyama T, Nagahama Y, Okubo K, Yamauchi F, Yoshikawa M. Saponin and sapogenol. XLII. Structures and acetyl-soyasaponins A1, A2, and A3, astringent partially acetylated bisdesmosides of soyasapogenol A, from American soybean, the seeds of Glycine max Merrill. Chem Pharm Bull. 1988;36:2819–2828. [Google Scholar]
- Kosambi DD. The estimation of map distance from recombination values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- Kudou S, Tonomura M, Tsukamoto C, Shimoyamada M, Uchida T, Okubo K. Isolation and structural elucidation of the major genuine soybean saponin. Biosci Biotechnol Biochem. 1992;56:142–143. doi: 10.1271/bbb.56.142. [DOI] [PubMed] [Google Scholar]
- Kudou S, Tonomura M, Tsukamoto C, Uchida T, Sakabe T, Tamura N, Okubo K. Isolation and structural elucidation of DDMP-conjugated soyasaponins as genuine saponins from soybean seeds. Biosci Biotechnol Biochem. 1993;57:546–550. [Google Scholar]
- Kurosawa Y, Takahara H, Shiraiwa M. UDP-glucuronic acid: soyasapogenol glucuronosyltransferase involved in saponin biosynthesis in germinating soybean seeds. Planta. 2002;215:620–629. doi: 10.1007/s00425-002-0781-x. [DOI] [PubMed] [Google Scholar]
- Okubo K, Iijima M, Kobayashi Y, Yoshikoshi M, Uchida T, Kudou S. Components responsible for the undesirable taste of soybean seeds. Biosci Biotech Biochem. 1992;56:99–103. [Google Scholar]
- Shibuya M, Nishimura K, Yasuyama N, Ebizuka Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010;584:2258–2264. doi: 10.1016/j.febslet.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Shimoyamada M, Kudo S, Okubo K, Yamauchi F, Harada K. Distribution of saponin constituents in some varieties of soybean plant. Agric Biol Chem. 1990;54:77–81. [Google Scholar]
- Shimoyamada M, Harada K, Okubo K. Saponin composition in developing soybean seed (Glycine max (L.) Merrill, cv. Mikuriyaao) Agric Biol Chem. 1991;55:1403–1405. [Google Scholar]
- Shiraiwa M, Yamauchi F, Harada K, Okubo K. Inheritance of “Group A saponin” in soybean seed. Agric Biol Chem. 1990;54:1347–1352. [PubMed] [Google Scholar]
- Shiraiwa M, Kudo S, Shimoyamada M, Harada K, Okubo K. Composition and structure of “group A saponin” in soybean seed. Agric Biol Chem. 1991a;55:315–322. [PubMed] [Google Scholar]
- Shiraiwa M, Harada K, Okubo K. Composition and content of saponins in soybean seed according to variety, cultivation year and maturity. Agric Biol Chem. 1991b;55:323–331. [PubMed] [Google Scholar]
- Takada Y, Sayama T, Kikuchi A, Kato S, Tatsuzaki N, Nakamoto Y, Suzuki A, Tsukamoto C, Ishimoto M. Genetic analysis of variation in sugar chain composition at the C-22 position of group A saponin in soybean, Glycine max (L.) Merrill. Breed Sci. 2010;60:3–8. [Google Scholar]
- Taniyama T, Nagahama Y, Yoshikawa M, Kitagawa I. Saponin and sapogenol. XLIII. Acetyl-soyasaponins A4, A5, and A6, new astringent bisdesmosides of soyasapogenol A, from Japanese soybean, the seeds of Glycine max Merrill. Chem Pharm Bull. 1988;36:2829–2839. [Google Scholar]
- Topping DL, Storer GB, Calvert GD, Illman RJ, Oakenfull DG, Weller RA. Effect of dietary saponins on fecal bile acids and neutral sterols, plasma lipids, and lipoprotein turnover in the pig. Am J Clin Nutr. 1980;33:783–786. doi: 10.1093/ajcn/33.4.783. [DOI] [PubMed] [Google Scholar]
- Tsukamoto C, Kikuchi A, Kudou S, Harada K, Kitamura K, Okubo K. Group A acetyl saponin-deficient mutant from the wild soybean. Phytochemistry. 1992;31:4139–4142. [Google Scholar]
- Tsukamoto C, Kikuchi A, Harada K, Kitamura K, Okubo K. Genetic and chemical polymorphisms of saponins in soybean seed. Phytochemistry. 1993;34:1351–1356. doi: 10.1016/0031-9422(91)80028-y. [DOI] [PubMed] [Google Scholar]
- Tsukamoto C, Shimada S, Igita K, Kudou S, Kokubun M, Okubo K, Kitamura K. Factors affecting isoflavone content in soybean seeds: changes in isoflavones, saponins, and composition of fatty acids at different temperatures during seed development. J Agric Food Chem. 1995;43:1184–1192. [Google Scholar]
- Tsukamoto C, Yoshiki Y. Soy saponin. In: Sugano M, editor. Soy in Health and Disease Prevention. CRC Press, Taylor & Francis Group; New York, USA: 2006. pp. 155–172. [Google Scholar]
- Voorrips RE. MapChart: Software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93:77–78. doi: 10.1093/jhered/93.1.77. [DOI] [PubMed] [Google Scholar]