Abstract
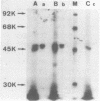
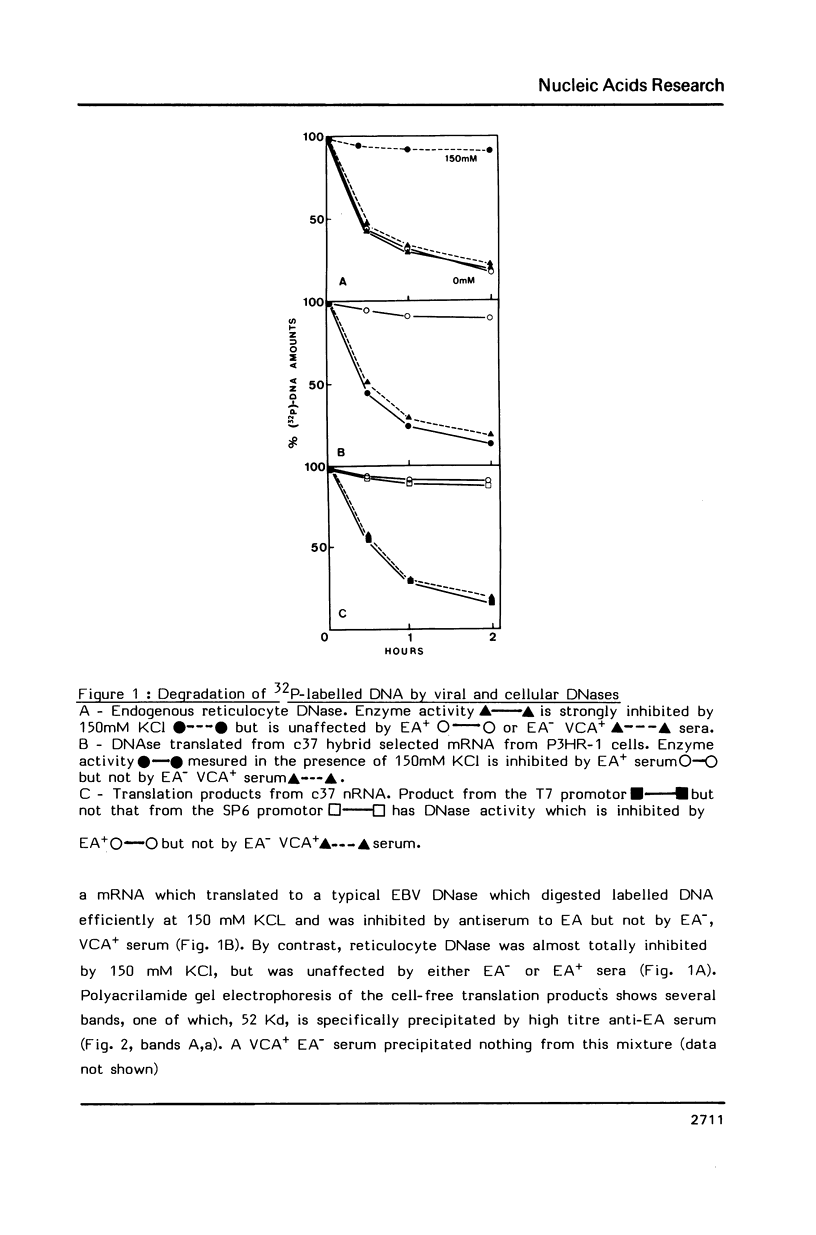
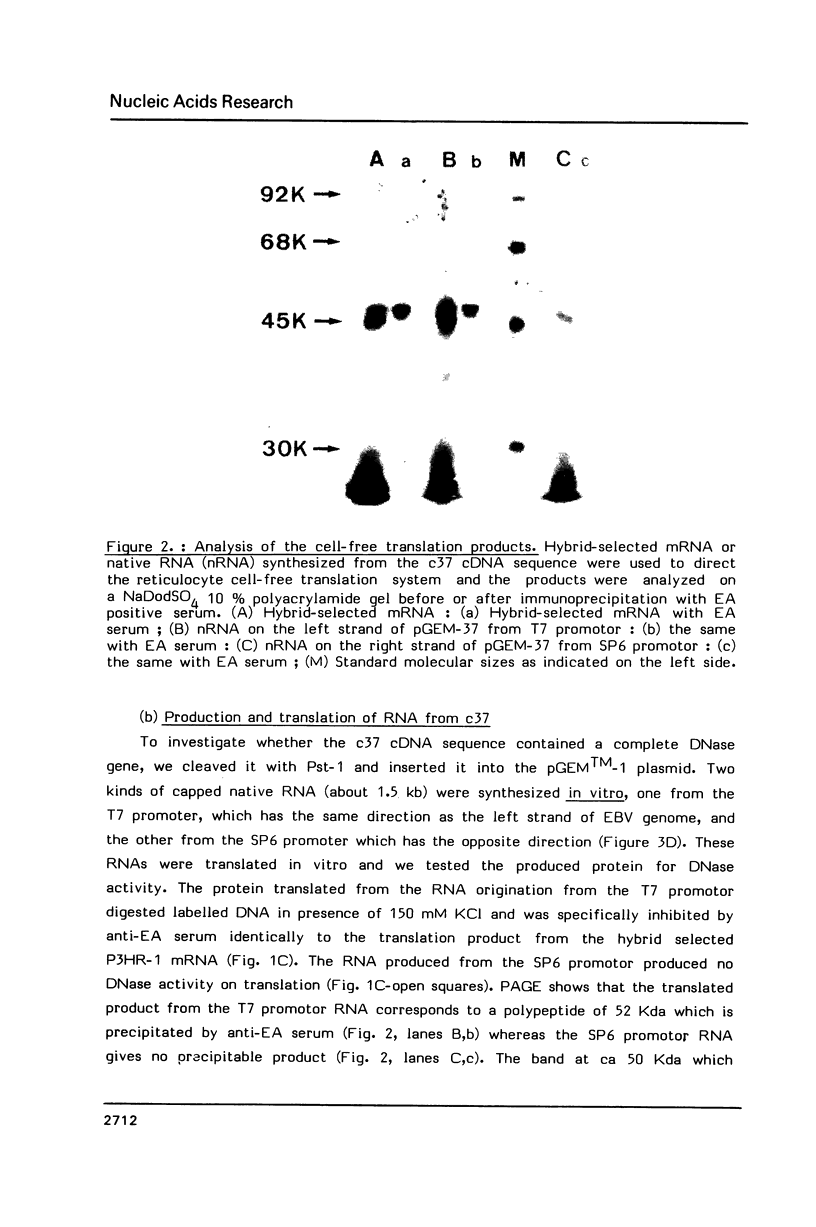
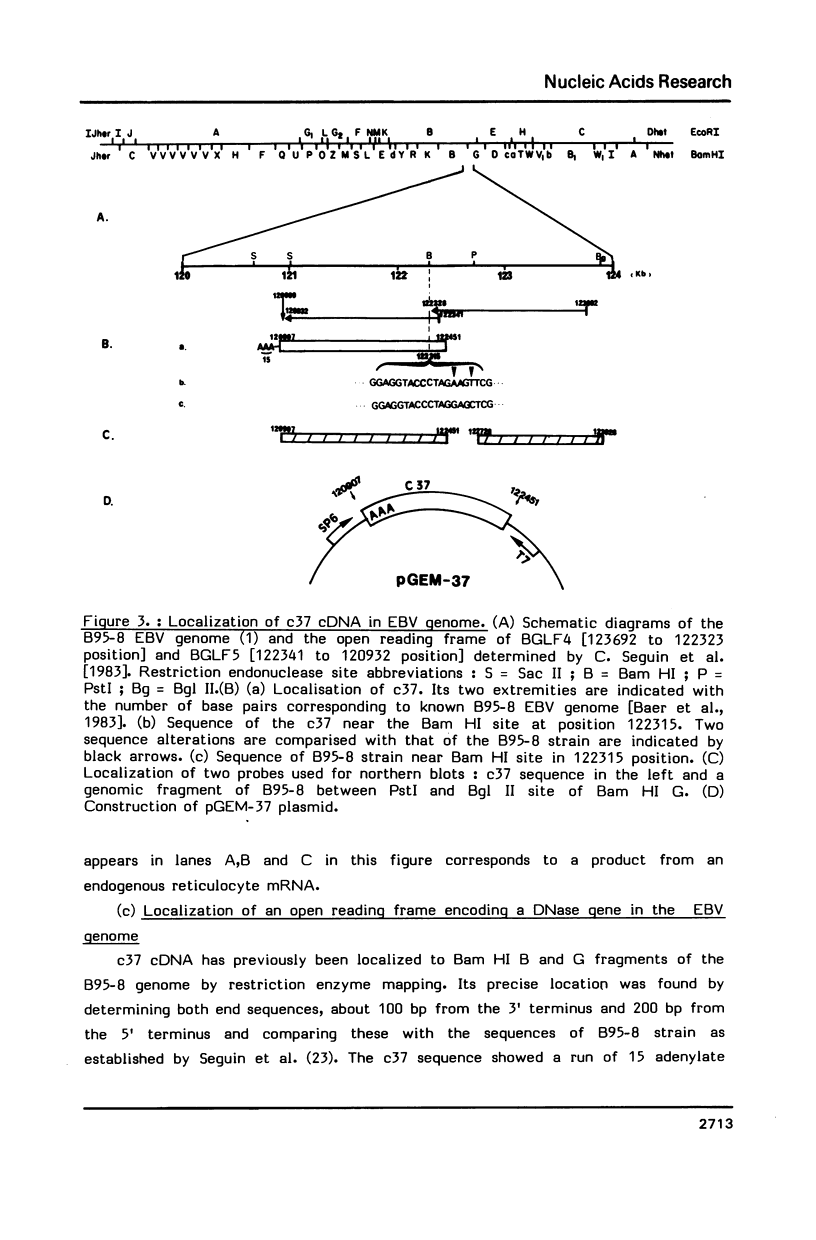
We have recently obtained 18 distinct cDNA clones representing different genes expressed in the early phase of EBV infection. One of them, c37, which is situated at the position 12907-122451 in the B95-8 viral genome, is shown here to code for a viral desoxyribonuclease [DNase]. Cell free translation of c37-selected messenger RNA yielded a protein of about 52 KDa which was immunoprecipitated by a high EA titer serum from nasopharyngeal carcinoma patient. This protein showed a DNase activity which was resistant to high salt concentrations (150 to 300 mM KCl) and was specifically neutralized by EA positive serum. These properties are typical of the EBV-specific DNase activity that we recently described in chemically induced EBV-transformed lymphoid cells. The same results were obtained on cell-free translation of the native RNA synthesized in vitro from pGEM-37 plasmid containing the entire c37 cDNA sequence (1.53 Kb). These data indicate that the BGLF5 open reading frame contained in c37 encodes for the EBV-specific DNase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Hoffmann P. J., Glaser R. Studies on the activity of DNase associated with the replication of the Epstein-Barr virus. Virology. 1980 Jan 30;100(2):334–338. doi: 10.1016/0042-6822(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Francke B., Garrett B. The effect of a temperature-sensitive lesion in the alkaline DNase of herpes simplex virus type 2 on the synthesis of viral DNA. Virology. 1982 Jan 15;116(1):116–127. doi: 10.1016/0042-6822(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Heller M., Dambaugh T., Kieff E. Epstein-Barr virus DNA. IX. Variation among viral DNAs from producer and nonproducer infected cells. J Virol. 1981 May;38(2):632–648. doi: 10.1128/jvi.38.2.632-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M., Kieff E. Mapping of polypeptides encoded by the Epstein-Barr virus genome in productive infection. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5698–5702. doi: 10.1073/pnas.79.18.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E., Dambaugh T., Heller M., King W., Cheung A., van Santen V., Hummel M., Beisel C., Fennewald S., Hennessy K. The biology and chemistry of Epstein-Barr virus. J Infect Dis. 1982 Oct;146(4):506–517. doi: 10.1093/infdis/146.4.506. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manet E., Chevallier A., Zhang C. X., Ooka T., Chavrier P., Daillie J. Construction and use of cDNA clones for the mapping and identification of Epstein-Barr virus early P3HR-1 mRNAs. J Virol. 1985 May;54(2):608–614. doi: 10.1128/jvi.54.2.608-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Frame M. C. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the exonuclease gene and neighbouring genes. Nucleic Acids Res. 1986 Apr 25;14(8):3435–3448. doi: 10.1093/nar/14.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Calender A. Effects of arabinofuranosylthymine on Epstein-Barr virus replication. Virology. 1980 Jul 15;104(1):219–223. doi: 10.1016/0042-6822(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Ooka T., Calender A., de Turenne M., Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983 Apr;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., De Turenne M., De The G., Daillie J. Epstein-Barr virus-specific DNase activity in nonproducer Raji cells after treatment with 12-o-tetradecanoylphorbol-13-acetate and sodium butyrate. J Virol. 1984 Feb;49(2):626–628. doi: 10.1128/jvi.49.2.626-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T. The molecular biology of Epstein-Barr virus. Biomed Pharmacother. 1985;39(2):59–66. [PubMed] [Google Scholar]
- Resnick M. A., Sugino A., Nitiss J., Chow T. DNA polymerases, deoxyribonucleases, and recombination during meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2811–2817. doi: 10.1128/mcb.4.12.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Sugino A., Nitiss J., Chow T. DNA polymerases, deoxyribonucleases, and recombination during meiosis in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2811–2817. doi: 10.1128/mcb.4.12.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saemundsen A. K., Kallin B., Klein G. Effect of n-butyrate on cellular and viral DNA synthesis in cells latently infected with Epstein-Barr virus. Virology. 1980 Dec;107(2):557–561. doi: 10.1016/0042-6822(80)90326-8. [DOI] [PubMed] [Google Scholar]
- Strobel-Fidler M., Francke B. Alkaline deoxyribonuclease induced by herpes simplex virus type 1: composition and properties of the purified enzyme. Virology. 1980 Jun;103(2):493–501. doi: 10.1016/0042-6822(80)90206-8. [DOI] [PubMed] [Google Scholar]
- Séguin C., Farrell P. J., Barrell B. G. DNA sequence and transcription of the BamHI fragment B region of B95-8 Epstein-Barr virus. Mol Biol Med. 1983 Oct;1(3):369–392. [PubMed] [Google Scholar]
- van Santen V., Cheung A., Hummel M., Kieff E. RNA encoded by the IR1-U2 region of Epstein-Barr virus DNA in latently infected, growth-transformed cells. J Virol. 1983 May;46(2):424–433. doi: 10.1128/jvi.46.2.424-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]