Abstract
RNA silencing refers collectively to diverse RNA-mediated pathways of nucleotide-sequence-specific inhibition of gene expression. It has been used to analyze gene function and engineer novel traits in various organisms. Here, we review the application of RNA silencing in soybean. To produce soybean lines, in which a particular gene is stably silenced, researchers have frequently used a transgene that transcribes inverted repeats of a target gene segment. Suppression of gene expression in developing soybean embryos has been one of the main focuses of metabolic engineering using transgene-induced silencing. Plants that have enhanced resistance against diseases caused by viruses or cyst nematode have also been produced. Meanwhile, Agrobacterium rhizogenes-mediated transformation has been used to induce RNA silencing in roots, which enabled analysis of the roles of gene products in nodulation or disease resistance. RNA silencing has also been induced using viral vectors, which is particularly useful for gene function analysis. So far, three viral vectors for virus-induced gene silencing have been developed for soybean. One of the features of the soybean genome is the presence of a large number of duplicated genes. Potential use of RNA silencing technology in combination with forward genetic approaches for analyzing duplicated genes is discussed.
Keywords: epigenetic changes, metabolic engineering, post-transcriptional gene silencing, RNA interference, soybean (Glycine max), transgene, virus-induced gene silencing
Introduction
Gene silencing is one of the regulatory mechanisms of gene expression in eukaryotes, which refers to diverse RNA-guided sequence-specific inhibition of gene expression, either at the post-transcriptional or transcriptional level (reviewed by Brodersen and Voinnet 2006, Vaucheret 2006). Post-transcriptional gene silencing (PTGS) was first discovered in transgenic petunia plants whose flower color pattern was changed as a consequence of overexpression of a gene that encodes the key enzyme for anthocyanin biosynthesis in 1990 (Napoli et al. 1990, van der Krol et al. 1990). Similar phenomena have also been reported for plants transformed with various genes, which include virus resistance of plants that have gene or gene segments derived from the viral genome (reviewed by Baulcombe 1996, Wilson 1993). Because of these findings, gene silencing is thought to have developed to defend against viruses. Several lines of research in plants indicated that double-stranded RNA (dsRNA) is crucial for RNA degradation (Metzlaff et al. 1997, Waterhouse et al. 1998). The potency of dsRNA to induce gene silencing was demonstrated in Caenorhabditis elegans by injecting dsRNA into cells in 1998 (Fire et al. 1998), and the phenomenon was termed RNA interference (RNAi).
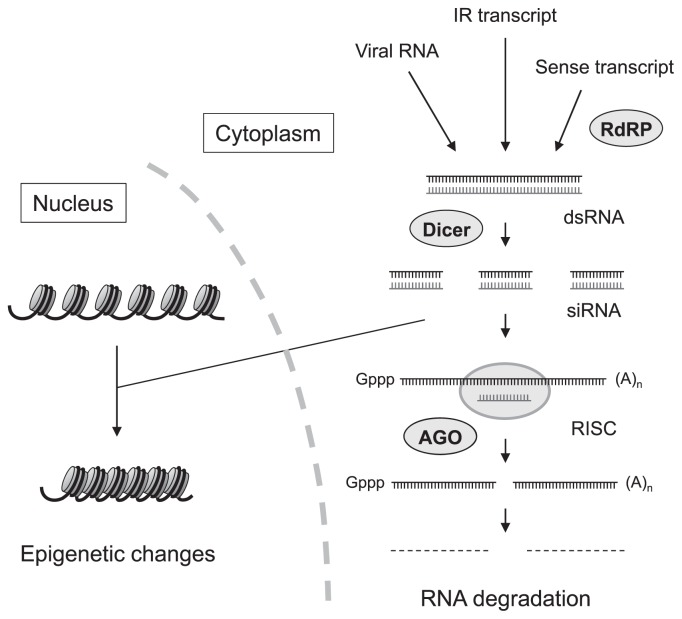
Subsequent genetic and biochemical analyses in several organisms revealed that PTGS and RNAi share the same pathway and consist of two main processes: (i) processing of dsRNA into 20–26-nt small RNA molecules (short interfering RNA; siRNA) by an enzyme called Dicer that has RNaseIII-like endonuclease activity; (ii) cleavage of RNA guided by siRNA at a complementary nucleotide sequence in the RNA-induced silencing complex (RISC) containing the Argonaute (AGO) protein (reviewed by Matzke et al. 2001). The formation of dsRNA from single-stranded sense RNA was explained by the synthesis of its complementary strand by RNA-dependent RNA polymerase (RdRP). This process provides templates for Dicer cleavage that produces siRNAs and consequently allows amplification of silencing (reviewed by Baulcombe 2004) (Fig. 1). siRNA is responsible for not only induction of sequence-specific RNA degradation but also epigenetic changes involving DNA methylation and histone modification in the nucleus, which leads to transcriptional gene silencing (TGS) (reviewed by Matzke et al. 2009). It has become evident that siRNA plays a role in systemic silencing as a mobile signal (Dunoyer et al. 2010, Molnar et al. 2010). In addition to siRNA, small RNA molecules called micro RNAs (miRNAs) are also involved in negative regulation of gene expression (reviewed by Mallory and Vaucheret 2006). These gene silencing phenomena that are induced by sequence-specific RNA interaction are collectively called RNA silencing (reviewed by Matzke et al. 2004, Voinnet 2002).
Fig. 1.
Pathways of RNA silencing used to engineer novel traits in plants. Posttranscriptional gene silencing is triggered by dsRNA. Transcripts from transgenes that have an IR sequence can form dsRNA. Sense transcripts can produce dsRNA through the synthesis of complementary strand by RdRP. The replication intermediate or duplex structures formed within single-stranded RNA of the viral genome can also provide dsRNA. These dsRNAs are processed into siRNAs by the endonuclease Dicer. The siRNA is loaded into the RISC complex that contains AGO and guides the RISC complex to the mRNA by base-pairing. The RISC complex cuts the mRNA, which is subsequently degraded. siRNA can also induce epigenetic changes involving DNA methylation and/or changes in histone modification in the nucleus. These changes can convert nucleosomes to a more tightly packed structure, thereby transcription is repressed. Abbreviations: IR, inverted repeat; RdRP, RNA-dependent RNA polymerase; dsRNA, double-stranded RNA; siRNA, short interfering RNA; RISC, RNA-induced silencing complex; AGO, Argonaute.
RNA silencing plays an important role in many biological processes including development, stability of the genome, and defense against invading nucleic acids such as transgenes and viruses (reviewed by Baulcombe 2004, Matzke et al. 2009, Vaucheret 2006). It can also be used as a tool for analyzing specific gene functions and producing new features in organisms including plants (reviewed by Frizzi and Huang 2010, Kanazawa 2008, Mansoor et al. 2006). Here, we review the application of RNA silencing in the genetic analysis and molecular breeding of soybean [Glycine max (L.) Merrill].
Methods of transgene-induced RNA silencing in soybean
Engineering novel traits through RNA silencing in soybean has been done using transgenes or virus vectors: examples are listed in Tables 1, 2, 3. RNA silencing in some transgenic soybean plants was induced by introducing a transgene that transcribes sense RNA homologous to a gene present in the plant genome, a phenomenon termed co-suppression (Napoli et al. 1990). This type of silencing was first discovered in transgenic petunia plants that had silencing of CHS-A for chalcone synthase (Napoli et al. 1990, van der Krol et al. 1990), in which mRNA transcribed from both CHS-A transgene and endogenous CHS-A gene was degraded. When sense transcripts from a transgene trigger RNA degradation, the pathway is also referred to as sense (S)-PTGS (Brodersen and Voinnet 2006). To obtain plants that have RNA silencing of a particular gene target, it is possible to generate co-suppressed plant lines as a byproduct of a transformation to overexpress the gene under the control of a strong promoter. However, a more promising method to induce RNA degradation is to transform plants with a construct comprising an inverted repeat (IR) sequence of the target gene, which forms dsRNA upon transcription (IR-PTGS) (Helliwell and Waterhouse 2005, Wesley et al. 2001). This idea was based on the understanding of general mechanisms of RNA silencing in which dsRNA triggers the reaction of RNA degradation. The majority of transgene-induced RNA silencing in soybean have actually been done using such an IR construct (Table 1). IR-PTGS can also be induced when multiple transgenes are integrated in the same site in the genome in an inverted orientation and fortuitous read-through transcription over the transgenes produces dsRNA.
Table 1.
Metabolic engineering through transgene-induced RNA silencing in soybean
| Target gene | Method or construct | Promoter | Transformation | Tissues assayed | Effect | Reference |
|---|---|---|---|---|---|---|
| Fatty acid desaturase gene FAD2-1 | S-PTGS | β-conglycinin | Particle bombardment | Seed | Increase in oleic acid content | Kinney 1996 |
| β-conglycinin α and α′ subunit genes | S-PTGS | β-conglycinin | Particle bombardment | Seed | Changes in seed protein composition | Kinney et al. 2001 |
| β-glucuronidase (GUS) gene | S-PTGS | CaMV 35S | Particle bombardment | Leaf and flower | Lack of GUS expression | Reddy et al. 2003 |
| Gly m Bd 30 K gene | S-PTGS | β-conglycinin α subunit | Particle bombardment | Seed | Reduced Gly m Bd 30 K | Herman et al. 2003 |
| Flavanone 3-hydroxylase genea | S-PTGS | Kti3 | Particle bombardment | Seed | Increased isoflavone | Yu et al. 2003 |
| Isoflavone synthase genes IFS1 and IFS2 | IR-PTGS | FMV | A. rhizogenesc | Hairy root | Reduced isoflavone and enhanced susceptibility of P. sojae | Subramanian et al. 2005 |
| Thioredoxin gene | IR-PTGS | CaMV 35S | A. rhizogenesc | Hairy root | Suppression of root nodule development | Lee et al. 2005 |
| Isoflavone synthase genes IFS1 and IFS2 | IR-PTGS | Ubiquitin | A. rhizogenesc | Hairy root | Suppression of root nodule development | Subramanian et al. 2006 |
| Myo-inositol-1-phosphate synthase gene | IR-PTGS | CaMV 35S | Particle bombardment | Seed | Absence of seed development and reduced phytic acid | Nunes et al. 2006 |
| Senescence-associated receptor-like kinase gene | IR-PTGS | CaMV 35S | A. tumefaciensd | Leaf | Retarded leaf senescence | Li et al. 2006 |
| Delta 15 desaturase geneb | IR-PTGS | β-conglycinin α′ subunit | Particle bombardment | Seed | Production of arachidonic acid | Chen et al. 2006 |
| Multidrug resistance-associated protein (MRP) ATP-binding cassette (ABC) transporter gene | IR-PTGS | Kti3 | Particle bombardment | Seed | Reduced phytic acid | Shi et al. 2007 |
| Chalcone synthase gene CHS6 and isoflavone synthase gene IFS2 | S-PTGS | CsVMV (CvMV) | A. rhizogenesc | Hairy root | Reduced isoflavone and coumesterol and increased growth of F. solani | Lozovaya et al. 2007 |
| Chalcone reductase and isoflavone synthase genes | IR-PTGS | CsVMV (CvMV) | A. rhizogenesc | Hairy root | Suppression of resistance against P. sojae and cell death | Graham et al. 2007 |
| Seed oil body protein 24-kDa oleosin gene | IR-PTGS | Oleosin 24-kD isoform A | Particle bombardment | Seed | Changes in seed oil body size and slow growth of the plant | Schmidt and Herman 2008 |
| Fatty acid desaturase gene GmFAD3 | IR-PTGS | Glycinin | A. tumefaciensd | Seed | Reduced linolenic acids | Flores et al. 2008 |
| Fatty acid desaturase gene GmFAD2 | IR-PTGS | Lectin | A. tumefaciensd | Seed | Increased oleic acid | Wang and Xu 2008 |
| Lipoxygenase genes LOX9 and LOX10 | IR-PTGS | CaMV 35S | A. rhizogenesc | Hairy root | No effect on root nodule development | Hayashi et al. 2008 |
| Glutathione S-transferase gene GST9 | IR-PTGS | CsVMV (CvMV) | A. rhizogenesc | Hairy root | Reduced nitrogenase activity and increased oxidatively damaged proteins | Dalton et al. 2009 |
| Ecto-apyrase gene GS52 | IR-PTGS | FMV | A. rhizogenesc | Hairy root | Suppression of root nodule development | Govindarajulu et al. 2009 |
| FW2.2-like gene GmFWL1 | IR-PTGS | FMV | A. rhizogenesc | Hairy root | Suppression of root nodule development | Libault et al. 2010 |
| Leucine-rich repeat transmembrane receptor kinase gene | amiRNAe | Ubiquitin-3 | A. rhizogenesc | Hairy root | Suppression of root production and no effect on resistance to cyst nematode | Melito et al. 2010 |
| Fatty acid desaturase gene FAD2-1 | Intron IR-PTGSf | β-conglycinin α′ subunit | A. tumefaciensd | Seed | Changes in fatty acid composition | Wagner et al. 2011 |
| MYB transcription factor gene GmMYB176 | IR-PTGS | CaMV 35S | A. rhizogenesc | Hairy root | Reduced isoflavonoids | Yi et al. 2010 |
| Amino aldehyde dehydrogenase gene | IR-PTGS | CaMV 35S | A. tumefaciensd | Callus | Biosynthesis of 2-acytyl-1-pyrroline | Arikit et al. 2011 |
| Glycinin A1bB2 subunit and FAD-2 genes | IR-PTGS | Glycinin | Particle bombardment | Seed | Changes in seed protein composition | Schmidt et al. 2011 |
| β-amyrin synthase genes GmBAS1 and GmBAS2 | IR-PTGS | β-conglycinin α′ subunit | Particle bombardment | Seed | Reduced saponin | Takagi et al. 2011 |
| Phospholipase D gene SPLDα | IR-PTGS | β-conglycinin α′ subunit | Particle bombardment | Seed | Changes in phospholipid and triacyl- glycerol composition | Lee et al. 2011 |
The silencing-inducing plasmid was co-bombarded with a plasmid having an expression cassette of the gene encoding a chimeric protein of the maize C1 and R transcription factors.
The silencing-inducing cassette was introduced together with the cassettes that express the delta 5 desatuarase, delta 6 desaturase, and GLELO elongase genes located on the same plasmid.
Agrobacterium rhizogenes-mediated root transformation.
Agrobacterium tumefaciens-mediated transformation.
Gene silencing induced by artificial microRNA.
Gene silencing induced by transcribing inverted repeats of intron.
Abbreviations: PTGS, posttranscriptional gene silencing; S-PTGS, sense-PTGS; IR-PTGS, inverted repeat-PTGS; CaMV, Cauliflower mosaic virus; Kti3, Kunitz trypsin inhibitor 3; FMV, Figwort mosaic virus; CsVMV (CvMV), Cassava vein mosaic virus; P. sojae, Phytophthora sojae; F. solani, Fusarium solani.
Table 2.
Enhancement of disease resistance through transgene-induced RNA silencing targeted to pathogens in soybean
| Target gene | Construct | Promoter | Transformation method | Reference |
|---|---|---|---|---|
| Soybean mosaic virus, CP gene and 3′ UTR | cDNA | CaMV 35S | A. tumefaciensa | Wang et al. 2001 |
| Bean pod mottle virus, CP gene | cDNA | CaMV 35S | Particle bombardment | Reddy et al. 2001c |
| Soybean dwarf virus, CP gene | cDNA IR | CaMV 35S | Particle bombardment | Tougou et al. 2006 |
| Soybean dwarf virus, CP gene | cDNA | CaMV 35S | Particle bombardment | Tougou et al. 2007 |
| Soybean mosaic virus, CP gene | cDNA | CaMV 35S | Particle bombardment | Furutani et al. 2006, 2007 |
| Cyst nematode (Heterodera glycines), major sperm protein gene | cDNA IR | Arabidopsis ACT2 | Particle bombardment | Steeves et al. 2006 |
| H. glycines genes Cpn-1, Y25 and Prp-17 | cDNA IR | CaMV 35S | A. rhizogenesb | Li et al. 2010 |
| Root-knot nematode (Meloidogyne incognita) TP and MSP genes | cDNA IR | FMV | A. rhizogenesb | Ibrahim et al. 2011 |
Agrobacterium tumefaciens-mediated transformation.
Agrobacterium rhizogenes-mediated root transformation.
The mechanism of virus resistance in this report could be mainly brought about by the expressed CP protein rather than through RNA silencing. However, we could not exclude the possibility of the involvement of RNA silencing in the phenomenon because no data of the level of viral RNA or CP mRNA in the virus-infected plants is presented.
Abbreviations: CP, coat protein; IR, inverted repeat; CaMV, Cauliflower mosaic virus; ACT2, actin 2; FMV, Figwort mosaic virus; TP, tyrosine phosphatase; MSP, mitochondrial stress-70 protein precursor.
Table 3.
Virus-induced gene silencing in soybean
| Virus | Target gene | Tissues assayed | Effect | Reference |
|---|---|---|---|---|
| Bean pod mottle virus | Phytoene desaturase gene | Leaf | Photo-bleaching | Zhang and Ghabrial 2006, Zhang et al. 2010 |
| Stearoyl-acyl carrier protein-desaturase gene | Leaf, stem, flower, root and seed | Reduced oleic acid, increased stearic acid and SA and resistance to pathogens | Kachroo et al. 2008 | |
| RAR1 and SGT1 | Leaf | Compromised resistance against SMV and P. syringae | Fu et al. 2009 | |
| Actin gene | Leaf and root | Severe mosaic, leaf deformation, stunting and reduced SMV accumulation | Zhang et al. 2009 | |
| Rebosomal protein genes Rps6 and Rps13 | Leaf and root | Very severe foliar symptoms and stunted root growth | ||
| Mpk4A and Mpk4B | Leaf and root | Stunting | ||
| Sgt1A and Sgt1B | Leaf and root | Mild symptom similar to the empty vector-infected control | ||
| Candidate genes for soybean rust resistance | Leaf | Compromised resistance against P. pachyrhizi | Meyer et al. 2009 | |
| Candidate genes for soybean rust resistance | Leaf | Compromised resistance against P. pachyrhizi | Pandey et al. 2011 | |
| Fatty acid desaturase gene FAD3 | Root, stem, leaf, petiole and seed | Increased seed size and susceptibility to P. syringae and BPMV accumulation | Singh et al. 2011 | |
| Cucumber mosaic virus | Chalcone synthase gene | Seed coat and leaf | Loss of pigmentation in seed coat | Nagamatsu et al. 2007 |
| Chalcone synthase gene | Seed (cotyledon) | Reduced isoflavone | ||
| Flavonoid 3′-hydroxylase gene | Leaf | Reduced quercetin | ||
| Flavonoid 3′-hydroxylase gene | Leaf and pubescence | Changes in pubescence color | Nagamatsu et al. 2009 | |
| GmTFL1b | Node, pod, and root | Reduced node number | Liu et al. 2010 | |
| Apple latent spherical virus | Phytoen desaturase gene | Leaf, pod, seed coat and embryo | Photo-bleaching | Yamagishi and Yoshikawa 2009 |
| Isoflavone synthase 2 gene | Seed (cotyledon) | Reduced isoflavone content |
Abbreviations: SA, salicylic acid; RAR1, required for Mla12-mediated resistance; SGT1, suppressor of G2 allele of skp1; SMV, Soybean mosaic virus; P. syringae, Pseudomonas syringae; Mpk, mitogen-activated protein (MAP) kinase; TFL, terminal flower; P. pachyrhizi, Phakopsora pachyrhizi; BPMV, Bean pod mottle virus.
An interesting finding reported in soybean is that RNA silencing is induced by a transgene that transcribes inverted repeats of a fatty acid desaturase FAD2-1A intron (Wagner et al. 2011). This result is contrary to the earlier belief that RNA silencing is a cytoplasmic event and intron does not trigger RNA degradation, which has been shown, for example, by using viral vector in plants (Ruiz et al. 1998) or by dsRNA injection to C. elegans cells (Fire et al. 1998), although irregular nuclear processing of primary transcripts associated with PTGS/RNAi has been reported previously (Metzlaff et al. 2000). The FAD2-A1 intron-induced RNA silencing led to the understanding that RNA degradation can take place in the nucleus (Hoffer et al. 2011). Although whether RNA degradation in the nucleus is inducible for other genes or in other plants is not known, this phenomenon is intriguing because the involvement of nuclear events has been assumed for amplification of RNA silencing via transitivity (Vermeersch et al. 2010) or intron-mediated suppression of RNA silencing (Christie et al. 2011).
Transcribing a transgene with a strong promoter tends to induce RNA silencing more frequently than that with a weak promoter (Que et al. 1997). For obtaining a higher level of transcription in soybean plants, the Cauliflower mosaic virus (CaMV) promoter has been used as in other plant species. Seed-specific promoters, such as those derived from the genes encoding subunits of β-conglycinin, glycinin, or Kunitz trypsin inhibitor, have also been used in soybean to induce seed-specific silencing, one feature that is exploited for metabolic engineering in soybean.
A gene construct that induces RNA silencing has been introduced to the soybean genome using either Agrobacterium tumefaciens infection or particle bombardment, which can produce stable transgenic soybean lines that have altered traits. In addition, RNA silencing can be induced in soybean roots using A. rhizogenes-mediated transformation, which has been used for gene functional analysis. Methods for soybean transformation are reviewed in another article of this issue (Yamada et al. 2012).
Metabolic engineering of soybean plants by transgene-induced RNA silencing
Because soybean seeds are valued economically for food and oil production, most modifications to transgenic soybean plants using RNA silencing are focused on seed components. Metabolic pathways in developing seeds have been targeted in terms of altering nutritional value for human or animals, e.g., changing seed storage protein composition (Kinney et al. 2001, Schmidt et al. 2011), and reducing phytic acids (Nunes et al. 2006, Shi et al. 2007), saponin (Takagi et al. 2011) or allergens (Herman et al. 2003) (Table 1). Metabolic engineering has also targeted oil production (Chen et al. 2006, Flores et al. 2008, Kinney 1996, Lee et al. 2011, Schmidt and Herman 2008, Wagner et al. 2011, Wang and Xu 2008) (Table 1). These modifications were done by inhibiting a step in a metabolic pathway to decrease a product. On the other hand, RNA silencing can also be used to increase the concentration of a specific metabolite. For example, Yu et al. (2003) has produced transgenic soybeans that contain more isoflavone. They induced the activation of genes involved in phenylpropanoid pathway by introducing a transcription factor gene and by blocking a competing branch pathway via co-suppression. Similarly, Artkit et al. (2011) demonstrated that RNA silencing of the amino aldehyde dehydrogenase gene induced the biosynthesis of a volatile compound, 2-acetyl-1-pyrroline, in soybean calli, an outcome expected from studies in rice.
RNA silencing can be induced efficiently in soybean roots using A. rhizogenes-mediated root transformation. This method has been used for analyzing roles of gene products in nodule development and/or function, which occurs as a consequence of interaction between legume plants and the nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum (Dalton et al. 2009, Govindarajulu et al. 2009, Hayashi et al. 2008, Lee et al. 2005, Libault et al. 2010, Subramanian et al. 2006). The hairy root system was also used for analyzing roles of a MYB transcription factor in isoflavonoid biosynthesis (Yi et al. 2010).
Transgene-induced RNA silencing has also been induced in leaf tissues for the β-glucuronidase gene (Reddy et al. 2003) or the senescence-associated receptor-like kinase gene (Li et al. 2006).
Disease resistance of soybean plants by transgene-induced RNA silencing
Another focus of modifying soybean plants through RNA silencing is resistance against diseases, particularly to those caused by viruses. The effects of gene silencing in plants were first used to develop resistance to viral diseases, even though the mechanism was not clear at the time. Resistance to viruses was achieved by transforming plants with genes or segments of genes derived from viruses and was referred to as pathogen-derived resistance (reviewed by Baulcombe 1996, Goldbach et al. 2003, Prins and Goldbach 1996, Wilson 1993). The resistance did not need protein translated from the transgene (Mueller et al. 1995, Sijen et al. 1996, Smith et al. 1994), which led to the understanding that RNA is the factor that conferred resistance to the plants and that the enhanced resistance is acquired via a mechanism analogous to that involved in co-suppression. Use of transgene-induced RNA silencing for plants to acquire resistance against viruses has been reported for various combinations of plants and viruses (reviewed by Baulcombe 1996, Goldbach et al. 2003, Mansoor et al. 2006). Using this strategy, soybean plants resistant to Soybean mosaic virus (SMV; Furutani et al. 2006, 2007, Wang et al. 2001), or Soybean dwarf virus (Tougou et al. 2006, 2007) have been produced (Table 2).
In addition to resistance against a virus, transgenic soybean plants resistant to cyst nematode (Heterodera glycines) have also been produced using RNA silencing (Steeves et al. 2006), in which an inverted repeat of the major sperm protein gene from cyst nematode was transcribed from the transgene. RNA silencing was elicited in cyst nematode after nematode ingestion of dsRNA molecules produced in the soybean plants; as a consequence, reproductive capabilities of the cyst nematode were suppressed. The effects of RNA silencing on controlling H. glycines (Li et al. 2010) or root-knot nematode (Meloidogyne incognita) (Ibrahim et al. 2011) infection have been assayed in soybean roots using A. rhizogenes-mediated transformation. On the other hand, this root transformation method has also been used for analyzing a role of host genes in resistance against diseases caused by Phytophthora sojae (Graham et al. 2007, Subramanian et al. 2005), Fusarium solani (Lozovaya et al. 2007) or cyst nematode (Melito et al. 2010).
VIGS as a powerful tool to analyze gene function
Although transgenes that express a virus-derived gene or gene segment can confer enhanced resistance against virus, plants intrinsically have the ability to cope with viruses. When plants are infected with an RNA virus, dsRNA of the viral genome is degraded by the infected plants (Al-Kaff et al. 1998, Covey et al. 1997). The dsRNA in the virus-infected cells is thought to be the replication intermediate of the viral RNA (Lu et al. 2003) or a duplex structure formed within single-stranded viral RNA (Molnar et al. 2005). The viral genomic RNA can be processed into siRNAs, then targeted by the siRNA/RNase complex. In this scenario, if a nonviral segment is inserted in the viral genome, siRNAs would also be produced from the segment. Therefore, if the insert corresponds to a sequence of the gene encoded in the host plant, infection by the virus results in the production of siRNAs corresponding to the plant gene and subsequently induces loss of function of the gene product. This fact led to the use of a virus vector as a source to induce silencing of a specific gene in the plant genome, which is referred to as virus-induced gene silencing (VIGS; Kumagai et al. 1995, Purkayastha and Dasgupta 2009, Ruiz et al. 1998). So far, at least 11 RNA viruses and five DNA viruses were developed as a plant virus vector for gene silencing, as listed previously (Kanazawa 2008). Three vectors are now available in soybean: those based on Bean pod mottle virus (BPMV; Zhang and Ghabrial 2006), Cucumber mosaic virus (CMV; Nagamatsu et al. 2007) and Apple latent spherical virus (ALSV; Yamagishi and Yoshikawa 2009) (Table 3).
An advantage of VIGS is its ease for making a gene construct and introducing nucleic acids to cells. In addition, the effect of silencing can be monitored within a short time after inoculating plants with the virus. Because of these features, VIGS is suitable for gene function analysis (reviewed by Burch-Smith et al. 2004, Lu et al. 2003, Metzlaff 2002) and has been used for gene identification via downregulating a candidate gene(s) responsible for a specific phenomenon in soybean. When Nagamatsu et al. (2007) tested VIGS on the putative flavonoid 3′-hydroxylase (F3′H) gene, the content of quercetin was decreased relative to kaempferol in the upper leaves after viral infection, which indicated that the putative gene actually encodes the F3′H protein. Nagamatsu et al. (2007) also demonstrated that VIGS of CHS genes resulted in lack of pigmentation in the seed coat tissues. Similarly, VIGS was used to confirm that the GmTFL1b gene, a candidate gene for the genetically identified locus Dt1, actually controls the determinate habit of soybean plants (Liu et al. 2010). VIGS has also been used to identify genes involved in resistance of soybean plants against pathogens such as SMV, BPMV, Pseudomonas syringae or Phakopsora pachyrhizi (Fu et al. 2009, Kachroo et al. 2008, Meyer et al. 2009, Pandey et al. 2011, Singh et al. 2011).
Specific features of VIGS
The extent of the induction of silencing is not equivalent between different portions of virus-infected plants because induction of the silencing is associated with propagation of the virus whose extent is often different in different parts of the host plants. This conditional nature of VIGS may have both positive and negative aspects in terms of using the technology for functional genomics. Although the instability may be a negative aspect of VIGS, it may in turn be an advantage by allowing observation of phenotypic changes caused by the dysfunction of a gene whose complete loss of expression is lethal to the plant (Lu et al. 2003). In fact, phenotypic changes have been induced by VIGS of the gene for proliferating cell nuclear antigen (Peele et al. 2001) and RNA polymerase II (Gosselé et al. 2002), for which null mutants cannot be retrieved by conventional or insertional mutagenesis approaches. Similarly, the effect of downregulation of the actin and ribosomal protein genes was detected using VIGS in soybean (Zhang et al. 2009).
VIGS has also been applied to genes whose products have a function and/or accumulate during seed development. Whether genes are actually downregulated in developing embryo or donwnregulated in other tissues and the level of transported products is decreased in seeds is intriguing. Yamagishi and Yoshikawa (2009) showed that RNA silencing of the phytoene desaturase and isoflavone synthase 2 genes actually occurs in soybean embryos, resulting in photo-bleaching and a decrease in isoflavone content, respectively, by the ALSV vector. A decrease in isoflavone content in soybean embryos has also been induced by the CMV vector (Nagamatsu et al. 2007).
Modification and optimization of VIGS in soybean
When VIGS is used to analyze the function of a gene, viral infection itself might be a problem depending on the target gene. Symptoms of virus infection indicate that gene expression in the infected cells has been affected. If a gene with expression affected by viral infection is chosen as the target of VIGS, the effect of VIGS might not appear as a specific effect caused by the sequence-specific degradation of the RNA, but a nonspecific effect of the viral infection might also be involved. Accordingly, efforts are sometimes needed to reduce the extent of nonspecific effects of viral infection and simultaneously efficiently induce VIGS. In this respect, when a new combination of plant species and virus vector is used, it is often necessary to control the efficiency of viral infection and symptom production to optimize the induction of VIGS (Kanazawa 2008).
Symptomless viral infection of soybean has been achieved using CMV (Nagamatsu et al. 2007) and ALSV (Yamagishi and Yoshikawa 2009). In case of CMV infection, a pseudorecombinant virus that consists of RNA components derived from different CMV strains were used to establish symptomless infection of the virus (Nagamatsu et al. 2007). Moreover, VIGS that accompanies neither severe viral symptoms nor outward phenotypic changes has been achieved by targeting the F3′H gene in soybean, while the flavonoid content was successfully modified by the VIGS (Nagamatsu et al. 2007).
For infection of soybean plants with RNA viruses, tissues of young plants are inoculated with in-vitro-generated transcripts of the viral genome or with the sap or RNA extracted from an infected leaf of other plants. Infection of soybean plants with ALSV was done by the following method. Plasmids containing ALSV cDNA were inoculated onto Chenopodium quinoa leaves. RNA extracted from the leaves was then introduced into cotyledons of soybean seedlings through particle bombardment-mediated delivery (Yamagishi and Yoshikawa 2009). For infection of soybean plants with CMV, the virus was first propagated in Nicotiana benthamiana plants: leaves of N. benthamiana were rub-inoculated with the in-vitro-generated transcripts of viral cDNA. Then, the primary leaves of soybean plants were inoculated with the sap from an infected leaf of the N. benthamiana plant (Nagamatsu et al. 2007). Infection of soybean plants with in-vitro-generated transcripts was also possible (Kanazawa et al., unpublished data). A similar method was also used for infection of BPMV (Zhang and Ghabrial 2006). The method of infection of soybean plants with BPMV has been modified as follows. Zhang et al. (2009) made DNA constructs in which the cDNA of the BPMV RNAs is transcribed under the control of the CaMV 35S promoter. They achieved infection of soybean plants with the virus through particle bombardment-mediated delivery of these DNA constructs to facilitate VIGS experiments. Zhang et al. (2010) also modified the BPMV vector and overcame its constraint that target sequences must be expressed as fusion proteins with a viral polypeptide.
Use of viral infection as a tool to “diagnose” an RNA-silencing-induced phenotype
Another interesting aspect of the use of viruses for the study of RNA silencing in plants is the function of a virus-encoded suppressor protein of RNA silencing. These suppressor proteins affect viral accumulation in plants. The ability of the suppressor protein to allow viral accumulation is due to its inhibition of RNA silencing by preventing the incorporation of siRNAs into RISCs or by interfering with RISCs (reviewed by Silhavy and Burgyan 2004). It has been known that the lack of brown pigmentation in the seed coat of soybean is caused by naturally occurring CHS RNA silencing (Senda et al. 2004, reviewed by Senda et al. 2012). When a soybean plant that has a yellow seed coat is infected with CMV, the seed coat restores pigmentation (Senda et al. 2004). This phenomenon is due to the activity of gene silencing suppressor protein called 2b encoded by the CMV. This example typically indicates that, using the function of viral suppressor protein, we can “diagnose” whether an observed phenotypic change in a plant is caused by RNA silencing. A similar phenomenon has also been detected in maize (Della Vedova et al. 2005) and petunia (Koseki et al. 2005), both of which have phenotypic changes through naturally occurring RNA silencing of an endogenous gene.
RNA silencing as a tool to understand regulatory mechanisms of biological phenomenon associated with mRNA level of a gene
RNA silencing of a particular gene is also useful for analyzing biological phenomena, in particular those involving the effect of a difference in the mRNA level of the gene. For example, the regulatory mechanisms of pigmentation in soybean pubescence was analyzed using VIGS of the F3′H gene, whose function is necessary for pigmentation of soybean pubescence. Silencing did not result in lack of pigmentation when plants were grown in normal greenhouse conditions, but plants lacked pigmentation when grown in controlled conditions; the steady-state mRNA level of the F3′H gene was reduced to ca. 5% of that of greenhouse-grown plants (Nagamatsu et al. 2009). VIGS in the controlled conditions resulted in a further decrease in the mRNA level, which led to the discovery that a threshold mRNA level of the F3′H gene was associated with the pigmented pubescence (Nagamatsu et al. 2009).
Future prospects of the use of RNA silencing in soybean
1. Stability and heritability of RNA silencing
Induction of transgene-mediated RNA silencing can be affected by various factors such as structure, copy number, or expression level of the transgene, environmental conditions or developmental stages of the plant (Majewski et al. 2009, and references therein). In addition, induction of transgene-mediated RNA silencing can be destabilized during cell proliferation and appears to be re-initiated in each generation (Furutani et al. 2007, Mitsuhara et al. 2002). However, transgene-mediated RNA silencing can induce a strong, tissue-specific or ubiquitous silencing and is suitable for producing plants in which one or more genes are stably silenced in the presence of the transgene as far as the transgene is capable of inducing the silencing. On the other hand, because of elimination of viruses during meiosis, VIGS is basically transient and is confined to the plants in which the virus is inoculated. An exceptional VIGS vector applicable to soybean is ALSV: it can transmit to the next generation and induce silencing across generations (Yamagishi and Yoshikawa 2009).
Heritable silencing can be induced in plants via induction of epigenetic changes by CMV, although the virus does not transmit to the subsequent generation (Kanazawa et al. 2011a, 2011b). Gene silencing through transcriptional repression can be induced by dsRNA targeted to a gene promoter (reviewed by Matzke et al. 2004). However, until recently, no plant has been produced that harbors an endogenous gene that remains silenced in the absence of promoter-targeting dsRNA. We have reported for the first time that TGS can be induced by targeting dsRNA to the endogenous gene promoters in petunia and tomato plants, using a CMV-based vector and that the induced gene silencing is heritable (Kanazawa et al. 2011a). Efficient silencing depended on the function of the 2b protein encoded in the vector, which facilitates epigenetic modifications through the transport of siRNA to the nucleus (Kanazawa et al. 2011a). The efficiency of promoter-targeted silencing also depends on features of promoter RNA segments (e.g., length and nucleotide composition) (Otagaki et al. 2011). An advantage of the RNA-mediated TGS using the CMV vector is that the progeny plants do not have any transgene because the virus is eliminated during meiosis. Plants that are produced by this system have altered traits but do not carry a transgene, thus constituting a novel class of modified plants (Kanazawa et al. 2011b). Because of the availability of the vector in soybean, VIGS can potentially be used for producing such novel class of plants in soybean as well.
2. RNA silencing as a tool to analyze duplicated genes
One feature of the soybean genome is the presence of a large number of duplicated genes. Soybean is thought to be derived from an ancestral plant(s) with a tetraploid genome, and as a consequence, large portions of the soybean genome are duplicated (Shoemaker et al. 1996), with nearly 75% of the genes present in multiple copies (Schmutz et al. 2010). In addition, genes in the soybean genome are sometimes duplicated in tandem (e.g., Kong et al. 2010, Matsumura et al. 2005, Schlueter et al. 2008, Yoshino et al. 2002). Our recent studies have indeed shown functional redundancy of duplicated genes in soybean (Kanazawa et al. 2009, Liu et al. 2008). Such gene duplication can be an obstacle to producing mutants by conventional methods of mutagenesis. In this regard, the gene silencing technique is particularly useful because it allows silencing of multiple cognate genes having nucleotide sequence identity.
In addition, it is of interest to understand whether duplicated genes have identical or diversified functions, which may depend on the time after duplication event and/or the selection pressure on the genes. To analyze the functions of each copy of the duplicated genes, we need to silence a specific copy of the duplicated genes. In plants, siRNAs promote production of secondary siRNAs from 5′ and/or 3′ of the initially targeted region via production of dsRNA by RdRP. These secondary siRNAs can lead to silencing of a secondary target that is not directly targeted by the primary silencing trigger (reviewed by Voinnet 2008). Studies so far have indicated that such a spread of RNA silencing, called transitive RNA silencing, does not occur with the majority of endogenous genes, although it can happen to a transgene (Vermeersch et al. 2010, and references therein). Assuming the lack of transitive RNA silencing, it is possible to induce silencing of a specific copy of a duplicated gene. Targeting a region specific for each copy, e.g., the 3′ UTR, can induce silencing of the gene copy only, whereas targeting a region conserved in duplicated gene copies can induce silencing of the multiple gene copies simultaneously. Such selective RNA silencing was successful in a gene family of rice (Miki et al. 2005) and this strategy may work for analyzing functional diversification of duplicated genes in any plant species. Considering the presence of a large number of gene-level duplication and/or chromosomal segmental duplication, it should also be noted that naturally occurring gene silencing may be discovered in soybean in the future in addition to CHS silencing, which is manifested as a visibly altered phenotype (Senda et al. 2004, 2011).
3. Potential targets and methods
Tolerance to abiotic stress and fertility control through RNA silencing have been reported for various plants (Mansoor et al. 2006). These traits can be a future target of RNA silencing in soybean. Attempts to induce RNA silencing only in seeds will increase because genes responsible for the synthesis of seed components are sometimes essential for normal vegetative growth, whose downregulation in nonseed tissues might have deleterious effects on plant growth. A typical example is the embryo-specific silencing of a transporter gene, which results in reduction of phytic acid content in soybean seeds without inducing undesirable agronomic characters associated with phytic acid reduction (Shi et al. 2007).
A recently introduced approach to suppress gene expression in plants is the use of artificial miRNAs (amiRNAs; also called synthetic miRNAs; reviewed by Frizzi and Huang 2010, Ossowski et al. 2008). This approach involves modification of plant miRNA sequence to target specific transcripts, originally not under miRNA control, and down-regulation of gene expression via specific cleavage of the target RNA. This method has been applied to target viral RNA (Niu et al. 2006) and transcripts of endogenous genes in plants (Alvarez et al. 2006, Schwab et al. 2006). In soybean, Melito et al. (2010) have used amiRNA to downregulate the leucine-rich repeat transmembrane receptor-kinase gene. Considering that miRNA has been extensively studied in soybean (e.g., Song et al. 2011), amiRNA can be the method of choice for RNA silencing in soybean. Because of its specificity, this method will be useful for silencing a limited copy of duplicated genes.
Such a reverse genetic approach may also be supplemented by forward genetic approaches already done in soybean such as high linear energy transfer radiation-based mutagenesis, e.g., irradiation of ion beam (Arase et al. 2011) and fast neutron (Bolon et al. 2011), which potentially bring about a large deletion in the genome. Similarly, a gene tagging system using the Ds transposon (Mathieu et al. 2009) has also been developed in soybean. A combination with these different approaches will increase the applicability of RNA silencing.
Acknowledgements
We thank the editorial board members of this special issue, especially Jun Abe and Masao Ishimoto, for giving us the opportunity to write this review and discussing the focus of it. We thank Kenji Nakahara for helpful discussions on the virus resistance of transgenic plants. We also thank Chikara Masuta for our cooperative researches of RNA silencing cited in this review. We are grateful to coworkers of our research projects and to many colleagues for interesting discussion. Our work is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Literature Cited
- Al-Kaff NS, Covey SN, Kreike MM, Page AM, Pinder R, Dale PJ. Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science. 1998;279:2113–2115. doi: 10.1126/science.279.5359.2113. [DOI] [PubMed] [Google Scholar]
- Alvarez JP, Pekker I, Goldshmidt A, Blum E, Amsellem Z, Eshed Y. Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell. 2006;18:1134–1151. doi: 10.1105/tpc.105.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase S, Hase Y, Abe Y, Kasai M, Yamada T, Kitamura K, Narumi I, Tanaka A, Kanazawa A. Optimization of ion-beam irradiation for mutagenesis in soybean: effects on plant growth and production of visibly altered mutants. Plant Biotechnol. 2011;28:323–329. [Google Scholar]
- Arikit S, Yoshihashi T, Wanchana S, Uyen TT, Huong NT, Wongpornchai S, Vanavichit A. Deficiency in the amino aldehyde dehydrogenase encoded by GmAMADH2, the homologue of rice Os2AP, enhances 2-acetyl-1-pyrroline biosynthesis in soybeans (Glycine max L.) Plant Biotechnol J. 2011;9:75–87. doi: 10.1111/j.1467-7652.2010.00533.x. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. Mechanisms of pathogen-derived resistance to viruses in transgenic plants. Plant Cell. 1996;8:1833–1844. doi: 10.1105/tpc.8.10.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Bolon Y-T, Haun WJ, Xu WW, Grant D, Stacey MG, Nelson RT, Gerhardt DJ, Jeddeloh JA, Stacey G, Muehlbauer GJ, et al. Phenotypic and genomic analyses of a fast neutron mutant population resource in soybean. Plant Physiol. 2011;156:240–253. doi: 10.1104/pp.110.170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Voinnet O. The diversity of RNA silencing pathways in plants. Trends Genet. 2006;22:268–280. doi: 10.1016/j.tig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Matsui K, Ogawa M, Oe M, Ochiai M, Kawashima H, Sakuradani E, Shimizu S, Ishimoto M, Hayashi M, et al. Expression of Δ6, Δ5 desaturase and GLELO elongase genes from Mortierella alpina for production of arachidonic acid in soybean [Glycine max (L.) Merrill] seeds. Plant Sci. 2006;170:399–406. [Google Scholar]
- Christie M, Croft LJ, Carroll BJ. Intron splicing suppresses RNA silencing in Arabidopsis. Plant J. 2011 doi: 10.1111/j.1365-313X.2011.04676.x. (in press) [DOI] [PubMed] [Google Scholar]
- Covey SN, Al-Kaff NS, Lángara A, Turner DS. Plants combat infection by gene silencing. Nature. 1997;385:781–782. [Google Scholar]
- Dalton DA, Boniface C, Turner Z, Lindahl A, Kim HJ, Jelinek L, Govindarajulu M, Finger RE, Taylor CG. Physiological roles of glutathione S-transferases in soybean root nodules. Plant Physiol. 2009;150:521–530. doi: 10.1104/pp.109.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Vedova CB, Lorbiecke R, Kirsch H, Schulte MB, Scheets K, Borchert LM, Scheffler BE, Wienand U, Cone KC, Birchler JA. The dominant inhibitory chalcone synthase allele C2-Idf (Inhibitor diffuse) from Zea mays (L.) acts via an endogenous RNA silencing mechanism. Genetics. 2005;170:1989–2002. doi: 10.1534/genetics.105.043406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P, Gregory S, Himber C, Meyer D, Takeda A, Carrington JC, Voinnet O. Small RNA duplexes function as mobile silencing signals between plant cells. Science. 2010;238:912– 916. doi: 10.1126/science.1185880. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Flores T, Karpova O, Su X, Zeng P, Bilyeu K, Sleper DA, Nguyen HT, Zhang ZJ. Silencing of GmFAD3 gene by siRNA leads to low a-linolenic acids (18: 3) of fad3-mutant phenotype in soybean [Glycine max (Merr.)] Transgenic Res. 2008;17:839–850. doi: 10.1007/s11248-008-9167-6. [DOI] [PubMed] [Google Scholar]
- Frizzi A, Huang S. Tapping RNA silencing pathways for plant biotechnology. Plant Biotechnol J. 2010;8:655–677. doi: 10.1111/j.1467-7652.2010.00505.x. [DOI] [PubMed] [Google Scholar]
- Fu DQ, Ghabrial S, Kachroo A. GmRAR1 and GmSGT1 are required for basal, R gene–mediated and systemic acquired resistance in soybean. Mol Plant Microbe Interact. 2009;22:86–95. doi: 10.1094/MPMI-22-1-0086. [DOI] [PubMed] [Google Scholar]
- Furutani N, Hidaka S, Kosaka Y, Shizukawa Y, Kanematsu S. Coat protein gene-mediated resistance to soybean mosaic virus in transgenic soybean. Breed Sci. 2006;56:119–124. [Google Scholar]
- Furutani N, Yamagishi N, Hidaka S, Shizukawa Y, Kanematsu S, Kosaka Y. Soybean mosaic virus resistance in transgenic soybean caused by post-transcriptional gene silencing. Breed Sci. 2007;57:123–128. [Google Scholar]
- Goldbach R, Bucher E, Prins M. Resistance mechanisms to plant viruses: an overview. Virus Res. 2003;92:207–212. doi: 10.1016/s0168-1702(02)00353-2. [DOI] [PubMed] [Google Scholar]
- Gosselé V, Faché I, Meulewaeter F, Cornelissen M, Metzlaff M. SVISS—a novel transient gene silencing system for gene function discovery and validation in tobacco plants. Plant J. 2002;32:859–866. doi: 10.1046/j.1365-313x.2002.01471.x. [DOI] [PubMed] [Google Scholar]
- Govindarajulu M, Kim S-Y, Libault M, Berg RH, Tanaka K, Stacey G, Taylor CG. GS52 ecto-apyrase plays a critical role during soybean nodulation. Plant Physiol. 2009;149:994–1004. doi: 10.1104/pp.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TL, Graham MY, Subramanian S, Yu O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonoids suppresses race-specific resistance and hypersensitive cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007;144:728–740. doi: 10.1104/pp.107.097865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Gresshoff PM, Kinkema M. Molecular analysis of lipoxygenases associated with nodule development in soybean. Mol Plant Microbe Interact. 2008;21:843–853. doi: 10.1094/MPMI-21-6-0843. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Waterhouse PM. Constructs and methods for hairpin RNA-mediated gene silencing in plants. Methods Enzymol. 2005;392:24–35. doi: 10.1016/S0076-6879(04)92002-2. [DOI] [PubMed] [Google Scholar]
- Herman EM, Helm RM, Rudolf J, Kinney AJ. Genetic modification removes an immunodominant allergen from soybean. Plant Physiol. 2003;132:36–43. doi: 10.1104/pp.103.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer P, Ivashuta S, Pontes O, Vitins A, Pikaard C, Mroczka A, Wagner N, Voelker T. Posttranscriptional gene silencing in nuclei. Proc. Natl. Acad. Sci. USA. 2011;108:409–414. doi: 10.1073/pnas.1009805108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HMM, Alkharouf NW, Meyer SLF, Aly MAM, El-Din AEKYG, Hussein EHA, Matthews BF. Post-transcriptional gene silencing of root-knot nematode in transformed soybean roots. Exp Parasitol. 2011;127:90–99. doi: 10.1016/j.exppara.2010.06.037. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Fu DQ, Havens W, Navarre D, Kachroo P, Ghabrial SA. An oleic acid–mediated pathway induces constitutive defense signaling and enhanced resistance to multiple pathogens in soybean. Mol Plant Microbe Interact. 2008;21:564–575. doi: 10.1094/MPMI-21-5-0564. [DOI] [PubMed] [Google Scholar]
- Kanazawa A. RNA silencing manifested as visibly altered phenotypes in plants. Plant Biotechnol. 2008;25:423–435. [Google Scholar]
- Kanazawa A, Liu B, Kong F, Arase S, Abe J. Adaptive evolution involving gene duplication and insertion of a novel Ty1/copia-like retrotransposon in soybean. J Mol Evol. 2009;69:164–175. doi: 10.1007/s00239-009-9262-1. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Inaba J, Shimura H, Otagaki S, Tsukahara S, Matsuzawa A, Kim BM, Goto K, Masuta C. Virus-mediated efficient induction of epigenetic modifications of endogenous genes with phenotypic changes in plants. Plant J. 2011a;65:156–168. doi: 10.1111/j.1365-313X.2010.04401.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Inaba J, Kasai M, Shimura H, Masuta C. RNA-mediated epigenetic modifications of an endogenous gene targeted by a viral vector: a potent gene silencing system to produce a plant that does not carry a transgene but has altered traits. Plant Signal Behav. 2011b;6:1090–1093. doi: 10.4161/psb.6.8.16046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney AJ. Development of genetically engineered soybean oils for food applications. J. Food Lipids. 1996;3:273–292. [Google Scholar]
- Kinney AJ, Jung R, Herman EM. Cosuppression of the α subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum–derived protein bodies. Plant Cell. 2001;13:1165–1178. doi: 10.1105/tpc.13.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Liu B, Xia Z, Sato S, Kim B, Watanabe S, Yamada T, Tabata S, Kanazawa A, Harada K, et al. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiol. 2010;154:1220–1231. doi: 10.1104/pp.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseki M, Goto K, Masuta C, Kanazawa A. The star-type color pattern in Petunia hybrida ‘Red Star’ flowers is induced by sequence-specific degradation of chalcone synthase RNA. Plant Cell Physiol. 2005;46:1879–1883. doi: 10.1093/pcp/pci192. [DOI] [PubMed] [Google Scholar]
- Kumagai MH, Donson J, della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. Sci. USA. 1995;92:1679– 1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-Y, Shin K-H, Kim Y-K, Suh J-Y, Gu Y-Y, Kim M-R, Hur Y-S, Son O, Kim J-S, Song E, et al. Induction of thioredoxin is required for nodule development to reduce reactive oxygen species levels in soybean roots. Plant Physiol. 2005;139:1881–1889. doi: 10.1104/pp.105.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Welti R, Schapaugh WT, Trick HN. Phospholipid and triacylglycerol profiles modified by PLD suppression in soybean seed. Plant Biotechnol J. 2011;9:359–372. doi: 10.1111/j.1467-7652.2010.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Todd TC, Oakley TR, Lee J, Trick HN. Host-derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines Ichinohe. Planta. 2010;232:775–785. doi: 10.1007/s00425-010-1209-7. [DOI] [PubMed] [Google Scholar]
- Li X-P, Gan R, Li P-L, Ma Y-Y, Zhang L-W, Zhang R, Wang Y, Wang NN. Identification and functional characterization of a leucine-rich repeat receptor-like kinase gene that is involved in regulation of soybean leaf senescence. Plant Mol Biol. 2006;61:829–844. doi: 10.1007/s11103-006-0052-5. [DOI] [PubMed] [Google Scholar]
- Libault M, Zhang XC, Govindarajulu M, Qiu J, Ong YT, Brechenmacher L, Berg RH, Hurley-Sommer A, Taylor CG, Stacey G. A member of the highly conserved FWL (tomato FW2.2-like) gene family is essential for soybean nodule organogenesis. Plant J. 2010;62:852–864. doi: 10.1111/j.1365-313X.2010.04201.x. [DOI] [PubMed] [Google Scholar]
- Liu B, Kanazawa A, Matsumura H, Takahashi R, Harada K, Abe J. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics. 2008;180:995–1007. doi: 10.1534/genetics.108.092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Watanabe S, Uchimiya T, Kong F, Kanazawa A, Xia Z, Nagamatsu A, Arai M, Yamada T, Kitamura K, et al. Soybean stem growth habit gene Dt1 is an orthologue of Arabidopsis TFL1. Plant Physiol. 2010;153:198–210. doi: 10.1104/pp.109.150607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya VV, Lygin AV, Zernova OV, Ulanov AV, Li S, Hartman GL, Widholm JM. Modification of phenolic metabolism in soybean hairy roots through down regulation of chalcone synthase or isoflavone synthase. Planta. 2007;225:665–679. doi: 10.1007/s00425-006-0368-z. [DOI] [PubMed] [Google Scholar]
- Lu R, Martin-Hernandez AM, Peart JR, Malcuit I, Baulcombe DC. Virus-induced gene silencing in plants. Methods. 2003;30:296–303. doi: 10.1016/s1046-2023(03)00037-9. [DOI] [PubMed] [Google Scholar]
- Majewski P, Wołoszyńska M, Jańska H. Developmentally early and late onset of Rps10 silencing in Arabidopsis thaliana: genetic and environmental regulation. J Exp Bot. 2009;60:1163–1178. doi: 10.1093/jxb/ern362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory AC, Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006;38(Suppl):S31–36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- Mansoor S, Amin I, Hussain M, Zafar Y, Briddon RW. Engineering novel traits in plants through RNA interference. Trends Plant Sci. 2006;11:559–565. doi: 10.1016/j.tplants.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Mathieu M, Winters EK, Kong F, Wan J, Wang S, Eckert H, Luth D, Paz M, Donovan C, Zhang Z, et al. Establishment of a soybean (Glycine max Merr. L) transposon-based mutagenesis repository. Planta. 2009;229:279–289. doi: 10.1007/s00425-008-0827-9. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Watanabe S, Harada K, Senda M, Akada S, Kawasaki S, Dubouzet EG, Minaka N, Takahashi R. Molecular linkage mapping and phylogeny of the chalcone synthase multigene family in soybean. Theor Appl Genet. 2005;110:1203–1209. doi: 10.1007/s00122-005-1950-7. [DOI] [PubMed] [Google Scholar]
- Matzke M, Matzke AJM, Kooter JM. RNA: guiding gene silencing. Science. 2001;293:1080–1083. doi: 10.1126/science.1063051. [DOI] [PubMed] [Google Scholar]
- Matzke M, Aufsatz W, Kanno T, Daxinger L, Papp I, Mette MF, Matzke AJM. Genetic analysis of RNA-mediated transcriptional gene silencing. Biochim Biophys Acta. 2004;1677:129–141. doi: 10.1016/j.bbaexp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Matzke M, Kanno T, Daxinger L, Huettel B, Matzke AJM. RNA-mediated chromatin-based silencing in plants. Curr Opin Cell Biol. 2009;21:367–376. doi: 10.1016/j.ceb.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Melito S, Heuberger AL, Cook D, Diers BW, MacGuidwin AE, Bent AF. A nematode demographics assay in transgenic roots reveals no significant impacts of the Rhg1 locus LRR-Kinase on soybean cyst nematode resistance. BMC Plant Biol. 2010;10:104. doi: 10.1186/1471-2229-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzlaff M. RNA-mediated RNA degradation in transgene-and virus-induced gene silencing. Biol Chem. 2002;383:1483–1489. doi: 10.1515/BC.2002.170. [DOI] [PubMed] [Google Scholar]
- Metzlaff M, O’Dell M, Cluster PD, Flavell RB. RNA-mediated degradation and chalcone synthase A silencing in petunia. Cell. 1997;88:845–854. doi: 10.1016/s0092-8674(00)81930-3. [DOI] [PubMed] [Google Scholar]
- Metzlaff M, O’Dell M, Hellens R, Flavell RB. Developmentally and transgene regulated nuclear processing of primary transcripts of chalcone synthase A in petunia. Plant J. 2000;23:63–72. doi: 10.1046/j.1365-313x.2000.00793.x. [DOI] [PubMed] [Google Scholar]
- Meyer JDF, Silva DCG, Yang C, Pedley KF, Zhang C, van de Mortel M, Hill JH, Shoemaker RC, Abdelnoor RV, Whitham SA, et al. Identification and analyses of candidate genes for Rpp4-mediated resistance to asian soybean rust in soybean. Plant Physiol. 2009;150:295–307. doi: 10.1104/pp.108.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Itoh R, Shimamoto K. RNA silencing of single and multiple members in a gene family of rice. Plant Physiol. 2005;138:1903–1913. doi: 10.1104/pp.105.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhara I, Shirasawa-Seo N, Iwai T, Nakamura S, Honkura R, Ohashi Y. Release from post-transcriptional gene silencing by cell proliferation in transgenic tobacco plants: possible mechanism for noninheritance of the silencing. Genetics. 2002;160:343–352. doi: 10.1093/genetics/160.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár A, Csorba T, Lakatos L, Váralleyay É, Lacomme C, Burgyán J. Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J Virol. 2005;79:7812–7818. doi: 10.1128/JVI.79.12.7812-7818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science. 2010;328:872–875. doi: 10.1126/science.1187959. [DOI] [PubMed] [Google Scholar]
- Mueller E, Gilbert J, Davenport G, Brigneti G, Baulcombe DC. Homology-dependent resistance: transgenic virus resistance in plants related to homology-dependent gene silencing. Plant J. 1995;7:1001–1013. [Google Scholar]
- Nagamatsu A, Masuta C, Senda M, Matsuura H, Kasai A, Hong JS, Kitamura K, Abe J, Kanazawa A. Functional analysis of soybean genes involved in flavonoid biosynthesis by virus-induced gene silencing. Plant Biotechnol J. 2007;5:778–790. doi: 10.1111/j.1467-7652.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- Nagamatsu A, Masuta C, Matsuura H, Kitamura K, Abe J, Kanazawa A. Down-regulation of flavonoid 3′-hydroxylase gene expression by virus-induced gene silencing in soybean reveals the presence of a threshold mRNA level associated with pigmentation in pubescence. J Plant Physiol. 2009;166:32–39. doi: 10.1016/j.jplph.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu QW, Lin SS, Reyes JL, Chen KC, Wu HW, Yeh SD, Chua NH. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat Biotechnol. 2006;24:1420–1428. doi: 10.1038/nbt1255. [DOI] [PubMed] [Google Scholar]
- Nunes ACS, Vianna AGR, Cuneo F, Amaya-Farfán J, de Capdeville G, Rech EL, Aragão FJL. RNAi-mediated silencing of the myo-inositol-1-phosphate synthase gene (GmMIPS1) in transgenic soybean inhibited seed development and reduced phytate content. Planta. 2006;224:125–132. doi: 10.1007/s00425-005-0201-0. [DOI] [PubMed] [Google Scholar]
- Ossowski S, Schwab R, Weigel D. Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J. 2008;53:674–690. doi: 10.1111/j.1365-313X.2007.03328.x. [DOI] [PubMed] [Google Scholar]
- Otagaki S, Kawai M, Masuta C, Kanazawa A. Size and positional effects of promoter RNA segments on virus-induced RNA-directed DNA methylation and transcriptional gene silencing. Epigenetics. 2011;6:681–691. doi: 10.4161/epi.6.6.16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Yang C, Zhang C, Graham MA, Horstman HD, Lee Y, Zabotina OA, Hill JH, Pedley KF, Whitham SA. Functional analysis of the asian soybean rust resistance pathway mediated by Rpp2. Mol Plant Microbe Interact. 2011;24:194–206. doi: 10.1094/MPMI-08-10-0187. [DOI] [PubMed] [Google Scholar]
- Peele C, Jordan CV, Muangsan N, Turnage M, Egelkrout E, Eagle P, Hanley-Bowdoin L, Robertson D. Silencing of a meristematic gene using geminivirus-derived vectors. Plant J. 2001;27:357–366. doi: 10.1046/j.1365-313x.2001.01080.x. [DOI] [PubMed] [Google Scholar]
- Prins M, Goldbach R. RNA-mediated virus resistance in transgenic plants. Arch Virol. 1996;141:2259–2276. doi: 10.1007/BF01718629. [DOI] [PubMed] [Google Scholar]
- Purkayastha A, Dasgupta I. Virus-induced gene silencing: a versatile tool for discovery of gene functions in plants. Plant Physiol Biochem. 2009;47:967–976. doi: 10.1016/j.plaphy.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Que Q, Wang HY, English JJ, Jorgensen RA. The frequency and degree of cosuppression by sense chalcone synthase transgenes are dependent on transgene promoter strength and are reduced by premature nonsense codons in the transgene coding sequence. Plant Cell. 1997;9:1357–1368. doi: 10.1105/tpc.9.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MSS, Ghabrial SA, Redmond CT, Dinkins RD, Collins GB. Resistance to Bean pod mottle virus in transgenic soybean lines expressing the capsid polyprotein. Phytopathol. 2001;91:831–838. doi: 10.1094/PHYTO.2001.91.9.831. [DOI] [PubMed] [Google Scholar]
- Reddy MSS, Dinkins RD, Collins GB. Gene silencing in transgenic soybean plants transformed via particle bombardment. Plant Cell Rep. 2003;21:676–683. doi: 10.1007/s00299-002-0567-4. [DOI] [PubMed] [Google Scholar]
- Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937– 946. doi: 10.1105/tpc.10.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter JA, Scheffler BE, Jackson S, Shoemaker RC. Fractionation of synteny in a genomic region containing tandemly duplicated genes across Glycine max, Medicago truncatula, and Arabidopsis thaliana. J Hered. 2008;99:390–395. doi: 10.1093/jhered/esn010. [DOI] [PubMed] [Google Scholar]
- Schmidt MA, Herman EM. Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant. 2008;1:910–924. doi: 10.1093/mp/ssn049. [DOI] [PubMed] [Google Scholar]
- Schmidt MA, Barbazuk WB, Sandford M, May G, Song Z, Zhou W, Nikolau BJ, Herman EM. Silencing of soybean seed storage proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiol. 2011;156:330–345. doi: 10.1104/pp.111.173807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda M, Masuta C, Ohnishi S, Goto K, Kasai A, Sano T, Hong JS, MacFarlane S. Patterning of virus-infected Glycine max seed coat is associated with suppression of endogenous silencing of chalcone synthase genes. Plant Cell. 2004;16:807–818. doi: 10.1105/tpc.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda M, Kurauchi T, Kasai A, Ohnishi S. Suppressive mechanism of seed coat pigmentation in yellow soybean. Breed Sci. 2012;61:523–530. doi: 10.1270/jsbbs.61.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang H, Schellin K, Li B, Faller M, Stoop JM, Meeley RB, Ertl DS, Ranch JP, Glassman K. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol. 2007;8:930–937. doi: 10.1038/nbt1322. [DOI] [PubMed] [Google Scholar]
- Shoemaker RC, Polzin K, Labate J, Specht JE, Brummer EC, Olson T, Young N, Concibido V, Wilcox J, Tamulonis JP, et al. Genome duplication in soybean (Glycine subgenus Soja) Genetics. 1996;144:329–338. doi: 10.1093/genetics/144.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T, Wellink J, Hiriart J-B, van Kammen A. RNA-mediated virus resistance: role of repeated transgenes and delineation of targeted regions. Plant Cell. 1996;8:2277–2294. doi: 10.1105/tpc.8.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy D, Burgyán J. Effects and side-effects of viral RNA silencing suppressors on short RNAs. Trends Plant Sci. 2004;9:76–83. doi: 10.1016/j.tplants.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Singh AK, Fu D-Q, El-Habbak M, Navarre D, Ghabrial S, Kachroo A. Silencing genes encoding omega-3 fatty acid desaturase alters seed size and accumulation of Bean pod mottle virus in soybean. Mol Plant Microbe Interact. 2011;24:506–515. doi: 10.1094/MPMI-09-10-0201. [DOI] [PubMed] [Google Scholar]
- Smith HA, Swaney SL, Parks TD, Wernsman EA, Dougherty WG. Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of non-essential RNAs. Plant Cell. 1994;6:1441–1453. doi: 10.1105/tpc.6.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song QX, Liu YF, Hu XY, Zhang WK, Ma B, Chen SY, Zhang JS. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol. 2011;11:5. doi: 10.1186/1471-2229-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves RM, Todd TC, Essig JS, Trick HN. Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct Plant Biol. 2006;33:991–999. doi: 10.1071/FP06130. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Graham MY, Yu O, Graham TL. RNA interference of soybean isoflavone synthase genes leads to silencing in tissues distal to the transformation site and to enhanced susceptibility to Phytophthora sojae. Plant Physiol. 2005;137:1345–1353. doi: 10.1104/pp.104.057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006;48:261–273. doi: 10.1111/j.1365-313X.2006.02874.x. [DOI] [PubMed] [Google Scholar]
- Takagi K, Nishizawa K, Hirose A, Kita A, Ishimoto M. Manipulation of saponin biosynthesis by RNA interference-mediated silencing of β-amyrin synthase gene expression in soybean. Plant Cell Rep. 2011 doi: 10.1007/s00299-011-1091-1. (in press) [DOI] [PubMed] [Google Scholar]
- Tougou M, Furutani N, Yamagishi N, Shizukawa Y, Takahata Y, Hidaka S. Development of resistant transgenic soybeans with inverted repeat-coat protein genes of soybean dwarf virus. Plant Cell Rep. 2006;25:1213–1218. doi: 10.1007/s00299-006-0186-6. [DOI] [PubMed] [Google Scholar]
- Tougou M, Yamagishi N, Furutani N, Shizukawa Y, Takahata Y, Hidaka S. Soybean dwarf virus-resistant transgenic soybeans with the sense coat protein gene. Plant Cell Rep. 2007;26:1967–1975. doi: 10.1007/s00299-007-0404-x. [DOI] [PubMed] [Google Scholar]
- van der Krol AR, Mur LA, Beld M, Mol JNM, Stuitje AR. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990;2:291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes Dev. 2006;20:759–771. doi: 10.1101/gad.1410506. [DOI] [PubMed] [Google Scholar]
- Vermeersch L, De Winne N, Depicker A. Introns reduce transitivity proportionally to their length, suggesting that silencing spreads along the pre-mRNA. Plant J. 2010;64:392–401. doi: 10.1111/j.1365-313x.2010.04335.x. [DOI] [PubMed] [Google Scholar]
- Voinnet O. RNA silencing: small RNAs as ubiquitous regulators of gene expression. Curr Opin Plant Biol. 2002;5:444–451. doi: 10.1016/s1369-5266(02)00291-1. [DOI] [PubMed] [Google Scholar]
- Voinnet O. Use, tolerance and avoidance of amplified RNA silencing by plants. Trends Plant Sci. 2008;13:317–328. doi: 10.1016/j.tplants.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Wagner N, Mroczka A, Roberts PD, Schreckengost W, Voelker T. RNAi trigger fragment truncation attenuates soybean FAD2-1 transcript suppression and yields intermediate oil phenotypes. Plant Biotechnol J. 2011;9:723–728. doi: 10.1111/j.1467-7652.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Xu Y. Hypocotyl-based Agrobacterium-mediated transformation of soybean (Glycine max) and application for RNA interference. Plant Cell Rep. 2008;27:1177–1184. doi: 10.1007/s00299-008-0535-8. [DOI] [PubMed] [Google Scholar]
- Wang X, Eggenberger AL, Nutter FW, Jr, Hill JH. Pathogen-derived transgenic resistance to soybean mosaic virus in soybean. Mol Breed. 2001;8:119–127. [Google Scholar]
- Waterhouse PM, Graham MW, -B M. Virus resistance and gene silencing in plants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci USA. 1998;95:13959–13964. doi: 10.1073/pnas.95.23.13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijk PA, et al. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 2001;27:581–590. doi: 10.1046/j.1365-313x.2001.01105.x. [DOI] [PubMed] [Google Scholar]
- Wilson TMA. Strategies to protect crop plants against viruses: pathogen-derived resistance blossoms. Proc. Natl. Acad. Sci USA. 1993;90:3134–3141. doi: 10.1073/pnas.90.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Takagi K, Ishimoto M. Recent advance in soybean transformation and their application to molecular breeding and genomic analysis. Breed Sci. 2012;61:480–494. doi: 10.1270/jsbbs.61.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Yoshikawa N. Virus-induced gene silencing in soybean seeds and the emergence stage of soybean plants with Apple latent spherical virus vectors. Plant Mol Biol. 2009;71:15–24. doi: 10.1007/s11103-009-9505-y. [DOI] [PubMed] [Google Scholar]
- Yi J, Derynck MR, Li X, Telmer P, Marsolais F, Dhaubhadel S. A single-repeat MYB transcription factor, GmMYB176, regulates CHS8 gene expression and affects isoflavonoid biosynthesis in soybean. Plant J. 2010;62:1019–1034. doi: 10.1111/j.1365-313X.2010.04214.x. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Kanazawa A, Tsutsumi K, Nakamura I, Takahashi K, Shimamoto Y. Structural variation around the gene encoding the α subunit of soybean β-conglycinin and correlation with the expression of the α subunit. Breed Sci. 2002;52:285–292. [Google Scholar]
- Yu O, Shi J, Hession AO, Maxwell CA, McGonigle B, Odell JT. Metabolic engineering to increase isoflavone biosynthesis in soybean seed. Phytochemistry. 2003;63:753–763. doi: 10.1016/s0031-9422(03)00345-5. [DOI] [PubMed] [Google Scholar]
- Zhang C, Ghabrial SA. Development of Bean pod mottle virus–based vectors for stable protein expression and sequence-specific virus-induced gene silencing in soybean. Virology. 2006;344:401–411. doi: 10.1016/j.virol.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Zhang C, Yang C, Whitham SA, Hill JH. Development and use of an efficient DNA-based viral gene silencing vector for soybean. Mol Plant Microbe Interact. 2009;22:123–131. doi: 10.1094/MPMI-22-2-0123. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bradshaw JD, Whitham SA, Hill JH. The development of an efficient multipurpose bean pod mottle virus vector set for foreign gene expression and RNA silencing. Plant Physiol. 2010;153:52–65. doi: 10.1104/pp.109.151639. [DOI] [PMC free article] [PubMed] [Google Scholar]