To Charles Darwin the suddenness of the angiosperm appearance and their rapid rise to dominance in the fossil record was both a “perplexing phenomenon” to “those who believe in extremely gradual evolution” and an “abominable mystery” (1). It has been 125 years since Darwin's letter to Heer and for most of this time the investigation of the mystery has been in the domain of comparative morphology, traditional taxonomy, and the fossil record, principally of pollen and leaves. These approaches have failed to illuminate the mystery that has now grown to include major aspects of angiosperm phylogeny, evolutionary success, and origin (2). But lately, exciting new methods and data are available that have enormous potential to resolve this set of problems. New kinds of fossils have been discovered from critical times (2–4); new algorithms are available for the rapid comparative analysis of all kinds of data (5), and molecular genetics is providing data on nucleic acid sequences and homeotic genes like the MADS family (6–12). These allow invaluable insights into homology of floral organs.
How then have these changes in the landscape of evolutionary biology affected our understanding of the set of problems often grouped under “Darwin's Abominable Mystery?” And, for that matter, are these problems of sufficient stature to be worthy of this continuing attention? With respect to the latter, the answer is yes. The angiosperms dominate the terrestrial biota with between 300,000 and 400,000 species (13). They are vital sources of foods and drugs and are the primary constituents of the tropical rain forests, and they provide important three-dimensional structural definition for terrestrial ecosystems at most latitudes. In addition to addressing one of the greatest fundamental gaps in our understanding of evolutionary history, an understanding of precise relationships within the angiosperms would have remarkable practical value and relevance. It would allow a better understanding of species distributions and their ecological implications. It would facilitate more efficient phylogenetic context-guided searches for natural drugs and provide a precise framework within which to intelligently direct and ethically evaluate the inevitable, if controversial, bioengineering of plants for agricultural and medicinal purposes. Finally, knowledge of relationships has the potential for allowing more informed decision making on biodiversity conservation issues by permitting comparisons of the explicit uniqueness of taxa in situations involving difficult choices.
Molecular data have provided the potential to transcend subjective limitations on assessments of homology in morphological features at the very time when new algorithms provide tools necessary for large, previously intractable, data sets to be analyzed very quickly (5). Only 2 years ago it seemed that a consensus, based on molecular data, would emerge on angiosperm relationships within 10 years (14). One year later the New York Times reported that “evolutionary biologists have at last answered a question so difficult that Darwin himself called it the ‘abominable mystery”’ (15). Actually the article referred to a consensus of independent studies of extant angiosperms. This consensus included four independent analyses that pointed to the same result: Amborella at the base of the flowering plants (7–12). Although not all scientists were convinced that the matter of the basal angiosperm was settled, the response was dramatic with one morphologist heralding the identification of a basal angiosperm as “the answer” (15), even though it conflicted with his own previous analyses (16).
Now a year later, in this issue of PNAS Barkman et al. (17) report yet another but more detailed and extensive analysis of genes from all three compartments and includes and accommodates the discovery of an additional copy of the atpA gene in Amborella. The results of the Barkman et al. analysis suggest a different and significant basal arrangement of flowering plants with Nymphaea, a water lily, sharing the first branch with Amborella. This difference has implications for identifying an angiosperm ancestor. It suggests that such an ancestor might share characters with Nymphaea as well as with Amborella—a decided contrast because Nymphaea has vessels and bisexual flowers (13).
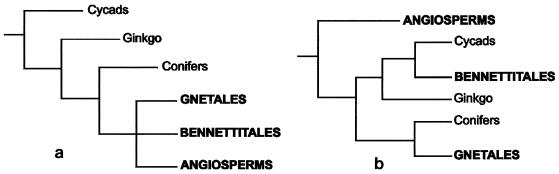
But even when The New York Times was highlighting the “consensus” on angiosperm relations (15), another well-established consensus, on angiosperm relations to other seed plants, was beginning to erode. Virtually all morphology-based analyses of seed plants have included a monophyletic group consisting of the angiosperms + Gnetales + Bennettitales—the so-called anthophytes (16, 18, 19). However, even 2 years ago gene sequence data were suggesting that that Gnetales might more properly be placed with the remainder of the gymnosperms and that any valid structural/life cycle gnetalean similarities to angiosperms were convergent (20, 21). Subsequent research has supported this conclusion based on genomic studies and analyses of MADS box genes (6, 22). Now, morphology-based and combined morphology/molecular analyses of seed plant relationships promise to further alter the landscape of seed plant relationships by removing the Bennettitales from the anthophytes and eliminating the anthophyte concept (23) (Fig. 1). This analysis differs from previous ones in the broader sampling of extant cycads, the more precise analysis of fossil bennettitalean characters, and the treatment of poorly understood fossils purported to be links between Bennettitales and Gnetales despite their ambiguous character associations. The results suggest that the Bennettitales are actually rather basal seed plants and sister group to the cycads (Fig. 1) despite the superficial similarity of the reproductive structures to those of classically archetypal angiosperms.
Figure 1.
(a) A typical morphology-based phylogeny of existing seed plants (plus the extinct Bennettitales) illustrating (in bold type) the anthophyte clade. (b) A composite phylogeny illustrating the realignment of Bennettitales and Gnetales based on ITS and new morphological data (21).
Where then do we stand? A more resolved and, as a result of the recent Barkman et al. study, a more compelling picture of relationships within the flowering plants is now emerging based on molecular data. And although the results of this most recent analysis are not consistent with phylogenies based on morphological data sets (16, 19, 24), there is no consensus among the exclusively morphology-based sets. With both Nymphaea and Amborella at the base of the latest angiosperm tree, a wider range of characters might be expected in archetypal angiosperms or angiosperm sister groups than that implied by the previous consensus on Amborella alone. What of complementary projections derived from analyses of seed plant relationships? Do these analyses point toward likely archetypal angiosperms or angiosperm ancestors? No. Not when we incorporate the recent analyses challenging the validity of the anthophytes. Instead, angiosperms become more distantly related to all existing seed plants, leaving a gap populated only by extinct taxa that may or may not be represented in the fossil record.
That brings us full circle to the kind of evidence that first allowed Darwin to recognize the perplexing phenomenon in the first place: the fossil record. How does the record impinge on our understanding of within angiosperm relationships, angiosperm success, and angiosperm origins? An improved understanding of the angiosperm fossil record has resulted from critical analysis of the leaf and pollen records (25), but most dramatically from a new emphasis on well-preserved fossil angiosperm flowers (2, 3, 26). One obvious application of these new data and resultant pattern would be to test hypotheses of phylogeny based on extant taxa for correspondence with the observed temporal progression of angiosperm taxa in the fossil record. There is no exact congruence between any angiosperm phylogeny based on existing taxa and angiosperm fossil history. It is too early to make too much of this because the pace of paleobotanical discovery is forcing ongoing reassessments of pattern. There is rough consistency between the fossil record and the Barkman et al. phylogeny, but this is also true for some other estimations of flowering plant relationships. Regrettably, there is no confirmation of the basal angiosperm taxon based on an unequivocal progression of discrete identifiable taxa early in angiosperm history. Instead, reliable evidence of early Cretaceous angiosperms suggests a rapid initial diversification with “eudicots” immediately following magnoliids (2). Among earliest recognizable taxa are the families Chloranthaceae and Winteraceae (14). These families (and others) are basal in several morphology-derived phylogenies. Nonetheless, more recent discoveries of Barremian (circa 130 million years before present) Nympheaceae and Amborella-like flowers by E. M. Friis and her colleagues† are consistent with the results reported in the Barkman et al. paper. Further support for Barkman et al. comes in the form of recently discovered flowers very similar to those of the modern genus Nymphaea in Turonian deposits from New Jersey. Their modern aspect even 90 million years before present is consistent with an earlier appearance of the family (Fig. 2). Monocots, though, are an exception to the rough correspondence between fossil history of angiosperms and virtually all hypotheses of angiosperm phylogeny based on modern taxa. Although such analyses suggest that the monocots diverged from dicots relatively early in flowering plant history, recent critical review of monocot leaf and fossil records suggests that there are no bona fide Lower Cretaceous examples (26, 27). The first reliably identified monocots are Turonian in age (26) and, surprisingly, they are flowers of several genera of the modern family Triuridaceae (26, 27), diminutive saprophytic plants with very simple vegetative structure. This family includes the modern genus Lacandonia that is distinguished by having flowers with carpels borne outside of the stamens. More data will be necessary before the implications of this discovery can be fully appreciated.
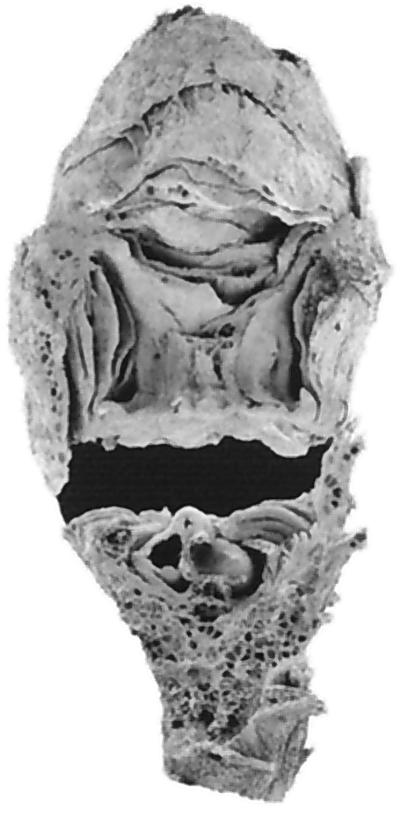
Figure 2.
A three-dimensionally preserved Cretaceous (Turonian) flower similar to modern Nymphaea (scanning electron micrograph by Jennifer Svitko).
New fossil floral data reveal a dramatic modernization of the angiosperms by the Turonian (90 million years before present, ref. 4). Had Darwin witnessed such a pattern, he might have been even more astonished by the rapid ascension of flowering plants. Yet this very attribute of the fossil history of angiosperms holds clues to another aspect of the perplexing phenomenon: why the angiosperms are so successful. Flowers provide a record of mode of pollination in addition to revealing a precise knowledge of taxonomic affinity. In conjunction with a remarkably improved fossil record of insects (28), the history of floral form provides a more precise knowledge of the timing of angiosperm-pollinator relationships and thus of angiosperm diversification vs. insect diversification. A correlation between these events has been presumed since the time of Saporta (1) especially in light of speciation promoting aspects of certain kinds of insect pollination (29). And this relationship, essentially unique to angiosperms, has been considered one of the foundations of relative angiosperm success. Thus, assertions of an apparent disjunction between angiosperm and anthophilous insect radiations based on fossil evidence were surprising (30) and called for a reconsideration of the significance of insect pollinators. However, these assertions do not hold up in the face of new fossil evidence (4, 28). The pattern of angiosperm radiation is consistent with the pattern of anthophilous insect radiation and the pattern of appearance of derived floral characters and taxa specifically associated with the most advanced anthophilous insects (Fig. 3; ref. 31). There is a compelling similarity between the rate of floral innovation/million years and the rate of angiosperm diversification during the Cenomanian/Turonian interval coinciding with the first occurrences of many derived insect pollinators.
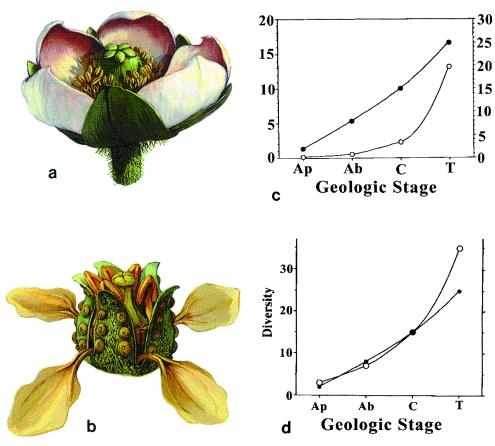
Figure 3.
(a) A reconstruction of Paleoclusia, a Cretaceous relative of modern Clusiaceae, a family closely associated with meliponine bee pollinators. (b) A reconstruction of an extinct (Cretaceous, Turonian) ericalean flower typical of a complex that includes several adaptations for pollination by derived anthophilous insects clawed petals and pollen in polyads. (Reconstructions by Michael Rothman.) (c) Appearance of floral innovations (●) during the Aptian (Ap)-Albian (Ab)-Cenomanian (C)-Turonian (T) interval vs. appearance of new floral characters/million years (○). (d) Angiosperm radiation in the Aptian–Turonian interval (based on ref. 32; ○) vs. rate of appearance of new floral characters during the same interval (●).
New molecular and fossil data combined with new analytical tools are improving our understanding of angiosperm history and success. But one aspect of the “abominable” mystery has become even more enigmatic in the face of recent advances: the identity of taxa transitional to the flowering plants. Given the evident distance between angiosperms and other seed plants and missing fossil intermediates, there will be a new emphasis on discovery of new and careful evaluation of existing fossil data. With no taxon universally accepted as transitional between angiosperms and any other group of seed plants, attention inevitably will turn to plausible fossil groups and the search for deeper homologues among extant conifers. Mesozoic pteridosperms, and Caytonia in particular, are among those known extinct taxa that have been considered closely related to the angiosperms. Although this possibility is supported by some recent analyses (16), others suggest that the apparent similarities are analogous, breaking down under critical examination (19). The paucity of characters and quality of preservation of Caytonia fossils makes it difficult to objectively resolve this discrepancy even though the hypotheses supporting the juxtaposition of Caytoniales with the flowering plants are elegant and appealing (16). Other, better preserved, but incompletely known Mesozoic pteridosperms, the Corystosperms, are now being considered as possible angiosperm ancestors in light of hypotheses derived from insights into MADS box genes (33). It is simply too early to evaluate this possibility based on the available fossil evidence and in the absence of more compelling data from comparative genetic studies. Identification of transitional taxa would be aided by early angiosperms that retained characters linking them to other seed plants, but the earliest fossil angiosperms are comparable to modern families with one exception, Archaefructus (34). Although the age of this taxon is not firmly established (it could be as old as the latest Upper Jurassic, but is no younger than Lower Cretaceous), the mixture of characters is interesting and potentially illuminating because it is a mosaic of characters now found in more than one family of angiosperms and as it becomes better understood, it may have even greater implications.
There is now little doubt that many of the elements of Darwin's mystery are within reach, there is some irony in the fact that despite stunning progress on many fronts, ultimate resolution still depends on the stochastic nature of fossil discovery.
Footnotes
See companion article on page 13166.
Friis, E. M., Pedersen, K. R. & Crane, P. R. Sixth Conference, International Organization of Paleobotany, July 31–August 3, 2000, Qinhuangdao, China, 36–37.
References
- 1.Darwin C. In: More Letters form Charles Darwin. Darwin F, Seward A C, editors. Vol. 2. New York: Appleton; 1903. pp. 239–240. and 20–22. [Google Scholar]
- 2.Crane P R, Friis E M, Pedersen K R. Nature (London) 1995;374:27–33. [Google Scholar]
- 3.Dilcher D L, Crane P R. Ann Mo Bot Gard. 1984;71:351–383. [Google Scholar]
- 4.Crepet W L. Rev Paleob Palyn. 1996;90:339–359. [Google Scholar]
- 5.Nixon K C. Cladistics. 1999;15:407–414. doi: 10.1111/j.1096-0031.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 6.Theissen G, Kim J T, Saedler H. J Mol Evol. 1996;43:484–516. doi: 10.1007/BF02337521. [DOI] [PubMed] [Google Scholar]
- 7.Mathews S, Donoghue M J. Science. 1999;286:947–950. doi: 10.1126/science.286.5441.947. [DOI] [PubMed] [Google Scholar]
- 8.Qiu Y-l, Lee J, Bernasconi Q F, Soltis D E, Soltis P E, Zanis M, Zimmer E A, Chen Z, Savolainen V, Chase M W. Nature (London) 1999;402:404–407. doi: 10.1038/46536. [DOI] [PubMed] [Google Scholar]
- 9.Soltis P S, Soltis D E, Chase M W. Nature (London) 1999;402:402–404. doi: 10.1038/46528. [DOI] [PubMed] [Google Scholar]
- 10.Nandi O I, Chase M W, Endress P K. Ann Mo Bot Gard. 1998;85:137–212. [Google Scholar]
- 11.Donoghue M J, Mathews S. Mol Phy Evol. 1998;9:489–500. doi: 10.1006/mpev.1998.0511. [DOI] [PubMed] [Google Scholar]
- 12.Soltis D E, Soltis P S, Mort M E, Chase M W, Salvolainen V, Hoot S B, Morton C M. Syst Biol. 1998;47:32–42. doi: 10.1080/106351598261012. [DOI] [PubMed] [Google Scholar]
- 13.Cronquist A. The Evolution and Classification of Flowering Plants. New York: Columbia Univ. Press; 1981. [Google Scholar]
- 14.Crepet W L. Science. 1998;282:1653–1654. [Google Scholar]
- 15.Yoon, C. K. (10–29-1999) New York Times, pp. A1, A26.
- 16.Doyle J A. Int J Plant Sci. 1996;157:S3–S39. [Google Scholar]
- 17.Barkman T J, Chenery G, McNeal J R, Lyons-Weiler J, dePamphilis C W. Proc Natl Acad Sci USA. 2000;97:13166–13171. doi: 10.1073/pnas.220427497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane P R. Ann Mo Bot Gard. 1985;72:716–793. [Google Scholar]
- 19.Nixon K C, Crepet W L, Stevenson D, Friis E M. Ann Mo Bot Gard. 1994;81:484–533. [Google Scholar]
- 20.Goremykin V, Bobrova V K, Pahnke J, Troitsky A, Antonov A, Martin W. Mol Biol Evol. 1996;13:383–396. doi: 10.1093/oxfordjournals.molbev.a025597. [DOI] [PubMed] [Google Scholar]
- 21.Bobrova V K, Goremykin V, Troitski A, Valékho-Roman K M, Antonov A. Zh Obshch Biol. 1995;56:645–661. [PubMed] [Google Scholar]
- 22.Bowe L M, Coat G, dePamphilis C W. Proc Natl Acad Sci USA. 2000;97:4092–4097. doi: 10.1073/pnas.97.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crepet, W. L. & Nixon, K. C. (2000) Bot. Rev., in press.
- 24.Loconte H, Stevenson D W. Brittonia. 1990;42:197–211. [Google Scholar]
- 25.Hickey L J, Doyle J A. Bot Rev. 1977;43:3–104. [Google Scholar]
- 26.Gandolfo M A, Nixon K C, Crepet W L, Stevenson D W, Friis E M. Nature (London) 1998;394:532–533. [Google Scholar]
- 27.Gandolfo M A, Nixon K C, Crepet W L. In: Monocots: Systematics and Evolution. Wilson K L, Morrison D A, editors. Melbourne: Commonwealth Scientific and Industrial Research Organization; 2000. pp. 44–51. [Google Scholar]
- 28.Grimaldi D A. Ann Mo Bot Gard. 1999;86:373–406. [Google Scholar]
- 29.Grant V. Am J Bot. 1950;37:294–296. [Google Scholar]
- 30.Labandeira C C, Sepkoski J J., Jr Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- 31.Crepet W L, Nixon K C. Am J Bot. 1998;85:1122–1133. [PubMed] [Google Scholar]
- 32.Niklas K J, Tiffney B H, Knoll A H. In: Phanerozoic Diversity Patterns. Valentine J W, editor. Princeton, NJ: Princton Univ. Press; 1985. pp. 97–128. [Google Scholar]
- 33.Frohlich M W, Parker D S. Sys Bot. 2000;25:155–170. [Google Scholar]
- 34.Sun G, Dilcher D L, Zheng S, Zhou Z. Science. 1998;282:1693. doi: 10.1126/science.282.5394.1692. [DOI] [PubMed] [Google Scholar]