Abstract
The Wnt/β-catenin pathway, which signals through the Frizzled (Fzd) receptor family and several coreceptors, has long been implicated in cancer. Here we demonstrate a therapeutic approach to targeting the Wnt pathway with a monoclonal antibody, OMP-18R5. This antibody, initially identified by binding to Frizzled 7, interacts with five Fzd receptors through a conserved epitope within the extracellular domain and blocks canonical Wnt signaling induced by multiple Wnt family members. In xenograft studies with minimally passaged human tumors, this antibody inhibits the growth of a range of tumor types, reduces tumor-initiating cell frequency, and exhibits synergistic activity with standard-of-care chemotherapeutic agents.
Keywords: differentiation, cancer stem cell, pancreatic, breast, lung
Investigation into the role of the Wnt pathway in human tumors has been hampered by a lack of therapeutic agents able to inhibit Wnt signaling. The Wnt pathway is complex, with a large number of known ligands, receptors, coreceptors, and regulatory components. Members of the Wnt family have been shown to induce several distinct signaling events, including activation of the β-catenin signaling (termed the “canonical pathway”) as well as other signaling cascades, including the planar cell-polarity pathway and the Ca+2 pathway (1–5). Lack of a detailed understanding of the receptor-ligand specificities of the various pathway components and their relationship to the Wnt-mediated signaling pathways has also hindered development of therapeutic agents. However, mutations in the Wnt signaling pathway occur in most cases of human colon cancer, and active signaling has been noted in multiple major tumor types, highlighting this pathway as a potentially promising target for the development of new anticancer agents (6, 7). In recent years, several small molecules that impact the Wnt pathway have been identified. The nonsteroidal anti-inflammatory compound Sulindac and the antibiotic salinomycin have been reported to possess ability to down-regulate β-catenin (8, 9). Inhibitors of the poly-ADP ribosylating enzymes tankyrase 1 and tankyrase 2 also have been found to be inhibitors of Wnt signaling, by a mechanism that results in stabilization of axin and the β-catenin destruction complex (10). However, these agents also impact other signaling events beyond the Wnt pathway. To date, highly potent small molecule agents that specifically inhibit Wnt signaling have not been reported.
An alternative approach to developing agents that block Wnt signaling is to focus on the extracellular receptor–ligand interactions. There are 19 human Wnts and 10 Frizzled (Fzd) receptors. In addition, Wnt signaling through Fzds involves two coreceptors, LRP5 and LRP6, as well as other coreceptors, such as ROR and Ryk. Although this substantial complexity and functional redundancy makes the prospect of developing effective inhibitors more challenging, it also offers the potential for the identification of agents that block only portions of Wnt signaling. This selectivity relative to potential intracellular signaling components may offer advantages in safety and therapeutic index. Previous studies have indicated that use of soluble Wnt inhibitors and decoy receptor molecules can impact tumor growth (11, 12). In this article we demonstrate the ability to generate a potent Wnt inhibitory antibody that functions by binding to select Fzd receptors, and that this antibody is active across a range of human tumor types.
Results
Identification of an Antagonistic Wnt Pathway Antibody That Targets Multiple Fzd Receptors.
Antibodies to the Fzd receptors were identified using phage display and were subsequently screened for the ability to inhibit Wnt3A signaling in a cell-based assay. Through this approach, an antibody that was initially isolated by ability to bind to FZD7, OMP-18R5, was found to block most β-catenin signaling in response to Wnt3A (Fig. 1A). In subsequent experiments it was also found to inhibit β-catenin signaling in response to each of the Wnt family members that were active in these reporter studies (Fig. 1B). OMP-18R5 also reduced both Wnt3A-induced accumulation of β-catenin and phosphorylation of LRP6 (Fig. 1C). OMP-18R5 did not inhibit β-catenin signaling in response to intracellular pathway activation with the GSK3 inhibitor compound BIO (6-bromoindirubin-3′-oxime) (Fig. S1A) and also did not inhibit activation of a Notch pathway reporter in response to the Notch ligand DLL4 (Fig. S1B). The OMP-18R5 anti-Fzd antibody was also screened by flow cytometry with cells overexpressing each individual Fzd. OMP-18R5 was found to bind 5 of the 10 Fzd receptors: FZD1, FZD2, FZD5, FZD7, and FZD8 (Fig. 2A and Fig. S2). Interestingly, each of these receptors has been previously implicated in canonical Wnt signaling (12–17). The epitope on the Fzd proteins bound by OMP-18R5 was mapped through a survey of introduced amino acid substitutions within FZD8, several of which eliminated OMP-18R5 binding (Fig. 2B and Fig. S3). OMP-18R5 binds to a discontinuous epitope that spans a “cleft” region that is apparent in the reported crystal structure (18) of mouse Fzd8 (Fig. 2C). Interestingly, the residues lining the base of this cleft are highly conserved across the Fzd family, suggesting that this site is functionally important. To determine whether OMP-18R5 directly blocked the ability of Wnt to interact with Fzd, binding studies were conducted. OMP-18R5 blocked Wnt3A binding to Fzd5 ECD, suggesting that a mechanism by which OMP-18R5 inhibits Wnt signaling may involve the direct inhibition of Wnt binding (Fig. 2D and Fig. S4).
Fig. 1.
Anti-Fzd antibody OMP-18R5 inhibits Wnt signaling. (A) OMP-18R5 antibody inhibits recombinant Wnt3A signaling as assessed by β-catenin responsive TOP-FLASH luciferase reporter and a FOP-FLASH control reporter. (B) OMP-18R5 antibody (50 nM) inhibits the ability of several Wnts to induce canonical signaling. Shown is relative luciferase signal normalized to control Renilla luciferase and relative to signal in the absence of Wnt (no Wnt). Bars represent the mean ± SD. (C) OMP-18R5 blocks phosphorylation of LRP6 and the accumulation of active β-catenin. HEK-293 cells were treated with purified Wnt3A and OMP-18R5, as indicated. Cells were lysed in the presence of phosphatase inhibitor and Western blot analysis was performed. OMP-18R5 inhibited the induction of phosphorylated (Ser1490) LRP6 by Wnt3A and attenuated the Wnt induced accumulation of β-catenin and unphosphorylated (Ser37, Thr41) β-catenin.
Fig. 2.
Anti-Fzd antibody OMP-18R5 binds to 5 of the 10 human Fzd receptors and inhibits Wnt binding. (A) Binding curves of OMP-18R5 to cells that overexpress the indicated Fzd. Binding was assessed by flow cytometry analysis with cells transfected with cDNA encoding the indicated FZD protein. (B) Epitope mapping of OMP-18R5 to FZD8. Expression vectors comprising N-terminal FLAG-tagged FZD8 variants encoding the indicated amino acid substitution were transiently transfected along with GFP and OMP-18R5 binding was assessed by flow cytometry. (C) Surface rendering of the cysteine-rich domain of FZD8 (18), highlighting important residues involved in OMP-18R5 binding in green and highly conserved residues in pink. (D) Binding of OMP-18R5 to FZD inhibits the interaction of Wnt with FZD. Wnt3A, FZD5-Fc and OMP-18R5 were coincubated as indicated, and then OMP-18R5 and FZD5-Fc were removed by protein A immunoprecipitation. Supernatant was then assayed for Wnt activity in an 8xTCF luciferase reporter assay.
Wnt Pathway Blockade Inhibits the Growth of Human Tumor Xenografts.
The impact of inhibiting Wnt/β-catenin signaling on tumor growth was assessed using human tumor xenografts in mice. These models were conducted with minimally passaged human tumors (19, 20) (Table S1). OMP-18R5 treatment resulted in inhibition of growth in several types of human tumors, including breast, pancreatic, colon [wild-type adenomatous polyposis coli (APC) and β-catenin] and lung tumors (Fig. 3). Striking synergy was observed when OMP-18R5 was combined with several standard-of-care chemotherapeutic agents, including taxol in nonsmall-cell lung cancer and breast cancer models, irinotecan in colon cancer models, and gemcitabine in pancreatic cancer models (Fig. 3 B, C, E, and G–I). OMP-18R5 had activity in 6 of 11 pancreatic tumors, 3 of 6 breast tumors, and 7 of 8 nonsmall-cell lung tumors tested. OMP-18R5 did not display activity in colon tumors harboring APC or β-catenin mutations, but was highly active in a colon tumor with wild-type APC and β-catenin. OMP-18R5 also inhibited the growth of the established cell line PA-1 (derived from a human teratocarcinoma), which had previously shown to be inhibited by FZD8-fc protein (12) (Fig. 3F). In tumor recurrence studies (Fig. 3 G and H), OMP-18R5 treatment induced an extended delay in the regrowth of tumors following a treatment with high-dose chemotherapy, whereas tumors treated with control antibody demonstrated rapid regrowth.
Fig. 3.
Inhibition of canonical Wnt signaling inhibits tumor growth as monotherapy and in combination with chemotherapy. Results of nine tumor models: colon tumor C28 (A), breast tumor T3 (B), lung NSCLC Lu24 (C), pancreatic tumor PN8 (D), breast tumor PE13 (E), teratocarcinoma line PA1 (F), pancreatic tumor PN4 (G), breast tumor PE13 (H), and colon tumor C28 (I). Mean tumor volumes with SEs are presented (n = 10). (G and H) Duration of chemotherapy and antibody treatment is indicated by brackets. Asterisks denote statistical significance P < 0.02 (* vs. control Ab; ** vs. chemotherapy alone).
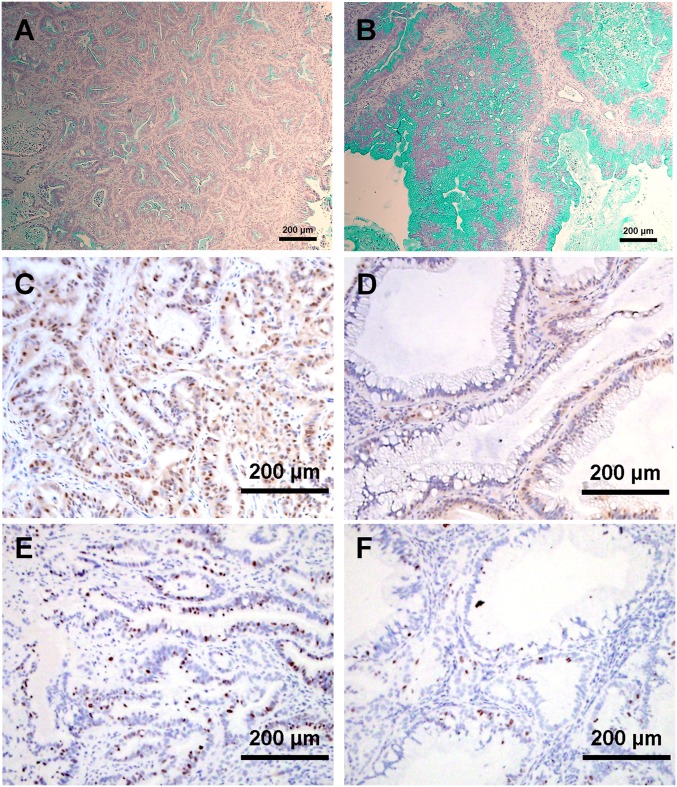
Several tumors showed synergistic response to OMP-18R5 and chemotherapy. Gene-expression analysis of the response of breast tumor PE-13 to OMP-18R5 alone or in combination with taxol indicated that OMP-18R5 and taxol induced opposing changes in the expression of numerous genes involved in stress responses, as well as ABC family transporters, suggesting Wnt pathway inhibition may alter cellular responses to taxol treatment (Fig. S5). mRNA for several genes previously associated with breast tumor tumorigenicity (20–22), including CD44, ALDH1A1, SOX1, and SOX2, were decreased by OMP-18R5. Similarly, OMP-18R5 also displayed synergy with gemcitabine in pancreatic tumors. OMP-18R5 was noted to induce changes in the histopathology of the pancreatic tumors. In PN4, a pancreatic tumor with a moderately differentiated ductal phenotype, the ducts were greatly enlarged and the cells lining the ducts were larger following OMP-18R5 treatment. Staining with Alcian blue, to reveal mucin expression, revealed large increases in mucin content (Fig. 4 A and B). Immunohistochemical (IHC) staining also revealed reduced nuclear β-catenin levels following OMP-18R5 treatment. (Fig. 4 C and D). Proliferation, as measured by Ki-67 staining, was also reduced by OMP-18R5 treatment (Fig. 4 E and F). Quantitative PCR of known targets of Wnt/β-catenin signaling Wnt target genes and genes associated with pancreatic lineages was performed (Fig. 5). Down-regulation of AXIN2 and osteopontin (SPP1) was observed in tumors treated with OMP-18R5, consistent with inhibition of Wnt signaling (23, 24). In addition, in these pancreatic xenografts, multiple genes within the mucin family and certain cytokeratins were induced by OMP-18R5. Gemcitabine treatment resulted in elevated mRNA for several genes associated with epithelieal-mesenchymal transition, including fibronectin, vimentin, slug, and snail. These effects were blocked by combined treatment with OMP-18R5.
Fig. 4.
Inhibition of Wnt signaling in pancreas tumor induces mucininous differentiation and decreased nuclear β-catenin and cellular proliferation. Shown is staining of sections of pancreatic tumor PN4 with Alcian blue to highlight mucin (A and B), and with anti–β-catenin (C and D) and with anti-Ki-67 to indicate proliferating cells (E and F). Sections are from tumors following treatment (41 d) with control antibody (A, C, and E) or OMP-18R5 (B, D, and F).
Fig. 5.
Expression changes induced by Wnt pathway inhibition. Shown is the relative mRNA expression (normalized to control antibody and to human control mRNA GAPDH) for the indicated human genes from pancreatic PN4 tumors treated with OMP-18R5, gemcitabine, or the combination. Data shown is average from four tumors in each treatment group analyzed individually. Shown are genes with statistically significant (Student’s t test P < 0.05) expression difference in response to OMP-18R5 or combination treatment relative to either control or gemcitabine treatment arms) with the exception of CDH1 which is included for completeness.
Targeting Fzd Receptors Reduces Tumorigenicity.
Several studies have suggested that Wnt signaling plays a key role in mediating self-renewal essential for tumor initiating cells (25–27). To assess the impact of OMP-18R5 on tumor-initiating cell frequency, limiting dilution studies were conducted wherein defined numbers of human cells from treated or control tumors were assessed for their ability to initiate tumor growth after secondary transplant into a new cohort of mice in the absence of further treatment. OMP-18R5 treatment of the pancreatic tumor PN4 (Fig. 6A) and the breast tumor PE13 (Fig. 6B) reduced the tumor-initiating cell frequency of the tumor cells by threefold. Neither gemcitabine treatment of the pancreatic tumor nor taxol treatment of the breast tumor reduced tumorigenic cell frequency (in fact, taxol treatment increased tumorigenicity, P < 0.05). In contrast, the combination of OMP-18R5 and chemotherapeutic agents reduced tumorigenicity by 10-fold in both pancreas and breast tumors.
Fig. 6.
Inhibition of Wnt reduces tumorigenicity and induces the presence of nontumorigenic cells. Limiting dilution analysis of the ability of human cells isolated from treated pancreatic PN4 tumor (A) and breast tumor PE13 (B) to initiate tumor growth in the absence of further treatment following secondary transplant to a new cohort of mice. The primary tumor was treated with control antibody or OMP-18R5 or chemotherapy, as indicated. The tumor initiating frequency (TIC) refers to the average number of cells determined to be required to cause tumor growth in the recipient cohort.
Intracellular Blockade of the Canonical Wnt Pathway Inhibits Tumor Growth.
The OMP-18R5 antibody blocks Wnt signaling by binding to multiple Fzd receptors, suggesting a requirement for activation of the canonical Wnt pathway during tumor growth. Although OMP-18R5 is a human IgG2 isotype—an isotype that mediates little effector function, such as complement mediated cytotoxicity or antibody dependent cell killing—and further that these studies were performed in immunocompromised mice, it is conceivable that immune function contributes to OMP-18R5 efficacy. To probe Wnt pathway involvement further, we tested the impact of an alternative approach to Wnt pathway inhibition. Overexpression of the intracellular Wnt pathway component Axin leads to destabilization of β-catenin and inhibition of canonical signaling (28). We find that introduction of Axin into tumors by lentivirus-mediated transduction substantially abrogated tumor growth (Fig. S6), suggesting importance of canonical Wnt pathway signaling within the tumor cells.
Discussion
The role of the Wnt-Fzd pathway in cancer has been the subject of investigation for nearly three decades following the initial observation that mouse mammary tumor virus retroviral integration events resulting in inappropriate Wnt1 expression could result in murine mammary gland tumors (29). Recognition of the importance of APC and β-catenin mutations in colon cancer has provided strong evidence that the pathway plays a major role in the genesis of at least one major type of human cancer (30, 31). Although not as frequently mutated in other tumor types, increased Wnt pathway activation and epigenetic silencing of Wnt antagonists has been noted in several tumor types (7, 32–37).
In the present study, we provide evidence that it is possible to generate potent inhibitors of canonical Wnt signaling by simultaneous targeting of several Fzd receptors. The antibody described in this study binds to five distinct Fzd receptors through a conserved epitope. Interestingly, this epitope is distinct from the region of FZD8, previously implicated in Wnt binding by alanine scanning mutation analysis (18). The structure of Wnt has not yet been reported, but both Wnt and the antibody are substantially larger proteins than the relatively small cysteine-rich domain of Fzd receptors, so it is possible that steric hindrance prevents simultaneous binding of Wnt and antibody. Our data indicate that blockade of Wnt signaling can inhibit growth in a variety of human tumor types. Inhibition of tumor growth was noted in lung, breast, colon, and pancreatic tumors. Interestingly, there was strong synergy observed with several chemotherapy agents, particularly taxol. Gene-expression analysis suggests that rather than “enhancing” the magnitude of taxol-induced effects, the combination with OMP-18R5 actually inhibits some taxol-induced gene changes and may therefore be altering cellular response to taxol treatment. The robust ability of overexpressed axin within tumors to also inhibit tumor growth is evidence that the tumor cells themselves are dependent upon this pathway.
Generally, single-agent activity was observed to be significant, but modest relative to the efficacy observed in combination with chemotherapy. This finding may reflect the fact that OMP-18R5 does not target all Wnt pathway receptors. However, it has previously been demonstrated that robust Wnt inhibition by adenovirus-mediated delivery of the pan-Wnt pathway inhibitor dickkopf 1 (DKK1) results in profound inhibition of gastrointestinal tract homeostasis with disruption of the crypt-villus epithelial cell layer (38). Because of the high degree of evolutionary conservation in this pathway, the OMP-18R5 binds to both human and mouse Fzd receptors. In fact, within the cysteine-rich domain FZD1, -2, -7, and -8 are identical between human and mouse, and FZD5 has only two amino acid changes that do not impact OMP-18R5 binding (Fig. S2). There was no apparent impact of OMP-18R5 on the gastrointestinal tract at the doses used in these studies. At very large doses (100 mg/kg) we did observe an intestinal colitis that resembled, although was less severe, the results reported with DKK1. OMP-18R5 did however substantially inhibit the expression of several known Wnt target genes in the liver (Fig. S7) (39).
OMP-18R5 reduces tumor cell proliferation and tumor-initiating cell frequency. The reduction in tumor-initiating cell frequency was quantified using limiting-dilution tumorigenicity assays. This functional assay measures in vivo tumorigenicity and makes no assumption about the frequency, FACS marker profile, or heterogeneity of the tumor-initiating cell population. A strength of this approach is that it can be applied to any tumor. In contrast, cell-surface markers may vary in different tumors and their expression may be influenced by environment, which changes in response to drug treatment. OMP-18R5 reduced tumor-initiating cell frequency both as a single agent and in combination with chemotherapy. In contrast neither gemcitabine nor taxol reduced tumorigenicity as single agents. Taxol treatment actually resulted in an increase in tumor-initiating cell frequency, consistent with previous reports that tumor-initiating cells are selectively resistant to various chemotherapeutics and radiation (40, 41).
In conclusion, we have developed a unique antibody that antagonizes the Wnt signaling pathway. Targeting the Wnt pathway through by blockade of selected members of the Fzd receptor family may provide an efficacious approach for the treatment of a broad range of tumors.
Materials and Methods
All animal work was performed according to OncoMed Pharmaceutical's Institutional Animal Care and Use Committee guidelines.
Antibody Generation and Reporter Studies.
OMP-18R5 was generated by panning the HuCAL GOLD phage-display library (MorphoSys) with recombinant FZD7 protein (42). DNA fragments encoding the fAb generated from the phage display library were subcloned into a full-length human IgG2 expression vector. The antibody was expressed in CHO cells and purified. The ability of Wnt to activate T-cell factor (TCF)-dependent transcription was assessed by transient transfection of HEK293 responder cells with the TOP-FLASH/FOP-FLASH luciferase reporter assay and a Renilla luciferase transfection control reporter, followed by treatment with purified Wnt3A (R&D Systems) or 24-h coculture (1:1) exposure to HEK293 cells transiently transfected with expression vectors encoding human Wnt proteins, and then assayed using the Dual-Glo luciferase assay reporter system (Promega). The TOP-FLASH reporter was synthesized and includes eight copies of a TCF binding site (AGATCAAAGG) upstream of a minimal promoter and firefly luciferase. BIO was obtained from Sigma-Aldrich. The ORF cDNA encoding human Fzd, Wnt, Smoothened, and Notch2 proteins were isolated by PCR or synthesized (DNA2.0). The Notch luciferase reporter assay was performed essentially as previously described (19). Briefly, PC3 cells were transfected with an expression vector encoding full-length human Notch2 and an 8xCBF1-luciferase reporter vector and a Renilla luciferase vector as a transfection control. Cells were plated in wells coated with 100 ng of human DLL4 (R&D Systems) and exposed to the indicated antibodies at 10 μg/mL and then assayed 18 h later using the Dual-Glo luciferase system. Fzd-Fc fusion proteins were produced in baculovirus and contained the N-terminal extracellular domain of human Fzd receptors fused to the CH2-CH3 domains of human IgG1. Lentiviral vectors containing CMV-mouse AXIN-IRES-GFP or CMV-IRES-GFP cassettes were generated by trans-complementation in HEK 293 cells transiently cotransfected with the plasmid containing the vector genome along with the gag-pol, rev, and VSV-G env packaging constructs. For lentiviral transduction studies, tumor cells were isolated as single cells and cultured (43) in the presence of lentivirus for 24 h. Transduced cells were then isolated by flow cytometry gating on positive GFP expression.
Histology and IHC, and Western Blottings.
Formalin-fixed and paraffin-embedded tumors were sectioned at 4-μm thickness. Sections were stained with Alcian blue as recommended by the manufacturer (Poly Scientific). The IHC analysis used anti–β-catenin (mAb2081, Millipore; 1:100) and anti–Ki-67 (sp6, Vector Laboratories; 1:200) with optimal cutting temperature embedded frozen-tumor sections. For phospho-LRP6 and β-catenin Western blot analysis, HEK-293 cells were cultured in 2% (vol/vol) FBS containing DMEM for 4 h in the presence of 400 ng/mL recombinant Wnt3A (R&D Systems) and 50 μg/mL OMP-18R5, as indicated. Cells were lysed in lysis buffer (Invitrogen) containing 1 tablet/10 mL PhosSTOP phosphatase inhibitor (Roche). Western blots were probed with α-LRP6 (Cell Signaling; C47E12), α-phospho LRP6 (Ser1490; Cell Signaling), α-β-catenin (BD Transduction Laboratories; 610154), α−“active”-β-catenin (unphosphorylated Ser37, Thr-41; clone 8E7; Millipore).
Xenograft Models.
The establishment of minimally passaged human tumor xenograft models and determination of tumor initiating cell frequency was performed as previously described (19, 44). Briefly, human tumors xenograft models were established by subcutaneous implantation of patient-derived solid tissue fragments in NOD/SCID mice. Established tumors were subsequently disassociated and single-cell suspensions of tumor cells were frozen at −80C. Tumors for subsequent experiments were established by serial implantation of frozen cell stocks. For tumorigenicity studies, single-cell suspensions from control and treated tumors were incubated with biotinylated α-mouse CD45-biotin and α-mouse H2Kd on ice for 30 min followed by addition of streptavidin-labeled magnetic beads to remove murine stromal cells. Human tumor cells were collected, counted, and diluted, and then injected subcutaneously in NOD/SCID mice. Tumor growth was monitored for up to 3 mo. Cancer stem cell frequency was determined using L-Calc Version 1.1 software program (StemCell Technologies). OMP18R5 was dosed at 10 mg/kg twice weekly, except as indicated, and was dosed at 20 mg/kg once weekly in Fig. 4G and 15 mg/kg twice weekly in Fig. 4C. Taxol (paclitaxel) was dosed at 10 mg/kg once weekly except for Fig. 4H, which was dosed at a maximum tolerated dose of 20 mg/kg twice weekly. Gemcitabine was dosed at 50 mg/kg twice weekly. Irinotecan was dosed at 7.5 mg/kg twice weekly. All agents were administered intraperitoneally.
Data Analysis.
Data are expressed as mean ± SEM. Differences in mean values between groups were analyzed by nonparametric t test. Tumor-initiating cell frequency was determined using Poisson distribution statistics and L-Calc software.
Supplementary Material
Acknowledgments
We thank many people at OncoMed for their contributions to this work, including Jorge Monteon, Paul Sauer, Peter Stathis, Ian Scott, Min Wang, Daniel Croom, Jim Evans, Xiaomei Song, and Michael Mulkerrin. Research funding was provided by OncoMed Pharmaceuticals.
Footnotes
Conflict of interest statement: All of the authors are employees of OncoMed Pharmaceuticals, which provided research funding. R.N. is a member of the OncoMed Scientific Advisory Board and holds stock in the company.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120068109/-/DCSupplemental.
References
- 1.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: From flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y. Wnt/Planar cell polarity signaling: A new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 4.James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: Signals, core proteins, and effectors. Methods Mol Biol. 2008;468:131–144. doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- 5.Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinert G, et al. Sulindac sulfide reverses aberrant self-renewal of progenitor cells induced by the AML-associated fusion proteins PML/RARα and PLZF/RARα. PLoS ONE. 2011;6:e22540. doi: 10.1371/journal.pone.0022540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu D, et al. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, et al. Blockade of Wnt signaling inhibits angiogenesis and tumor growth in hepatocellular carcinoma. Cancer Res. 2009;69:6951–6959. doi: 10.1158/0008-5472.CAN-09-0541. [DOI] [PubMed] [Google Scholar]
- 12.DeAlmeida VI, et al. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer Res. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- 13.Gazit A, et al. Human Frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18:5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- 14.Bhat RA, Stauffer B, Della Pietra A, Bodine PV. Wnt3-Frizzled 1 chimera as a model to study canonical Wnt signaling. J Cell Biochem. 2010;109:876–884. doi: 10.1002/jcb.22447. [DOI] [PubMed] [Google Scholar]
- 15.Verkaar F, van Rosmalen JW, Smits JF, Blankesteijn WM, Zaman GJ. Stably overexpressed human Frizzled-2 signals through the beta-catenin pathway and does not activate Ca2+-mobilization in Human Embryonic Kidney 293 cells. Cell Signal. 2009;21:22–33. doi: 10.1016/j.cellsig.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Holmen SL, Robertson SA, Zylstra CR, Williams BO. Wnt-independent activation of beta-catenin mediated by a Dkk1-Fz5 fusion protein. Biochem Biophys Res Commun. 2005;328:533–539. doi: 10.1016/j.bbrc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Ueno K, et al. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dann CE, et al. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 19.Hoey T, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell. 2009;5:168–177. doi: 10.1016/j.stem.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright MH, et al. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Tanani MK, et al. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Yan D, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta-catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on beta-catenin signalling. Nature. 2008;452:650–653. doi: 10.1038/nature06835. [DOI] [PubMed] [Google Scholar]
- 26.Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 27.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 28.Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 29.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 30.Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinfeld B, et al. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 32.DiMeo TA, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–158. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Kanzaki H, et al. Single nucleotide polymorphism of the AXIN2 gene is preferentially associated with human lung cancer risk in a Japanese population. Int J Mol Med. 2006;18:279–284. [PubMed] [Google Scholar]
- 35.Klarmann GJ, Decker A, Farrar WL. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics. 2008;3:59–63. doi: 10.4161/epi.3.2.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazieres J, et al. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- 37.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Kuhnert F, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benhamouche S, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 42.Rothe C, et al. The human combinatorial antibody library HuCAL GOLD combines diversification of all six CDRs according to the natural immune system with a novel display method for efficient selection of high-affinity antibodies. J Mol Biol. 2008;376:1182–1200. doi: 10.1016/j.jmb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Dylla SJ, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.