The work by Takahashi et al. (1) recently reported a negative association between brainstem serotonin transporters and personality traits such as honesty and rejection to unfairness. They interpret their locus within brainstem to include the dorsal raphe nucleus (DRN) (1). Other than DRN, the median raphe nucleus (MRN) also projects to the forebrain and may be equally important in the serotonergic modulation of cortical activity. Hence, several studies have focused on these two midbrain raphe nuclei with respect to receptor and transporter distributions or anatomical connections. Small nuclei such as DRN and MRN are, however, exceptionally difficult to localize in human imaging research in vivo, such as positron emission tomography (PET).
The works of Baker et al. (2, 3) provided insight into the distribution and location of serotonergic neurons within MRN and DRN in the postmortem human brain (Fig. 1 A and B). Rostrocaudally, the DRN extends at the level of accessory oculomotor nucleus to the motor trigeminal nucleus. At the level of the trochlear nucleus ∼7–5 mm above the isthmus, the DRN exhibits its fountain-shaped appearance in a transverse section with a ventrodorsal and mediolateral extension of about 5 mm. The DRN above the isthmus includes the majority of neurons, with its subparts containing the greatest number of serotonergic neurons. At the level of the isthmus, the DRN narrows extensively to just 0.5 mm in the mediolateral extension and nestles up against the fourth ventricle. Farther caudally, the DRN divides into two staves and extends 14 mm below the level of the isthmus. For reliably delineating the DRN, a circular region of interest (ROI) may be placed at the level between superior and inferior colliculus and not extending 6 mm in diameter in a transverse plain in two slices. The ROI then may include up to 14 voxels given a voxel size of 2 × 2 × 2 mm of a PET image in MNI (Montreal Neurological Institute) standard space (Fig. 1C). In comparison, the MRN in the pontine tegmentum extends rostrocaudally from the level of the decussation of the superior cerebellar peduncle (1 mm above isthmus) down to the motor trigeminal nucleus ∼18 mm below isthmus. Most serotonergic neurons of the MRN lie within the first 10 mm below the level of the isthmus. In a transverse plain, the MRN appears with a ventrodorsal extension of about 6 mm and a mediolateral extension of about 2 mm. A reliable delineation of MRN may, therefore, provide a ROI in two slices within the rostral one-third of the pons, not extending 8 voxels.
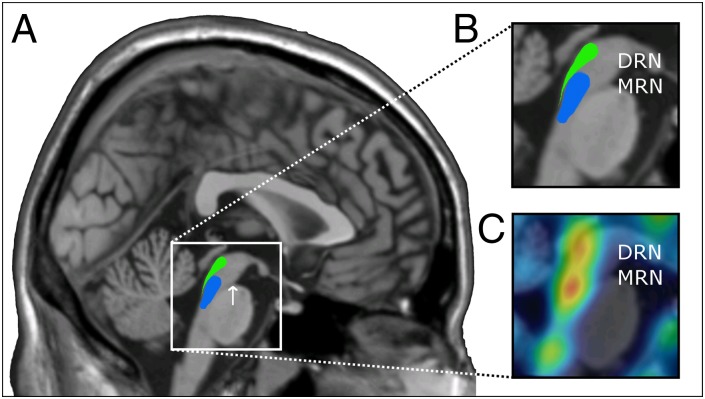
Fig. 1.
(A) Delineation of DRN (green) and MRN (blue) on a sagittal plane (x = 1 mm MNI space) of an anatomical MR image based on postmortem data from the work by Baker et al. (2, 3). The white arrow indicates the isthmus. (B) Magnification of midbrain and rostral pontine tegmentum. (C) PET mean image of serotonin-1A receptor binding within midbrain and brainstem, superimposed on the MR image. Red indicates high receptor binding. Note the two local maxima indicating the two different raphe nuclei. For the differentiaion of raphe nuclei based on Fluorodeoxyglucose-PET, see ref. 5. PMOD 3.3 (PMOD Technologies) was used for image fusion.
Given figure 2a in the work by Takahashi et al. (1), we propose that they measured serotonin transporters within the MRN rather than the DRN and suggest that MRN may be easily mistaken in imaging studies as DRN. DRN and MRN differ in several aspects, including receptor distributions and afferent and efferent connections (4), which highlights the importance of differentiating and identifying them as two separate nuclei in neuroimaging research.
Footnotes
Conflict of interest statement: G.S.K., M.S. and R.L. declare no conflict of interest. A.H. is recipient of a DOC (doctoral program) fellowship of the Austrian Academy of Sciences at the Department of Psychiatry and Psychotherapy.
References
- 1.Takahashi H, et al. Honesty mediates the relationship between serotonin and reaction to unfairness. Proc Natl Acad Sci USA. 2012;109:4281–4284. doi: 10.1073/pnas.1118687109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker KG, Halliday GM, Törk I. Cytoarchitecture of the human dorsal raphe nucleus. J Comp Neurol. 1990;301:147–161. doi: 10.1002/cne.903010202. [DOI] [PubMed] [Google Scholar]
- 3.Baker KG, et al. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. doi: 10.1002/syn.890070407. [DOI] [PubMed] [Google Scholar]
- 4.Lechin F, van der Dijs B, Hernández-Adrián G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: Relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Son YD, et al. Glucose metabolism of the midline nuclei raphe in the brainstem observed by PET-MRI fusion imaging. NeuroImage. 2012;59:1094–1097. doi: 10.1016/j.neuroimage.2011.09.036. [DOI] [PubMed] [Google Scholar]