Abstract
In the liver of female mice, the transcriptional activity of estrogen receptor (ER) α oscillates in phase with the 4-d-long estrous cycle. Here systemic, genome-wide analysis demonstrates that ER tetradian oscillation is necessary to generate pulses of expression in genes for fatty acid and cholesterol synthesis. This ER-dependent metabolic programming changes with pregnancy and after cessation of ovarian function due to age or surgical menopause, suggesting that ER signaling is optimized to coordinate liver functions with the energetic requirements of each reproductive stage. Alterations of amplitude and frequency of the tetradian cycle, as observed after surgical menopause, age, or specific ablation of the hepatic Igf-1 gene, are associated with liver fat deposition. Appropriate hormone replacement therapy reinstating the oscillatory activity of liver ER prevents the effect of surgical menopause on fat deposition in liver.
Keywords: energy metabolism, estrogen action, steroid hormone physiology, selective estrogen receptor modulators
Estrogen receptor (ER) α (or ESR1 or NR3A) is a transcription factor regulated by estrogens and nonestrogenic substances. ERα is highly expressed in reproductive organs and is indispensable for female reproductive functions (1). ERα is also present in most nonreproductive organs, where its role is currently object of intense investigation because of the large number of dysfunctions associated with the postmenopause and affecting the metabolic, cardiovascular, and immune systems (2–5).
Application of the ER responsive element (ERE)-Luc reporter mouse, a transgenic mouse in which the luciferase reporter is driven by a promoter carrying multiple ERE copies (6), identified the liver as the organ in which ERs are most transcriptionally active (7, 8). Further studies demonstrated that hepatic ERα transcriptional activity oscillates with the estrous cycle (tetradian oscillation) and is regulated by several compounds, including growth factors and nutritional proteins (9). This latter mechanism is instrumental for the synthesis of the circulating insulin-like growth factor 1 (IGF-1) necessary for the progression of the estrous cycle (9).
These studies pointed to the hepatic ERα as a sensor of nutrient availability and a switch able to block the reproductive cycle in the event of malnutrition. On the other hand, a large body of evidence indicates that ERs are important regulators of several aspects of liver energy metabolism. Phenotypic analysis of mutant mice, including ERα (ERKO), ERβ, and aromatase KO mice, demonstrated that ERs play a pivotal role in the regulation of many processes related to the control of energy homeostasis, including energy expenditure, insulin sensitivity, and fat distribution (1, 10–13). In addition, investigation of the genetic programs controlled by hepatic ERs performed by comparing the transcriptomes of vehicle- and 17β-estradiol (E2)-treated ovariectomized (ovx) mice (14) established the potential for ER to recognize and regulate the transcription of numerous genes involved in fatty acid and glucose metabolism. The conspicuous body of data demonstrating the control of hepatic ERs over large genetic programs relevant for liver lipogenesis, gluconeogenesis, and lipid transport, along with the fact that the ER transcriptional activity may be modulated by estrogenic and nonestrogenic molecules, led us to hypothesize a role of liver ERs as peripheral coordinators of energy homeostasis in response to reproductive cues. Indeed, liver ERs would be able to recognize the transition to a different reproductive stage (characterized by changes in specific circulating hormones) and to adapt the hepatic metabolism to the energy requirements of each stage by selecting the most appropriate genetic program. To test this hypothesis, we verified by genome-wide analyses whether the subtle oscillations of estrogens occurring during the estrous cycle were sufficient to influence liver gene expression, and examined the role played by ERα in this event. Given previous findings on the importance of nutrient- and ER-dependent secretion of hepatic IGF-1 for the progression of the estrous cycle (9) and on the role of IGF-1 on the transcriptional regulation of hepatic unliganded ERα (7), we expanded our study to include mice with impaired IGF-1 synthesis (liver Igf1−/−, or LID, mice) (15). Our findings demonstrate the involvement of ERs in the pulsatile synthesis of fatty acids and cholesterol in liver, and the importance of the maintenance of such oscillation to limit fat deposition in the hepatic tissues.
Results
Gene Expression Microarray Analysis to Evaluate the Influence of the Estrous Cycle on Liver Transcriptional Activity.
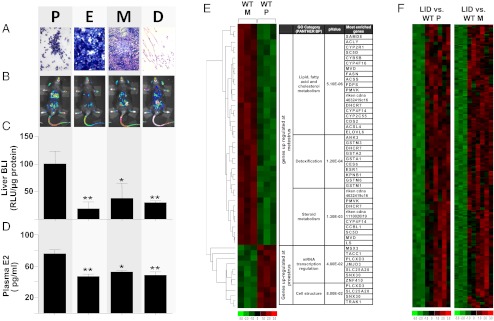
Liver transcriptome was analyzed at proestrus (P) and metestrus (M), the phases of the reproductive cycle characterized by high and low levels of circulating E2, respectively (Fig. 1 A–D). Global microarray gene expression led to the identification of 54 genes differentially expressed in the two phases of the cycle (Fig. 1E). Gene Ontology analysis carried out with the DAVID annotation tool (16) showed a clear functional difference in the genes expressed at each phase. At M, the up-regulated genes were principally connected with the metabolism of fatty acids, cholesterol, steroids, and detoxification, whereas at P we found genes relevant for transcriptional regulation and cell structure (Fig. 1E). In contrast, our analysis of LID mice found no significant functional difference in the populations of hepatic mRNAs at P and at M (Fig. 1F and Table S1). In particular, the genes involved in energy metabolism were not turned off at P, as was observed in WT mice. Thus, the alterations of the cycle due to the ablation of the hepatic Igf-1 gene had significant consequences for the liver’s ability to reprogram its transcription in relation to the stage of the reproductive cycle. This was surprising, because LID mice cycle and are fertile; however, compared with WT mice, in these mice the range of oscillation of circulating E2 is less pronounced (43-59 pg/mL vs. 48–78 pg/mL) and the length of the cycle is almost doubled (Fig. S1).
Fig. 1.
Characterization of the genes expressed differentially at proestrus (P) and metestrus (M) in mice. (A) Cytochemistry representative of vaginal smears stained at the different phases of the estrous cycle. P, proestrus; E, estrus; M, metestrus; D, diestrus. (B) In vivo bioluminescence (luciferase) imaging of intact female mice acquired at the different phases of the estrous cycle. Pseudocolor images report the level of activity of the ER in the various body areas. (C) Semiquantitative analysis of luciferase emission from hepatic tissue. Values were calculated as relative light units (RLU) and normalized over the amount (μg) of proteins in the sample. (D) E2 serum content. Both luciferase emission and E2 assays were performed on 3-mo- old cycling ERE-Luc mice; n = 3–6 mice for each phase. Data are shown as mean ± SEM. Statistics were calculated by one-way ANOVA followed by Bonferroni’s post hoc test. **P < 0.01; *P < 0.05 for comparison with proestrus animals. (E and F) Affymetrix GeneChip array data analyzed by hierarchical clustering using the dChip software. Green represents underexpressed genes; red, overexpressed genes. WT M, WT mouse liver samples harvested at metestrus; WT P, WT mouse liver samples harvested at proestrus; LID, liver-specific IGF-1–deficient mouse liver samples. The threshold for considering a change significant was a ≥1.5-fold difference in RNA levels with P ≤0.01. The table in E represents the most significant Gene Ontology (GO) functional annotation (Panther Biological Process category) of the differentially expressed genes.
Identification of Liver ER DNA-Binding Sites at Proestrus and Metestrus.
To evaluate the involvement of hepatic ERα in the establishment of the differential genetic programs found at P and M, we subjected liver extracts from WT and LID females in the two phases of the cycle to chromatin immunoprecipitation (ChIP) using ERα-specific antibodies, followed by hybridization to mouse whole-genome tiling arrays. Computational analysis indicated that the largest proportion of the ERα-binding sites was located in intergenic regions (P, 51%; M, 58%; LID, 53%), and approximately one-fourth were found in introns (P, 27%; M, 27%; LID, 37%). In WT mice, the sequences localized within 3 kb upstream of coding regions were higher at P (18%) than at M (12%), and in LID mice, the percentage of promoter-proximal sequences was significantly lower than in WT mice (5%) (Figs. S2A and S3A). In WT mice, a total of 919 ERα-binding sites located within 20 kb of the transcriptional start site of genes were identified. Comparing P and M revealed that only 8% of all binding sites (n = 74) were bound by ERα at both phases of the reproductive cycle, whereas 366 sites were bound by ERα only at P and 479 were bound only at M (Fig. S2B). RT-PCR analysis of the samples from WT mice confirmed that the sequences identified by the tiling array were indeed enriched compared with the input sample (Fig. S3B), and that ERα was recruited differently in P and M. Clustering analysis by DAVID bioinformatics was consistent with the previous microarray study; in WT mice at P, none of the sequences ChIP by ERα were in proximity to genes involved in lipid metabolism, whereas several of the sequences identified at M were associated with lipid metabolic processes. Interestingly, both P and M demonstrated a significant number of genes relevant for reproductive functions (Table S2). In LID mice livers, a total of 1,111 ERα-binding sites were identified (Fig. S2B). Again, in line with the microarray analysis, no significant functional differences for genes in the proximity of ERα-binding sites were seen at P and M; thus, the ChIP data were combined for analysis. In these mice, a large percentage of the ERα-binding sites in liver were located proximal to genes involved in lipid metabolism. In contrast to WT mice, in LID mice ERα did not bind sequences in the vicinity of genes involved in reproductive functioning.
We next examined the nature of ERα-bound motifs. We found that the ER recognized a wide variety of motifs in which the ERE was not predominant (Table S3). To better identify the networking of the transcription factor motifs in ERα DNA-binding regions, we applied Ingenuity Pathway Analysis computational methods to the different experimental settings. In WT mice at P, ERα was associated with responsive elements networking around STAT5 included in the reproductive system function network (Fig. S4A); conversely at M, the focal points of ER activity were hepatocyte nuclear factor (HNF) proteins participating in a network specialized in lipid metabolism (Fig. S4B). Interestingly, the motifs recognized by ERα in LID mice at P and M also had HNF proteins as focal elements; however, these were categorized in the hepatic system disorders and lipid metabolism network (Fig. S4C). This analysis further strengthened the view of an effect of the cycle on ERα transcriptional programs. At P, ERα is associated mainly with sequences involved in reproductive system activities, and at M it is associated mainly with sequences relevant for metabolic functions. In LID mice, the significant decrease in circulating IGF-1 leads ERα to associate primarily with gene networks active in hepatic steatosis. Overall, these results led us to conclude that (i) in regularly cycling mice, ERα plays an instrumental role in programming liver gene expression and ensuring that genes involved in energy metabolism are expressed only in selected phases of the reproductive cycle, and that (ii) liver IGF-1 synthesis is part of this programming, as demonstrated by the lack of clear fluctuation of gene expression in liver in its absence.
Estrous Cycle Plays a Pivotal Role in Regulation of Liver Lipid Metabolism.
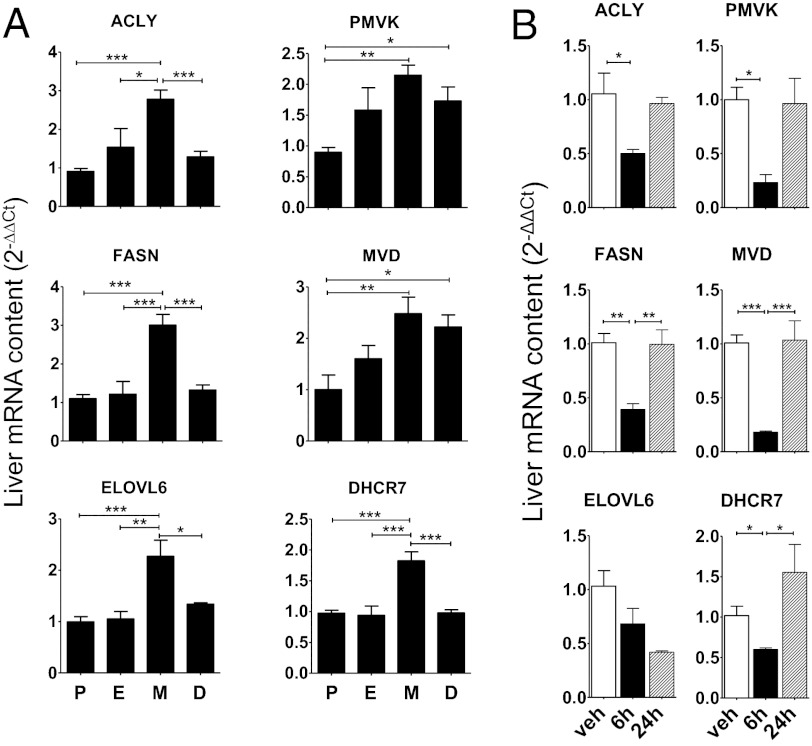
To obtain a quantitative estimate of the fluctuations of expression of the genes involved in energy metabolism, we measured the levels of mRNAs previously identified as differentially expressed at P and M in liver extracts. The quantitative analysis was carried out by RT-PCR on mRNAs encoding key lipogenesis-regulator enzymes: ACLY (involved in the supply of cytosolic acetyl-CoA for de novo lipogenesis), FASN (reportedly involved in body weight regulation), and ELOVL6 (which plays a crucial role in obesity-induced insulin resistance). We also measured core regulatory enzymes of cholesterogenesis, including PMVK and MVD (key enzymes in the early steps of the cholesterol biosynthesis) and DHCR7 (which plays a major role in the final step of cholesterol biosynthesis) (Fig. S5). In healthy, cycling mice, the relative content of these mRNAs changed significantly during the cycle and was lowest at P and highest at M (Fig. 2A). The finding that the expression of these genes was lowest when the levels of circulating E2 were highest suggested that ERα acted as a repressor. This was confirmed by the observation of significantly higher liver content of all mRNAs except MVD at 2 wk after ovx than at P (Fig. S6), reaching levels similar to those seen at M. In addition, hormone replacement therapy (HRT) with E2 (50 μg/kg i.p.) was associated with a rapid decrease (6 h) in the liver content of ACLY, FASN, PMVK, MVD, and DHCR7 mRNAs; as expected with administration of the readily catabolized E2, the effect of HRT disappeared by 24 h after the cessation of treatment. The time course of the effect of E2 was different for the gene encoding the ELOVL6 enzyme; its mRNA level was significantly lower than that in controls only at 24 h after treatment (Fig. 2B).
Fig. 2.
Quantitative analysis of liver content of mRNA encoding enzymes for cholesterol and fatty acid metabolism during the estrous cycle. Measure of mRNA content of selected enzymes encoding for cholesterol and fatty acid metabolism in liver of 3-mo-old WT mice at the different phases of the estrous cycle (A) and WT ovx mice (B) treated either with vehicle (veh) or 50 μg/kg of E2, harvested at 6 h and 24 h posttreatment. Vehicle livers harvested at 6 h and 24 h showed no variation. Data are shown as mean ± SEM; n = 3–6 mice for each phase. Statistics were calculated by one-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison with proestrus (A) and vehicle-treated (B) animals.
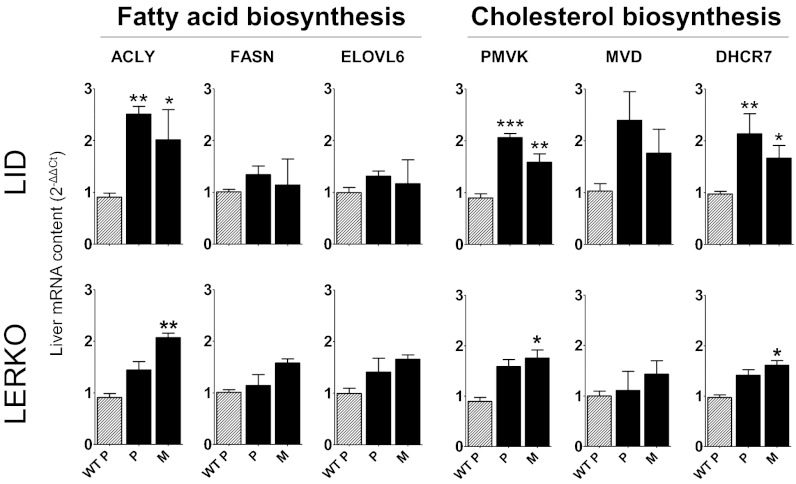
To confirm the association between ablation of the Igf-1 gene in liver and a lack of oscillation of energy-related genes during the cycle, we repeated the study using LID mice (Fig. 3). We found no significant change in gene expression between P and M. In both phases of the cycle, the concentrations of these mRNAs were never higher than those measured in WT mice at M; the levels of mRNAs encoding ACLY, PMVK, and DHCR7, but not those encoding FASN, ELOVL6, and MVD, were higher in LID mice than in WT mice at P. The involvement of ERα in the oscillations of these mRNAs has been confirmed in a study of liver extracts from mice with liver-specific ERKO (LERKO) (9). As expected, no cycle-related oscillation was found; similar to the findings in LID mice, LERKO mice exhibited slightly higher levels of ACLY, ELOVL6, and DHCR7 compared with WT mice at P, but higher levels of no enzymes at M (Fig. 3). These findings indicate that during a regular 4-d-long estrous cycle, activation of liver ERα at P is associated with repressed synthesis of the enzymes responsible for fatty acid and cholesterol metabolism, which is then reset when circulating estrogens are decreased (estrous, E, and M). This observation is consistent with the view that liver ERα participates in the metabolic adaptations necessary to satisfy energy requirements for egg maturation or for continuation of the cycle in the absence of fertilization.
Fig. 3.
Measure of mRNA content of selected enzymes encoding for de novo synthesis of fatty acids and cholesterol in liver of 3-mo-old LID and LERKO mice compared with WT mice at proestrus (WT P). Data are shown as mean ± SEM; n = 3–6 mice for each phase. Statistics were calculated by one-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison with WT at proestrus.
Liver ERα-Dependent Transcriptional Programs: Potential Biological Significance.
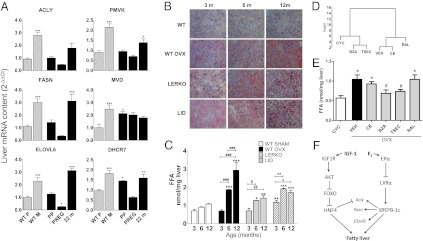
To further investigate the ability of liver ERα to regulate hepatic energy metabolism in relation to different reproductive stages, we measured the expression of ERα-regulated genes in female mice at 20 d of age (prepuberal), during pregnancy (17th day of gestation), and at 22 mo of age, when the cycle is arrested in permanent diestrus (D) (Fig. 4A). Before puberty, all mRNAs for fatty acid metabolism tested were present at the low concentrations found at P in adult mice, only two of the mRNAs involved in cholesterol biosynthesis were expressed at higher levels (MVD, +120% and DHCR7, +50% vs. intact mice at P). In pregnant mice, expression of both fatty acid and cholesterol biosynthetic genes was quite low, for Elovl6 was even significantly lower than in mice at P.
Fig. 4.
Liver lipid metabolism is affected by age and reproductive stage. (A) Measurement of mRNA content of selected enzymes encoding for de novo synthesis of fatty acids and cholesterol in liver of WT mice harvested at metestrus (WT M), from 20-d-old prepuberty (PP), 3-mo-old pregnant (17 dpc; PREG), and 22-mo-old (22 m) mice, compared with WT mice at proestrus (WT P). Data are shown as mean ± SEM; n = 3–6 mice for each phase. Statistics were calculated by the two-tailed t test. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison with WT P. (B) Representative results of Oil Red O-stained frozen sections of liver harvested from WT sham (WT), ovx WT (WT OVX), LERKO, and LID mice at 3, 6, and 12 mo of age. The red color represents neutral lipids. (C) FFA content of hepatic tissue harvested from WT, WT OVX, LERKO, and LID mice at 3, 6, and 12 mo of age. Data are shown as mean ± SEM; n = 3–6 mice for each phase. Statistics were calculated by one-way ANOVA followed by Bonferroni’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001 for comparison with WT. ###P < 0.001 for comparison between WT OVX of different ages. §P < 0.05; §§P < 0.01 for comparison between LERKO of different ages. °P < 0.05; °°P < 0.01 for comparison between LID of different ages. Reinstatement of ER tetradian cycle by appropriate HRT prevents the effect of ovariectomy. (D) Dendrogram analysis of the efficacy of selected HRT. The distances between branch lengths represent the distances between the physiology model (CYC) and the surgical menopause model (VEH). The efficacy of HRT is measured by its ability to mimic ER activity in the cycling mice. (E) FFA content of hepatic tissue harvested from intact cycling mice (CYC) and from ovx mice treated for 21 d with vehicle (VEH) and selected HRT. CE, conjugated estrogen; TSEC, tissue-selective estrogen complex; RAL, raloxifene. Data are shown as mean ± SEM; n = 6–8 mice per treatment. Statistics were calculated by the two-tailed t test. *P < 0.05 for comparison with ovx mice; #P < 0.05 for comparison with vehicle-treated mice. Livers of cycling mice were collected at metestrus, and the experiment was repeated twice. (F) Proposed mechanism of liver ERα modulation of fatty acid biosynthetic genes.
In old, acyclic mice, levels of most of the mRNAs tested were high, but never significantly higher than those at M. Particularly high were FASN (+220% vs. P), ELOVL6 (+215% vs. P) PMVK (+38% vs. P) and DHCR7 (+85% vs. P). These findings were quite consistent with the concentrations of these mRNAs in ovx mice (Fig. S6). These results indicate that the reproductive stage has repercussions for the enzymes for fatty acid and cholesterol synthesis controlled by ERα in the liver.
Alteration of Physiological Tetradian Oscillatory Pattern of Enzymes for Lipid Synthesis Is Associated with Fat Deposits in Liver.
Our data indicated tight regulation by the estrous cycle on the expression of genes for lipid and cholesterol synthesis. The expression of these genes did not oscillate with absent or altered ovarian function and ablation of the hepatic gene encoding ERα. Given the biological importance of oscillations in the maintenance of homeostasis, we next investigated possible pathological consequences of the absence of oscillations in the expression of liver fatty acid and cholesterol synthetic enzymes. To do so, we measured lipid deposits in liver by Oil Red O staining in WT mice before and after ovx and in intact LID and LERKO mice at 3, 6, and 12 m of age (Fig. 4B). We found no staining in WT cycling mice up to age 12 mo; ovx performed at age 2.5 mo had no effect in young mice, but a significant effect in mice aged 6 and 12 mo. A significant effect of age was observed in both LERKO and LID mice, with Oil Red O staining directly proportional to age. Finally, LID mice exhibited increased Oil Red O staining in young (3-mo-old) mice as well. Quantitative biochemical analysis of liver free fatty acid (FFA) content (Fig. 4C) yielded results consistent with the staining and demonstrated that long-term ovariectomy induces a major accumulation of lipids in liver. These observations lead us to conclude that in female mice, the oscillatory expression of lipid synthetic enzymes is necessary for a proper control of lipid production.
In a previous report (17), we explained how the disruption of physiological liver ER oscillatory activity caused by surgical menopause can be restored by long-term treatment with specific HRT. In particular, we demonstrated that ovx induced a significant decrease in the oscillation of ER in liver and intestine and disrupted the synchrony of the oscillations between these two organs. Long-term treatment with bazedoxifen (BZA) and TSEC [BZA with conjugated estrogen (CE)], but not with CE alone or raloxifen (RAL) were able to reinstate ER oscillatory activity in these two organs at a level and rhythm compatible with that observed in intact, cycling mice. Thus, to confirm the relevance of ER rhythmic oscillations on lipid accumulation, we ovariectomized 2-mo-old ERE-Luc mice for 4–5 wk before initiating a long-term treatment (21 d) with vehicle, CE, BZA, TSEC, and RAL at dosages previously shown to induce a proper oscillation of ER in liver and intestine. We then measured luciferase activity in liver and intestine, and used Fourier transformation to measure the frequency of the oscillations induced by the treatments. We next evaluated the synchrony of the peaks of ER activity in liver and intestine, and analyzed the data obtained by agglomerative hierarchical clustering to identify the classes of compounds that most closely mimicked the ER oscillatory behavior characteristic of cycling mice (18). The results clearly indicated that ovariectomy changed the oscillatory pattern of ER significantly (as indicated by the Manhattan distance from the cycling mice), and that CE and RAL did not significantly improve the distance from the cycling controls (Fig. 4D), demonstrating their inability to induce a physiological oscillation. Conversely, BZA and TSEC were found to cluster with cycling mice, indicating the ability to reinstate oscillation more similar to that of cycling mice than that of ovx mice. This finding confirmed our previous findings in liver (17) as well as in other organs (18). Our measurements of liver FFA content revealed significantly higher levels of lipids in ovx mice treated with vehicle for 21 d (+84% vs. intact cycling mice); among the HRTs, only TSEC and BZA prevented liver FFA accumulation. When HRT did not reinstate the correct oscillatory pattern with CE and RAL, lipid accumulation occurred (Fig. 4E).
Discussion
The main finding of the present study is that liver ERα transcriptional activity oscillates in strict correlation with the estrous cycle and plays a major role in the maintenance of a discontinuous, oscillatory expression of the genes involved in fat metabolism. The liver is an extremely plastic organ in which all of the molecules relevant for energy metabolism are rapidly synthesized and catabolized to ensure a tight association between energy production and the needs of the whole organism. Thus, to respond to each change in the reproductive stages, it is conceivable that female liver contains a molecular sensor able to decode the endocrine changes that characterize each reproductive stage, and to translate these subtle hormonal signals into activation of precise gene networks that provide the required energy. ERα’s unique versatility as a sensor and an effector of molecular signaling make it an excellent candidate for this role. It is well known that structurally and functionally quite diverse molecules, such as steroids, peptide hormones, pollutants, ions, and amino acids, may regulate the transcriptional activity of ERα (19–22), and that ERα transcriptional programs may be modulated in relation to the changes in levels of circulating hormones associated with each given reproductive stage (23, 24). The nature of the signal perceived by ERα in fact imposes well-defined spatial conformations, enabling its interaction with the specific coregulators necessary for the selection of a precise gene network (25).
Here we propose that regulation of energy metabolism by ERα is tightly regulated by reproductive functions, as indicated by several findings. First, in adult mice the expression of the genes for fatty acid and cholesterol synthesis oscillated synchronously with the cycle, possibly in the function of potential egg fertilization. Second, in prepuberal female mice there was opposite expression of the two sets of genes. The high cholesterol synthesis may be related to the needs of a still-growing organism. Third, in the later stages of pregnancy, when circulating estrogen levels reach the highest point (26), there was a dramatic decrease of expression of all enzymes, possibly because at day 17 of pregnancy, the fetus acquires the ability to produce its own cholesterol (27). Finally, and most relevantly, in the absence of a cycle due to age or surgical menopause, expression of most of the genes studied was not oscillatory and was generally set to accumulate the mRNAs at levels significantly higher than those at proestrus. Most relevant is the fact that a long-term dysregulation of the estrous cycle and tetradian ERα oscillatory activity (due to, e.g., ovariectomy, aging, altered IGF-1 signaling) resulted in accumulation of fat deposits in liver. In LID and LERKO mice, the severity of lipid deposition was inversely proportional to the extent of synchrony between the estrous cycle and production of mRNAs for fatty acid/cholesterol synthesis.
Research on biological rhythms has established that oscillations are important biological devices (28) essential to health. Biological oscillations are maintained by a set of transcription/translation feedback loops (29, 30) or pulsatile secretion of hormones (31, 32). Oscillation in the activity of transcription factors is well established and has been proposed as a common feature for gene regulation. It is well recognized that ER signaling is temporally organized at multiple levels. In the constant presence of the ligand, ERα undergoes very rapid cycles of coregulator recruitment and transcription (33, 34). In liver, ERα activity is regulated by periodic food intake (8). Daily, the circadian clock protein PER2, after induction by ERα, inhibits the receptor activity by physical interaction (35). Finally, the estrous cycle induces an infradian activation of the receptor with a pulse that is species-specific and in mouse is tetradian, lasting 4 d. In analogy with what is observed for circadian rhythm, we have shown that the central oscillator—in this case, the ovaries—has a overall role in maintaining harmonic ERα rheostasis (rhythmic steady state) (36), yet in each tissue ERα is able to oscillate autonomously (17). Previous studies have shown that the adequate frequency and intensity of nuclear receptor signaling is required for the selection of specific transcriptional networks. Our study suggests that the tetradian activity of liver ERα is important in readying the receptor to adjust to the changes of energy requirements in relation to the reproductive stage. These findings are particularly relevant for the understanding of the etiology of metabolic disorders and hepatic lipid metabolism associated with impaired ovarian functions (e.g., polycystic ovary syndrome or the cessation of the reproductive cycle) (37), indicating that selective ER modulators (SERMs) may reinstate ER oscillatory activity. This suggests a revision of current rationales for treating postmenopause problems, taking into account the periodic nature of ER signaling to ensure the more efficacious use of estrogens.
The mechanism underlying the oscillation in the production of energy metabolites has not yet been elucidated. Data from our laboratory and others show that LXRα is a target for ERα (38) and in liver the synthesis of liver X receptor (LXR) α is regulated by the estrous cycle and is significantly increased in LERKO mice. On the other hand, IGF-1 is known to regulate the activity of HNF4, a transcription factor relevant for fatty acid deposition (39); we believe that the physiological changes in circulating estrogens and IGF-1 favor the different pathways regulating lipid synthesis, which will alternate in response to these two hormones (Fig. 4F). Our analysis of the motifs immunoprecipitated with ERα supports the view of a significant change in the receptor’s interactions with different transcription factors during the cycle. Of course, this view is highly simplified, and we do not rule out the possible involvement of other paracrine factor or alimentary cues in the final control of liver lipid metabolism. For the differential effects of the HRTs tested here, we can only speculate that each of them may act differently on ERα and modulate LXR and FOXO-HNF4 transcriptional activity, thus opposing the ovx-induced fatty acid deposition in liver.
In conclusion, the present study points to a previously unreported role for liver ERα in the temporal orchestration of lipid metabolism to ensure a continuous, dynamic equilibrium between energy metabolism and reproduction.
Methods
Animals.
Studies were done using female heterozygous C57BL/6 ERE-Luc and mutants with liver-specific ablation of ERα and IGF-1 genes (LERKO and LID mice) generated as specified in SI Methods. Animal care and all experiments were in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National institutes of Health in accordance with the European Guidelines for Animal Care and Use of Experimental Animals.
Bioluminescence-Based Imaging and Luciferase Assays.
In vivo bioluminescence imaging and luciferase assays were carried out as described previously (40).
Microarray and Tiling Array Analysis.
Microarray analysis was carried out using the GeneChip Mouse Genome 430 2.0 Array (Affymetrix) and scanned with the GeneChip Scanner 3000 (Affymetrix). For the tiling array, fixed liver tissues were disaggregated and immunoprecipitated with ERα antibody MC-20 (Santa Cruz Biotechnology). Labeled products (8 μg) were hybridized to the Affymetrix mouse tiling 2.0R array set.
Histochemistry.
For histochemical analyses, 7-μm frozen sections were prepared with a Microm HM-500M cryostat and mounted onto poly-l-lysine–coated glass slides before staining with Oil Red O lipid stain at room temperature for 60 min. Counterstaining was done with hematoxylin.
RNA Extraction and Retro-Transcription.
After harvesting, 30 mg of frozen liver tissue was weighed and used for RNA extraction with the Qiagen RNeasy Kit. cDNA was prepared as described previously (9).
Hepatic FFA Assay.
FFAs were extracted by homogenization of liver tissue in chloroform/1% Triton X-100, and quantitation was done with the BioVision enzyme-based Free Fatty Acid Quantification Kit, in accordance with the manufacturer’s instructions.
Phenetics of Drug Action.
ER oscillatory activity after SERM treatments was analyzed as described previously (18) and detailed in SI Methods.
Supplementary Material
Acknowledgments
We thank Shirley Liu (Dana Farber Cancer Institute) for initial identification of ERα-IP regions by MAT algorithms, Paolo Ciana for thoughtful discussions, Valeria Benedusi for a critical reading of the manuscript, and Clara Meda and Monica Rebecchi for technical assistance. This work was supported by grants from the European Community (IP CRESCENDO LSHM-CT-2005-018652 and STREP EWA LSHM-CT-2005-518245) and the National Institutes of Health (RO1 AG027713 granted to A.M., DK074967 granted to M.B.), and by Pfizer Pharmaceutical Co.
Footnotes
A.V., S.D.T., A.S., J.C., and M.B. declare no conflict of interest. A.M. has received grant support and consulting fees from Pfizer.
*This Direct Submission article had a prearranged editor.
Database deposition: The data reported in this paper have been deposited in ArrayExpress (experiment nos. E-MEXP-3504 and E-MEXP-3505).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205797109/-/DCSupplemental.
References
- 1.Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 2.Bryzgalova G, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, et al. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherwin BB, Phillips S. Estrogen and cognitive functioning in surgically menopausal women. Ann N Y Acad Sci. 1990;592:474–475. [Google Scholar]
- 5.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: New players in diabetes mellitus. Trends Mol Med. 2006;12:425–431. doi: 10.1016/j.molmed.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Ciana P, et al. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol. 2001;15:1104–1113. doi: 10.1210/mend.15.7.0658. [DOI] [PubMed] [Google Scholar]
- 7.Ciana P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9:82–86. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- 8.Ciana P, et al. Estrogenic activities in rodent estrogen-free diets. Endocrinology. 2005;146:5144–5150. doi: 10.1210/en.2005-0660. [DOI] [PubMed] [Google Scholar]
- 9.Della Torre S, et al. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13:205–214. doi: 10.1016/j.cmet.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso-Magdalena P, et al. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryzgalova G, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295:E904–E912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones ME, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao H, Fält S, Sandelin A, Gustafsson JA, Dahlman-Wright K. Genome-wide identification of estrogen receptor alpha-binding sites in mouse liver. Mol Endocrinol. 2008;22:10–22. doi: 10.1210/me.2007-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakar S, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 17.Della Torre S, et al. The conundrum of estrogen receptor oscillatory activity in the search for an appropriate hormone replacement therapy. Endocrinology. 2011;152:2256–2265. doi: 10.1210/en.2011-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rando G, et al. An innovative method to classify SERMs based on the dynamics of estrogen receptor transcriptional activity in living animals. Mol Endocrinol. 2010;24:735–744. doi: 10.1210/me.2009-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nettles KW, et al. Structural plasticity in the oestrogen receptor ligand-binding domain. EMBO Rep. 2007;8:563–568. doi: 10.1038/sj.embor.7400963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun A, et al. Discovering small-molecule estrogen receptor α/coactivator binding inhibitors: High-throughput screening, ligand development, and models for enhanced potency. ChemMedChem. 2011;6:654–666. doi: 10.1002/cmdc.201000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carascossa S, Dudek P, Cenni B, Briand PA, Picard D. CARM1 mediates the ligand-independent and tamoxifen-resistant activation of the estrogen receptor alpha by cAMP. Genes Dev. 2010;24:708–719. doi: 10.1101/gad.568410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bondesson M, et al. A CASCADE of effects of bisphenol A. Reprod Toxicol. 2009;28:563–567. doi: 10.1016/j.reprotox.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Barkley MS, Geschwind II, Bradford GE. The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod. 1979;20:733–738. doi: 10.1095/biolreprod20.4.733. [DOI] [PubMed] [Google Scholar]
- 24.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Brzozowski AM, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 26.Yen S. In: Endocrine-Metabolic Adaptations in Pregnancy. Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. Yen S, Jaffe R, Barbieri R, editors. Philadelphia: WB Saunders; 1991. pp. 936–981. [Google Scholar]
- 27.Belknap WM, Dietschy JM. Sterol synthesis and low-density lipoprotein clearance in vivo in the pregnant rat, placenta, and fetus: Sources for tissue cholesterol during fetal development. J Clin Invest. 1988;82:2077–2085. doi: 10.1172/JCI113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai TY, et al. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–129. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duguay D, Cermakian N. The crosstalk between physiology and circadian clock proteins. Chronobiol Int. 2009;26:1479–1513. doi: 10.3109/07420520903497575. [DOI] [PubMed] [Google Scholar]
- 31.Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology. 1976;98:562–570. doi: 10.1210/endo-98-3-562. [DOI] [PubMed] [Google Scholar]
- 32.Vujovic N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am J Physiol Regul Integr Comp Physiol. 2008;295:R355–R360. doi: 10.1152/ajpregu.00498.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Métivier R, et al. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- 34.Stenoien DL, et al. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor alpha-coactivator complexes in living cells. Mol Cell Biol. 2001;21:4404–4412. doi: 10.1128/MCB.21.13.4404-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP. The clock gene Per2 links the circadian system to the estrogen receptor. Oncogene. 2007;26:7916–7920. doi: 10.1038/sj.onc.1210585. [DOI] [PubMed] [Google Scholar]
- 36.Mrosovsky N. Rheostasis: The Physiology of Change. USA: Oxford Univ Press New York; 1990. [Google Scholar]
- 37.Völzke H, et al. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594–595. doi: 10.1136/gut.2006.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundholm L, et al. Gene expression profiling identifies liver X receptor alpha as an estrogen-regulated gene in mouse adipose tissue. J Mol Endocrinol. 2004;32:879–892. doi: 10.1677/jme.0.0320879. [DOI] [PubMed] [Google Scholar]
- 39.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maggi A, Rando G. Reporter mice for the study of intracellular receptor activity. Methods Mol Biol. 2009;590:307–316. doi: 10.1007/978-1-60327-378-7_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.