Abstract
Objects grasped by an agent have a value not only for the acting agent, but also for an individual observing the grasping act. The value that the observer attributes to the object that is grasped can be pivotal for selecting a possible behavioral response. Mirror neurons in area F5 of the monkey premotor cortex have been suggested to play a crucial role in the understanding of action goals. However, it has not been addressed if these neurons are also involved in representing the value of the grasped object. Here we report that observation-related neuronal responses of F5 mirror neurons are indeed modulated by the value that the monkey associates with the grasped object. These findings suggest that during action observation F5 mirror neurons have access to key information needed to shape the behavioral responses of the observer.
Keywords: macaque monkey, ventral premotor cortex, reward, social interaction
Mirror neurons are a class of neurons originally discovered in the ventral premotor cortex (area F5) (1–3) and later found in the inferior parietal lobule (area PFG) of the macaque monkey (4, 5). Their distinctive property is to discharge both during the execution of a given motor act as well as during the observation of a similar motor act performed by others. Mirror neurons are thought to be involved in understanding the goals of motor acts of others by transforming the representation of a motor act coded visually into its motor representation (6–10). In accordance with this view, mirror neurons exhibit a substantial degree of abstraction from the details of the observed motor act. Thus, they typically respond to the observation of a motor act, independent of its direction (1, 2), the type of effector used (11, 12), or the type of sensory information describing it (13, 14). At first glance, one might expect that mirror neuron responses depend exclusively on the observed motor act. However, this is not necessarily the case. For example, we recently showed that the responses of a subset of F5 mirror neurons to observed motor acts were modulated by the “operational” distance between the observer and the observed actor, thus suggesting that different responses might depend on the ability of the observer to act on the object or interfere or not with the action unfolding in front of him (15). This dependency of the neuronal responses on the operational relationship between the two involved agents suggested that this subgroup of mirror neurons might actually contribute to providing a link between action interpretation and response selection. However, the selection of an appropriate response to the actions of others may also require the assessment of the value that the object on which the observed action is performed has for the observer. As this value also depends on the specific needs, preferences, and desires of the observing monkey, it is commonly referred to as “subjective” value.
Previous studies reported that mirror neurons become active both when the observed agent acts on food or on 3D objects devoid of particular interest for the monkey. Hence, mirror neurons do not necessarily seem to require food to be activated (2, 4, 16). No study, however, has specifically addressed this issue and, in particular, no experiments assessing whether the subjective value of the grasped object may influence the intensity of mirror neuron responses during action observation have been carried out.
In the present study, we addressed this question in three experiments. In the first experiment we compared the responses of mirror neurons to the observation of grasping food with the observation of grasping nonfood objects. In the second experiment, we compared the neuronal responses to the observation of grasping objects associated with reward or not. In the third experiment, we examined whether the responses of mirror neurons were differently modulated when the target objects assumed specific reward value for the monkey. Our results show that a large subset of mirror neurons is influenced by the subjective value of the object on which the observed action is performed.
Results
Identification of Mirror Neurons.
Single neuron activity was recorded from area F5 (Fig. 1A) of two monkeys. The motor properties of the recorded neurons were assessed and neurons that exhibited a statistically significant increase of their discharge with respect to baseline in association with active grasping movements were selected (for details, see ref. 17 and Experimental Procedures). Then, we determined whether these grasping neurons also responded to the observation of grasping carried out by the experimenter. The properties of the ones that were responsive, henceforth defined as mirror neurons, were studied in the three experiments reported below.
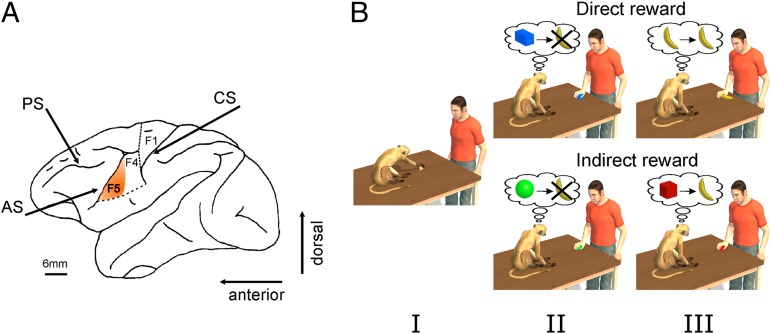
Fig. 1.
Recording region and paradigm. (A) Lateral view of the left hemisphere of the monkey brain. The highlighted sector denotes the part of the ventral premotor cortex (area F5) from which neurons were recorded (AS, arcuate sulcus; CS, central sulcus; PS, principal sulcus). (B) Schematic view of the experimental paradigm. In each session we first tested the motor responses of neurons during active movements of the monkey (I). The visual responses of these neurons were further tested with the experimenter executing goal-directed motor acts in front of the monkey. Direct Reward Condition: The experimenter grasped either nonfood (II) or food objects (III). After a food object was grasped, the monkey was rewarded by delivering a raisin in a randomly chosen subset of trials. No reward was given following the grasping of a nonfood object (a metal object). Indirect Reward Condition: The experimenter grasped two objects. One object was randomly followed by a reward (typically a raisin), whereas the other object was not associated with any reward (for further details, see Experimental Procedures). Note that the color of the nonrewarded object used in experiments was actually gray. In the figure it is rendered green to make it easier to distinguish it from the hand. Please also note that the shape and size of the objects shown do not correspond to their actual properties. For details about shape and size, please refer to the Experimental Procedures.
Experiment 1.
In the first experiment (Fig. 1B, direct reward) the experimenter grasped a neutral object devoid of any reward value for the monkey or, alternatively, food objects (typically a raisin) that were handed over to the monkey at the end of a randomly chosen subset (20–30%) of the trials after the end of the experimenter’s motor act. Both food and nonfood objects were grasped using a precision grip. Exp. 1 was based on 149 mirror neurons, preferring grasping. These neurons represented about half (50%) of F5 grasping neurons recorded in this experiment (n = 299).
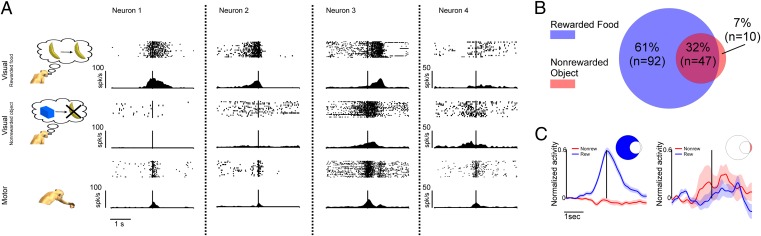
Fig. 2A compares the visual responses of four mirror neurons recorded in this experiment. All four neurons were activated by the execution of grasping. However, their visual responses exhibited clearly different patterns dependent on which type of object the observed motor act was aiming at. Neurons 1 and 2 were selectively activated by observed motor acts directed at food items. In contrast, their activity during the observation of motor acts aiming at nonfood objects did not differ from baseline activity. Neuron 3 was activated by the observation of goal-directed motor acts independent of the type of targeted object (food or nonfood). Finally, the neuron shown in the right column of Fig. 2A (neuron 4) exhibited a clear response to the observation of a motor act directed to the nonfood object, but it gave only a weak response to motor acts directed to food objects.
Fig. 2.
Responses of exemplary mirror neurons and population activity during the observation of actions predicting later rewards or their absence. (A) Examples of neurons tested in Exp. 1. All four neurons responded to active movements of the monkey. Their visual responses exhibited either a preference for the grasping of a food object (neurons 1 and 2), no preference for the type of object (food or nonfood objects) on which actions were executed (neuron 3), or a preference for the grasping of the nonfood object (neuron 4). (B) Venn diagram illustrating the number of mirror neurons significantly activated in the rewarded and the nonrewarded conditions. The intersection of the two circles represents neurons whose discharges exhibited no statistically significant difference between the two experimental conditions. Neurons not contained in the intersection responded with a significantly stronger discharge during one of the two experimental conditions. (C) Normalized population responses of mirror neurons exhibiting selectivity for either food or nonfood conditions. The shaded regions represent SEM. Vertical lines in A and C represent the time of contact between the experimenter’s hand and the object.
The responses of 68% of the studied mirror neurons (n = 102) were influenced by the type of the observed grasped object (food or nonfood): 61% (n = 92) were more strongly activated when the grasping action was directed at food objects. Conversely, a much smaller subgroup of 7% neurons (n = 10) showed a preference for acts on nonfood objects. The remaining 47 neurons (32%) responded to the observation of motor acts independent of whether the object was eatable or not (Fig. 2B). The two subpopulations of mirror neurons showing preference for food and nonfood objects, respectively, did not differ with regard to the temporal dynamics of their response. In both groups the peak discharges occurred at approximately the time when the hand touched the object (Fig. 2C).
These results suggest that there are two distinct subsets of mirror neurons in area F5, a less numerous one lacking preference for object value and a more numerous one exhibiting such preference. A possible explanation of this difference is that mirror neuron responses distinguish motor acts directed at food as such from those directed at nonfood objects. Alternatively, it is possible that the results obtained were because of differences in the subjective value of the observed object, independent of whether the object was food or not. To distinguish between these two possible interpretations, we carried out a second experiment (Exp. 2).
Experiment 2.
In Exp. 2 (Fig. 1B, indirect reward) the monkey observed motor acts carried out on two nonfood objects (a large gray cylinder and a small red cylinder). Both objects were grasped using a power grip. None of these acts had a reward value for the monkey before the experiment. During neuron testing, motor acts on one of them (the red cylinder) were rewarded by a piece of dry food. The reward was given to the monkey at the end of a subset of randomly selected trials (20–30%). In contrast, motor acts on the other object (gray cylinder) were never rewarded. A total of 227 F5 neurons with motor responses to grasping were studied in Exp. 2. Of these neurons, 87 [of which n = 36 also studied in the first experiment (Fig. S1)] exhibited visual responses to grasping motor acts aiming at least at one of the used objects.
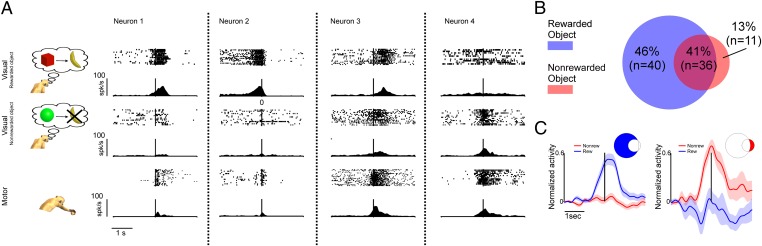
Fig. 3A illustrates examples of four mirror neurons tested by presenting motor acts on the two objects. Their motor responses are also illustrated. Only neuron 3 exhibited visual responses largely independent of whether the grasped object was associated with a reward or not. In contrast, the activity of neurons 1, 2, and 4 was modulated according to the presence or absence of the reward. Neurons 1 and 2 were more strongly activated by the object associated with a reward, whereas neuron 4 was more strongly activated by the object not associated with reward.
Fig. 3.
Responses of exemplary mirror neurons and population activity during the observation of actions predicting later rewards or their absence. (A) Examples of the four types of neurons recorded in Exp. 2. (B) Venn diagram. (C) Normalized population activity. Same conventions as Fig. 2.
About half of the tested mirror neurons (46%, n = 40) exhibited stronger visual responses when the observed motor acts were performed on rewarded objects (Fig. 3B). In contrast, 11 neurons (13%) showed a stronger activity for nonrewarded objects. The remaining neurons (41%, n = 36) responded to the visual presentation of motor acts independent of whether the motor act was associated or not to a reward. Similar to neurons studied in Exp. 1, the two subpopulations of mirror neurons tested in Exp. 2 did not differ with respect to the temporal dynamics of their responses. In both groups, the peak discharge occurred at approximately the time when the hand touched the object (Fig. 3C).
These findings support a modulating role of the subjective value of the observed object, independent of whether the grasped object was food (Exp. 1) or not (Exp. 2).
In the final experiment (Exp. 3), we tested whether mirror neurons reflect differences in subjective value in a continuous rather than a categorical manner.
Experiment 3.
This experiment was carried out on one of the two monkeys (monkey E). The experiment consisted of a series of blocks of trials with each block characterized by the experimenter grasping one out of two possible nonfood objects (a big cylinder and a small cylinder, respectively; see Experimental Procedures for details). Independent of the object chosen, on a given block the motor act directed at the object could be associated with three types of consequences for the observing monkey: (i) the delivery of a highly relished food (a freshly cut piece of fruit), (ii) the delivery of a less relished food (dry pellet chow that the monkey often refused), or (iii) no reward at all. Before each block the experimenter let the monkey know what kind of consequence to expect in the upcoming block by showing the appropriate food item or, alternatively, an empty hand in the case of blocks without any reward. To reinforce the type of motor act–reward association in blocks with reward, in the first trial of the block the monkey was given the food associated with the given block. Subsequently, in that block the monkey received further rewards in a randomly chosen subset of trials.
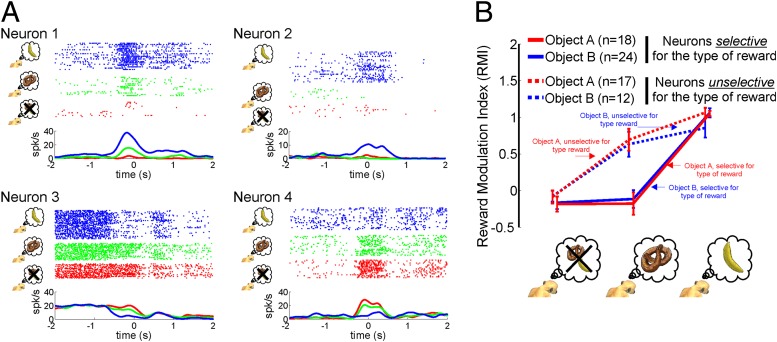
Fig. 4A shows examples of neurons whose discharge rate depended on the subjective value of the object on which the observed motor act was performed. Neuron 1 exhibited a monotonic excitatory dependency on the subjective value, with the strongest responses to the motor act associated with the favorite reward. Neuron 2 gave a response only to the motor act associated with the favorite reward. Neuron 3 showed an inhibitory response modulation with the strongest inhibition elicited by the motor act associated with the favorite reward. Finally, neuron 4 exhibited a graded dependency on the subjective value of the motor act, with the strongest response for blocks lacking rewards.
Fig. 4.
Impact of different subjective values on the visual responses of mirror neurons. (A) Exemplary mirror neurons recorded in the Exp. 3. Neuron 1 showed a monotonic excitatory modulation, with the strongest response to the most relished reward (symbolized by a banana and blue coloring). Neuron 2 exhibited a response only for the most relished reward. Neuron 3 showed a monotonic inhibitory modulation (less-relished reward, indicated by the pretzel and green coloring). Finally, neuron 4 demonstrated again a monotonic excitatory modulation but, unlike neuron 1, its strongest response was in the nonrewarded condition (red coloring). (B) The plot shows average ± SE data, based on an index (RMI) that captures the normalized absolute size of the reward associated discharge. Neurons showing a significant difference between the favorite and the less relished reward were classified as “selective for the type of reward.” The remaining neurons were classified as “unselective for the type of reward” (see Experimental Procedures).
About 50% (Table S1) of the F5 mirror neurons tested in Exp. 3, preferring grasping, exhibited a significant effect of the subjective value (P < 0.05 Kruskall–Wallis test). Post hoc comparisons (U test, P < 0.05 Bonferroni-corrected) revealed that a large number of the value-sensitive mirror neurons (about 35%) (Table S2) showed selectivity for the favorite reward condition (i.e., response in the favorite-reward condition significantly larger than the responses in the less-relished reward and no-reward conditions and, at the same time, no difference between the latter two). Only a few (less than 5%) preferred the less-relished reward or, alternatively, the absence of a reward (about 10%). Finally, the remaining 50% of the mirror neurons exhibiting a significant effect of subjective value did not show preference for a particular condition, independent of the choice of the other conditions contributing to the post doc (U test) comparisons (neurons labeled as “response dependent on the reward but unselective” in Table S2).
To come up with a population measure of the influence of the subjective value on the neuronal responses, we calculated a reward modulation index (RMI; see Experimental Procedures) for the subset of neurons (n = 35 for object A and n = 36 for object B) that, according to the aforementioned post hoc test, showed a significant difference between the favorite reward and the absence of a reward. The RMI assumes similar values independent of whether a neuron prefers the absence of reward or the favorite reward. Fig. 4B plots the mean RMI as function of reward type, separately for neurons distinguishing significantly (U test, P < 0.05 Bonferroni-corrected) between the favorite reward and the less-relished reward and those that do not. Fig. 4B demonstrates that less than half of the subset of neurons responded to the absence or availability of a reward without caring about the subjective value of the reward. The other neurons, on the other hand, showed a monotonic dependency on subjective value as indicated by significantly larger responses to the favorite compared with the less-relished reward.
Discussion
The results of the experiments reported here clearly indicate that the visual responses of mirror neurons are modulated by the subjective value that the observed object, on which the motor act was performed, has for the observer. Furthermore, the visual responses of most mirror neurons were stronger if the observed motor act was associated with the most relished reward, although a minority of mirror neurons seemed to prefer objects not followed by rewards or, alternatively, by less-relished rewards.
There is rich evidence that in many parts of the brain, reward may influence the neuronal discharge during goal-directed movements (18). This finding is also true for the frontal lobe, for which it has been demonstrated that in tasks with rewards of variable size, there is stronger activity when the monkey anticipates a larger reward (19–22). There is now agreement that this neuronal modulation may be determined either by the value of the expected reward or by the degree of motivation/attention induced by the expectation of reward (see ref. 23). Experiments in which attempts were made to differentiate between these two variables showed that the neurons in both orbitofrontal cortex (19–21) and in cingulate sulcus (PFcs) (see ref. 22) seem to represent the reward value. In contrast, reward-related signals in lateral prefrontal cortex, the frontal eye fields, and premotor cortex suggest that these areas use reward for motivational purposes or for allocation of attentional resources (19–21, 24).
In general, a role of motivation for reward-associated responses may be assumed in paradigms, in which differences in the value of expected rewards are able to shape the monkey’s task-related behavior. However, this was clearly not the case in our experiments. Our monkeys passively observed object-directed motor acts performed by others, the consequences of which in terms of subsequent rewards for the monkey were not influenced by the monkey’s behavior. In other words, monkeys did not have to engage in any active behavior to get the reward and only trials in which the monkey was observing the action were considered (see Experimental Procedures). As a consequence, in the case of the category of neurons showing a stronger discharge for the observation of rewarded stimuli, this discharge increase can hardly be attributed directly to motivation, defined as a signal preparing motor activity needed to fulfill a task.
It is more difficult to rule out a role of attention as an explanation of our findings. In fact, the presence of food in the first experiment could have increased the monkey´s attention toward the motor act performed by the experimenter on the food. The same explanation might also hold for Exp. 2, in which neutral objects associated with reward assumed the same value as food. Similarly, in Exp. 3 the monkey could have paid more attention to motor acts on objects associated with the most relished food. This finding could explain the strongest discharge found in most neurons during the observation of these motor acts. The use of blocked paradigms might have reinforced this effect.
However, there are two observations suggesting that in the experiments reported here, the subjective value of the observed object rather than attention, may have been the variable modulating mirror neurons responses. First, the time monkeys spent in scrutinizing the visual area in which actions unfolded was typically the same in all sessions, independent of the specific object involved and the specific reward expectations (see the analysis of eye position discussion in the SI Text for a detailed description of the oculomotor behavior). This finding suggests that there was no difference in the amount of attention in the different tasks. Second, more than 15% of the neurons (Table S2) had a preference for the less-relished reward or the absence of reward, although one might expect the favorite reward to maximally boost attention and attention-related response. For these reasons we think that the observed modulation of the response was indeed mostly because of the subjective value associated with the object rather than to modifications of the level of attention.
In conclusion, our results suggest that the discharge of F5 mirror neurons is influenced by the information on the value that the object targeted by the motor act has for the observer. This information may help the observer to interpret the action meaning. This interpretation fits previous data (4, 16), showing that during observation of a grasping act embedded in different actions serving different goals, mirror neurons discharge differently depending on the final goal of the observed action. This finding has prompted the idea that the differential discharge would allow the observer to understand the motor intention of the observed agent. Understanding the action goal requires access to object semantics (e.g., food vs. non-eatable object) as well as contextual information. The subjective value assigned to the object is an element contributing to the comprehension of others’ intentions. Thus, the present data, showing an influence of subjective value, might provide a possible explanation of how the monkey mirror neuron system could contribute to reading the intention of an observed agent. However, we emphasize that this interpretation remains speculative because it relies on the untested assumption that the value attributions of the monkey observer and the human actor are the same.
Experimental Procedures
Subjects, Surgery, and Recording Methods.
Two male monkeys (Macaca mulatta) were used in the experiments. During the experiments, the monkeys sat comfortably in a primate chair. The movements of the left arm were restrained by a gentle gauze bandage; the right arm was free to move. Surgery and recording methods followed previous descriptions (15). All animal preparations and procedures fully complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the local ethics committee (Regierungspraesidium Tuebingen).
Motor Task.
To identify the motor properties of the neurons recorded from area F5, we trained monkeys to grasp and lift three small metallic objects presented at constant locations within their workspace (Condition I in Fig. 1) as described previously (15, 17).
Visual Task.
Visual responses of mirror neurons were tested by having the experimenter executing motor acts on food or nonfood objects in front of the monkey.
In all of the experiments, the position of the experimenter grasping and lifting the objects, as well as the position of the objects with respect to the monkey’s body, were the same across all experimental conditions. The objects were always outside the monkey reaching distance and they were presented for at least 4 s before the action began. Trials in which the monkey was not directing his gaze toward the seen motor act were aborted and not considered for further analysis.
In the first experiment the experimenter grasped with a precision grip, in different blocks, either a piece of food (dry food, typically a raisin) or, alternatively, a nonfood object (a gray small thin rectangular plate with dimensions = 8 × 1 × 1 cm). In randomly selected trials the experimenter rewarded the monkey, giving it the piece of food he had grasped. The monkey was never rewarded in the blocks in which the experimenter grasped nonfood objects.
In the second experiment two nonfood objects, differing in their colors and sizes, were used: a large gray cylinder with the diameter of 5 cm and height of 5 cm, and a red small cylinder of 2 cm of diameter and 2 cm of height. Both objects were grasped with whole-hand grip. As in Exp. 1, a block design was used. The red object predicted a later reward that was given to the monkey on a randomly chosen subset of trials. In contrast, the gray object was never rewarded.
In the Exp. 3 the experimenter grasped one out of two nonfood objects with a whole-hand grip. The objects were: a black big cylinder, 5 cm of diameter and 5 cm of height (object A), or a black small cylinder, 2 cm of diameter and 2 cm of height (object B). For a given block of trials, monkeys observed motor acts directed always at one of the two objects, associated for the whole duration of the block with specific consequences for the monkey. There could be three types of consequences, chosen at random for a given block, either the delivery of a highly relished food item (a freshly cut piece of fruit) at the end of the trial, the delivery of a less-relished food item (dry pellet chow that the monkey usually refused), or no reward delivery. Each experiment consisted of three blocks, the order of which was pseudorandomized. Each block contained trials of the same type (n = 10 or more). Every block started with the experimenter showing the future reward to the monkey or, in the case of blocks without reward, showing his empty hand. In the food-related blocks, the food, after its presentation, was hidden from the monkey’s sight. Then the action stimulus was presented and, at the end of randomly chosen trials (20–30%), the monkey was rewarded with preferred food, less-relished food, or was not rewarded. To reinforce the type of motor act-reward association in blocks with reward, in the first trial the experimenter offered the monkey the food associated with the given block.
Analysis of Single Neuron Responses.
To be included in the analysis, a single unit had to be recorded long enough to allow a minimum of eight valid trials for each condition of both the motor and visual task. However, it must be emphasized that, on average, the number of valid trials per neuron, considered for statistical analysis, was considerably higher (median ≥ than 16 trials). Statistical comparisons were based on nonparametric tests as the data were frequently not normally distributed (P < 0.1, Shapiro test).
The identification of neurons responding during the execution and the observation of grasping motor acts followed procedures previously described (15, 17). Briefly, to investigate the visual selectivity, the responses of each neuron during the observation of motor acts executed by the experimenter were divided into four phases: (i) baseline, before initiation of the experimenter’s hand movement (from 2,000 ms to 1,500 ms before hand-object contact); (ii) approaching (from 500 ms before to hand-object contact); (iii) grasping (from hand object-contact until its lifting); and (iv) lifting (interval of 500 ms starting with the lifting of the object). The selectivity with respect to baseline was assessed by comparing the mean firing rates in the various phases by a Kruskal–Wallis test (P < 0.05 with Bonferroni-correction for multiple comparisons). Only neurons showing the same activity in the baseline period between conditions (P > 0.05, U test) were considered. The preference for a condition of each visually selective neuron was assessed by first subtracting the baseline activity from the phases in which the neuron showed its visual responses and then by comparing the “best” phase (i.e., the phase showing the largest difference with respect to baseline) between conditions (P < 0.05, U test).
Reward Modulation Index.
To provide a population measure of the influence on neuronal responses of the manipulation of the monkey’s reward association we used a RMI similar to a previously described index (15). For each neuron that distinguished significantly between the no-reward and the favorite-reward condition, the discharge rates for all of the three experimental conditions (no reward, less-relished reward, and favorite reward) were normalized by dividing them by the discharge rate in the preferred condition (i.e., either the no-reward or the favorite-reward condition) after having subtracted the respective baseline activity and, in the case of neurons exhibiting a preference for the no-reward condition, by subtracting the normalized discharge rate from 1. Neurons were grouped based on the difference between the favorite and the less relished reward (P < 0.05, U test with Bonferroni correction): neurons showing a significant difference between the two were classified as “selective for the type of reward.” The other neurons were classified as “unselective for the type of reward.” A cumulative RMI was obtained by averaging across neurons.
Recording of Eye Position.
Eye movement records sampled at 1 kHz were stored continuously during experimental sessions at a sampling rate of 12.5 kHz, and off-line down-sampled to a frequency of 50 Hz for further analysis. Only traces of eye movements occurring from 2 s before the contact of the experimenter’s hand with the target object until 2 s after the contact were considered for further analysis.
Saccades were detected by isolating periods in which the eye velocity exceeded a threshold of 150° per second. Periods of fixations were identified as the periods between consecutive saccades.
To compare the fixation patterns across different experimental conditions, we computed for each condition the 2D distribution of eye position in the fronto-parallel plane in bins of 1° relative to the center of the action movie. This distribution allowed us to figure out which parts of the seen action attracted the attention of the monkey. We tested if the fixation distributions for the rewarded and nonrewarded conditions differed by resorting to bootstrapping statistics (SI Text).
Supplementary Material
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 550-C10, GI 305/4-1) and The Werner Reichardt Centre for Integrative Neuroscience.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205553109/-/DCSupplemental.
References
- 1.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 3.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 4.Fogassi L, et al. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 5.Rozzi S, Ferrari PF, Bonini L, Rizzolatti G, Fogassi L. Functional organization of inferior parietal lobule convexity in the macaque monkey: Electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectonic areas. Eur J Neurosci. 2008;28:1569–1588. doi: 10.1111/j.1460-9568.2008.06395.x. [DOI] [PubMed] [Google Scholar]
- 6.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 7.Kraskov A, Dancause N, Quallo MM, Shepherd S, Lemon RN. Corticospinal neurons in macaque ventral premotor cortex with mirror properties: A potential mechanism for action suppression? Neuron. 2009;64:922–930. doi: 10.1016/j.neuron.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 9.Rizzolatti G, Fogassi L, Gallese V. Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol. 2002;12:149–154. doi: 10.1016/s0959-4388(02)00308-2. [DOI] [PubMed] [Google Scholar]
- 10.Gazzaniga MS. The Cognitive Neurosciences. 4th Ed. Cambridge, MA: MIT Press; 2009. p. xvii. 1294 pp. [Google Scholar]
- 11.Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17:212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- 12.Umiltà MA, et al. When pliers become fingers in the monkey motor system. Proc Natl Acad Sci USA. 2008;105:2209–2213. doi: 10.1073/pnas.0705985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keysers C, et al. Audiovisual mirror neurons and action recognition. Exp Brain Res. 2003;153:628–636. doi: 10.1007/s00221-003-1603-5. [DOI] [PubMed] [Google Scholar]
- 14.Kohler E, et al. Hearing sounds, understanding actions: Action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- 15.Caggiano V, Fogassi L, Rizzolatti G, Thier P, Casile A. Mirror neurons differentially encode the peripersonal and extrapersonal space of monkeys. Science. 2009;324:403–406. doi: 10.1126/science.1166818. [DOI] [PubMed] [Google Scholar]
- 16.Bonini L, et al. Ventral premotor and inferior parietal cortices make distinct contribution to action organization and intention understanding. Cereb Cortex. 2009;20:1372–1385. doi: 10.1093/cercor/bhp200. [DOI] [PubMed] [Google Scholar]
- 17.Caggiano V, et al. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Curr Biol. 2011;21:144–148. doi: 10.1016/j.cub.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 19.Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- 20.Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- 21.Roesch MR, Olson CR. Neuronal activity related to anticipated reward in frontal cortex: Does it represent value or reflect motivation? Ann N Y Acad Sci. 2007;1121:431–446. doi: 10.1196/annals.1401.004. [DOI] [PubMed] [Google Scholar]
- 22.Wallis JD, Kennerley SW. Heterogeneous reward signals in prefrontal cortex. Curr Opin Neurobiol. 2010;20:191–198. doi: 10.1016/j.conb.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maunsell JH. Neuronal representations of cognitive state: Reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Pardo-Vazquez JL, Leboran V, Acuña C. Neural correlates of decisions and their outcomes in the ventral premotor cortex. J Neurosci. 2008;28:12396–12408. doi: 10.1523/JNEUROSCI.3396-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.