Abstract
A low fat oxidative capacity has been linked to muscle diacylglycerol (DAG) accumulation and insulin resistance. Alternatively, a low fat oxidation rate may stimulate glucose oxidation, thereby enhancing glucose disposal. Here, we investigated whether an ethyl-2-[6-(4-chlorophenoxy)hexyl]-oxirane-2-carboxylate (etomoxir)-induced inhibition of fat oxidation leads to muscle fat storage and insulin resistance. An intervention in healthy male subjects was combined with studies in human primary myotubes. Furthermore, muscle DAG and triacylglycerol (TAG), mitochondrial function, and insulin signaling were examined in etomoxir-treated C57bl6 mice. In humans, etomoxir administration increased glucose oxidation at the expense of fat oxidation. This effect was accompanied by an increased abundance of GLUT4 at the sarcolemma and a lowering of plasma glucose levels, indicative of improved glucose homeostasis. In mice, etomoxir injections resulted in accumulation of muscle TAG and DAG, yet improved insulin-stimulated GLUT4 translocation. Also in human myotubes, insulin signaling was improved by etomoxir, in the presence of increased intramyocellular lipid accumulation. These insulin-sensitizing effects in mice and human myotubes were accompanied by increased phosphorylation of AMP-activated protein kinase (AMPK). Our results show that a reduction in fat oxidation leading to accumulation of muscle DAG does not necessarily lead to insulin resistance, as the reduction in fat oxidation may activate AMPK.
Obesity is a serious metabolic disorder characterized by excessive fatty acid (FA) accumulation, giving rise to many obesity-related complications including insulin resistance and type 2 diabetes (1, 2). Skeletal muscle plays an important role in the pathophysiology of this disease because it is responsible for the majority of basal and insulin-stimulated glucose uptake (3, 4). Randle et al. (5) postulated more than 40 y ago that increased FA oxidation, resulting from high circulating free fatty acid (FFA) levels, would impair insulin-stimulated muscle glucose utilization. In this model, the availability of lipids as a fuel source impairs the use of glucose as a substrate through inhibition of key glycolytic enzymes. In fact, strategies to limit fat oxidation have been used for diabetes treatment. For example, inhibitors of carnitine palmitoyl transferase 1 (CPT1) like ethyl-2-[6-(4-chlorophenoxy)hexyl]-oxirane-2-carboxylate (etomoxir) have initially been shown to effectively lower blood glucose levels in diabetic patients (6, 7). Similarly, short-term treatment of diabetic rats with etomoxir resulted in favorable changes in fasting plasma glucose, FFA, and triacylglycerol (TAG) concentrations (8, 9).
In contrast to these findings and to the Randle hypothesis, studies from the last two decades revealed that the development of muscle insulin resistance associates with a decreased rather than increased fat oxidative capacity (10). Indeed, numerous studies have shown that type 2 diabetes patients display a diminished oxidative capacity (11, 12). A reduction in (fat) oxidative capacity has been proposed to be responsible for accumulation of fat and FA intermediates in skeletal muscle. Especially the accumulation of diacylglycerol (DAG) has been causally linked to the development of muscle insulin resistance via an inhibitory effect on insulin signaling (13, 14). Likewise, type 2 diabetes patients are characterized by elevated muscle lipid content and a low mitochondrial capacity (15, 16). In that sense, inhibiting fat oxidation may specifically have negative effects on insulin-stimulated glucose metabolism and hence not be an ideal strategy to improve insulin sensitivity. In this study we examined the hypothesis that blocking fat oxidation by inhibiting CPT1 pharmacologically with etomoxir (17, 18) has beneficial effects on muscle glucose handling in non–insulin-stimulated conditions, but would impair insulin signaling due to the accumulation of intramyocellular lipids. To this end, we analyzed muscle biopsies of a human study and performed additional experiments in vitro using primary human myotubes and in vivo using mice to examine the underlying mechanisms in more detail. The human data revealed that in non–insulin-stimulated conditions after etomoxir administration, glucose oxidation is increased at the expense of fat oxidation. Interestingly, this was accompanied by increased membrane GLUT4 and lowered plasma glucose levels. In mice, we show that blocking fat oxidation indeed leads to the accumulation of muscle DAG and TAG. Surprisingly, this does not impede insulin signaling. Our data in mice and in human primary myotubes revealed that these effects are most likely attributed to activation of AMPK. Jointly, these results suggest that the relationship between fat oxidation, DAG accumulation, and insulin sensitivity is less straightforward than has been previously proposed.
Results
Etomoxir Treatment of Humans.
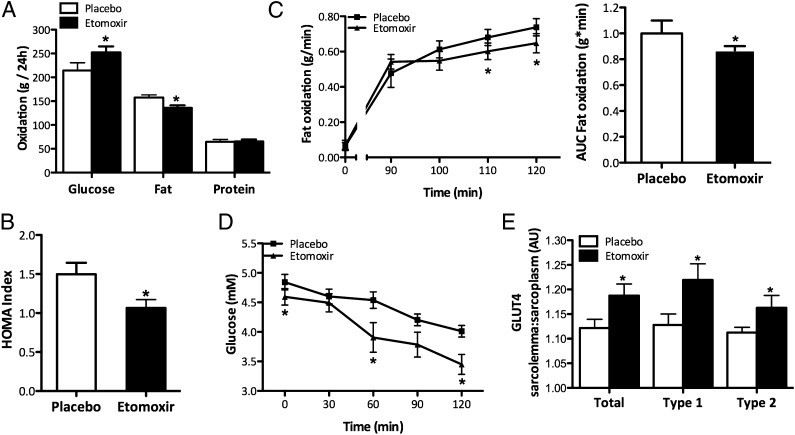
As reported previously (19), etomoxir treatment did not affect 24-h energy expenditure (11.01 ± 0.25 vs. 10.90 ± 0.41 MJ/24 h in etomoxir vs. placebo, P > 0.05), but reduced 24-h fat oxidation and increased 24-h glucose oxidation compared with placebo in humans (P < 0.05, Fig. 1A). Fasting plasma TAG levels were reduced after etomoxir (416.4 ± 96.5 vs. 733.3 ± 131.6 μmol/L in etomoxir vs. placebo), and no treatment effects were observed in plasma FFA and glycerol concentrations (FFA: 487.4 ± 75.6 vs. 381.5 ± 44.1 μmol/L in etomoxir vs. placebo, P > 0.05 and glycerol: 88.7 ± 10.7 vs. 100.8 ± 25.2 μmol/L in etomoxir vs. placebo, P > 0.05). Measurement of the homeostasis model assessment (HOMA) index revealed that etomoxir treatment improved insulin sensitivity (P < 0.05, Fig. 1B), mainly due to lower fasting plasma glucose concentration, (4.60 ± 0.14 vs. 4.85 ± 0.13 mmol/L in etomoxir vs. placebo, P < 0.05), with nonsignificant differences in fasting insulin concentrations (5.5 ± 0.4 vs. 6.7 ± 0.8 microunits/mL in etomoxir vs. placebo, P > 0.05). Subjects also performed a 2-h cycling protocol at 50% of their maximal workload. During exercise, fat oxidation was reduced (P < 0.05, Fig. 1C), blood glucose levels markedly decreased (P < 0.05, Fig. 1D), and plasma lactate levels increased at the end of exercise (2.71 ± 0.36 vs. 2.02 ± 0.28 in etomoxir vs. placebo, P < 0.05), suggesting reduced reliance on fat as a substrate. Given that etomoxir affected fasting plasma glucose levels and increased 24-h glucose oxidation, we analyzed human muscle biopsies for the abundance of GLUT4 in the membrane and sarcoplasm. Etomoxir administration significantly increased the relative fraction of GLUT4 associated with the membrane by 50% compared with placebo (Fig. 1E). This effect was observed both in type 1 and type 2 muscle fibers, although it tended to be more pronounced in type 1 fibers (Fig. 1E). The relative fraction of GLUT4 near the membrane tended to correlate with 24-h glucose oxidation (r = +0.77, P = 0.07) and correlated strongly with 24-h glucose balance (r = −0.94, P < 0.005).
Fig. 1.
The effect of etomoxir in healthy males on (A) Twenty-four hour glucose, fat, and protein oxidation rates (modified from ref. 33). (B) HOMA index. (C) Fat oxidation during a 2-h cycling protocol, represented as rates (Left) and area under the curve (in percentage, Right). (D) Blood glucose values during a 2-h cycling protocol. (E) Relative fraction of total GLUT4 associated with the sarcolemma and separated for type 1 and 2 muscle fibers. Data are expressed as mean + SEM (n = 10). *P < 0.05 etomoxir vs. placebo.
Skeletal Muscle Mitochondrial Metabolism in Mice upon Etomoxir Administration.
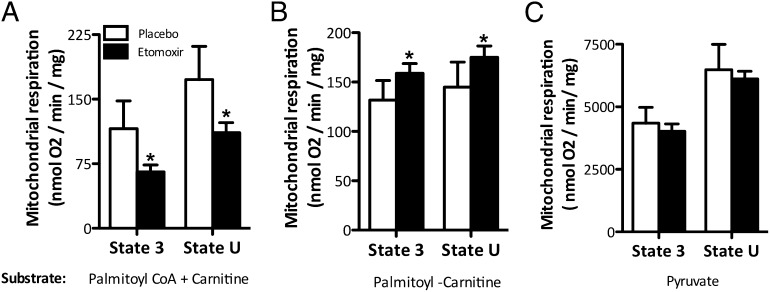
The underlying mechanism by which etomoxir might affect glucose homeostasis could not be further investigated in human muscle biopsies, due to limited material. Therefore, we used mice to investigate whether etomoxir treatment indeed reduced the entry of FAs into the mitochondria. Etomoxir treatment did not affect food intake or body weight gain (Fig. S1). Mice were killed after 8 d of etomoxir treatment. Separate addition of palmitoyl-CoA and carnitine, thus requiring CPT1, resulted in a significantly decreased state-3 mitochondrial respiration in mitochondria isolated from muscle of etomoxir-treated mice (P < 0.05, Fig. 2A). Similarly, maximal respiratory capacity upon addition of the chemical uncoupler trifluorocarbonylcyanide phenylhydrazone (FCCP) was significantly lower in the etomoxir- compared with the placebo-treated animals (P < 0.05, Fig. 2A). To investigate whether the effect of etomoxir treatment on inhibiting fat oxidation was solely due to inhibition of CPT1 and not a reduced overall mitochondrial function because of lipotoxicity, we further investigated mitochondrial function with palmitoyl-carnitine (which does not require CPT1) and pyruvate as substrates. Remarkably, both state-3 respiration and maximal mitochondrial respiratory capacity with palmitoyl-carnitine as a substrate were significantly elevated upon etomoxir treatment compared with placebo (P < 0.05, Fig. 2B). When using pyruvate as a substrate, both state-3 respiration and the maximal respiratory capacity of the isolated mitochondria were not affected by etomoxir treatment (P > 0.05, Fig. 2C). No differences in β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity were detected between etomoxir and placebo groups (41.49 ± 6.00 vs. 45.17 ± 10.31 units/g protein, P > 0.05 in etomoxir vs. placebo), indicating similar β-oxidative capacity. Western blotting of the oxidative phosphorylation (OXPHOS) complexes revealed no differences in expression of total OXPHOS levels [30.06 ± 10.05 vs. 31.48 ± 8.69 arbitrary units (AU), P > 0.05 in etomoxir vs. placebo].
Fig. 2.
Effect of etomoxir on the respiratory rates of freshly isolated skeletal muscle mitochondria using a two-chamber oxygraph. Mitochondria were incubated in a respiration media containing malate. State-3 respiration (upon substrates and ADP) was achieved by addition of (A) palmitoyl CoA + carnitine, (B) palmitoyl-carnitine, and (C) pyruvate as different substrates. Maximal oxygen flux (state U) was obtained by titration of the chemical uncoupler FCCP in the presence of oligomycin. Values are expressed in nmol/mg mitochondrial protein/min (mean + SEM) (placebo n = 6, etomoxir n = 5). *P < 0.05 etomoxir vs. placebo.
Intramyocellular Lipid Content in Mice upon Etomoxir Administration.
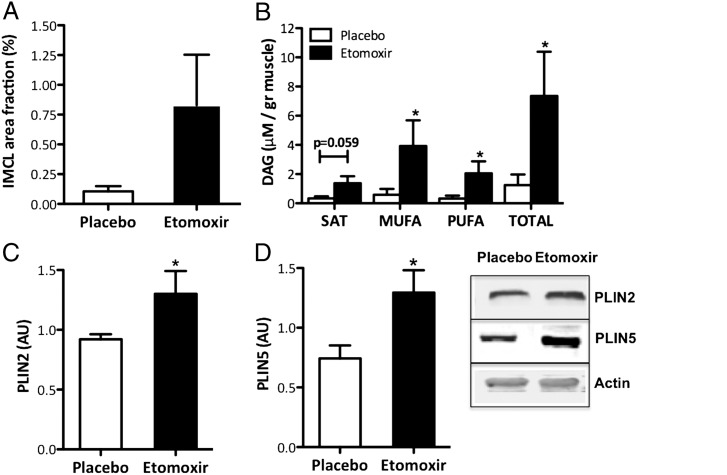
Quantification of intramyocellular lipid storage in mouse tibialis anterior muscle revealed that etomoxir treatment resulted in a 3.6-fold higher intramyocellular lipid content (P = 0.06, Fig. 3A), consistent with an inhibitory effect of etomoxir on fat oxidation. Furthermore, etomoxir treatment resulted in a 5.9-fold increase in skeletal muscle total DAG content (P < 0.05, Fig. 3B), which was mainly accounted for by increases in poly- and monounsaturated FAs in the DAG fraction [saturated (SAT): P = 0.059, monounsaturated fatty acid (MUFA): P < 0.05, and polyunsaturated fatty acid (PUFA): P < 0.05]. Consistent with the increase in muscle fat content, the protein expression of perilipin 2 (PLIN2) and perilipin 5 (PLIN5), two lipid droplet coating proteins suggested to be involved in lipid droplet metabolism, were also induced by etomoxir treatment (P < 0.05, Fig. 3 C and D).
Fig. 3.
Effect of etomoxir in mice on (A) IMCL levels and (B) total DAG levels in tibialis anterior muscle and saturated (SAT), monounsaturated (MUFA), and polyunsaturated (PUFA) fractions. Western blotting of the lipid droplet coating proteins (C) PLIN2 and (D) PLIN5 in tibialis anterior muscle of etomoxir-treated mice. Data are expressed as mean + SEM (placebo n = 6, etomoxir n = 5). *P < 0.05 etomoxir vs. placebo.
Muscle Insulin Signaling upon Etomoxir Administration.
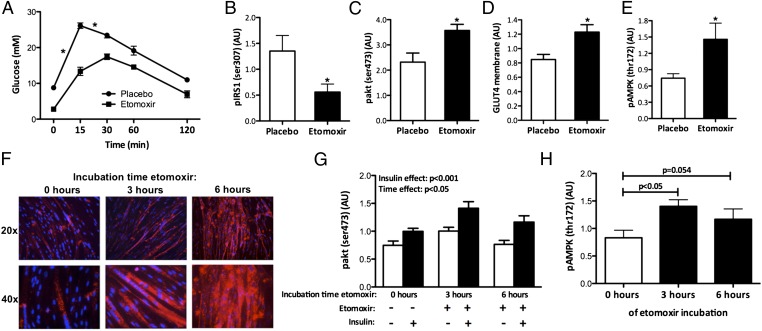
Given the increase in muscle TAG and DAG, we next investigated whether the etomoxir-induced intramyocellular lipid accumulation impeded glucose homeostasis in mice. I.p. glucose tolerance tests revealed that both basal and early postprandial plasma glucose levels were markedly reduced in etomoxir-treated mice, with a less pronounced increase in blood glucose levels upon glucose injection (Fig. 4A). Next, we determined the effect of etomoxir treatment on skeletal muscle insulin signaling by injecting a bolus of insulin 10 min before killing the animals. Interestingly, despite the pronounced accumulation of intramyocellular lipids, IRS1 serine phosphorylation on residue 307, a marker of muscle insulin resistance, was decreased rather than increased with the etomoxir treatment upon in vivo insulin stimulation (P < 0.05, Fig. 4B). PKCθ protein content in the membrane, a direct measurement of DAG-induced insulin resistance, was not increased by etomoxir treatment (0.89 ± 0.16 vs. 1.13 ± 0.17 AU, P > 0.05 in etomoxir vs. placebo). Furthermore, phosphorylation of Akt, a downstream target of IRS, was increased rather than decreased by etomoxir treatment (P < 0.05, Fig. 4C). In line with this finding, insulin-stimulated GLUT4 translocation was also increased in the etomoxir group compared with placebo (P < 0.05, Fig. 4D), indicating that insulin signaling was improved by etomoxir, even in the face of elevated DAG levels. We next investigated whether the improvements in glucose homeostasis in the etomoxir-treated animals, might be attributed to increased AMPK activation. Indeed, phosphorylation of AMPK on Thr172 was increased upon etomoxir treatment (P < 0.05, Fig. 4E).
Fig. 4.
Effect of etomoxir on (A) whole-body glucose tolerance measured by an ipGTT (2 g/kg bw glucose) in mice. Effect of etomoxir on the expression of several proteins in the insulin signaling cascade was investigated in tibialis anterior muscle of mice. Western blotting of (B) pIRS1 (Ser307), (C) pAkt, and (D) GLUT4. (E) Phosphorylation of AMPK was examined by Western blotting in mouse tibialis anterior muscle. Human myotubes were incubated with 100 μM etomoxir and (F) IMCL accumulation is shown after 0, 3, and 6 h of etomoxir incubation. (G) Western blotting of pAkt in human myotubes incubated with etomoxir for 0, 3, and 6 h in the absence or presence of insulin. (H) pAMPK protein expression upon 0, 3, and 6 h of etomoxir incubation in human myotubes, measured by Western blotting. Representative Western blots are shown in Fig. S2. Data are expressed as mean + SEM (n = 10 per group for mice experiments, n = 6–8 per group for cell experiments) *P < 0.05 etomoxir vs. placebo.
Taken together, these results suggest that inhibition of fat oxidation, by blocking mitochondrial entry of fatty acids, does not necessarily lead to muscle insulin resistance. Rather, despite accumulation of fat in skeletal muscle, it may even increase muscle insulin sensitivity via a mechanism that possibly involves the activation of AMPK. To investigate whether similar effects can be observed in humans, we treated human primary myotubes with etomoxir. First, we confirmed that etomoxir treatment promotes intramyocellular lipid accumulation (Fig. 4F). Regardless of the pronounced fat accumulation, insulin-stimulated Akt phosphorylation was not impaired by etomoxir treatment (Fig. 4G), and etomoxir led to phosphorylation of AMPK on Thr172 after 3 h of incubation (Fig. 4H).
Discussion
High-fat availability plays a prominent role in the onset of skeletal muscle insulin resistance. Whereas Randle was the first to postulate that elevated fat oxidation causes reduced insulin-stimulated glucose uptake and thereby impedes insulin sensitivity, over the years more and more researchers discounted this simple model of such a reciprocal regulation of FA oxidation and glucose disposal. Rather, the idea evolved that a low FA oxidative capacity is causally related to the development of insulin resistance, via the accumulation of FA intermediates, which interfere at several distinct steps in the insulin signaling cascade. For example, acute elevation of circulating lipids leads to the accumulation of DAG in muscle and thereby impairs insulin action (20). We here show that blocking the entry of FAs into the mitochondria by etomoxir increased glucose uptake and oxidation at the expense of fat oxidation; both in non–insulin-stimulated as well as insulin-stimulated conditions. Interestingly, the accumulation of muscle TAG and DAG, as a result of etomoxir administration, did not negatively affect muscle insulin signaling.
Here, we show that blocking fat oxidation by etomoxir in healthy male subjects on a high-fat diet, clearly improved 24-h glucose oxidation in the non–insulin-stimulated state at the expense of a lower fat oxidation. HOMA index suggests improved insulin sensitivity upon etomoxir, which was mainly due to lower plasma glucose values. Our results confirm previous human and animal studies showing improved glucose oxidation and decreased blood glucose concentrations upon etomoxir administration in the non–insulin-stimulated state (6–9). Consistent with improved glucose oxidation, etomoxir interestingly induced an increase in the sarcolemmal GLUT4 fraction in the non–insulin-stimulated state. This increase is remarkable as GLUT4 translocation is considered to occur upon muscle contraction and/or insulin stimulation. This finding suggests that inhibition of FA oxidation at the level of CPT1 does not only increase glucose oxidation through substrate competition at the TCA cycle, but also induces a signal leading to GLUT4 translocation. Our observation that GLUT4 translocation was more pronounced in type 1 compared with type 2 muscle fibers is consistent with the suggestion that etomoxir-induced CPT1 inhibition (which is believed to be more manifest in type 1 muscle fibers) induces a signal mediating GLUT4 translocation. Our data point toward activation of AMPK as a possible signal that facilitated the etomoxir-induced GLUT4 translocation. This notion is supported by our findings in human primary myotubes and in mice that inhibition of fat oxidation by blocking mitochondrial entry of FAs indeed activates AMPK. This activation of AMPK may be directly due to either reduced fat oxidation or disturbances of myocellular energy or glucose homeostasis, as reviewed before (21, 22). However, this finding extends previous findings showing that a reduction in fat oxidation, via the genetic manipulation of malonyl-CoA dehydrogenase (MCD), leads to an improvement in glucose homeostasis. In these studies, either AMPK was not measured (23) or was unaffected (24). Why AMPK was not activated in these previous studies cannot be deduced from the present results, but together these studies do show that a mild reduction in mitochondrial fatty acid entry promotes glucose homeostasis.
In the last two decades, the prevailing model to explain the development of skeletal muscle insulin resistance suggests that the accumulation of FA intermediates in muscle—due to an imbalance between fat oxidation and FA availability—inhibits insulin signaling, thereby leading to reduced insulin-stimulated GLUT4 translocation. We therefore also tested whether an etomoxir-induced reduction in fat oxidation would increase muscle lipid intermediates and impair insulin signaling in mice. We confirmed that etomoxir administration solely affected CPT1 and not overall mitochondrial function as respiration of a FA substrate coupled to carnitine, thus bypassing CPT1, did not decrease but even increased the respiration rates. The decreased fat oxidation in etomoxir-treated animals was paralleled with increased storage of muscle lipid intermediates. Both muscle TAG and DAG levels were increased after etomoxir treatment, and also PLIN2 and PLIN5, lipid droplet coating proteins involved in lipid droplet metabolism, were increased by etomoxir treatment. Remarkably however, whole body glucose tolerance and muscle-specific insulin signaling were not negatively affected in the etomoxir-treated mice compared with the control group. Thus, insulin-stimulated GLUT4 translocation, as well as Akt phosphorylation were increased upon etomoxir treatment. In general, in conditions of lipid-induced insulin resistance both the insulin receptor as well as IRS1 are negatively regulated by serine phosphorylation, which opposes phosphorylation on tyrosine residues, and thus impedes activation of downstream targets, including Akt and GLUT4 (25). However, the insulin signaling cascade was not hampered by serine 307 phosphorylation of IRS1, or increased presence of PKCθ at the membrane, despite elevated DAG levels. We did confirm these findings in human primary myotubes by showing that a marked increase in intramyocellular lipid accumulation upon etomoxir treatment did not negatively affect insulin signaling.
Although there are studies describing that activation of AMPK can antagonize insulin action in muscle (26, 27), in other cases, the insulin and AMPK signaling pathways work in the same direction, particularly in processes that regulate plasma glucose levels (28). Thus, it has been shown that Akt and Akt substrate of 160 kDa (AS160) are important downstream substrates involved in AMPK-mediated glucose uptake (29), and AMPK activation could thereby overcome fat-induced insulin resistance. However, in our study not only Akt phosphorylation was increased after etomoxir treatment of primary human myotubes and mice, but also the upstream activator of Akt, IRS1 serine 307 phosphorylation, was decreased in etomoxir-treated mice. In that respect, Jakobsen et al. (30) reported a direct interaction between AMPK and the most upstream component of the insulin signaling cascade, IRS1, in mouse C2C12 myotubes preincubated with 5-amino-1-beta-D-ribofuranosyl-imidazole-4-carboxamide (AICAR), which correlated with a 65% increase in insulin-stimulated IRS1-associated PI3 kinase activity. It could therefore be postulated that the increased AMPK phosphorylation that we observed upon etomoxir treatment might contribute to the improved insulin-stimulated insulin signaling.
As explained above, even though it seems possible that the insulin signaling and AMPK pathway can work synergistically in improving muscle glucose uptake, it is still remarkable that the increased accumulation of lipid intermediates in muscle upon etomoxir did not negatively impact insulin signaling substrates. Elevated levels of the lipotoxic derivate DAG are generally considered to cause lipid-induced insulin resistance (13). However, we previously reported that elevated muscle DAG levels are not per se accompanied by skeletal muscle insulin resistance. That is, we showed that myocellular DGAT1 overexpression, an enzyme responsible for the conversion of DAG into inert TAG, improved skeletal muscle insulin sensitivity, yet increased muscle DAG levels (31). This phenomenon of improved insulin sensitivity coupled to increased storage of DAG in muscle was also reported in endurance-trained athletes (32). These findings indicate that the role of the individual lipid intermediates in causing muscle lipid-induced insulin resistance is not straightforward and seems to depend on several aspects, including cellular energy status and the balance between lipid availability and oxidation. Overall, our data suggest that blockade of fat oxidation at the level of CPT1 by administration of etomoxir results in improved insulin sensitivity through increased GLUT4-induced glucose uptake both in non–insulin- as well as insulin-stimulated conditions. Our results show that a reduction in fat oxidation leading to the accumulation of muscle DAG and TAG levels does not necessarily lead to muscle insulin resistance, probably due to activation of AMPK.
Materials and Methods
Human Subjects.
Healthy, lean young men [age 26.7 ± 2.8 y, body mass index (BMI) 21.8 ± 0.4 kg/m2, n = 10] participated in the study. The medical ethics committee of Maastricht University approved the study, and subjects gave their written informed consent. Each subject underwent two treatments in randomized order, as described previously (33). A detailed description of the study design can be found in SI Materials and Methods.
Blood Analyses.
Plasma nonesterified FAs, glycerol, glucose, and lactate were measured with enzymatic assays automated on a Cobas Fara/Mira. Insulin concentration was determined using a radioimmunoassay (RIA) (Linco Research).
Muscle Biopsy and Analysis.
Muscle biopsies were taken from the midthigh region from Musculus vastus lateralis according to the Bergström technique (34), freed from blood and any visible connective and adipose tissue, embedded in Tissue-Tek (Tissue-Tek), and directly frozen in melting isopentane.
Immunofluorescence Assay.
Fresh cryosections (5 μm) from human biopsies were stained for simultaneous identification of fiber type and subcellular GLUT4 content. Triple immunofluorescence was performed to discriminate between type 1 and 2 fibers, simultaneously with the basement membrane protein laminin to identify the sarcolemma and GLUT4 (35, 36).
Animals.
Thirty-one 14-wk-old male C57BL6 mice were housed individually in an environmentally controlled laboratory (temperature 22 ± 1 °C, relative humidity 55 ± 2%, 12:12 h light-dark cycle). Mice were allowed ad libitum access to a 45% high-fat diet (D01060502; Research Diets) for a period of 14 d.
On day 6 of the diet intervention, mice were randomly divided into two groups (n = 15 for etomoxir and n = 16 for placebo). One group received a daily i.p. injection of the CPT1 inhibitor, etomoxir [20 mg/kg body weight (bw) dissolved in 0.9% NaCl (wt/vol); purchased from Sigma-Aldrich] during the remaining 8 d of the diet intervention. The control group received a daily i.p. injection of an equivalent volume of 0.9% (wt/vol) NaCl. On day 14, 10 animals of the etomoxir group and placebo group were subjected to an i.p. glucose tolerance test. A bolus of 2 g/kg bw of glucose was injected after a 6-h fast and blood was collected before (t = 0) and 15, 30, 60, and 120 min after the bolus. On day 15, 10 mice of the placebo and etomoxir group received an i.p. injection of insulin (10 units/kg) 10 min before killing. All mice were anesthetized by a mixture of 79% (vol/vol) of CO2 and 21% (vol/vol) of O2, followed by cervical dislocation. Anterior tibialis muscle from both hindlimbs was rapidly dissected, frozen in liquid nitrogen-cooled isopentane, and stored at −80 °C until further analyses. The remaining 5 animals in the etomoxir group and the 6 remaining animals in the placebo group were killed without an insulin bolus. Part of the muscles of these animals were used for mitochondrial respiration, the other part was used for intramyocellular lipid content (IMCL) and DAG determination. All experiments were approved by the institutional animal care and use committee of the Maastricht University and complied with the principles of laboratory animal care.
Cell Culture Experiments.
Samples of M. vastus lateralis from five healthy, sedentary nondiabetic male subjects (age 52.6 ± 2.8 y, n = 5) were obtained by muscle biopsy using the Bergström technique (34) and cultured as described before (37). Differentiated myotubes were treated with 100 μM etomoxir (Sigma) for 0, 3, and 6 h in the presence of 30 μM oleate conjugated to essential fatty acid free BSA (ratio BSA:palmitate 1:2.5). Cells were harvested posttreatment and lysed in Nonidet P-40 lysis buffer [62.5 mM Tris-HCL, 2% (vol/vol) Nonidet P-40, 1.25 mM EDTA, protease and phosphatase inhibitors].
Intramyocellular Lipid Content.
Cryosections (5 μm) from the midbelly region of the tibialis anterior muscle of mice were stained for neutral lipids with Oil Red O staining as described previously (36). Intramyocellular lipid content was expressed per cell surface area. Fully differentiated myotubes were fixed with 3.7% (vol/vol) formaldehyde in PBS for 20 min. Thereafter, slides were incubated in 0.3% (wt/vol) Oil Red O in 60% (vol/vol) triethylphosphate for 30 min. Subsequently, slides were mounted using mowiol. DAPI was included with the mowiol to allow visualization of nuclei. Slides were examined using a Nikon E800 fluorescence microscope (Uvikon). Digital images were captured and processed using Lucia G/F 5.49 image analysis software (Nikon).
DAG Measurement.
Total lipids were extracted from frozen mouse tibialis anterior muscle and DAG content was determined as described previously (31).
Mitochondrial Isolation and Oxygen Consumption.
Isolation and oxygen consumption measurements of mouse skeletal muscle mitochondria were performed using a two-chamber oxygraph (Oroboros Instruments), as described previously (38). Briefly, mitochondria were incubated in respiration medium containing (100 mM sucrose, 20 mM K+-TES (PH 7.2), 50 mM KCl, 2 mM MgCl2, 1 mM EDTA, 4 mM KH2PO4, 3 mM malate, and 0.1% of fatty acid free BSA). Pyruvate (5 mM) was added as a carbohydrate-derived substrate. Palmitoyl-carnitine (50 μM) and palmitoyl-CoA (50 μM) + carnitine (2 μM) were used as fatty acid substrates, with the latter being CPT1 dependent. After addition of substrates, ADP (450 mM) was added to initiate state-3 respiration, followed by oligomycin (1 mg/mL) to block ATP synthesis (state-4 respiration). Maximal oxygen flux (state uncoupled) was obtained by titration of the chemical uncoupler FCCP.
β-HAD Enzyme Activity.
Activity of β-HAD, as a marker for β-oxidative capacity was measured in tibialis anterior muscle homogenates of mice, as described previously (39). Protein concentration was determined using a Bio-Rad protein assay kit according to manufacturer instructions.
Western Blotting.
The Western blots in mouse tibialis anterior muscle and human myotubes were performed with antibodies against PLIN5 (GP31; Progen), PLIN2 (GP41; Progen), OXPHOS (MS601; MitoSciences), Akt and pAkt (Ser473) (9271 and 9272, respectively; Cell Signaling Technology, Bioké), pIRS (Ser307) (2381S; Cell Signaling), PKCθ (2059; Cell Signaling), GLUT4 (sc-1608; Santa Cruz, Bio-Connect), and AMPK and pAMPK (Thr172) (2532 and 2531, respectively; Cell Signaling). Detailed information on the procedures can be found in SI Materials and Methods.
Statistics.
Data are presented as mean ± SEM. In the human trial, differences between etomoxir and placebo were analyzed pairwise with Student t tests. Pearsons correlation coefficients were calculated to determine the relationship between selected variables. Differences between animal groups were analyzed by a nonparametric t test. The i.p. glucose tolerance test was analyzed by means of factorial repeated measures ANOVA to determine the time, group, and time × group effect. For the human myotubes experiments, the differences were determined by univariate ANOVA followed by Bonferroni post hoc tests when applicable. For the analysis of pAkt in myotubes, factorial ANOVA was used to determine the effect of incubation time and insulin. The accepted level of statistical significance was P < 0.05 for all analyses. All calculations were done using the Statistical Package for the Social Sciences (SPSS 16.0 software).
Supplementary Material
Acknowledgments
This study was funded by Top Institute (TI) Food and Nutrition. TI Food and Nutrition, formerly known as Wageningen Center for Food Sciences (WCFS), is a unique public/private partnership that generates vision on scientific breakthroughs in food and nutrition, resulting in the development of innovative products and technologies that respond to consumer demands for safe, tasty, and healthy foods. Partners are major Dutch food companies and research organizations. VICI Grant 918.96.618, VIDI Grant 917.66.359, and VENI Grant 916.11.136 for innovative research from the Netherlands Organization for Scientific Research supports the work of P.S., M.K.C.H., and V.B.S.-H., respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206868109/-/DCSupplemental.
References
- 1.Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann OP, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001;50:2579–2584. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 4.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 5.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 6.Hübinger A, Knode O, Susanto F, Reinauer H, Gries FA. Effects of the carnitine-acyltransferase inhibitor etomoxir on insulin sensitivity, energy expenditure and substrate oxidation in NIDDM. Horm Metab Res. 1997;29:436–439. doi: 10.1055/s-2007-979072. [DOI] [PubMed] [Google Scholar]
- 7.Hübinger A, Weikert G, Wolf HP, Gries FA. The effect of etomoxir on insulin sensitivity in type 2 diabetic patients. Horm Metab Res. 1992;24:115–118. doi: 10.1055/s-2007-1003271. [DOI] [PubMed] [Google Scholar]
- 8.Barnett M, Collier GR, O’Dea K. The longitudinal effect of inhibiting fatty acid oxidation in diabetic rats fed a high fat diet. Horm Metab Res. 1992;24:360–362. doi: 10.1055/s-2007-1003335. [DOI] [PubMed] [Google Scholar]
- 9.Barnett M, Habito R, Cameron-Smith D, Yamamoto A, Collier GR. The effect of inhibiting fatty acid oxidation on basal glucose metabolism in Psammomys obesus. Horm Metab Res. 1996;28:165–170. doi: 10.1055/s-2007-979153. [DOI] [PubMed] [Google Scholar]
- 10.Phielix E, Mensink M. Type 2 diabetes mellitus and skeletal muscle metabolic function. Physiol Behav. 2008;94:252–258. doi: 10.1016/j.physbeh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Colberg SR, Simoneau JA, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest. 1995;95:1846–1853. doi: 10.1172/JCI117864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav. 2008;94:242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Phielix E, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 17.Declercq PE, et al. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987;262:9812–9821. [PubMed] [Google Scholar]
- 18.Agius L, Meredith EJ, Sherratt HS. Stereospecificity of the inhibition by etomoxir of fatty acid and cholesterol synthesis in isolated rat hepatocytes. Biochem Pharmacol. 1991;42:1717–1720. doi: 10.1016/0006-2952(91)90507-2. [DOI] [PubMed] [Google Scholar]
- 19.Hinderling VB, Schrauwen P, Langhans W, Westerterp-Plantenga MS. The effect of etomoxir on 24-h substrate oxidation and satiety in humans. Am J Clin Nutr. 2002;76:141–147. doi: 10.1093/ajcn/76.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 22.Zhang BB, Zhou G, Li C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Bouzakri K, et al. Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes. 2008;57:1508–1516. doi: 10.2337/db07-0583. [DOI] [PubMed] [Google Scholar]
- 25.Zick Y. Uncoupling insulin signalling by serine/threonine phosphorylation: A molecular basis for insulin resistance. Biochem Soc Trans. 2004;32:812–816. doi: 10.1042/BST0320812. [DOI] [PubMed] [Google Scholar]
- 26.Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–1274. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- 27.Beauloye C, et al. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- 28.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson HK, et al. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–46916. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- 31.Timmers S, et al. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS ONE. 2011;6:e14503. doi: 10.1371/journal.pone.0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amati F, Dube JJ, Coen PM, Goodpaster BH. Diacylglycerol and ceramide content in skeletal muscle insulin resistance: The next chapter in the story of good and bad lipids? Obesity (Silver Spring) 2009;17(Supplement 2):S71. [Google Scholar]
- 33.Schrauwen P, et al. Etomoxir-induced increase in UCP3 supports a role of uncoupling protein 3 as a mitochondrial fatty acid anion exporter. FASEB J. 2002;16:1688–1690. doi: 10.1096/fj.02-0275fje. [DOI] [PubMed] [Google Scholar]
- 34.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 35.Borghouts LB, Schaart G, Hesselink MK, Keizer HA. GLUT-4 expression is not consistently higher in type-1 than in type-2 fibres of rat and human vastus lateralis muscles; an immunohistochemical study. Pflugers Arch. 2000;441:351–358. doi: 10.1007/s004240000431. [DOI] [PubMed] [Google Scholar]
- 36.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–68. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 37.Sparks LM, et al. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS ONE. 2011;6:e21068. doi: 10.1371/journal.pone.0021068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabben M, et al. The effect of UCP3 overexpression on mitochondrial ROS production in skeletal muscle of young versus aged mice. FEBS Lett. 2008;582:4147–4152. doi: 10.1016/j.febslet.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 39.den Hoed M, Hesselink MK, van Kranenburg GP, Westerterp KR. Habitual physical activity in daily life correlates positively with markers for mitochondrial capacity. J Appl Physiol. 2008;105:561–568. doi: 10.1152/japplphysiol.00091.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.