Abstract
Numerosity, the number of elements in a set, is a most abstract quantitative category. As such, it is independent of the sensory modality of its elements, i.e., supramodal. Because neuronal numerosity selectivity had never been compared directly across different sensory modalities, it remained elusive if and where single neurons encode numerosity irrespective of the items’ modality. Here, monkeys were trained to discriminate both the number of auditory sounds and visual items within the same session. While the monkeys performed this task, the activity of neurons was recorded in the lateral prefrontal cortex and ventral intraparietal sulcus, structures critically involved in numerical cognition. Groups of neurons in both areas encoded either the number of auditory pulses, visual items, or both. The finding of neurons responding to numerosity irrespective of the sensory modality supports the idea of a nonverbal, supramodal neuronal code of numerical quantity in the primate brain.
Keywords: multimodal, number, association cortex, single-cell recordings
Nonverbal numerical competence, such as the estimation of set size (numerosity), is rooted in biological primitives that can also be explored in animals (1–3). Over the past years, the anatomical substrates and neuronal mechanisms of numerical cognition in primates have been unraveled down to the level of single neurons (4). Studies with behaviorally trained monkeys have identified a parieto-frontal network of individual neurons selectively tuned to the number of items. This cortical network is putatively homologous with the network endowing humans to estimate numerosity, count, and operate with number symbols (5–12).
At the level of individual neurons, electrophysiological studies so far exclusively investigated the representation of numerosity for a single modality (unimodal). Neurons in area 5 of the monkey posterior parietal cortex encode the numerosity of a series of self-performed actions (13, 14). The number of visual items is represented in areas lateral intraparietal area (LIP) (15, 16) and ventral intraparietal area (VIP) (15, 17, 18) of the intraparietal sulcus (IPS), as well as the lateral prefrontal cortex (PFC) of monkeys (19–24).
However, numerosity is a most abstract quantitative category (25). As such, numerical magnitude is independent of the spatiotemporal presentation formats within a modality. Three visual objects in a picture or three light flashes in a row are both instances of “three.” Within the visual modality, numerosity can be determined either at a single glance from the spatial layout of a multiple-item pattern (i.e., ∴) or enumerated successively across time in a temporal layout (i.e., • - • - • etc.). Recordings in monkeys suggest that neurons can represent the unimodal number of visual items across spatiotemporal presentation formats (17).
However, as an abstract magnitude, numerosity is also independent of sensory modality (“supramodal”). Three light flashes or three heart beats are again both instances of three. So far, it remained elusive whether and where single neurons might encode numerosity irrespective of the items’ modality, because numerosity selectivity had never been compared across different sensory modalities in one and the same experiment.
Results
Two rhesus monkeys were trained to discriminate the number of sequentially presented auditory and visual items using a delayed match-to-sample protocol (Fig. 1 A and B). The monkeys had to judge whether two successive task periods (first sample, then test) separated by a temporal delay contained the same number of (one to four) items. If so, the animals had to release a lever to earn a reward. In the sample period, numerosity was presented in one of two randomly chosen ways: either auditorily by a sequence of white-noise sounds (auditory sample protocol), or visually by single dots appearing one-by-one (visual sample protocol). The number of sequentially presented items in the sample phase had to be matched to the number of elements in multiple-dot test displays. Thus, only visual numerosities had to be assessed in the visual sample protocol (Fig. 1B), whereas the number of auditory events had to be matched to visual quantities in the auditory sample protocol (Fig. 1A). To eliminate a potentially confounding effect of temporal variables in the sample period, three sample conditions were used in all sessions for both the auditory and the visual protocols (Fig. 1 C–E and Table S1). These three conditions controlled for the total duration of the sample period, the duration of individual items and pauses in between, the total sensory energy, and the regularity (rhythm) of the item sequence.
Fig. 1.
Task protocols for the sequential delayed match-to-numerosity task (shown for example numerosity 3 only). (A) Auditory sample protocol. A trial started when the monkey grasped a lever. The monkey had to release the lever if the sample period and test display contained the same number of auditory and visual items, respectively, and continue holding it if they did not (probability of match/nonmatch condition = 0.5). The sample numerosity was cued by sequentially presented sounds separated by silent intervals. The temporal succession and duration of individual sounds (indicated by speaker icons) were varied within and across numerosities (see details in C–E and Table S1). Sequentially presented numerosities in the sample phase always had to be matched to numerosity in multiple-dot displays in the test phase. (B) Visual sample protocol. This protocol was the same as in A, with the exception that the numerosity in the sample phase was cued by the number of single dots. (C–E) Temporal stimulus conditions for individual items (sounds or dots) during the sample phase (see Table S1 for details). Item presentation (either sounds or dots) is indicated by square pulses. All three conditions, four numerosities and two modalities were pseudorandomly presented in each session.
Behavior.
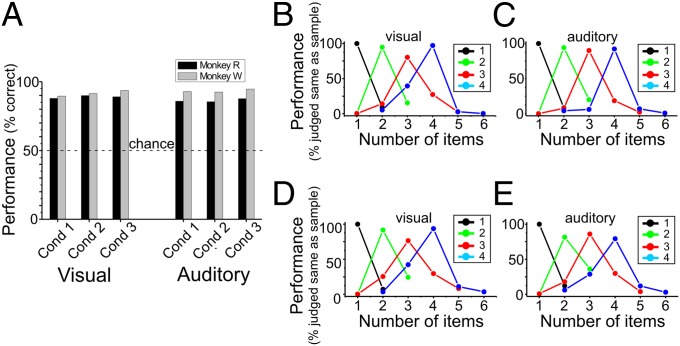
Both monkeys showed a high overall performance of 88% and 92% correct choices (Fig. 2A), significantly better than chance for all tested protocols and conditions (binomial test, P < 0.0001). Average performance was at the same level of accuracy for the visual (monkey R: 89%; monkey W: 91%) and auditory protocols (monkey R: 88%; monkey W: 92%). Importantly, the three temporal conditions within the unimodal and crossmodal protocol did not cause differences in discrimination performance in either monkey (Fig. 2A, cond 1 – cond 3). This result suggests that the monkeys were indeed judging numerosities in both modalities irrespective of temporal cues.
Fig. 2.
Behavior. (A) Discrimination accuracy of both monkeys (during recording sessions) for the three temporal conditions (cond 1–3) in the visual and auditory protocols. Chance performance was 50%. (B–E) Behavioral performance curves of monkey W (B and C) and monkey R (D and E) for the visual (B and D) and auditory protocol (C and E). Average performance across all three conditions (as defined in Table S1) is shown.
Fig. 2 B–E shows the detailed behavioral performance functions of both monkeys in the visual and auditory protocol averaged across all three temporal conditions. The functions indicate the probability that a monkey judged the numerosity in the test period as containing the same number of sounds/dots as the sample numerosity. The graphs illustrate that the animals made more errors when the nonmatch numerosities were adjacent to the sample and performed progressively better as numerical distance between match and nonmatch numerosities increased (numerical distance effect). For larger set sizes, the two numerosities had to be numerically more distant for performance to reach the level obtained with smaller quantities and closer numerical distance (numerical size effect). The distance effect and size effect were identical in both the visual (Fig. 2 B and D) and the auditory protocols (Fig. 2 C and E).
Neuronal Responses in the PFC and IPS.
The activity of a total of 432 single cells was recorded from two monkeys performing the auditory and visual numerosity discrimination tasks. Of those, 232 neurons were measured in the lateral prefrontal cortex (PFC), and 200 neurons were recorded from the ventral area of the intraparietal sulcus (VIP, Fig. 3A). Numerosities one to four were randomized from trial to trial and across sensory modalities to exclude any bias in the investigation of individual neuron’s responses.
Fig. 3.
Recording sites and neural responses. Data refer to the sample period only. (A) Lateral view of the left hemisphere of a monkey brain (Lower) and coronal section at the level of the dotted line (Upper, Horsley–Clark coordinates 0 mm anterior/posterior) indicating the topographical relationships of cortical landmarks. Colored areas in the prefrontal cortex and in the depth of the IPS mark the recording areas (drawings reconstructed from a structural MRI scan). ips, intraparietal sulcus; ls, lateral sulcus; ps, principal sulcus; sts, superior temporal sulcus. (B–D) Responses of an example PFC neuron selective to the auditory numerosity two in the sample period (only condition 3 is shown for clarity). (B Top) The temporal succession of individual items (square pulses represent single items). The corresponding discharges for many repetitions of the protocol are plotted as dot-raster histograms (Middle, each dot represents an action potential) and averaged spike density functions (Bottom, activity averaged and smoothed). The first 500 ms represent the fixation period. Colors correspond to the stimulation condition and the plotting of the neural data. Gray shaded areas represent item presentation. (C) Numerosity tuning functions indicating the mean activity of the neuron in B for each sound pulse in a sequence of four sounds in all three temporal conditions (see Table S1) (error bars represent SEM). For all temporal conditions, the neuron discharged maximally to the second sound, thus the neuron was tuned to numerosity two. (D) The same neuron shown in B was significantly tuned to numerosity two irrespective of whether the sample period showed two, three, or four sequential items (all three conditions pooled). (E and F) Average normalized numerosity tuning functions of PFC neurons to auditory (E) and visual (F) items. (G) Frequency histogram of sample numerosities preferred by selective PFC neurons. (H and I) Average normalized numerosity tuning functions of IPS neurons to auditory (H) and visual (I) items. (J) Frequency histogram of sample numerosities preferred by selective IPS neurons.
In the PFC, a proportion of the tested neurons (42 of 232 or 18%) showed activity that varied significantly with the number of sounds during sample presentation in the auditory protocol [two-way ANOVA, with factors “stimulus protocol” (three modifications) and “sample numerosity” (1–4 numerosities), P < 0.01]. Only two of these 42 neurons showed an additional stimulus prototocol effect (i.e., temporal sensitivity); significant factor interactions were absent in all neurons. The detailed responses of a PFC neuron tuned to two sound pulses is shown in Fig. 3B (only responses to condition 3 are shown for clarity). Irrespective of the timing of sound pulses, this neuron showed peak activity for the auditory numerosity “two” (its “preferred numerosity”) in all three temporal conditions, and a systematic drop-off of activity as the number of sounds in the sample period varied from the preferred value (Fig. 3C). This was true even in trials with two, three, or four consecutive sounds and varied sequence timing (Fig. 3D). Besides auditory numerosity, even more neurons in the PFC (67 of 232 or 29%) were tuned to the number of dots in the visual protocol (no other significance of main factors or interactions).
Similar response profiles were observed for all PFC neurons tuned to auditory or visual numerosities one, two, three, or four. The population tuning curves of PFC neurons tuned to auditory or visual numerosity are shown in Fig. 3 E and F, respectively. On average, cells showed peak activity for one of the auditory or visual quantities and a systematic drop-off of activity as the number of sample items varied from the preferred value. Numerosity one was the most frequent preferred numerosity in both the auditory or visual modality (Fig. 3G).
In the IPS, fewer neurons (20 of 200 or 10%) were significantly tuned to numerosity in the auditory protocol (two-factor ANOVA, P < 0.01), with one of those neurons also showing a significant protocol effect (no neuron showed significant interactions). About the same fraction of IPS neurons (22 of 200 or 11%) showed a significant maximum discharge to a certain number of dots in the visual protocol (only two of these neurons showed an additional stimulus protocol effect, no significant interactions), confirming our previous finding (17). Just as with PFC neurons, tuned IPS neurons showed peak tuning and, thus, a clear labeled-line code for both auditory (Fig. 3H) and visual numerosities (Fig. 3I). Similarly, the distribution of preferred numerosities was comparable, albeit based on much fewer selective neurons (Fig. 3J). The proportion of both auditory and visual numerosity-selective neurons was significantly larger in the PFC compared with the IPS (P < 0.05, Pearson χ2 test).
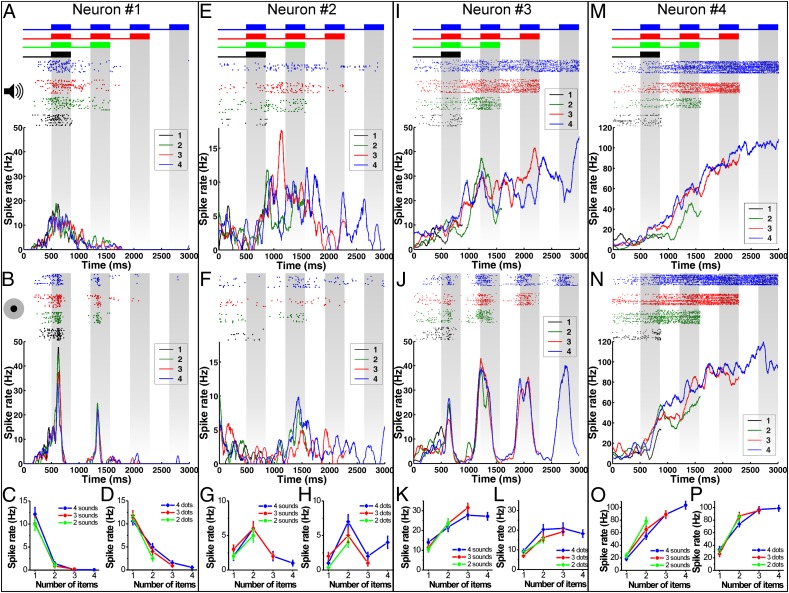
After the analysis of neurons signaling numerosity in the visual and auditory modality, I next tested whether a proportion of these neurons would also encode the number of items irrespective of stimulus modality. In the PFC, 25 (60%) of the 42 auditory and 67 visual numerosity-selective neurons, respectively, responded to both auditory and visual numerosity. Fig. 4 shows four example PFC neurons tuned to one (Fig. 4A), two (Fig. 4 E–H), three (Fig. 4 I–L), and four (Fig. 4 M–P) items in both modalities. The corresponding tuning functions of these four neurons for the number of sounds and dots illustrate that this tuning was similar in both modalities. Moreover, numerosity selectivity of such neurons was independent of the temporal presentation scheme or the number of overall sounds/dots presented in the sample period. In VIP, six neurons (30%) of the 20 auditory and 22 visual numerosity-selective neurons, respectively, were tuned in both the auditory and visual protocol and, thus, responded to quantitative information bimodally (Fig. 5A).
Fig. 4.
Bimodal responses to auditory/visual numerosities in the sample phase. (A–D) Example PFC neuron (neuron 1) selectively tuned to both auditory and visual numerosity one. (A) Responses of neuron 1 to the number of sound pulses. Layout same as in Fig. 2B. (Top) Illustration of stimulus sequence (only condition 3 is shown for clarity). (Middle) Corresponding dot-raster histogram resulting from the four numerosity sequences. (Bottom) Corresponding averaged spike-density function. Gray shaded areas indicate item presentation. (B) Responses of neuron 1 to visual items. The layout is the same as in A. (C) Numerosity tuning functions of neuron 1 to two, three, or four sounds sequences averaged across all three timing conditions. (D) Numerosity-tuning functions of neuron 1 to two, three, or four dot sequences averaged across all three timing conditions. (E–H) PFC neuron 2 tuned to both auditory and visual numerosity two. The layout is the same as for neuron 1. (I–L) PFC neuron 3 tuned to both auditory and visual numerosity three. The layout is the same as for neuron 1. (M–P) PFC neuron 4 tuned to both auditory and visual numerosity four. The layout is the same as for previous neurons.
Fig. 5.
Freuqency of supramodal numerosity-selective neurons in the sample phase. (A) Venn diagram illustrating the distributions of unimodal auditory, unimodal visual, and modality-independent (graphical overlap) numerosity-selective neurons. Numbers indicate the number of neurons per set, and percentages refer to the total number of recorded neurons in each area. (B) Frequency of neurons in PFC and VIP that had the same preferred numerosities in the visual and auditory protocols.
On average, the bimodal PFC neurons preferred the same numerosities in both the auditory and visual protocols, which was confirmed by a correlation analysis (r = 0.49, Spearman Rho, P = 0.013). Auditory and visual numerosity preferences of VIP neurons were also correlated (r = 0.92, Spearman Rho, P < 0.01). However, in the VIP only neurons with preferred numerosity one had the sample-preferred numerosity in both the visual and auditory protocol (Fig. 5B). In contrast, numerosity-selective PFC neurons with the same preferred numerosities in both the visual and auditory protocol had evenly distributed preferred numerosities from one to four (Fig. 5B). Thus, a substantial proportion of numerosity-selective PFC and VIP neurons encodes the number of items independently from the sensory modality, i.e., supramodally.
After offset of the sequence of auditory sounds or visual dots, the animals had full information about the cardinality of a set. Throughout the following delay period, they had to maintain the numerosity in mind and prepare to find the matching quantity in a test display. A proportion of PFC neurons (30 of 232 or 13%) was tuned to audiovisual numerosity in the delay phase (three-factor ANOVA, with numerosity, presentation protocol, and stimulus modality as factors, P < 0.01). Eight of the 30 selective neurons also showed a significant protocol effect, and 18 showed a significant modality effect. An example PFC neuron tuned to numerosity four is shown in Fig. 6 A–C. The highest spike rate was found whenever four items were shown, both in the auditory and the visual protocol. In addition to a main effect for numerosity, this example neuron also showed a clear main effect for stimulus modality, indicated by the rate offset between visual and auditory numerosity tuning functions (Fig. 6C). Such an additional effect of numerosity modality during the delay was found in most numerosity-selective neurons.
Fig. 6.
Neural responses of PFC and IPS neurons during the delay period. (A–C) A single PFC neuron (neuron a) tuned to numerosity four both in the auditory and visual protocol. (Neuron a is identical to neuron 4 in Fig. 4 M–P). The discharges of neuron a following auditory (A) or visual (B) sample phases (all three temporal conditions pooled) are plotted. (A Top and B Top) Color-coded dot-raster histograms, bottom panels are the corresponding spike density histograms. Time 0 ms represents onset of the delay period, which lasted 1,200 ms (see Fig. 1 A and B). (C) Tuning functions of neuron a to the auditory and visual protocol. (D–F) A single IPS neuron (neuron b) showing similar delay activity in the auditory (D) versus visual (E) presentation protocol, with two as the preferred numerosity (F). (G and H) Average normalized numerosity tuning functions of PFC neurons (G) and IPS neurons (H) in the delay period. (I and J) Normalized average tuning function across all preferred numerosities and selective neurons for the delay epoch in the PFC and IPS, respectively. Functions for correct (black lines) and error trials (red lines) are shown. Error bars indicate SEs across cells.
In the IPS, several neurons (20 of 200 or 10%) were significantly tuned to bimodal numerosity in the memory period (three-factorial ANOVA, P < 0.01). Four of the 20 IPS-selective neurons also showed a significant protocol effect, and 7 of the 20 neurons showed a significant modality effect. Fig. 6 D–F displays the detailed responses of a bimodal numerosity tuned IPS neuron. This neuron showed maximum activity to preferred numerosity two, without an additional modality effect (or any other effect), as indicated by the superimposed auditory and visual numerosity tuning functions (Fig. 6F).
The average response profiles of all numerosity-selective neurons during the delay period are shown in Fig. 6G for PFC neurons and Fig. 6H for IPS neurons. An examination of error trials suggested that the delay activity of both IPS and PFC neurons was directly related to the monkeys’ performance (Fig. 6 I and J). When monkeys made judgment errors, neural delay rate activity for the preferred numerosity in the IPS and PFC was significantly reduced by 16% and 26%, respectively, compared with that observed on correct trials (i.e., 100%; P < 0.05, Wilcoxon signed ranks test, two-tailed).
Discussion
Monkeys discriminated the number of sequentially presented (one to four) visual dots and sound pulses in random order within a given session. Groups of neurons in the VIP and PFC encoded either the number of auditory pulses, visual items, or both. The proportion of neurons responding to numerosity irrespective of modality supports the idea of a most abstract, supramodal neuronal code of numerical quantity in the primate brain.
Selectivity to the Number of Auditory Events.
In all current studies investigating the neuronal code of numerosity, monkeys either had to assess the number of visual items (16, 17, 19) or the number of actions (13, 14). The neuronal coding of auditory events had not been addressed so far. The behavioral data were consistent across the different presentation protocols, confirming that the monkeys judged the number of events and not nonnumerical cues. The discrimination performance showed a clear numerical distance and size effect, equivalent to unimodal visual discrimination (20). This result provides behavioral evidence that monkeys, just as humans, rely on an analog magnitude system that integrates across modalities (3, 26).
With 18% and 10% of numerosity-selective cells in the PFC and VIP, respectively, the frequency of auditory numerosity detectors in the macaque brain is comparable to the proportion of visual numerosity-selective neurons. Although it has been known that neurons in VIP (27, 28) and PFC respond to auditory stimulation (29, 30), this finding shows the capacity of VIP and PFC neurons to encode abstract auditory categories comparable to visual categories. This result emphasizes that VIP and the PFC play an important role not only in quantity processing, but also in nonspatial auditory cognition in general.
Supramodal Numerosity Detectors.
The IPS, a key node in basic quantity representations, has been shown to become activated to numbers in a supramodal fashion in humans. Eger et al. (31) performed fMRI while subjects were asked to detect numerals, letters, or colors in visual sequences or acoustic streams. To avoid confounds by response selection and associated cognitive states (such as attention), the authors analyzed the presentation of nontarget numerals (numerals that were not required to be detected) and compared them to nontarget letters or colors. The IPS was the only region that exhibited higher activation for numerals, both visually and acoustically. Therefore, numerical activation in the IPS seems to be supramodal (visual and auditory). Based on the temporal and spatial limitations of the BOLD-signal, however, fMRI cannot inform about the coding properties of individual neurons. Whether the brain encodes numerosity nonverbally based on intermingled populations of purely unimodal numerosity detectors, or rather implements supramodal numerosity-selective neurons remained an open question.
The single-cell recordings presented here indicate that some neurons in the primate frontal and parietal association cortex integrate numerosity across different modalities to form a supramodal categorical representation. This finding together with the previous identification of a population of neurons in VIP that represented the cardinality of a set irrespective of whether it had been cued in a spatial layout (i.e., multiple-dot patterns) or across time (i.e., single dots appearing one-by-one) (17) suggests a most abstract neuronal representation of numerosity: neurons encoding numerosity irrespective of the spatiotemporal presentation formats and the modality it is cued in. Such a modality-independent representation of numerosity has also been postulated based on psychophysical results in humans (32) and monkeys (26). Importantly, supramodally responding neurons cannot be explained by a simple learning effect because the animals were not trained to match sequential sounds to sequential dots.
PFC and VIP: Classical Multimodal Association Cortex.
Supramodal coding was found in both cortical key areas for numerical competence, the lateral PFC and VIP (4). A significantly larger proportion of both auditory and visual numerosity-selective neurons in addtion to a braod range of supramodally preferred numerosities in the PFC compared with VIP suggests a particularly strong involvement of PFC neurons in abstract numerosity coding early in phylogeny (22, 33). This correlates with recent studies investigating the development of numerical competence in children, emphasizing the important role of the PFC at early developmental stages (8, 34). Higher-level achievements, such as the associations of numerical values with numerical signs (33) or the planning of goal-directed behavior based on numerical information (35, 36), first seem to be accomplished in the frontal lobe, from which they may shift to the parietal areas with age and proficiency (4, 8).
As classical association cortices, both the PFC and the VIP are strategically situated for multimodal processing and crosstemporal integration (37–39). The auditory, visual, and tactile receptive fields of single neurons in the ventral intraparietal cortex can be encoded within a common reference frame (27, 28). Neurons in the PFC, in particular, encode magnitudes across different modalities (40, 41) and represent abstract magnitude decisions (42). Crossmodal neurons have been found in the PFC (43), and cells in this region are sensitive to the semantic congruency of multisensory communication components (44).
Putative Computational Advantage of Supramodal Numerosity Detectors.
The abstract, modality-independent (supramodal) numerosity detectors described here might provide a computational advantage: They could easily be linked to visual shapes or auditory sounds to establish symbolic representations of numbers in humans, such as numerals and number words. This is also postulated by the influential triple-code model (1). According to this framework, numerical cognition initially involves a lower step of modality-specific analysis, followed by a higher processing stage where these representations reach an abstract, amodal estimation module. The neurons described in this article could constitute a neuronal implementation of this module.
Materials and Methods
Setup.
Two male rhesus monkeys (Macacca mulatta) weighing 7.1 and 9.0 kg were used in this study. They were seated inside a double-walled, soundproof chamber (IAC) and faced a 15-inch flat screen monitor (1,024 × 768 pixels resolution, 75-Hz refresh rate) at a distance of 57 cm. A broadband speaker located above the monitor delivering the acoustic signals. Stimulus presentation and performance monitoring was accomplished using the NIMH Cortex program. The monkeys had to keep their gaze within 1.75° of the fixation point during sample presentation and the memory delay (monitored with an infrared eye tracking system, Iscan).
Stimuli.
In the auditory sample protocol (Fig. 1A), numerosity was cued by a sequence of white-noise sound pulses (65-dB SPL) with silent periods between individual pulses while the monkeys fixated a central spot displayed on a gray background (diameter: 6° of visual angle). In the visual sample protocol (Fig. 1B), successive black dots (diameter range 0.5–1.1° of visual angle) separated by short pauses were displayed centrally on a gray background. The items of the multiple-dot displays in the test phase (auditory and visual protocols) were randomly arranged (Fig. 1 A and B). Each quantity was tested with 100 different images per session. All four quantities were used in each session, and all displays were generated anew for each session by pseudorandomly shuffling relevant item features (e.g., position and size in the multiple-item displays) using custom-written Matlab (Mathworks) software.
In both the auditory and visual protocols, successive items were presented in three different temporal conditions (sequence patterns) to control for nonnumerical timing effects the monkeys could have exploited (Table S1 and Fig. 1 C–E). Within a given session, the two protocols and the three temporal conditions appeared in random order with equal probability. All four numerosities were presented in each session, resulting in a total of 24 different trial constellations [2 protocols(auditory/visual) × 3 temporal conditions × 4 numerosities].
Behavioral Protocol.
The visual and auditory numerosity protocols are depicted in Fig. 1 A and B. Three different temporal sample display conditions were used (see Fig. 1 C–E, Table S1, details in SI Materials and Methods). Trials were randomized and balanced across all relevant features (e.g., match vs. nonmatch, unimodal versus crossmodal, etc.).
Subjects and Recording Technique.
The monkeys were implanted with a head bolt to maintain the head in a constant position to allow for eye movement measurement. All surgeries were performed under sterile conditions while the animals were under general anesthesia. The animals received postoperative antibiotics and analgesics. All procedures were in accordance with the guidelines for animal experimentation approved by the Regierungspräsidium Tübingen, Germany.
Recordings were performed simultaneously from the lateral PFC (both banks of the principal sulcus) as well as in the depth of the IPS of both animals (Fig. 2A) using the MAP system (Plexon). Arrays of four to eight glass-coated tungsten microelectrodes (1–2 MOhm impedance) were inserted using a grid with 1-mm spacing. Recordings from the IPS were exclusively done at depths ranging from 9 to 13 mm below the cortical surface. Recording sites were localized using stereotaxic reconstructions from MR images. The (Horsley–Clark coordinates of the IPS recordings: 2 mm posterior to 3 mm anterior). Neurons were selected at random; no attempt was made to search for any task-related activity. Separation of single-unit waveforms was performed off-line (Offline Sorter, Plexon).
Behavioral Data Analysis.
The percentage of correct trials was taken as a measure of performance and used to construct behavioral performance functions (Fig. 2 B–D) (details in SI Materials and Methods). Analyses of performance data were carried out in Matlab (Mathworks) and Origin 7.5 (Origin Lab).
Neuronal Data Analysis.
The average spike rate in response to each individual item (sound pulse or dot, respectively) in the sample period was derived from a 250-ms interval after each item’s onset (details in SI Materials and Methods). The average discharge rates in response to individual items were used to create numerosity tuning curves (Fig. 3 C and D). For every item, the 250-ms analysis window was shifted by 60 ms to account for response latencies. For the delay period (Fig. 1 A and B), activity was averaged in a 1,000-ms interval starting 250 ms after delay onset.
To determine numerosity-selectivity during the sample period, a two-factorial analysis of variance (ANOVA, P < 0.01) was calculated separately for the auditory and visual protocol with “numerosity” (1–4) and “stimulation conditions” (three conditions, see Fig. 1 C–E and Table S1) as factors. In the delay period, a three-factorial ANOVA was computed with numerosity (1–4), stimulation conditions (three conditions), and “sample modality” (auditory or visual) as factors. A neuron was judged to be numerosity selective in the sample phase if it showed a main effect of numerosity in the auditory or visual protocol in the four-items sequence. A neuron was judged to be numerosity selective in the delay period if it showed a main effect of numerosity during the memory delay (three-factorial ANOVA, P < 0.01).
To derive averaged numerosity-filter functions, the tuning functions of individual neurons were normalized by setting the maximum activity following the most preferred quantity to 100% and the activity following the least preferred quantity to 0%. Pooling the resulting normalized tuning curves resulted in averaged numerosity-filter functions. Statistical tests were calculated in Matlab (MathWorks) and SPSS (SPSS Inc.).
Supplementary Material
Acknowledgments
I thank Simon N. Jacob for proofreading the manuscript. This work was supported by the DFG.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204580109/-/DCSupplemental.
References
- 1.Dehaene S. Varieties of numerical abilities. Cognition. 1992;44:1–42. doi: 10.1016/0010-0277(92)90049-n. [DOI] [PubMed] [Google Scholar]
- 2.Nieder A. Counting on neurons: The neurobiology of numerical competence. Nat Rev Neurosci. 2005;6:177–190. doi: 10.1038/nrn1626. [DOI] [PubMed] [Google Scholar]
- 3.Brannon EM. The representation of numerical magnitude. Curr Opin Neurobiol. 2006;16:222–229. doi: 10.1016/j.conb.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieder A, Dehaene S. Representation of number in the brain. Annu Rev Neurosci. 2009;32:185–208. doi: 10.1146/annurev.neuro.051508.135550. [DOI] [PubMed] [Google Scholar]
- 5.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 6.Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex. 2001;11:350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- 7.Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S. Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron. 2004;44:547–555. doi: 10.1016/j.neuron.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Ansari D, Garcia N, Lucas E, Hamon K, Dhital B. Neural correlates of symbolic number processing in children and adults. Neuroreport. 2005;16:1769–1773. doi: 10.1097/01.wnr.0000183905.23396.f1. [DOI] [PubMed] [Google Scholar]
- 9.Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53:293–305. doi: 10.1016/j.neuron.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Jacob SN, Nieder A. Notation-independent representation of fractions in the human parietal cortex. J Neurosci. 2009a;29:4652–4657. doi: 10.1523/JNEUROSCI.0651-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob SN, Nieder A. Tuning to non-symbolic proportions in the human frontoparietal cortex. Eur J Neurosci. 2009b;30:1432–1442. doi: 10.1111/j.1460-9568.2009.06932.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacob SN, Vallentin D, Nieder A. Relating magnitudes: The brain's code for proportions. Trends Cogn Sci. 2012;16:157–166. doi: 10.1016/j.tics.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Sawamura H, Shima K, Tanji J. Numerical representation for action in the parietal cortex of the monkey. Nature. 2002;415:918–922. doi: 10.1038/415918a. [DOI] [PubMed] [Google Scholar]
- 14.Sawamura H, Shima K, Tanji J. Deficits in action selection based on numerical information after inactivation of the posterior parietal cortex in monkeys. J Neurophysiol. 2010;104:902–910. doi: 10.1152/jn.01014.2009. [DOI] [PubMed] [Google Scholar]
- 15.Nieder A, Miller EK. A parieto-frontal network for visual numerical information in the monkey. Proc Natl Acad Sci USA. 2004;101:7457–7462. doi: 10.1073/pnas.0402239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roitman JD, Brannon EM, Platt ML. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 2007;5:e208. doi: 10.1371/journal.pbio.0050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieder A, Diester I, Tudusciuc O. Temporal and spatial enumeration processes in the primate parietal cortex. Science. 2006;313:1431–1435. doi: 10.1126/science.1130308. [DOI] [PubMed] [Google Scholar]
- 18.Tudusciuc O, Nieder A. Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. Proc Natl Acad Sci USA. 2007;104:14513–14518. doi: 10.1073/pnas.0705495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieder A, Freedman DJ, Miller EK. Representation of the quantity of visual items in the primate prefrontal cortex. Science. 2002;297:1708–1711. doi: 10.1126/science.1072493. [DOI] [PubMed] [Google Scholar]
- 20.Nieder A, Miller EK. Coding of cognitive magnitude: Compressed scaling of numerical information in the primate prefrontal cortex. Neuron. 2003;37:149–157. doi: 10.1016/s0896-6273(02)01144-3. [DOI] [PubMed] [Google Scholar]
- 21.Nieder A, Merten K. A labeled-line code for small and large numerosities in the monkey prefrontal cortex. J Neurosci. 2007;27:5986–5993. doi: 10.1523/JNEUROSCI.1056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diester I, Nieder A. Semantic associations between signs and numerical categories in the prefrontal cortex. PLoS Biol. 2007;5:e294. doi: 10.1371/journal.pbio.0050294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diester I, Nieder A. Complementary contributions of prefrontal neuron classes in abstract numerical categorization. J Neurosci. 2008;28:7737–7747. doi: 10.1523/JNEUROSCI.1347-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallentin D, Nieder A. Behavioral and prefrontal representation of spatial proportions in the monkey. Curr Biol. 2008;18:1420–1425. doi: 10.1016/j.cub.2008.08.042. [DOI] [PubMed] [Google Scholar]
- 25.Wiese H. Numbers, Language, and the Human Mind. Cambridge, UK: Cambridge Univ. Press; 2003. [Google Scholar]
- 26.Jordan KE, Maclean EL, Brannon EM. Monkeys match and tally quantities across senses. Cognition. 2008;108:617–625. doi: 10.1016/j.cognition.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlack A, Sterbing D’Angelo SJ, Hartung K, Hoffmann KP, Bremmer F. Multisensory space representations in the macaque ventral intraparietal area. J Neurosci. 2005;25:4616–4625. doi: 10.1523/JNEUROSCI.0455-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avillac M, Ben Hamed S, Duhamel JR. Multisensory integration in the ventral intraparietal area of the macaque monkey. J Neurosci. 2007;27:1922–1932. doi: 10.1523/JNEUROSCI.2646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romanski LM, Goldman-Rakic PS. An auditory domain in primate prefrontal cortex. Nat Neurosci. 2002;5:15–16. doi: 10.1038/nn781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russ BE, Orr LE, Cohen YE. Prefrontal neurons predict choices during an auditory same-different task. Curr Biol. 2008;18:1483–1488. doi: 10.1016/j.cub.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eger E, Sterzer P, Russ MO, Giraud AL, Kleinschmidt A. A supramodal number representation in human intraparietal cortex. Neuron. 2003;37:719–725. doi: 10.1016/s0896-6273(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 32.Kouider S, Dehaene S. Subliminal number priming within and across the visual and auditory modalities. Exp Psychol. 2009;56:418–433. doi: 10.1027/1618-3169.56.6.418. [DOI] [PubMed] [Google Scholar]
- 33.Nieder A. Prefrontal cortex and the evolution of symbolic reference. Curr Opin Neurobiol. 2009;19:1–10. doi: 10.1016/j.conb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Cantlon JF, et al. The neural development of an abstract concept of number. J Cogn Neurosci. 2009;21:2217–2229. doi: 10.1162/jocn.2008.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bongard S, Nieder A. Basic mathematical rules are encoded by primate prefrontal cortex neurons. Proc Natl Acad Sci USA. 2010;107:2277–2282. doi: 10.1073/pnas.0909180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallentin D, Bongard S, Nieder A. Numerical rule coding in the prefrontal, premotor, and posterior parietal cortices of macaques. J Neurosci. 2012;32:6621–6630. doi: 10.1523/JNEUROSCI.5071-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driver J, Noesselt T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron. 2008;57:11–23. doi: 10.1016/j.neuron.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein BE, Stanford TR. Multisensory integration: Current issues from the perspective of the single neuron. Nat Rev Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- 39.Cohen YE. Multimodal activity in the parietal cortex. Hear Res. 2009;258:100–105. doi: 10.1016/j.heares.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 41.Tudusciuc O, Nieder A. Contributions of primate prefrontal and posterior parietal cortices to length and numerosity representation. J Neurophysiol. 2009;101:2984–2994. doi: 10.1152/jn.90713.2008. [DOI] [PubMed] [Google Scholar]
- 42.Merten K, Nieder A. Active encoding of decisions about stimulus absence in primate prefrontal cortex neurons. Proc Natl Acad Sci USA. 2012;109:6289–6294. doi: 10.1073/pnas.1121084109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- 44.Sugihara T, Diltz MD, Averbeck BB, Romanski LM. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci. 2006;26:11138–11147. doi: 10.1523/JNEUROSCI.3550-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.