Abstract
Recent discoveries in Asia have greatly increased our understanding of the evolution of dinosaurs’ integumentary structures, revealing a previously unexpected diversity of “protofeathers” and feathers. However, all theropod dinosaurs with preserved feathers reported so far are coelurosaurs. Evidence for filaments or feathers in noncoelurosaurian theropods is circumstantial and debated. Here we report an exceptionally preserved skeleton of a juvenile megalosauroid, Sciurumimus albersdoerferi n. gen., n. sp., from the Late Jurassic of Germany, which preserves a filamentous plumage at the tail base and on parts of the body. These structures are identical to the type 1 feathers that have been reported in some ornithischians, the basal tyrannosaur Dilong, the basal therizinosauroid Beipiaosaurus, and, probably, in the basal coelurosaur Sinosauropteryx. Sciurumimus albersdoerferi represents the phylogenetically most basal theropod that preserves direct evidence for feathers and helps close the gap between feathers reported in coelurosaurian theropods and filaments in ornithischian dinosaurs, further supporting the homology of these structures. The specimen of Sciurumimus is the most complete megalosauroid yet discovered and helps clarify significant anatomical details of this important basal theropod clade, such as the complete absence of the fourth digit of the manus. The dentition of this probably early-posthatchling individual is markedly similar to that of basal coelurosaurian theropods, indicating that coelurosaur occurrences based on isolated teeth should be used with caution.
Keywords: feather evolution, Megalosauridae, Theropoda, Upper Jurassic
The discovery of Archaeopteryx in 1861 in Late Jurassic rocks in Southern Germany provided the first evidence of derived, avialan maniraptoran theropods with feathers (1). These remains long remained the only skeletal specimens with preserved feathers from the Mesozoic. In recent years, however, Mesozoic birds that preserve feathers and even nonavialan theropods with feathery body coverings have been found and are now phylogenetically and temporally widespread (2). Nearly all these specimens come from the Middle Jurassic to Early Cretaceous of eastern Asia, and all are coelurosaurs. Thus, there is a considerable phylogenetic gap between these animals and some basal ornithischians and pterosaurs, in which monofilamentous integumentary structures have been reported (3–5). The specimen reported here is significantly more basal in the evolutionary tree of theropods and thus represents the phylogenetically most basal theropod yet discovered with direct fossil evidence of a filamentous body covering. It furthermore is noteworthy in that it represents the most complete basal tetanuran theropod known to date and is one of very few complete early juvenile theropods known.
Systematic Paleontology
Dinosauria Owen, 1842; Theropoda Marsh, 1881; Tetanurae Gauthier, 1986; Megalosauroidea (Fitzinger, 1843); Sciurumimus albersdoerferi n. gen. and sp.
Holotype
Bürgermeister Müller Museum Solnhofen (BMMS) BK 11, a complete and exquisitely preserved skeleton of a juvenile individual preserved on a single slab.
Etymology
The genus name is from the scientific name of the tree squirrels, Sciurus, and mimos (Greek), meaning “mimic,” in reference to the bushy tail of the animal. The species epithet honors Raimund Albersdörfer, who made the specimen available for study.
Type Locality and Horizon
Rygol quarry, near Painten, Bavaria, Germany. Thin-bedded to laminated micritic limestones that are equivalent to the upper part of the Rögling Formation (6), Upper Kimmeridgian, Beckeri zone, Ulmense subzone, rebouletianum horizon (7, 8).
Diagnosis
Megalosauroid theropod with the following apomorphic characters: axial neural spine symmetrically “hatchet-shaped” in lateral view; posterior dorsal neural spines with rectangular edge anteriorly and lobe-shaped dorsal expansion posteriorly; anterior margin of ilium with semioval anterior process in its dorsal half.
Description and Comparisons
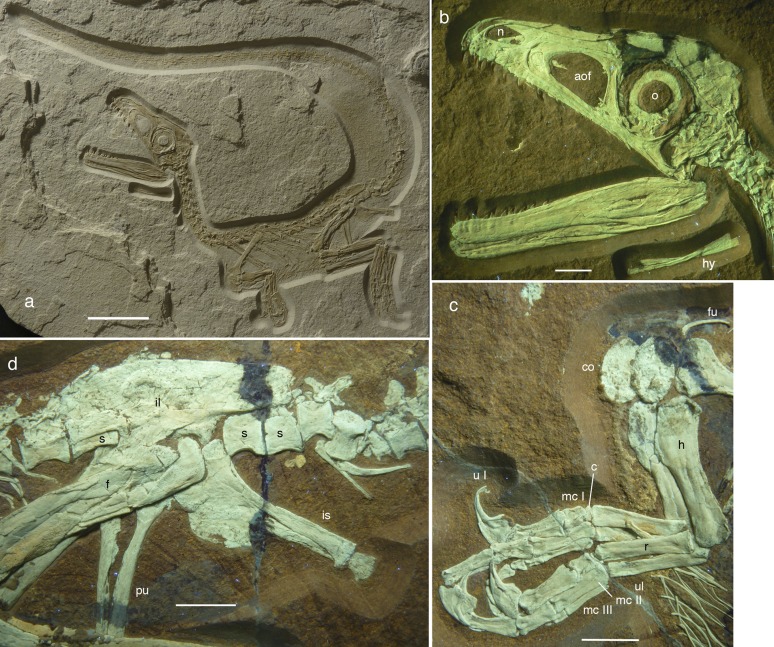
The specimen is preserved in complete articulation, lying on its right side (Fig. 1A). The skull (Fig. 1B) is relatively large, longer than the cervical vertebral series and 156% of the femur length. The skull is subtriangular in outline and is slightly more than twice as long as it is high. The nares are large, being ∼13% of the skull length. The orbit is the largest skull opening and encloses a complete scleral ring. The premaxillary body is considerably longer than high and meets a long anterior process of the maxilla below the naris. However, the latter bone is excluded from the narial margin by a robust posterior subnarial process of the premaxilla that contacts the nasal. The maxilla has a marked kink in the anterior margin of the ascending process dorsally and a large maxillary fenestra, which is closed medially (Figs. 1B and 2A), as in other megalosaurids (9, 10). A small premaxillary fenestra seems to be present under the overhanging anterior rim of the antorbital fossa. The maxillary antorbital fossa anterior to the antorbital fenestra accounts for ∼23% of the total length of the antorbital fossa, as in other basal tetanurans but unlike coelurosaurs, in which it typically accounts for 40% or more of the antorbital fossa (11). The lacrimal has a long, thin anterior process, which laterally forms a large lacrimal antorbital fossa that is continuous between the dorsal and ventral part of the vertical strut, in contrast to most theropods but similar to Torvosaurus (12). The jugal seems to be excluded from the antorbital fenestra by the maxilla and lacrimal, which are in contact with each other. The postorbital is slender and T-shaped, with the ventral process ending above the ventral margin of the orbit. The infratemporal fenestra was obviously high and narrow, although its borders are only partially preserved. A triangular area along the frontoparietal suture has been reconstructed as bone; this structure occupies almost the same position and area as the open frontoparietal gap in the hatchling theropod Scipionyx (13). Although partially obscured by reconstruction, the borders of the bones around this gap do not show clear signs of breakage, making the interpretation of this area as a similar structure probable. However, a similar reconstructed area within the frontal probably represents a damaged area rather than an unossified one. The quadratojugal is considerably higher than long and has a broad dorsal contact with the broad ventral process of the squamosal. A large quadrate foramen is present in the quadratojugal–quadrate suture. A broad and deep longitudinal fossa is present on the posterior face of the basioccipital bone below the occipital condyle (Fig. 2B), as seen in other megalosaurids and spinosaurids (10).
Fig. 1.
Juvenile megalosaurid Sciurumimus albersdoerferi (BMMS BK 11). (A) Overview of the limestone slab with the specimen as preserved. (B) Skull and hemimandibles under UV light in left lateral view. (C) Forelimbs under UV light. (D) Pelvic girdle under UV light. aof, antorbital fenestra; c, carpal; co, coracoid; f, femur; fu, furcula; h, humerus; hy, hyoid; il, ilium; is, ischium; mc, metacarpal; n, nares; o, orbit; pu, pubis; r, radius; s, sacral vertebra; u, ungual; ul, ulna. (Scale bars: 50 mm in A and 10 mm in B–D.)
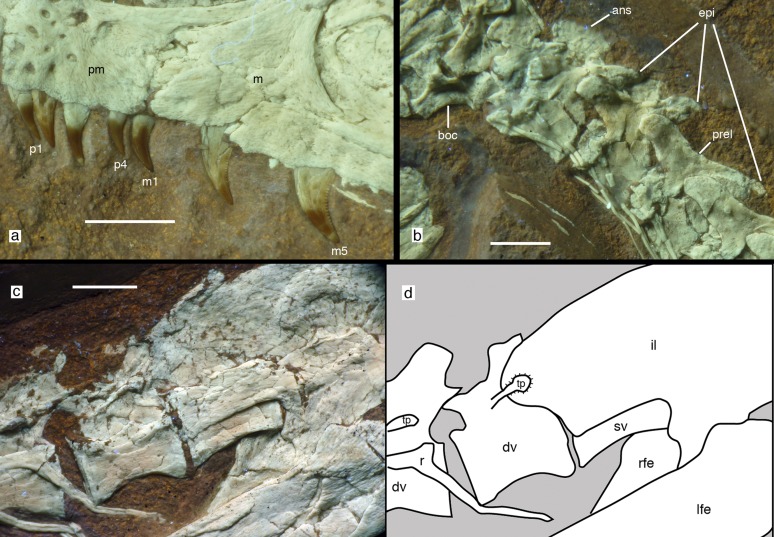
Fig. 2.
Anatomical details of Sciurumimus albersdoerferi. (A) Dentition of left premaxilla and anterior end of left maxilla. (B) Disarticulated occiput, atlas, axis, and anterior cervical vertebrae. (C and D) Posterior-most dorsal vertebrae and anterior part of ilium shown in a photograph (C) and in an interpretative drawing (D). All photographs were taken under UV light. ans, axial neural spine; boc, basioccipital; dv, dorsal vertebrae; epi, epipophyses; il, ilium; lfe, left femur; m, maxilla; m1 and m5, first and fifth maxillary tooth, respectively; p1and p4, first and fourth premaxillary tooth, respectively; pm, premaxilla; prel, prezygoepipophyseal lamina; r, rib; rfe, right femur; sv, sacral vertebra; tp, transverse process. (Scale bars: 5 mm in A and 10 mm in B–D.)
The anterior end of the dentary is slightly raised dorsally over the first three tooth positions; the medial side of the dentary shows two Meckelian foramina anteriorly, as seen in other basal theropods (10). An anteroventrally opening mylohyoid foramen is present along the ventral margin of the splenial. A large mandibular fenestra is present, and the retroarticular process is short and stout. The premaxilla bears four unserrated teeth, and the 11 maxillary and 12–14 dentary teeth are strongly recurved and bear serrations on the distal but not on the mesial carina (Fig. 2A).
There are 10 cervical and 13 dorsal vertebrae. As in many basal theropods, the axis lacks pleurocoels, but single, large pneumatic foramina are present in the remaining cervicals (Fig. 2B). Anterior cervical vertebrae might be slightly opisthocoelous, but the posterior cervicals seem amphiplatycoelous. Cervical neural arches have pronounced prezygoepipophyseal laminae and large, elongate epipophyses, which considerably overhang the postzygapophyses posteriorly (Fig. 2B). Anterior dorsal vertebrae have a well-developed ventral keel and bear pleurocoels, whereas posterior dorsals are apneumatic. Posterior dorsal vertebrae seem to have rather poorly developed neural arch lamination and backswept transverse processes. The neural spines of the posterior dorsal vertebrae are unusual in being very low anteriorly, with a squared anterior end and a lobe-shaped posterodorsal expansion posteriorly (Fig. 2 C and D). This expansion becomes more conspicuous in the posterior-most elements.
The sacrum consists of five vertebrae; the posterior ones are considerably shorter than the anterior sacrals. A total of 59 caudal vertebrae are preserved; a few elements probably are missing. Anterior caudal vertebrae lack ventral grooves or keels and have rather low, simple, posterodorsally directed neural spines. The exact position of the transition point cannot be established, but transverse processes are certainly absent posterior to caudal vertebra 20. Posterior caudals are elongate in shape and have short, bowed pre- and postzygapophyses, unlike the strongly elongate prezygapophyses in allosauroids and coelurosaurs. Chevrons are present in at least 36 vertebrae; they are simple rod-like structures in lateral view, without ventral anterior or posterior expansion. Slender gastralia are present, with the medial elements being longer and more robust than the lateral elements.
The scapula is more than 10 times longer than it is wide at its narrowest point, unlike the broader scapula in basal theropods, including megalosauroids but rather comparable to the scapulae in derived allosauroids and coelurosaurs (10, 11). It has a slight distal expansion that arises gradually from the shaft. The acromion process is expanded only moderately and gradually relative to the width of the shaft. The coracoid is oval, is shorter than it is high, and lacks a subglenoid process and a biceps tubercle, as seen in megalosaurids and spinosaurids. The left ramus of a small, slender furcula is exposed.
The forelimbs (Fig. 1C) are short and robust, as seen in some other megalosaurids (10), with a long manus accounting for ∼45% of the length of the forelimb. The humerus is short and robust, with a triangular internal tuberosity and a well-developed deltopectoral crest. The radius and ulna are considerably shorter than the humerus, and the ulna is anteroposteriorly expanded proximally to form a concave facet for the humerus anteriorly and a small but stout olecranon process posteriorly. The ulna is slightly more slender than the radius. A poorly ossified carpal is present and covers the proximal end of metacarpal I. The manus has three digits, with metacarpal I being less than half the length of metacarpal II and metacarpal III being shorter and considerably more slender than metacarpal II. There is no trace of a fourth metacarpal. Digit I is very robust, with phalanx I-1 exceeding the radius in width, as in compsognathids (14), and the ungual is more than half the length of the radius.
The ilium is elongate, with a gently curved dorsal margin and an undulate posterior end (Fig. 1D). There is no ventral hook anteriorly, but the anterior end has an unusual anterior “lip” dorsally (Fig. 2 C and D). The medial brevis shelf is not exposed in lateral view. The pubic peduncle is anteroposteriorly longer than the ischial peduncle, as in other tetanurans (11). The pubis is slender, longer than the ischium, and the shaft is straight, with a moderately expanded distal boot. The ischium is slightly expanded anteriorly distally, and the large, hatchet-shaped obturator process is not offset from the pubic peduncle. The femur is stout and has a wing-like lesser trochanter that is approximately half the height of the slender greater trochanter (Figs. 1D and 2 C and D). Tibia and fibula are slightly longer than the femur, and the fibula is distally expanded. The metatarsus is slender; metatarsals II and IV are of subequal length, and metatarsal V is transversely flat and anteriorly flexed. Metatarsal I is elongate and splint-like, rather than short and triangular as in most other tetanurans (11). In the foot, pedal ungual II is slightly larger than the other unguals.
Soft tissues are preserved in several areas of the skeleton (Fig. 3), and most seem to represent integumentary structures, with the possible exception of a short section of fossilized tissue along the posterior edge of the tibia, which might represent muscle tissue (Fig. 3E). The best soft tissue preservation is found on the tail, which preserves large patches of skin, especially on the ventral but also on the dorsal side, and very fine, long, hair-like filaments that correspond to type 1 feathers (2) dorsally in the anterior midsection (Fig. 3 C and D). The skin is smooth and, unlike Juravenator (15), does not show clear signs of scales. The feathers seem to be anchored in the skin and form a thick covering on the dorsal side of the tail and reach more than two and a half times the height of their respective caudal vertebrae. Shorter filaments are preserved on the ventral tail flank (Fig. 3C), above the middorsal vertebrae (Fig. 3B), and in a small patch on the ventral part of the body (Fig. 3F).
Fig. 3.
Soft tissue preservation in Sciurumimus. (A) Overview of skeleton under UV light, with position of magnifications in B–F indicated. (Scale bar: 50 mm.) (B) Fine filaments above the scapular region of the dorsal vertebral column. (C) Anterior midcaudal section with long dorsal filaments (upper white arrow), preserved skin (yellow patch), and fine filaments at the ventral lateral tail flank (lower white arrows). (D) Long filaments, anchored in the skin at the dorsal tail base. (E) Small section of possibly fossilized muscle tissue along the posterior edge of the tibia. (F) Small, fine filaments ventral to the gastralia in the abdominal area (arrows point to individual filaments). (G) Magnification of soft tissues dorsal to the ninth and 10th caudal vertebra. (H) Interpretative drawing showing possible follicles. Greenish white structures are bone, fine greenish lines above the vertebrae are preserved filaments, and yellow parts represent skin structures. Arrow in G points to a filament entering one of the vertical skin structures that might represent follicles. col, collagen fibers in the skin; fil, filaments; fo? possible follicles; tp, transverse process. All photographs were taken under UV light.
The protofeathers probably are monofilaments, because no branching patterns are visible in the well-preserved, long filaments above the tail; apparent branching patterns in a few places probably are the result of compaction of these structures (16). Because of the state of preservation, it cannot be established if these structures were hollow, like the filaments found in other dinosaurs (3, 14). The thickness of these filaments is ∼0.2 mm in the long filaments in the dorsal tail region and less in the shorter filaments at the tail flank, back, and belly of the animal; the filaments are comparable in size to the filamentous protofeathers found in Sinosauropteryx (14).
Discussion
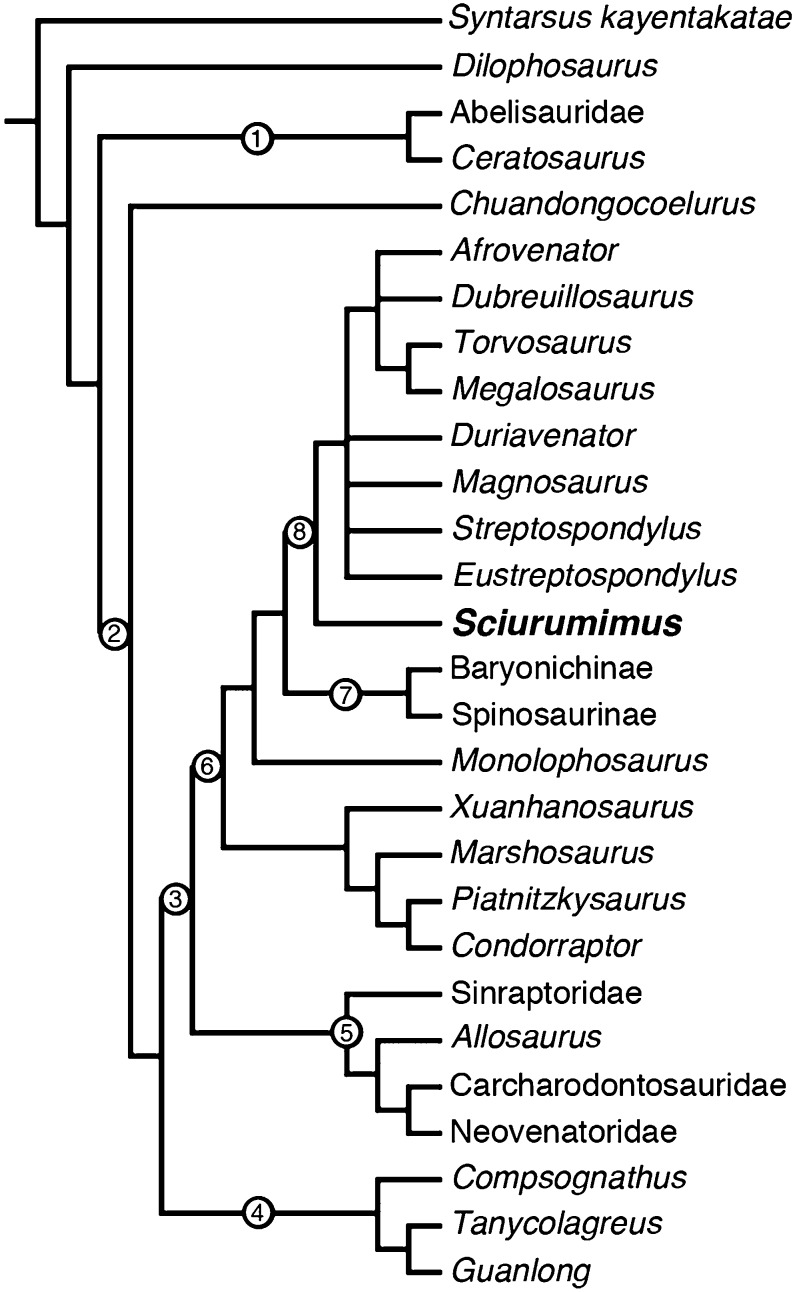
To establish the phylogenetic position of Sciurumimus, we carried out several analyses using three large recently published matrices (SI Text). Sciurumimus consistently was found to be a basal tetanuran and recovered as a basal megalosaurid within Megalosauroidea in the most detailed analysis of basal tetanuran interrelationships yet published (Fig. 4) (17). Synapomorphies of megalosauroids and more restricted ingroups present in Sciurumimus include an elongate anterior process of the maxillary body, a medially closed maxillary fenestra, a very slender anterior process of the lacrimal, a lateral blade of the lacrimal that does not overhang antorbital fenestra, the presence of a deep fossa ventral to the basioccipital condyle, a splenial foramen that opens anteroventrally, a slightly dorsally expanded anterior end of the dentary, a pronounced ventral keel in the anterior dorsal vertebrae, the absence of a posteroventral process of the coracoid, and an enlarged manual ungual I.
Fig. 4.
Phylogenetic position of Sciurumimus in the analysis of Benson et al. (17). Clade names: 1, Ceratosauria; 2, Tetanurae; 3, Carnosauria; 4, Coelurosauria; 5, Allosauroidea; 6, Megalosauroidea; 7, Spinosauridae; 8, Megalosauridae. Numbers on the stem indicate stem-based taxa, numbers on the node indicate node-based taxa.
Interestingly, the inclusion of Sciurumimus, without changes to any other codings, resulted in the recovery of a monophyletic Carnosauria that includes Megalosauroidea and Allosauroidea and represents the sister group to Coelurosauria. This result is in contrast to the vast majority of recent analyses, which depict the former two clades as successive sister taxa to coelurosaurs. Although this result certainly should be regarded with caution, given the early ontogenetic stage of Sciurumimus, this rather severe change to the phylogeny by the simple inclusion of an additional taxon highlights our still incomplete understanding of basal tetanuran evolution.
Sciurumimus represents the only complete megalosauroid known and helps clarify previously uncertain aspects of the anatomy of this group, such as the absence of a fourth digit in the manus. This absence highlights a surprisingly high level of homoplasy in this characteristic, given that the basal allosauroid Sinraptor (18), the neovenatorid Megaraptor (19), and the basal tyrannosaur Guanlong (20) retain a rudimentary fourth metacarpal, whereas most derived allosauroids (21, 22) and also coelurosaurs (e.g., 14) have only three metacarpals. These variations suggest either that the fourth digit was reduced several times independently or that a reduction of this structure at the base of tetanurans was reversed in some taxa, possibly atavistically.
Several characters indicating that the Sciurumimus albersdoerferi type specimen represents a very young, probably early-posthatchling, individual include the body proportions, with a very large skull and rather short hindlimbs, lack of fusion in the skeleton (unfused neurocentral sutures in all of the vertebral column, unfused sacral vertebrae, lack of fusion between elements of the braincase) (23), a coarsely striated bone-surface texture in all skeletal elements (24), and a very regular pattern of tooth development in the maxilla, possibly indicating that no teeth had been replaced (25). This regular pattern differs from that in perinates of more derived coelurosaurs, where there is considerable heterogeneity in among the teeth (26).
The dentition of Sciurumimus differs significantly from those of subadult or adult basal tetanurans in the slender and unserrated premaxillary teeth and strongly recurved maxillary teeth with only distal serrations. Given the rather uniform tooth morphology in most basal tetanurans (at least in respect to general morphology, such as tooth shape and presence and extent of serrations), the features seen here are regarded as juvenile characters. Thus, these differences support the assertion that juveniles of large theropod species fed on different prey items than adults (27). Conversely, this dentition is remarkably similar to that of basal coelurosaurs, which commonly have slender, more rounded premaxillary teeth that lack serrations (11, 28) and often have strongly recurved lateral teeth, frequently without mesial serrations in at least some teeth (14, 28, 29). This similarity might indicate that the dentition, as seen in compsognathids (14, 28, 30) and dromaeosaurids (29, 31), evolved by heterochronic processes or might reflect convergence resulting from similar prey preferences. This similarity also implies that the common practice of ascribing small, strongly recurved lateral teeth with reduced or no mesial serrations to dromaeosaurids or coelurosaurs in general (32–34) should be done with caution and that coelurosaur occurrences based on these tooth characters alone are of no use for inferring biogeographic or evolutionary patterns.
Sciurumimus is comparable in size to and basically is indistinguishable in proportions from the juvenile basal coelurosaur Juravenator (Table S1) (15, 35, 36). However, these taxa differ significantly in anatomical details (SI Text). Thus, if this observation is indicative of the condition in early-posthatchling theropods in general, these early-posthatchling theropods seem to have had remarkably similar proportions, and differences in allometric growth might account for the different body plans seen in adult theropods (37, 38). However, data on juvenile theropods are still very limited, and more information is needed to test this hypothesis.
The presence of type 1 feathers along the dorsal side of the tail, the ventral tail flank, and parts of the body in Sciurumimus show that the entire body of this animal was plumaged, as is the case in compsognathids (2). As a megalosaurid, Sciurumimus is the most basal theropod taxon yet reported with such integumentary structures and demonstrates that at least the juveniles of basal tetanurans had protofeathers. Sciurumimus thus helps bridge the considerable gap between basal ornithischians, for which monofilaments have been reported (4), and coelurosaurs, for which protofeathers [morphotype 1 (39)] or feathers generally seem to be present (2, 15, 40). As in tyrannosauroids (2), the preservation of scaly skin in adult basal tetanurans (41) therefore is no argument against the presence of feathers in this group in general, nor should the presence of scales in other dinosaur clades (2) be taken as such. Large adult dinosaurs might have lost feathers secondarily, just as today several groups of large mammals have lost hair. Furthermore, the joint presence of scales and filaments in some taxa (3, 15) indicates that the apparent lack of filaments in animals that preserve impressions of scaly skin in more coarse-grained sediments could be the result of taphonomic processes. Given that filaments in ornithischian dinosaurs (3, 4) are morphologically indistinguishable from protofeathers found in tetanurans and basal coelurosaurs, a filamentous body covering obviously represents the plesiomorphic state for dinosaurs in general, and, if one assumes that the hair-like structures of pterosaurs (5) are homologous structures, for ornithodiran archosaurs as well (42).
In the anterior midsection of the tail of Sciurumimus, the feathers seem to be anchored in the skin and are associated with dorsoventrally elongate skin structures (Fig. 3). Although collagen fibers in avian skin usually are oriented parallel to the body surface, these structures are perpendicular to the long axis of the body, and several show an elongated cup-shaped outline (Fig. 3). The only comparable structures in the avian skin are the follicles associated with the feathers (43), so we tentatively suggest that these structures might represent follicles. Thus, although several recent papers have argued that the origin of follicles was linked with the evolution of a rachis or barb ridges (2, 44, 45), Sciurumimus might present evidence for the hypothesis that follicles were associated with the origin of feathers (39). Furthermore, there is a meshwork of thin, elongated soft tissue structures below this outer layer (Fig. 3). These structures most likely represent collagen fibers within the stratum compactum of the dermis, which is characterized by a high density of collagen bundles in birds (43). The fibers clearly are different from the filaments in their orientation and their luminescence under filtered UV light and thus provide evidence against the interpretation of similarly arranged and oriented filaments in Chinese theropods as decaying collagen fibers (46, 47).
Supplementary Material
Acknowledgments
We thank Raimund Albersdörfer, who financed the excavations during which the specimen was found and made it available for study; Birgit Albersdörfer, the owner of the specimen; Joseph Schels and Wolfgang Häckel, who found and excavated the specimen; Jürgen Geppert, Wolfgang Häckel, and Stefan Selzer for the delicate preparation of the specimen; Wolfgang Rygol and the Kalkwerk Rygol GmbH & CoKG for permitting the excavations; Martin Röper and Monika Rothgaenger for introducing R. Albersdörfer to the fossiliferous layers at Painten; Martina Kölbl-Ebert for providing access to Juravenator for comparison; Adriana López-Arbarello and Richard Butler for many useful discussions; and Xu Xing and Roger Benson for critical revisions of the paper. This research was funded by Volkswagen Foundation Grant I/84 640 (to O.W.M.R) and by the American Museum of Natural History.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203238109/-/DCSupplemental.
References
- 1.Wellnhofer P. 2008. Archaeopteryx. Der Urvogel von Solnhofen [Archaeopteryx. The primary bird from Solnhofen] (Dr. Friedrich Pfeil, Munich). [in German]
- 2.Xu X, Guo Y. The origin and early evolution of feathers: insights from recent paleontological and neontological data. Vert PalAs. 2009;47:311–329. [Google Scholar]
- 3.Mayr G, Peters DS, Plodowski G, Vogel O. Bristle-like integumentary structures at the tail of the horned dinosaur Psittacosaurus. Naturwissenschaften. 2002;89:361–365. doi: 10.1007/s00114-002-0339-6. [DOI] [PubMed] [Google Scholar]
- 4.Zheng XT, You HL, Xu X, Dong ZM. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature. 2009;458:333–336. doi: 10.1038/nature07856. [DOI] [PubMed] [Google Scholar]
- 5.Unwin DM. The Pterosaurs from Deep Time. New York: Pi; 2005. [Google Scholar]
- 6.Zeiss A. Jurassic stratigraphy of Franconia. Stuttg Beitr Naturkd, B. 1977;31:1–32. [Google Scholar]
- 7.Link E, Fürsich FT. High resolution stratigraphy and microfacies analysis of the Upper Jurassic limestones of Painten, southern Franconian Alb. Archaeopteryx. 2001;19:71–88. German. [Google Scholar]
- 8.Schweigert G. Ammonite biostratigraphy as a tool for dating Upper Jurassic lithographic limestones from South Germany - first results and open questions. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2007;245:117–125. [Google Scholar]
- 9.Benson RBJ. A redescription of 'Megalosaurus' hesperis (Dinosauria, Theropoda) from the Inferior Oolite (Bajocian, Middle Jurassic) of Dorset, United Kingdom. Zootaxa. 2008;1931:57–67. [Google Scholar]
- 10.Benson RBJ. A description of Megalosaurus bucklandii (Dinosauria: Theropoda) from the Bathonian of the UK and the relationships of Middle Jurassic theropods. Zool J Linn Soc. 2010;158:882–935. [Google Scholar]
- 11.Rauhut OWM. The interrelationships and evolution of basal theropod dinosaurs. Spec Pap Palaeont. 2003;69:1–213. [Google Scholar]
- 12.Britt BB. Theropods of Dry Mesa Quarry (Morrison Formation, Late Jurassic), Colorado, with emphasis on the osteology of Torvosaurus tanneri. BYU Geol Stud. 1991;37:1–72. [Google Scholar]
- 13.Dal Sasso C, Maganuco S. Scipionyx samniticus (Theropoda: Compsognathidae) from the Lower Cretaceous of Italy. Mem Soc It Sci Nat Museo Civ Stor Nat Milano. 2011;37:1–281. [Google Scholar]
- 14.Currie PJ, Chen P. Anatomy of Sinosauropteryx prima from Liaoning, northeastern China. Can J Earth Sci. 2001;38:1705–1727. [Google Scholar]
- 15.Chiappe LM, Göhlich UB. Anatomy of Juravenator starki (Theropoda: Coelurosauria) from the Late Jurassic of Germany. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen. 2010;258:257–296. [Google Scholar]
- 16.Foth C. On the identification of feather structures in stem-line representatives of birds: Evidence from fossils and actuopalaeontology. Paläontologische Zeitschrift. 2012;86:91–102. [Google Scholar]
- 17.Benson RBJ, Carrano MT, Brusatte SL. A new clade of archaic large-bodied predatory dinosaurs (Theropoda: Allosauroidea) that survived to the latest Mesozoic. Naturwissenschaften. 2010;97:71–78. doi: 10.1007/s00114-009-0614-x. [DOI] [PubMed] [Google Scholar]
- 18.Currie PJ, Zhao X. A new carnosaur (Dinosauria, Theropoda) from the Jurassic of Xinjiang, People’s Republic of China. Can J Earth Sci. 1994;30:2037–2081. [Google Scholar]
- 19.Calvo JO, Porfiri JD, Veralli C, Novas FE, Poblete F. Phylogenetic status of Megaraptor namunhuaiquii Novas based on a new specimen from Neuquén, Patagonia, Argentina. Ameghiniana. 2004;41:565–575. [Google Scholar]
- 20.Xu X, et al. A basal tyrannosauroid dinosaur from the Late Jurassic of China. Nature. 2006;439:715–718. doi: 10.1038/nature04511. [DOI] [PubMed] [Google Scholar]
- 21.Gilmore GW. Osteology of the carnivorous dinosauria in the United States National Museum, with special reference to the genera Antrodemus (Allosaurus) and Ceratosaurus. B US Nat Mus. 1920;110:1–159. [Google Scholar]
- 22.Currie PJ, Carpenter K. A new specimen of Acrocanthosaurus atokensis (Theropoda, Dinosauria) from the Lower Cretaceous Antlers Formation (Lower Cretaceous, Aptian) of Oklahoma, USA. Geodiversitas. 2000;22:207–246. [Google Scholar]
- 23.Brochu CA. Closure of neurocentral sutures during crocodilian ontogeny: implications for maturity assessment in fossil archosaurs. J Vert Paleont. 1996;16:49–62. [Google Scholar]
- 24.Tumarkin-Deratzian AR, Vann DR, Dodson P. Bone surface texture as an ontogenetic indicator in long bones of the Canada goose Branta canadensis (Anseriformes: Anatidae) Zool J Linn Soc. 2006;148:133–168. [Google Scholar]
- 25.Dal Sasso C, Signore M. Exceptional soft-tissue preservation in a theropod dinosaur from Italy. Nature. 1998;392:383–387. [Google Scholar]
- 26.Bever GS, Norell MA. The perinate skull of Byronosaurus (Troodontidae) with observations on the cranial ontogeny of paravian theropods. American Museum Novitates. 2009;3657:1–51. [Google Scholar]
- 27.Farlow JO. Speculations about the diet and foraging behavior of large carnivorous dinosaurs. Am Midl Nat. 1976;95:186–191. [Google Scholar]
- 28.Stromer E. The teeth of Compsognathus and comments on the dentition of Theropoda. Cb Min Geol Palaeont B. 1934;1934:74–85. German. [Google Scholar]
- 29.Norell MA, et al. A new dromaeosaurid theropod from Ukhaa Tolgod (Ömnögov, Mongolia) Am Mus Novit. 2006;3545:1–51. [Google Scholar]
- 30.Peyer K. A reconsideration of Compsognathus from the Upper Tithonian of Canjuers, southeastern France. J Vert Paleont. 2006;26:879–896. [Google Scholar]
- 31.Xu X, Wu X. Cranial morphology of Sinornithosaurus millenii Xu et al. 1999 (Dinosauria: Theropoda: Dromaeosauridae) from the Yixian Formation of Liaoning, China. Can J Earth Sci. 2001;38:1739–1752. [Google Scholar]
- 32.Maganuco S, Cau A, Pasini G. First description of theropod remains from the Middle Jurassic (Bathonian) of Madagascar. Atti Soc It Sci Nat Museo Civ Stor Nat Milano. 2005;146:165–202. [Google Scholar]
- 33.van der Lubbe T, Richter U, Knötschke N. Velociraptorine dromaeosaurid teeth from the Kimmeridgian (Late Jurassic) of Germany. Acta Palaeontol Pol. 2009;54:401–408. [Google Scholar]
- 34.Knoll F, Ruiz-Omeñaca JI. Theropod teeth from the basalmost Cretaceous of Anoual (Morocco) and their palaeobiogeographical significance. Geol Mag. 2009;146:602–616. [Google Scholar]
- 35.Göhlich UB, Chiappe LM. A new carnivorous dinosaur from the Late Jurassic Solnhofen archipelago. Nature. 2006;440:329–332. doi: 10.1038/nature04579. [DOI] [PubMed] [Google Scholar]
- 36.Butler RJ, Upchurch P. Highly incomplete taxa and the phylogenetic relationships of the theropod dinosaur Juravenator starki. J Vert Paleont. 2007;27:253–256. [Google Scholar]
- 37.Erickson GM, et al. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature. 2004;430:772–775. doi: 10.1038/nature02699. [DOI] [PubMed] [Google Scholar]
- 38.Carr TD. Craniofacial ontogeny in Tyrannosauridae (Dinosauria, Coelurosauria) J Vert Paleont. 1999;19:497–520. [Google Scholar]
- 39.Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- 40.Norell MA, Xu X. Feathered dinosaurs. Annu Rev Earth Planet Sci. 2005;33:277–299. [Google Scholar]
- 41.Glut DF. Dinosaurs. The Encyclopedia. Supplement 3. NC: Mcfarland & Co, Jefferson; 2003. [Google Scholar]
- 42.Brusatte SL, et al. The origin and early radiation of dinosaurs. Earth Sci Rev. 2010;101:68–100. [Google Scholar]
- 43.Lucas AM, Stettenheim PR. Avian Anatomy. Integument, Part I & II. Washington: US Department of Agriculture; 1972. [Google Scholar]
- 44.Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zoolog B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- 45.Alibardi L, Toni M. Cytochemical and molecular characteristics of the process of cornification during feather morphogenesis. Prog Histochem Cytochem. 2008;43:1–69. doi: 10.1016/j.proghi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Feduccia A, Lingham-Soliar T, Hinchliffe JR. Do feathered dinosaurs exist? Testing the hypothesis on neontological and paleontological evidence. J Morphol. 2005;266:125–166. doi: 10.1002/jmor.10382. [DOI] [PubMed] [Google Scholar]
- 47.Lingham-Soliar T, Feduccia A, Wang X. A new Chinese specimen indicates that ‘protofeathers’ in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres. Proc Biol Sci. 2007;274:1823–1829. doi: 10.1098/rspb.2007.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.