Abstract
Purpose.
Clinical trials in glaucoma have often sought to predict whether a patient will progress or remain stable. This study proposes to combine and support results from earlier studies, forming a model to predict the actual rate of functional change in glaucoma.
Methods.
Data were taken from 259 eyes of 150 participants with early or suspected glaucoma in the ongoing Portland Progression Project. A total of 3854 study visits were available, each consisting of visual acuity, confocal scanning laser ophthalmoscopy (CSLO), and perimetry. The rate of functional change was calculated over each of 1541 series of six consecutive visits. Mixed effects models were formed to predict these rates using baseline perimetric measurements and CSLO parameters, together with IOP, age, and change in visual acuity through the series (to remove any confound from media changes).
Results.
Cup volume from CSLO was predictive of subsequent rate of functional change (P = 0.036), together with baseline mean deviation (P < 0.001) and pattern standard deviation (P = 0.097), age (P = 0.013), maximum IOP during the sequence (P = 0.004), and change in acuity during the sequence (P = 0.022). In a similar model, rim area was less predictive of functional change (P = 0.066).
Conclusions.
A larger optic cup and/or a more damaged visual field are predictive of more rapid perimetric sensitivity loss. The structural parameters most closely correlated with current functional status may not be the parameters that are most useful for predicting the future course of a patient's disease. Maximum IOP may be a more important risk factor than mean IOP over the same time period.
In glaucoma, larger optic cup size, greater current functional damage, and greater maximum IOP during follow-up are all predictive of a more rapid rate of functional change. The structural parameters most closely correlated with current functional status may not be the parameters that are most useful for predicting the future course of a patient's disease.
Introduction
Whether the vision of a patient with glaucoma will deteriorate rapidly or remain relatively stable is one of the most important questions in glaucoma management.1 However, predicting the rate of glaucomatous progression remains challenging. The course of the disease over time varies among patients and is disguised by considerable test variability.2–5 For patients, their hope in having their glaucoma managed is to prevent loss of visual function and its resultant impact on their quality of life and activities of daily living.6,7 Therefore, although structural testing plays a role in diagnosing and monitoring glaucoma, predicting the rate of functional change remains a high priority. Standard automated perimetry (SAP) is widely used to aid in this objective.8 The rate of future progression has been reported to be affected by various factors, including IOP,1,9–13 age,9–12,14 and ethnicity.11,14,15 Some of these findings have been disputed and remain controversial.1,12,15,16
In some patients, structural damage may be detected before functional loss with currently available tools17 and so may be predictive of future functional change, even though for other patients functional damage may be detected first.18–21 Measurements of structural parameters are generally better tolerated by patients than functional testing,22 making their use appealing. Confocal scanning laser ophthalmoscopy (CSLO) is one such test. The CSLO Ancillary Study to the Ocular Hypertension Treatment Study (OHTS) found that a decreased neuroretinal rim area, among other CSLO parameters, was predictive of reaching a primary open angle glaucoma (POAG) endpoint.23 However, endpoint-based analyses are dependent on the exact endpoint used, and provide limited information about the rate at which functional progression may occur. We therefore sought to determine the utility of CSLO parameters to predict both the current functional status and the rate of subsequent functional change. We have previously shown that the CSLO parameters most predictive of subsequent change may not be the same parameters most closely related to current status.24 In that study, we showed that while rim area was well correlated with the current mean deviation (MD) from SAP, cup volume and disc area were better correlated with the subsequent rate of change of MD.
It may also be expected that patients whose disease has already progressed to a stage at which functional loss is clinically detectable would be more likely to progress more rapidly than patients yet to develop any functional loss. However, estimates of the predictive value of initial perimetric testing vary. MD at baseline was reported to be predictive of the probability of subsequent progression in the Early Manifest Glaucoma Treatment trial (EMGT),9 but not in the OHTS11 or the Collaborative Normal-Tension Glaucoma Study.15 Baseline pattern standard deviation (PSD) was a significant predictor in OHTS.11 In the Advanced Glaucoma Intervention Study,9 baseline visual field status (field score) was not predictive of future progression. Each of these studies sought to classify subjects on a binary basis as “glaucoma/normal” or as “stable/progressing.”25 We have previously shown that both initial MD and initial PSD may be predictive of the rate of subsequent change in sensitivity, with MD providing significantly better predictability than PSD.26
Elevated IOP is a known risk factor for glaucomatous progression.1,9,27,28 Results for the predictive value of IOP fluctuation vary between studies.10,13,16,29–32 Progression has also been reported to be associated with the peak IOP33 and the range of IOP33,34 observed within the study period. We have previously shown that the maximum IOP observed may be more predictive of the rate of change than either mean IOP or IOP variability, based on an animal model of glaucoma.35
In this study, we proposed to validate those findings with a large longitudinal dataset and combine them into a more complete predictive model of the rate of glaucomatous progression. A random effects model was constructed using the entire sequence of available test results for each participant. Further modeling was conducted to predict the current functional status of participants, motivated by our previous results suggesting that the factors most related to current functional status may differ from the factors most predictive of the rate of subsequent change, with important consequences for the interpretation of cross-sectional and longitudinal studies.24 The overall goal of this work was to aid clinicians in assigning appropriate management strategies based on the best possible predictions of a patient's prognosis.
Methods
Data for this study were obtained from the Portland Progression Project, an ongoing longitudinal study of progression in participants with early and suspected glaucoma or risk factors for development of glaucoma, at Devers Eye Institute in Portland, Oregon. The protocol was approved by the Legacy Health Institutional Review Board. The study adheres to the tenets of the Declaration of Helsinki and complies with the Health Insurance Portability and Accountability Act of 1996. All participants provided written informed consent, after having the risks and benefits of participation explained to them.
Initially, participants were tested annually with a variety of structural and functional tests. Testing has since switched to 6-month intervals. Overall, the mean inter-visit interval was 331 days (median 357 days). At study entry, participants either had early glaucoma with visual field loss less severe than −6 decibels (dB) for MD or ocular hypertension (untreated IOP greater than 22 mm Hg) plus one or more risk factors for glaucoma as determined by their clinician (e.g., age >70, systemic hypertension, migraine, diet-controlled diabetes, peripheral vasospasm, African ancestry, or self-reported family history of glaucoma) and/or previously diagnosed glaucomatous optic neuropathy or suspicious optic nerve head appearance (cup–disc ratio asymmetry >0.2, neuroretinal rim notching or narrowing, disc hemorrhage).36,37 Participants with other diseases or medications likely to affect the visual field or those who had undergone ocular surgery (except for uncomplicated cataract surgery) were excluded. In addition, participants with visual acuity worse than 20/40 in either eye or with SAP MD worse than −6 dB at study entry were excluded to ensure that participants had no worse than mild glaucoma and only insignificant media change or cataract.
SAP visual field testing was performed using a Humphrey Field Analyzer II (HFA; Carl Zeiss Meditec Inc., Dublin, CA), employing the 24-2 testing pattern and conventional test procedures.8 The SITA standard algorithm38 was used for all testing. An optimal lens correction was placed before the tested eye, and the fellow eye was occluded with a translucent eye patch. All participants had previous experience with visual field testing prior to entering the study, and most had undergone multiple previous tests. CSLO optic nerve head scanning was performed using a Heidelberg Retina Tomograph Classic (HRT; Heidelberg Engineering, GmbH, Heidelberg, Germany) following standard operating procedures.39 IOP was measured using Goldmann Applanation Tonometry (Haag-Streit, Bern, Switzerland) with the participant seated at a slit lamp. The ages of the participants and their treatment status (whether or not they reported that they were taking IOP-reducing medication) were recorded, together with visual acuity.
The rate of change of MD (MDR) over each possible sequence of six consecutive visual field tests was calculated using linear regression of MD against test date. This resulted in multiple measures of MDR for some participants, based on overlapping windows of six consecutive visits (the highest number of visits per patient was 13, giving eight measures of MDR for each eye of those participants). For each different CSLO parameter, mixed effects models were constructed to predict MDR based on:
The value of the CSLO parameter at the first visit in the sequence
The MD from the first visual field test in the sequence
The PSD from the first visual field test in the sequence
The age of the participant at the start of the sequence
The mean IOP over the six visits
The maximum IOP recorded over the six visits
The rate of change of visual acuity over the six visits, in logMAR-equivalent units per year (included as a predictor to remove any effect of developing cataract on the MDR)
Because the mean and maximum IOP are highly correlated, only one or the other was included in the model. The intercept term was modeled as a Gaussian-distributed random effect in a hierarchical structure with two levels of nesting, to account for multiple overlapping sequences per eye and two eyes per participant. To account for the time series nature of the data, a first-order autoregressive correlation structure was used. Coefficient estimates from mixed effects models have been shown to be robust in the face of small sample sizes per cluster, as in this dataset, which had no more than two eyes within each “cluster” for an individual participant.40 To assess the suitability of this linear model, reduced models were formed to predict MDR based on all but one of the predictors, and residuals from these models were plotted against the values of the remaining predictor. These plots confirmed that no substantial nonlinearities existed for any of the predictors, and that the residuals in each case were approximately Gaussian. Predictive models were compared using Akaike's Information Criterion (AIC) to assess goodness-of-fit, assuming (2n − 1) degrees of freedom, where n = 6 was the number of predictors used in the model.
In order to determine the relation between CSLO parameters and the current status of the visual field, further mixed effects models were constructed to predict the MD based on each CSLO parameter in turn (with no other predictors included in the model), using the same correlation structure as above.
Results
A total of 3854 eligible visits were available for analysis, resulting in 1541 series of six consecutive visits that could be used to calculate MDR, from 259 eyes of 150 participants. Table 1 shows the characteristics of the included participants at their first included study visit. Forty-six percent of eyes did not receive IOP-lowering treatment during the study, 38% were treated throughout the study, and the remainder had at least one change in treatment status.
Table 1.
Baseline Characteristics of the Participants in the Study
|
|
Mean |
SD |
Range |
| Age (y) | 61.1 | 11.8 | 30.8–87.0 |
| Visual acuity (logMAR) | −0.007 | 0.083 | −0.10–0.50 |
| Mean deviation (dB) | −1.51 | 2.04 | −8.75–4.82 |
| Pattern standard deviation (dB) | 2.18 | 1.87 | 0.89–14.59 |
| Visual field index (%) | 98.6 | 4.22 | 64.5–100.0 |
| Rim area (mm2) | 1.51 | 0.35 | 0.78–2.57 |
| Cup volume (mm3) | 0.193 | 0.189 | 0.000–1.155 |
| Cup–disc area ratio | 0.306 | 0.156 | 0.00–0.65 |
| No. of eligible visits | 7.6 | 2.9 | 5–13 |
Table 2 shows the goodness-of-fit (as assessed using AIC) for models using each of the three different CSLO parameters, and either mean or maximum IOP, to predict MDR. The AIC represents the relative amount of information lost by using fitted values from a given model. Therefore, a lower AIC indicates a better fit to the data, and the difference between them can be interpreted as a relative probability of being an ideal fit. For example, asymptotically, the model using mean IOP and cup volume can be considered as being e(−148.1–286.0)/2 times as likely as the model using maximum IOP and cup volume to represent the perfect fit (i.e., the fit that minimizes the amount of information loss). Interpretation of the AIC remains controversial when applied to mixed effects models.41 However, these results suggest that the model using cup volume and maximum IOP may provide the best predictions of MDR.
Table 2.
Goodness-of-Fit of Different Candidate Models to Predict the Rate of Change of Mean Deviation
|
CSLO Parameter Used |
Rim Area |
Cup Volume |
Cup–Disc Ratio |
| AIC using mean IOP | −140.2 | 286.0 | 284.5 |
| AIC using maximum IOP | −95.5 | −148.1 | 269.5 |
Each cell contains the value of the AIC for that model. A lower AIC generally indicates a better fit to the data.
Table 3 shows the results of fitting a mixed effects model to predict MDR using each of three different CSLO parameters. The results strongly suggest that larger cup volume as measured by CSLO is predictive of more rapid subsequent functional deterioration. However, rim area was not found to be significant as a predictor of MDR. Indeed, the Pearson correlation between cup volume and MDR was −0.22, significantly stronger than the correlation between MDR and rim area (r = 0.18, comparison between correlations has P < 0.001 according to the Z2* statistic of Steiger42). A greater MD (better visual field status) at the start of the sequence was predictive of a more rapid MDR. This is an artefact caused by this first visit being part of the sequence used to calculate MDR. If the observed MD at this first visit is higher than the true MD, the MDR will be biased to being more negative. Therefore, this coefficient can be thought of as a “correction” for this source of bias. It should not be taken as evidence that a more severely damaged eye is less likely to progress rapidly. Our analysis cannot assess the predictive value of initial MD.26 Indeed, a worse (numerically greater) PSD at the start of the sequence was predictive of more rapid functional deterioration, although this did not always reach statistical significance. Greater age resulted in significantly more rapid deterioration, despite the fact that the rate of change was based on an age-corrected metric (MD). A greater maximum IOP measurement over the sequence was predictive of more rapid functional deterioration. When maximum IOP was replaced in the model by the mean IOP over the sequence, the fits generally did not improve, as was seen in Table 2. Indeed, when using cup volume as the CSLO parameter, maximum IOP was a significant predictor (P = 0.001, see Table 3), yet mean IOP in an equivalent model had P = 0.763. Decreasing visual acuity was also associated with decreasing MD, consistent with the expected effect of early cataract development on global visual field status. However, the fact that other predictors remained significant in the model indicates that not all of the change in MD can be explained by developing cataract.
Table 3.
Results of Fitting a Mixed Effects Model to the Data
|
CSLO Parameter Used: |
Rim Area |
Cup Volume |
Cup-Disc Ratio |
||||||
|
Coefficient |
SE |
P |
Coefficient |
SE |
P |
Coefficient |
SE |
P |
|
| CSLO parameter | 0.010 | 0.026 | 0.066 | −0.129 | 0.061 | 0.036 | −0.262 | 0.103 | 0.011 |
| MD at start of sequence | −0.073 | 0.006 | <0.001 | −0.081 | 0.006 | <0.001 | −0.111 | 0.008 | <0.001 |
| PSD at start of sequence | −0.013 | 0.008 | 0.093 | −0.013 | 0.008 | 0.097 | −0.076 | 0.010 | <0.001 |
| Age at start of sequence | −0.007 | 0.002 | 0.001 | −0.006 | 0.002 | 0.013 | −0.005 | 0.002 | 0.029 |
| Maximum IOP in sequence | −0.013 | 0.004 | 0.001 | −0.013 | 0.004 | 0.001 | −0.016 | 0.004 | <0.001 |
| Rate of change in visual acuity | −308 | 134 | 0.021 | −299 | 129 | 0.022 | −220 | 120 | 0.066 |
Each model uses one CSLO parameter (as indicated at the top of each column) together with other predictors to predict the rate of change of mean deviation (MD) over sequences of six tests, as described in the methods section. For each fixed effect included in the model, the fitted coefficient is shown together with its associated standard error (SE) and P value.
The HFA perimeter now produces a visual field index (VFI), which was designed to separate localized loss from generalized loss of sensitivity, on the assumption that the latter may not be caused by glaucoma. When the rate of change in VFI was used instead of MDR in models equivalent to those presented here, the rate of change of acuity was no longer a significant predictor (note that the CSLO parameters identified above were all still significant predictors of the rate of change of VFI in these models).
Table 4 shows the fitted regression coefficient and associated P value for all CSLO parameters considered (equivalent to and including the results in the row entitled “CSLO Parameter” in Table 3), together with the resultant AIC values summarizing the goodness-of-fit. As well as cup volume, other parameters related to the cup size (cup area, cup–disc ratio) as output by the HRT were predictive of the rate of functional change, with P < 0.05. However, cup volume provided the lowest AIC, indicating that the resultant model fit the data better. It can therefore be concluded that cup volume (and, to a lesser extent, rim area) provided information that was not available from the other predictors in the model, whereas the other parameters provided no additional information.
Table 4.
Significance of CSLO Parameters Predicting MDR
|
CSLO Parameter |
Coefficient |
SE |
P |
AIC |
| Disc area (mm2) | −0.023 | 0.035 | 0.518 | 277.4 |
| Rim area (mm2) | 0.010 | 0.026 | 0.066 | −95.5 |
| Cup area (mm2) | −0.092 | 0.039 | 0.017 | 272.2 |
| Cup–disc ratio | −0.262 | 0.103 | 0.011 | 269.5 |
| Rim volume (mm3) | 0.169 | 0.090 | 0.061 | 272.6 |
| Cup volume (mm3) | −0.129 | 0.061 | 0.036 | −148.1 |
| Mean cup depth (mm) | −0.006 | 0.013 | 0.636 | 279.5 |
| Max cup depth (mm) | −0.039 | 0.055 | 0.486 | 276.4 |
| RNFL thickness (mm) | 0.037 | 0.028 | 0.189 | 276.5 |
| RNFL cross-sectional area (mm2) | 0.038 | 0.040 | 0.337 | 276.6 |
Significance of each CSLO parameter in a mixed effects model predicting MDR that also incorporates other predictors as in the methods section and Table 3. For each parameter, the fitted coefficient is shown together with its associated standard error (SE) and P value, and AIC summarizing the goodness-of-fit.
Table 5 shows the results of using mixed effects models to predict the current functional status (MD) based on CSLO, in the same format as Table 4. In addition to the parameters that were found to be predictive of future functional change, the maximum cup depth was also predictive of current MD. Other parameters provided little information about the current state of the visual field in this dataset. Notably, a thinner rim was associated both with worse current function and worse subsequent rate of change, but this did not attain significance in either case.
Table 5.
Significance of CSLO Parameters Predicting Current MD
|
CSLO Parameter |
Coefficient |
SE |
P |
AIC |
| Disc area (mm2) | −0.148 | 0.120 | 0.216 | 5236 |
| Rim area (mm2) | 0.245 | 0.130 | 0.059 | 5234 |
| Cup area (mm2) | −0.485 | 0.139 | <0.001 | 5225 |
| Cup–disc ratio | −1.091 | 0.363 | 0.003 | 5226 |
| Rim volume (mm3) | 0.157 | 0.303 | 0.605 | 5235 |
| Cup volume (mm3) | −1.146 | 0.332 | 0.001 | 5223 |
| Mean cup depth (mm) | −0.003 | 0.052 | 0.947 | 5239 |
| Max cup depth (mm) | −0.689 | 0.181 | <0.001 | 5222 |
| RNFL thickness (mm) | −0.101 | 0.094 | 0.282 | 5237 |
| RNFL cross-sectional area (mm2) | −0.193 | 0.130 | 0.138 | 5235 |
Significance of each CSLO parameter in a mixed effects model predicting MD at the same visit. For each parameter, the fitted coefficient is shown together with its associated standard error (SE) and P value, and AIC summarizing the goodness-of-fit.
Discussion
It is not yet possible to accurately predict the future functional course that an individual patient with glaucoma will follow. There is too much variability among different patients. There is also too much test–retest variability in the current testing techniques (both structural and functional), some of which is due to limitations of the testing modalities, some of which may reflect true day-to-day variation, and some of which may be a results of the pathophysiology of the glaucomatous disease process.2–5 However, studies such as this can illuminate the risk factors for an increased rate of functional deterioration. Cross-validation of the results is hampered by the need for large datasets when identifying risk factors for a slowly and variably progressing disease. However, by looking at the results of multiple studies, the conclusions can be validated in a manner that is robust to differences in the study populations and protocols. This information could help clinicians to identify which patients are at highest risk of undergoing rapid functional loss, allowing earlier and more appropriate individualized management strategies to be implemented. Such high-risk patients should be followed more closely, in particular by conducting more frequent testing, even if they are apparently stable, to determine whether they need aggressive treatment or surgical intervention.
One of the main goals of this study was to find structural features that may be predictive of subsequent rapid functional deterioration. To accomplish this, CSLO parameters were considered individually, and the predictive value of each when combined with other predictors in a mixed effects model was assessed. Clinically, it is unlikely that one parameter alone would be considered when making management decisions. However, including multiple CSLO parameters in this analysis could cause useful information to be obscured by interactions among the different (correlated) parameters. This study elucidates the CSLO parameters that are independently predictive of functional change, after accounting for other non-CSLO sources of information. Notably, the CSLO parameters that were found to be good predictors of change could all be assessed by other means. A large cup is generally evident on stereophotographs of the optic nerve head, despite problems caused by the subjective nature of photo assessment, and should also be evident from radial or cube scans using optical coherence tomography.
Of the CSLO parameters output by the HRT, those related to the cup size (volume, area, and cup–disc ratio) were found to be predictive of more rapid functional change. This agrees with the broad findings of the OHTS CSLO ancillary study.23 Maximum cup depth was predictive of current functional status but was not predictive of the rate of subsequent change. One possible explanation for this finding would be a time lag between changes to different aspects of the optic nerve head morphology. For example, our findings would be consistent with a model wherein the cup enlarges early in the disease process (e.g., by expansion of the neural canal opening), followed by posterior displacement of the lamina cribrosa. This study cannot definitively show such a time lag, much less substantiate the causality of any such relation. However, it supports the principle that a measure that corresponds well with current functional status may not be the best measure for predicting progression.
As pointed out in the Results section, the fact that a greater MD (indicating less damage to the visual field) at the start of the sequence was predictive of a more rapid MDR is an anomalous artefact. When the first value in a sequence is higher, the least-square estimate of the slope that includes it will be more negative. Initial MD was included in the model specifically to correct for this bias. More notable is that a greater PSD (indicating more damage to the visual field) was predictive of a more rapid MDR. This is consistent with the hypothesis that a more severely damaged eye is likely to subsequently progress more rapidly. Although PSD does not increase monotonically with increasing damage later in the disease process (when MD is worse than around −15 dB), it does so at the early levels of damage observed in this study and encountered in the OHTS. The OHTS reported that a greater baseline PSD was predictive of an increased probability of conversion from ocular hypertension to POAG.27 We have previously shown that PSD is predictive of MDR26 and also that MD may in fact be an even better predictor than PSD. The latter finding cannot be verified using this analysis since the correlation between MD and MDR produces an insurmountable confound that hides any predictability that could be gained from using MD. However, we still believe that if this bias was not present, for example by using separate visual field tests from the same day to define baseline MD and the start of the sequence used to calculate MDR, a more negative MD would indeed be predictive of a worse MDR.
The maximum IOP observed during the sequence of tests was found to be predictive of MDR. High IOP was associated with an increased rate of functional deterioration, consistent with the results of clinical trials such as the OHTS11 and the EMGT.9 However, mean IOP within the sequence was not found to be significantly predictive, even though the two measures are highly correlated (r = 0.86, P < 0.001). Determining the manner in which increased IOP causes glaucomatous damage is hampered by the relative sparseness of the data, with IOP being measured only once per year or once per 6 months depending on whether it was collected early or late in the study. Such questions might only be definitively answered by the use of temporally rich IOP data such as provided by telemetry.43 However, our results agree with those from a primate model of glaucoma using far more frequent testing, whereby the maximum IOP was more predictive of the rate of progression than the mean IOP within the same time window.35 Even with those notable caveats, our results lend qualified support to the hypothesis that relatively short-term elevation of IOP to only moderate levels (less than 1-year duration) may be sufficient to drive more rapid visual field deterioration in glaucoma, without longer-term chronic IOP elevation.
Treatment status was not included as a predictor in the model. Treatment would be expected to be associated with an improvement in a participant's MDR. However, this is confounded by the fact that participants with more severe MDR are more likely to be prescribed IOP-lowering medication by their physician, causing a correlation between treatment and MDR. Including treatment status (defined as the proportion of the six visits at which the participant reported that they were taking IOP-lowering medication) results in a significantly positive regression coefficient (P < 0.001 in all models), even though this is an artefactual confound that is not informative and does not aid in predicting a patient's progression rate. Moreover, compliance with glaucoma medications has been reported to be relatively poor, and so it cannot be assumed that a participant is taking medication purely because it has been prescribed.44 The model presented here therefore implicitly assumes that the only effect of treatment is to lower IOP and that any beneficial effect on the progression rate will be captured by the effect of lower IOP on MDR.
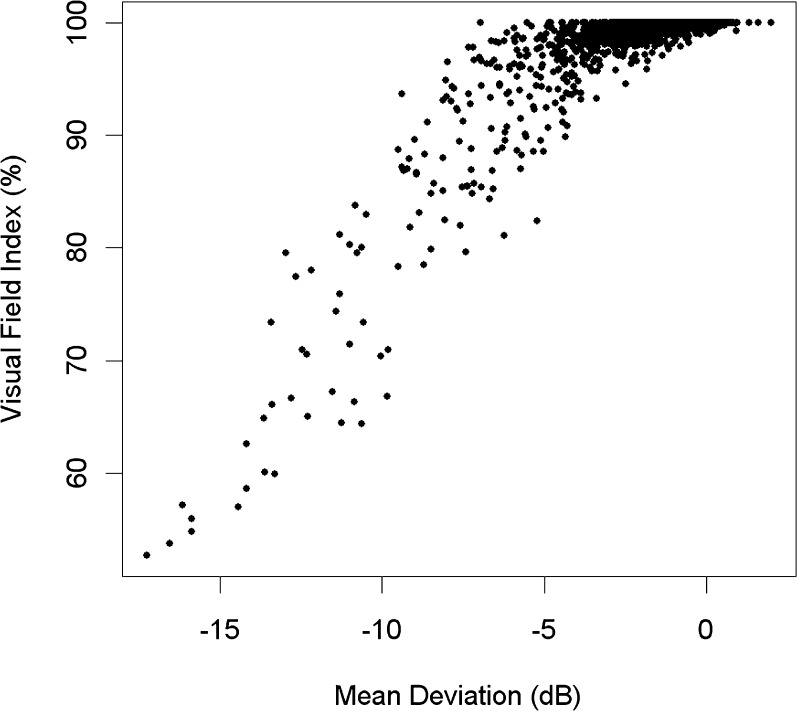
Rate of change of visual acuity was included in the model not because it is causally predictive of glaucomatous progression, but because developing cataract may be responsible for worsening MD in glaucoma patients. The inclusion of this term aims to remove this potential confound, enabling the effect of the other predictors to be better ascertained. This is probably an overly conservative correction for this confound since purely generalized loss and/or focal localized loss due to glaucoma may also reduce visual acuity, giving a relation with causality in both directions. Focal loss near the fovea can occur in glaucoma45 and could affect visual acuity. Generalized loss of sensitivity has been shown to be present early in the glaucomatous process.46–49 The HFA perimeter now produces the VFI, aiming to remove any effect of developing cataract from the assessment of glaucomatous functional status.50 Indeed, when the rate of change in VFI was used instead of MDR in models equivalent to those presented here, the rate of change of visual acuity was no longer a significant predictor. However, the VFI fails to detect generalized loss that is truly caused by glaucoma.51 Moreover, the VFI is unsuitable for assessment of patients with high-risk ocular hypertension or early glaucoma. It assumes that each field location has 100% of normal vision until it has progressed beyond the 5th percentile on the pattern deviation, and so even eyes with significant glaucomatous damage can have a VFI supposedly indicating 100% of normal vision (note that the VFI is reported on a percentage scale under the assumption that sensitivity declines linearly to 0 dB = 0% of normal vision, but there are relatively few field locations that have progressed to that extent in this dataset). This results in a “ceiling effect,” as seen in Figure 1. Due to the inability of VFI to detect visual field damage or change in very early glaucoma, it is preferable both for studies such as this and in clinical situations to instead use MDR while adjusting for any change in visual acuity when assessing functional progression.
Figure 1.
The relation between mean deviation and the visual field index (VFI) in the dataset, showing the “ceiling effect” that occurs when VFI = 100% even in the presence of early glaucomatous damage.
While mixed effects models provide an effective statistical technique for fitting clustered data such as these, they also present substantial challenges. There is no direct equivalent of the R2 statistic available that can be interpreted as saying that a model explains a certain proportion of the variability. Not least, this is because of confusion over whether the random effects (in this case, within-individual variability) should be included in this calculation. The number of degrees of freedom that should be used is also controversial. Therefore it is not possible to say that one model produces a significantly better fit than another because the underlying distributions remain undefined. This is an area of ongoing research in the statistical literature, now that advances in computational power have made mixed effects modeling more commonly used.41 However, the AIC can be compared between two candidate models to determine which fits the data better, provided that the outcome measure (in this case MDR) is the same and that the number of parameters being fit is the same (so that the degrees of freedom are the same in both cases, and so our choice to assume 2n − 1 degrees of freedom becomes irrelevant). It should also be noted that no adjustments were made for multiple comparisons despite several candidate models being considered. While formal “corrections” such as Bonferroni tend to be overly conservative, caution should be taken with the P values that only just reach conventional statistical significance.
Mixed effects linear models provide a useful method for extracting information concerning the risk factors for more rapid functional progression in early glaucoma. The results suggest that a larger optic cup and/or a more damaged visual field are predictive of more rapid sensitivity loss, that maximum IOP may be a more important risk factor than average IOP, and that adjusting for visual acuity could be an appropriate strategy to remove potential confounds due to developing cataract. Perhaps most importantly, the findings suggest that the structural parameters most closely correlated with current functional status may not be the parameters that are most useful for predicting the future course of a patient's disease.
Footnotes
Supported in part by National Institutes of Health Grants NEI R01-EY-03424 (CAJ) and NEI R01-EY-19674 (SD).
Disclosure: S.K. Gardiner, None; C.A. Johnson, None; S. Demirel, None
References
- 1.Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: The Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heijl A, Lindgren G, Olsson J. Test retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989;108:130–135 [DOI] [PubMed] [Google Scholar]
- 3.Piltz J, Starita R. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1990;109:109–110 [PubMed] [Google Scholar]
- 4.Henson D, Chaudry S, Artes P, Faragher E, Ansons A. Response variability in the visual field: comparison of optic neuritis, glaucoma, ocular hypertension, and normal eyes. Invest Ophthalmol Vis Sci. 2000;41:417–421 [PubMed] [Google Scholar]
- 5.Spry P, Johnson C, McKendrick A, Turpin A. Variability components of standard automated perimetry and frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2001;42:1404–1410 [PubMed] [Google Scholar]
- 6.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP. Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKean-Cowdin R, Wang Y, Wu J, Azen SP, Varma R. Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: The Los Angeles Latino Eye Study. Ophthalmology. 2008;115:941–948.e941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson D, Patella V.Automated Static Perimetry. 2 ed. St. Louis, MO: Mosby; 1999:147–159 [Google Scholar]
- 9.Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56 [DOI] [PubMed] [Google Scholar]
- 10.Nouri-Mahdavi K, Hoffman D, Coleman A, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635 [DOI] [PubMed] [Google Scholar]
- 11.Gordon M, Beiser J, Brandt J, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720 [DOI] [PubMed] [Google Scholar]
- 12.Friedman D, Wilson M, Liebmann J, Fechtner R, Weinreb R. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138:19–31 [DOI] [PubMed] [Google Scholar]
- 13.Hong S, Seong G, Hong Y. Long-term intraocular pressure fluctuation and progressive visual field deterioration in patients with glaucoma and low intraocular pressures after a triple procedure. Arch Ophthalmol. 2007;125:1010–1013 [DOI] [PubMed] [Google Scholar]
- 14.Broman A, Quigley H, West S, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drance S, Anderson D, Schulzer M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708 [DOI] [PubMed] [Google Scholar]
- 16.Bengtsson B, Leske M, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:205–209 [DOI] [PubMed] [Google Scholar]
- 17.Johnson C, Cioffi G, Liebmann J, Sample P, Zangwill L, Weinreb R. The relationship between structural and functional alterations in glaucoma: a review. Seminars in Ophthalmology. 2000;15:221–233 [DOI] [PubMed] [Google Scholar]
- 18.Artes P, Chauhan B. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–354 [DOI] [PubMed] [Google Scholar]
- 19.Chauhan B, McCormick T, Nicolela M, LeBlanc R. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001;119:1492–1499 [DOI] [PubMed] [Google Scholar]
- 20.Keltner JL, Johnson CA, Anderson DR, et al. The association between glaucomatous visual fields and optic nerve head features in the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113:1603–1612 [DOI] [PubMed] [Google Scholar]
- 21.Alencar LM, Zangwill LM, Weinreb RN, et al. A comparison of rates of change in neuroretinal rim area and retinal nerve fiber layer thickness in progressive glaucoma. Invest Ophthalmol Vis Sci. 2010;. Published ahead of print [DOI] [PMC free article] [PubMed]
- 22.Gardiner S, Demirel S. Assessment of patient opinions of different clinical tests used in the management of glaucoma. Ophthalmology. 2008;115:2127–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zangwill LM, Weinreb RN, Beiser JA, et al. Baseline topographic optic disc measurements are associated with the development of primary open-angle glaucoma: The Confocal Scanning Laser Ophthalmoscopy Ancillary Study to the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123:1188–1197 [DOI] [PubMed] [Google Scholar]
- 24.Gardiner S, Johnson C, Demirel S. Cup size predicts subsequent functional change in early glaucoma. Optom Vis Sci. 2011;88:1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caprioli J. The importance of rates in glaucoma. Am J Ophthalmol. 2008;145:191–192 [DOI] [PubMed] [Google Scholar]
- 26.Gardiner SK, Demirel S, Johnson CA. Perimetric indices as predictors of future glaucomatous functional change. Optom Vis Sci. 2011;88:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kass M, Heuer D, Higginbotham E, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713 [DOI] [PubMed] [Google Scholar]
- 28.Investigators AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440 [DOI] [PubMed] [Google Scholar]
- 29.Nouri-Mahdavi K, Hoffman D, Gaasterland D, Caprioli J. Prediction of visual field progression in glaucoma. Invest Ophthalmol Vis Sci. 2004;45:4346–4351 [DOI] [PubMed] [Google Scholar]
- 30.Caprioli J, Coleman AL. Intraocular pressure fluctuation: a risk factor for visual field progression at low intraocular pressures in the Advanced Glaucoma Intervention Study. Ophthalmology. 2008;115:1123–1129 [DOI] [PubMed] [Google Scholar]
- 31.Miglior S, Torri V, Zeyen T, Pfeiffer N, Vaz JC, Adamsons I. Intercurrent factors associated with the development of open-angle glaucoma in the European Glaucoma Prevention Study. Am J Ophthalmol. 2007;144:266–275 [DOI] [PubMed] [Google Scholar]
- 32.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefe's Arch Clin Exp Ophthalmol. 2005;243:513–518 [DOI] [PubMed] [Google Scholar]
- 33.Bergea B, Bodin L, Svedbergh B. Impact of intraocular pressure regulation on visual fields in open-angle glaucoma. Ophthalmology. 1999;106:997–1004 [DOI] [PubMed] [Google Scholar]
- 34.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–142 [DOI] [PubMed] [Google Scholar]
- 35.Burgoyne CF, Fortune B, Wang L, Downs JC, Gardiner SK. Intraocular pressure magnitude and fluctuation as predictors of rates of structural change in non human primate experimental glaucoma. (E-Abstract). Invest Ophthalmol Vis Sci. 2011;52:65820861486 [Google Scholar]
- 36.Gardiner SK, Johnson CA, Cioffi GA. Evaluation of the structure-function relationship in glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3712–3717 [DOI] [PubMed] [Google Scholar]
- 37.Spry P, Johnson C, Mansberger S, Cioffi G. Psychophysical investigation of ganglion cell loss in early glaucoma. J Glaucoma. 2005;14:11–18 [DOI] [PubMed] [Google Scholar]
- 38.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol. 1997;75:368–375 [DOI] [PubMed] [Google Scholar]
- 39.Fingeret M, Flanagan J, Lieberman J. (eds) The Heidelberg Retina Tomograph II Primer. Heidelberg, Germany: Heidelberg Engineering; 2005. [Google Scholar]
- 40.Bell BA, Ferron JM, Kromrey JD. Cluster size in multilevel models: the impact of sparse data structures on point and interval estimates in two-level models. Power. 2008;1122–1129
- 41.Liang H, Wu H, Zou GA. Note on conditional AIC for linear mixed-effects models. Biometrika. 2008;95:773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steiger J. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251 [Google Scholar]
- 43.Downs J, Burgoyne C, Liang Y, Sallee VA. New implantable system for telemetric IOP monitoring in nonhuman primates (NHP). Invest Ophthalmol Vis Sci. 2008;49:E-Abstract 2043 [Google Scholar]
- 44.Olthoff CMG, Schouten JSAG, van de Borne BW, Webers CAB. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology. 2005;112:953–961 [DOI] [PubMed] [Google Scholar]
- 45.Hood DC, Raza AS, de Moraes CGV, et al. Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henson D, Artes P, Chauhan B. Diffuse loss of sensitivity in early glaucoma. Invest Ophthalmol Vis Sci. 1999;40:3147–3151 [PubMed] [Google Scholar]
- 47.Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma: total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46:4600–4606 [DOI] [PubMed] [Google Scholar]
- 48.Artes PH, Chauhan BC, Keltner JL, et al. Longitudinal and cross-sectional analyses of visual field progression in participants of the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2010;128:1528–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grewal DS, Sehi M, Greenfield DS. Diffuse glaucomatous structural and functional damage in the hemifield without significant pattern loss. Arch Ophthalmol. 2009;127:1442–1448 [DOI] [PubMed] [Google Scholar]
- 50.Bengtsson B, Heijl AA. Visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353 [DOI] [PubMed] [Google Scholar]
- 51.Artes PH, Chauhan BC. Properties of the Statpac visual field index (VFI) (E-Abstract). Invest Ophthalmol Vis Sci. 2010;51:5494. [DOI] [PubMed] [Google Scholar]