This study investigated the response of metabolism and growth to fluctuating temperatures under carbon-limiting conditions (short days, low light). It is shown that biomass production is determined largely by the daytime temperature via its effect on photosynthesis. By contrast, the mobilization of starch and use of carbon for growth is compensated against changes in the night temperature.
Abstract
Diurnal cycles provide a tractable system to study the response of metabolism and growth to fluctuating temperatures. We reasoned that the response to daytime and night temperature may vary; while daytime temperature affects photosynthesis, night temperature affects use of carbon that was accumulated in the light. Three Arabidopsis thaliana accessions were grown in thermocycles under carbon-limiting conditions with different daytime or night temperatures (12 to 24°C) and analyzed for biomass, photosynthesis, respiration, enzyme activities, protein levels, and metabolite levels. The data were used to model carbon allocation and growth rates in the light and dark. Low daytime temperature led to an inhibition of photosynthesis and an even larger inhibition of growth. The inhibition of photosynthesis was partly ameliorated by a general increase in protein content. Low night temperature had no effect on protein content, starch turnover, or growth. In a warm night, there is excess capacity for carbon use. We propose that use of this capacity is restricted by feedback inhibition, which is relaxed at lower night temperature, thus buffering growth against fluctuations in night temperature. As examples, the rate of starch degradation is completely temperature compensated against even sudden changes in temperature, and polysome loading increases when the night temperature is decreased.
INTRODUCTION
Plants are continually exposed to changes in the ambient temperature. We know little about how metabolism and growth adjust to a fluctuating temperature regime, which features of the regime they respond to, and whether they always respond to temperature in the same way. Temperature affects almost all cellular and physiological processes, with the effect depending on the process (Larcher, 1995; Atkin and Tjoelker, 2003; Atkin et al., 2006). Photochemical processes are effectively temperature-insensitive, diffusion-based processes that vary as a function of the change in the absolute temperature (∼0.03-fold per 10°C change in the physiological range), enzyme activities typically decrease two- to threefold for each 10°C decrease in temperature (often termed the Q10; Larcher, 1995), and membrane-based processes are additionally affected by physical changes in the properties of lipids. Particular proteins or processes may be especially temperature sensitive, often in a nonlinear manner. The direct effects of temperature are often ameliorated by complex metabolic or physiological adjustments that, after a given time, improve plant performance at the new growth temperature. This process is often termed acclimation (Lambers et al., 1998). A better and predictive understanding of the response of metabolism to temperature is important to understand and model the effect of rising temperatures on plant growth, crop yield (Rajan and Blackman, 1975; Gent and Enoch, 1983; Murray, 1995; Frantz et al., 2004; Lawlor, 2005; Leakey et al., 2009), and global carbon (C) fluxes (King, 2005; Mahecha et al., 2010; Reich, 2010). A starting point for many such models is the temperature response of photosynthesis and respiration, which jointly define the whole-plant C budget.
It is well established that a sudden decrease in the temperature results in a lower rate of photosynthesis. Photosynthesis is a complex metabolic network. Limitations appear in subprocesses with a particularly large Q10 like Suc synthesis (Stitt and Grosse, 1988; Sharkey et al., 1995), while restrictions in the use of light energy lead to photoinhibition and oxidative stress (Niyogi, 1999; Nishiyama et al., 2006; Lawlor and Tezara, 2009). These direct negative effects are modified by acclimation. Growth at low temperature typically leads to a partial recovery of photosynthesis on a leaf area or fresh weight (FW) basis (Stitt and Hurry, 2002). Photosynthetic acclimation is relatively small in preformed leaves and larger in leaves that develop at the lower temperature (Strand et al., 1997; Campbell et al., 2007; Atkinson et al., 2010). The process includes an increase in leaf thickness and the overall protein concentration (Strand et al., 1999; Tjoelker et al., 1999; Loveys et al., 2002; Campbell et al., 2007). The latter is at least partly due to a decrease in vacuole size allowing the cytoplasm to occupy a larger proportion of the cellular volume (Huner et al., 1981; Griffith et al., 1985; Strand et al., 1999). The increase in leaf thickness and protein content support a general increase per unit leaf area in the capacity of proteins that are involved in photosynthesis (Strand et al., 1999; Stitt and Hurry, 2002). This rebalances temperature-dependent processes like electron transport, the Calvin-Benson cycle, and end-product synthesis with relatively temperature-insensitive physical processes like CO2 entry and light absorption (Stitt and Hurry, 2002). Acclimation also leads to specific changes in the activities of selected enzymes, including an increase in the capacity for Suc synthesis (Strand et al., 1997, 1999, 2003), and to a redistribution of phosphate between cellular compartments that allows a general increase in the levels of phosphorylated intermediates (Stitt and Hurry, 2002). While most studies of photosynthetic acclimation have been performed at 4 to 5°C, Usadel et al. (2008b) found an analogous, although smaller, increase in the overall protein concentration and shifts in the activities of enzymes at 12 to 17°C, indicating that acclimation occurs in response to less extreme temperatures.
Respiration plays an important role in the plant C budget, with 30 to 80% of the C that is fixed via photosynthesis being subsequently respired to CO2 (Hurd and Enoch, 1976; Amthor, 2000; Loveys et al., 2002). A short-term decrease of the temperature usually results in a marked decrease in respiration with Q10 values of 2 to 4 (ap Rees et al., 1988; Amthor, 1995; Atkin et al., 2005; Armstrong et al., 2007; Atkinson et al., 2007), although smaller Q10 values have been reported (Frantz et al., 2004). Growth at lower temperature leads to acclimation and recovery of respiration, although it usually does not recover to the rate observed at higher growth temperatures. Acclimation occurs in species from many functional groups (Tjoelker et al., 1999; Loveys et al., 2003; Atkin et al., 2005; Campbell et al., 2007). Like photosynthesis, respiratory acclimation is more marked in leaves that develop at the new temperature (Campbell et al., 2007). Several factors contribute, including the general increase in protein content (Atkin et al., 2005, 2008; Campbell et al., 2007; Tjoelker et al., 2008) and changes in the activities or levels of specific respiratory enzymes, in particular alternative oxidase and uncoupling protein (Armstrong et al., 2007; Campbell et al., 2007). Respiration is often subdivided into growth respiration (Rg) and maintenance respiration (Rm; Penning de Vries et al., 1974; Amthor, 2000). Rg refers to the CO2 that is released to provide energy for the biosynthesis of new biomass. The stoichiometry between Rg and biomass formation is thought to be relatively temperature independent (Penning de Vries et al., 1979). However, Rg can be altered as a result of temperature-dependent changes in biomass composition or the rate of growth (Frantz et al., 2004). For example, the increased protein content at low temperature is likely to result in higher growth costs and higher Rg because protein synthesis represents one of the most expensive components of the cellular budget (Penning de Vries, 1975; Warner, 1999; Hachiya et al., 2007; Pace and Manahan, 2007; Piques et al., 2009). Rm includes costs associated with turnover of proteins and other cellular components and with preservation of cellular function (e.g., pH and ionic gradients across membranes). It increases with rising temperature (Penning de Vries et al., 1979; Amthor, 2000).
Plants typically experience warm daytime temperatures, linked to the presence of sunlight and cooler nights. Little is known about how plants respond to fluctuating diurnal temperature regimes. Whole-plant studies have shown that the overall rate of growth is usually strongly dependent on daytime temperature and less dependent on the night temperature. Lower night temperature can lead to a decrease (Hussey, 1965; Rajan and Blackman, 1975), little or no change (Dale, 1965; Frantz et al., 2004), or an increase in growth (Gent and Enoch, 1983) with the response depending on the temperature range, photoperiod, and species (Rajan and Blackman, 1975). However, these earlier whole-plant studies did not provide any information about the diurnal timing of growth or the mechanisms that compensate growth against changes in the night temperature. Subsequent molecular and metabolic studies focused on the response to a sustained change in temperature. Recently Poiré et al. (2010) noted that decreasing the night temperature from 24 to 17°C had no effect on the rate or the diurnal timing of leaf expansion growth. While this implies that expansion growth at night is temperature compensated against a small decrease of the temperature, the underlying mechanisms remain unclear. It is also unclear if this holds only for expansion growth, which is mainly driven by water uptake, or also for the synthesis of cellular components.
Metabolism and growth ultimately depend on C that is photoassimilated in the light. However, growth can occur throughout the 24-h cycle (Geiger and Servaites, 1994; Wiese et al., 2007; Pantin et al., 2011; Yazdanbakhsh et al., 2011). This is possible because some C is stored in the light period and remobilized to support metabolism and growth at night (Geiger and Servaites, 1994; Geiger et al., 2000; Smith and Stitt, 2007). Many plants, including Arabidopsis thaliana, store much of their C as starch. Starch turnover is regulated by the circadian clock such that starch is almost, but not completely, degraded at the end of the night (EN; Graf et al., 2010; Graf and Smith, 2011). This response maximizes growth while avoiding premature exhaustion of starch, which would lead to C starvation and an inhibition of growth that persists for several hours into the next light period (Gibon et al., 2004b; Usadel et al., 2008a; Yazdanbakhsh et al., 2011). Thus, while daytime temperature will affect C assimilation, night temperature will influence utilization of C that was accumulated in the light.
The following experiments investigate the response of metabolism and growth in Arabidopsis to changes in the temperature imposed during the entire light/dark cycle, only in the light period, or only at night. The treatments were restricted to the range 12 to 24°C to avoid complicating effects due to high or low temperature stress. To maximize the importance of processes involved in C acquisition and allocation, the plants were grown in moderately C-limiting conditions (i.e., short days and limiting irradiance) (Gibon et al., 2009). The overall rate of growth was strongly dependent on the daytime temperature but insensitive to changes in the night temperature. Data from measurements of photosynthesis, respiration, and the dawn and dusk levels of starch, sugars, organic acids, and amino acids, which are the quantitatively most important transient stores of C, were used to model C allocation and growth rates in the light and dark periods and identify which processes are temperature compensated. The underlying mechanisms were investigated by profiling over 250 proteins with robotized enzyme assays (Gibon et al., 2004a) and tandem mass spectrometry (Piques et al., 2009), analyzing starch breakdown and polysome loading, the latter as an indicator of the rate of protein synthesis.
RESULTS
A Moderate Decrease in the Daily Growth Temperature Leads to a Decrease in Biomass and an Increase in Protein Levels and Major Metabolites
An initial experiment was performed with 20 Arabidopsis accessions to assess how reproducibly this reference species responds to a moderate decrease in temperature. The accessions were selected from a larger set of >100 genetically diverse accessions to maximize diversity for biomass in short-day conditions (Sulpice et al., 2009). General information about the accessions (passport data) is listed inSupplemental Data Set 1 online. The plants were grown in short-day conditions (8 h light/16 h dark) at a constant day/night temperature of 20 or 16°C. After 30 d, rosettes were harvested at the end of the day (ED; when C stores have accumulated to their diurnal maximum) and analyzed for rosette FW, total protein, starch, sugars, and amino acids (seeSupplemental Figure 1 andSupplemental Data Set 1 online). A decrease in growth temperature from 20 to 16°C resulted, on average, in a 10% decrease in rosette FW. Starch, total sugars, total amino acids, and total protein increased by an average of 11, 17, 20, and 21%, respectively. Analysis of variance (ANOVA) was performed to distinguish how much of the variation is determined by the genotype (G) or temperature (E) (seeSupplemental Data Set 1 online). G and E were highly significant for all traits at P < 0.01. The significance of their interaction was much lower, with only FW being significant at P < 0.01. As outlined in the Introduction, protein content increases during acclimation to low temperature. Previous studies found a significant negative correlation between biomass and protein content at 20°C in a set of over 100 accessions (Sulpice et al., 2009, 2010). This negative correlation was retained at 16°C, but shifted upwards (seeSupplemental Figure 1 online). These results show that moderate changes in temperature lead to coordinated changes in metabolism and growth, which are superimposed on genetic diversity for these traits.
Experimental Design to Separate Responses to Daytime and Night Temperature
For further experiments, three accessions (Columbia-0 [Col-0], Lipowiec-0 [Lip-0], and Burghaun-2 [Bu-2]) were chosen that exhibited contrasting biomass and protein content, but were not outliers (seeSupplemental Figure 1 and Supplemental Data Set 1 online). They were grown at a constant temperature of 24, 16, or 12°C in the daytime and the night or at a daytime temperature of 24°C and a night temperature of 16 or 12°C. These five growth regimes allow the following comparisons: (1) decreased temperature in both the daytime and the night (24°C/24°C versus 16°C/16°C versus 12°C/12°C), (2) decreased temperature only in the daytime (24°C/16°C versus 16°C/16°C and 24°C/12°C versus 12°C/12°C), and (3) decreased temperature only in the night (24°C/24°C versus 24°C/16°C versus 24°C/12°C). Temperature regimes that resulted in a higher night than daytime temperature were avoided. Rosettes were harvested 5 weeks after germination at ED and EN.
Biomass Depends on the Daytime Growth Temperature
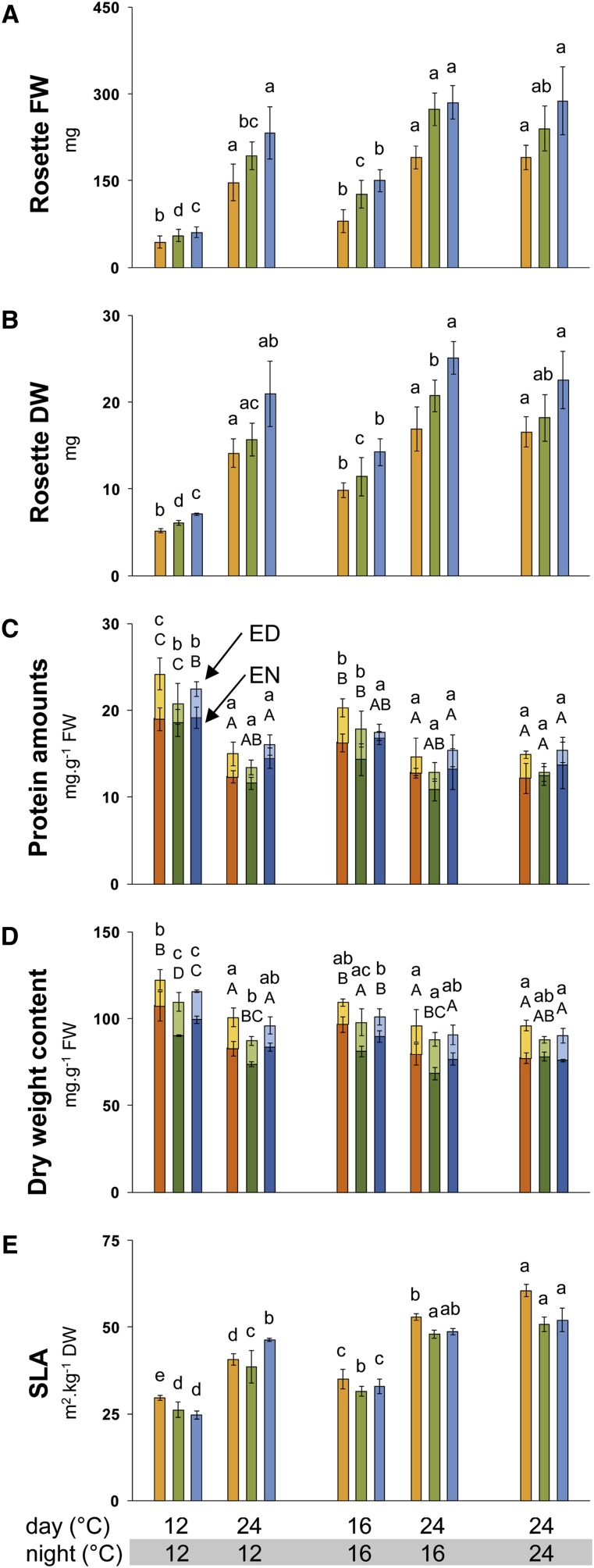
Biomass decreased on a FW (Figure 1A) and dry weight (DW) (Figure 1B) basis when the temperature was decreased across the entire 24-h cycle. On a DW basis, rosette biomass decreased by 37 to 40% and 66 to 69% in the 16°C/16°C and 12°C/12°C thermocycles, respectively, compared with the 24°C/24°C thermocycle. Rosette biomass decreased to a similar extent when only the daytime temperature was lowered: on a DW basis by 41 to 45% between 24°C/16°C and 16°C/16°C and by 61 to 66% between 24°C/12°C and 12°C/12°C. The decrease in biomass was much smaller when the temperature was only decreased at night. Compared with 24°C/24°C, biomass was unaltered in a 24°C/16°C thermocycle, and the apparent slight decrease in a 24°C/12°C thermocycle (7 to 15% on a DW basis) was not significant. This may still overestimate the impact of night temperature on growth because after illumination, ∼30 min elapsed until the temperature rose to the daytime value (data not shown). In summary, growth is dependent on the daytime temperature. This is especially striking as the plants were grown in short-day conditions, in which the night was twice as long as the light period.
Figure 1.
Rosette FW, Rosette DW, Protein Amounts, DWC , and SLA of Three Accessions Grown in Five Different Thermocycles.
Rosette FW (A), rosette DW (B), protein amounts (C), DWC (D), and SLA (E). Bu-2, orange bars; Col-0, green bars; Lip-0, blue bars. Plants were grown in five different thermocycles: 12°C/12°C (day/night), 24°C/12°C, 16°C/16°C, 24°C/16°C, and 24°C/24°C. Data represent the mean ± sd (n = 4). For (C) and (D), light colors are for ED and dark ones for EN. One-way ANOVA was used to identify potential candidates for a statistically significant difference between treatments separately for each of the three accessions and two time points. After ANOVA P value correction using Holm’s method (P < 0.05), individual contrasts were then identified in a post-hoc Tukey HSD test (P < 0.05). Significant differences are indicated by different letters within the same time point (for [C] and [D], lowercase for ED and uppercase for EN). Original data are provided inSupplemental Data Set 2 online.
Protein Content, Dry Matter Content, and Specific Leaf Area Depend on Daytime, but Not Night, Temperature
When the temperature was decreased for the entire 24-h cycle, protein content per unit FW increased by 18 to 35% (significant for Col-0 and Bu-2) in the 16°C/16°C thermocycle and by 43 to 59% (significant for all accessions) in the 12°C/12°C thermocycle compared with the 24°C/24°C thermocycle (Figure 1C). Protein showed a similar increase when only the daytime temperature was decreased (20 to 35% and 36 to 58%, respectively, in the 24°C/16°C versus 16°C/16°C and 24°C/12°C versus 12°C/12°C comparisons, significant for all accessions. By contrast, protein content remained unaltered when only the night temperature was varied.
Decreasing the temperature across the entire 24-h cycle led to a progressive and significant increase in dry weight content (DWC; defined as g DW per g FW), as did decreasing only the daytime temperature (Figure 1D). Decreasing only the night temperature had little effect on DWC. All three accessions showed the same response. The changes of DWC correlated with the changes in protein (R = 0.952, 0.884, and 0.951 for Bu-2, Col-0, and Lip-0, respectively) (seeSupplemental Figure 2A online). About 30% of the increase in DWC between the 24°C/24°C and 12°C/12°C thermocycles is due to the increase in protein (cf. Figures 1C and 1D). The remainder presumably is due to an increase in cell wall and other structural components. There was a small (5 to 25%) increase in protein content (Figure 1C) and DWC (Figure 1D) per unit FW at ED compared with EN in all temperature regimes and accessions, presumably reflecting a slight decrease in water content due to transpiration in the light and highlighting the precision of the sampling and analysis.
A decrease of the temperature across the entire light/dark cycle led to an increase in leaf thickness. Figure 1E shows the changes of specific leaf area (SLA; defined as leaf area per unit DW and recorded as m2 kg−1). SLA is the reciprocal of leaf thickness and defines how much leaf area is generated per unit biomass. SLA decreased by 50% between the 24°C/24°C and 12°C/12°C thermocycles. A decrease in SLA will lead to a decrease in the amount of light intercepted per unit leaf mass, as observed in many earlier studies of acclimation to low temperature (Strand et al., 1999; Tjoelker et al., 1999; Loveys et al., 2002; Campbell et al., 2007). A decrease of the daytime temperature also led to a strong decrease in SLA (∼30% in the 24°C/16°C versus 16°C/16°C and 50% in the 24°C/12°C versus 12°C/12°C, comparison). A decrease of only the night temperature led to a marginal decrease of the SLA (5 to 12%) between the 24°C/24°C and 24°C/16°C and a small but significant decrease (11 to 32%) between the 24°C/24°C and 24°C/12°C thermocycles.
Metabolite Levels
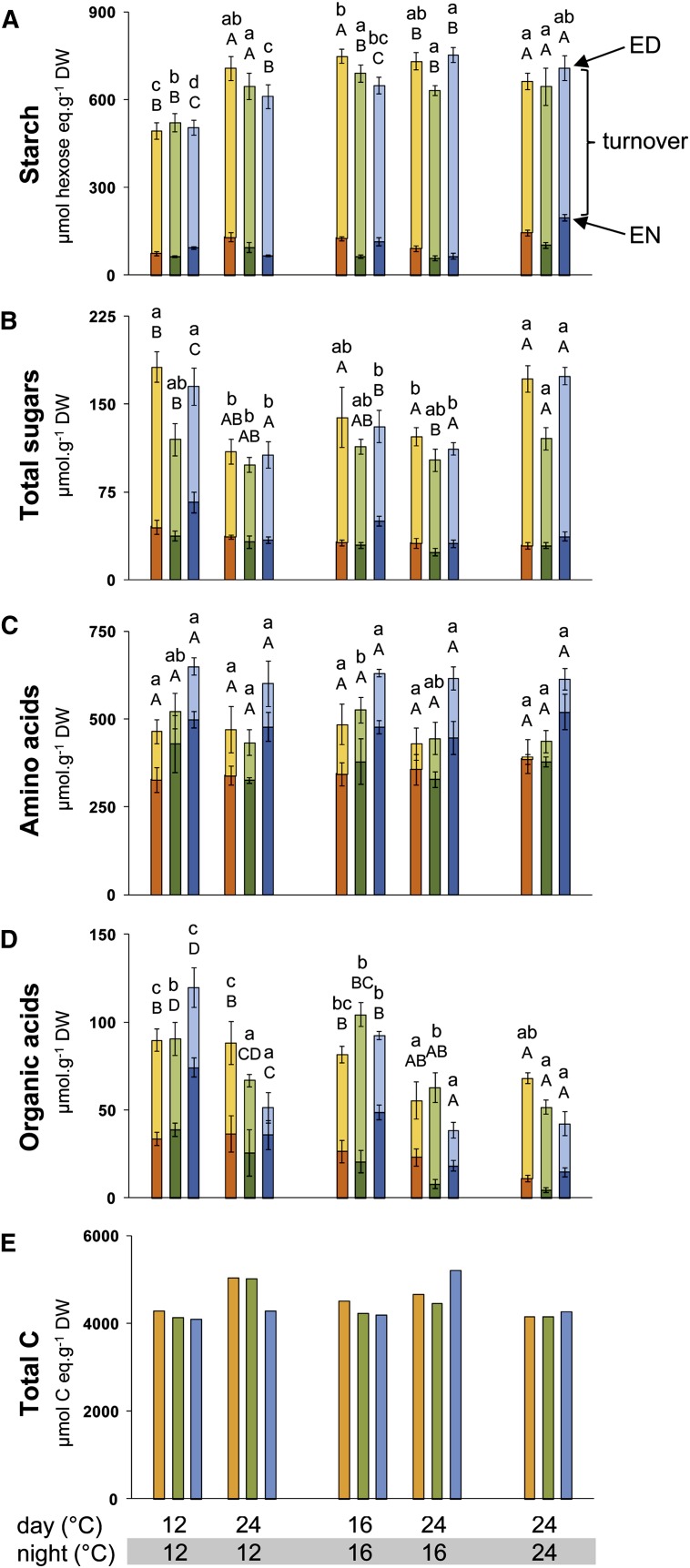
To provide information about C allocation, we analyzed the levels of major C metabolites (Gibon et al., 2006; Sulpice et al., 2009, 2010) at ED and EN. The metabolites analyzed included starch, which is by far the largest transient C store in Arabidopsis, Suc, Glc, and Fru (summarized as total sugars; seeSupplemental Data Set 2 online for individual values), the organic acids malate and fumarate, which can represent up to 15% of the transiently stored C in Arabidopsis (Chia et al., 2000; Pracharoenwattana et al., 2010; Zell et al., 2010), and total amino acids. Metabolite levels are expressed on a DW basis.
When the temperature was decreased across the entire light/dark cycle, starch levels at ED remained unaltered (Col-0 and Lip-0) or increased slightly (Bu-2) in the 16°C/16°C thermocycle and decreased slightly (10 to 20%) in the 12°C/12°C thermocycle compared with the 24°C/24°C thermocycle (Figure 2A). Starch was almost exhausted at EN in all three regimes. The residual starch at EN was highest in the 24°C/24°C thermocycle, intermediate in 16°C/16°C and lowest in the 12°C/12°C thermocycle. Total sugars (Glc, Fru, and Suc; Figure 2B) and amino acids (Figure 2C) were unaltered. Organic acids (Figure 2D) increased at ED in the 12°C/12°C and 16°C/16°C thermocycles compared with 24°C/24°C thermocycle. The amplitude of the diurnal changes (turnover) of these soluble metabolites between ED and EN was largest for organic acids and smallest for amino acids. However, the relative and absolute diurnal changes were much smaller than for starch.
Figure 2.
Metabolite Levels on a DW Basis in Plants Grown in Five Different Thermocycles.
Bu-2, orange bars; Col-0, green bars; and Lip-0, blue bars. Determinations were made at ED (light color) and EN (dark color) for starch (A), total sugars (B), amino acids (C), organic acids (D), and total C turnover (E). Total C turnover is the sum of the C in starch, sugars, organic acids, and amino acids turned over during the night. Data represent the mean ± sd (n = 4). Error bars are absent for (E) as the calculation was based on average values. One-way ANOVA was used to identify potential candidates for a statistically significant difference between treatments separately for each of the three accessions and two time points. After ANOVA P value correction using Holm’s method (P < 0.05), individual contrasts were then identified in a post-hoc Tukey HSD test (P < 0.05). They are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). Original data are provided inSupplemental Data Set 2 online.
When only the daytime temperature was decreased, starch at ED remained unchanged (Bu-2 and Col-0) or decreased slightly (Lip-0) in 24°C/16°C compared with 16°C/16°C and decreased in all three accessions in the 12°C/12°C compared with 24°C/12°C. Starch was almost exhausted at EN. Residual starch at EN was slightly, but significantly, lower (five of six accession-regime comparisons) in thermocycles with a lower daytime temperature. Total sugars did not change significantly at ED in the 16°C/16°C versus 24°C/16°C comparison but increased significantly in the 12°C/12°C versus 24°C/12°C comparison, whereas organic acids increased significantly at ED and sometimes significantly (two of six comparisons) at EN in both comparisons, and amino acids were unaltered.
When only the night temperature decreased, starch levels were unaltered at ED and slightly (significant in four of six comparisons) decreased at EN. Total sugars were decreased at ED and unaltered at EN, organic acids increased at ED and EN, and amino acids were unaltered. While some features resemble the response to a lower daytime temperature (e.g., near-complete turnover of starch in all temperature regimes; increased organic acids) others differ (e.g., starch at ED was slightly lower when the daytime temperature was decreased but not when the night temperature was decreased; total sugars at ED were higher when the daytime temperature was decreased and lower when the night temperature was decreased).
Diurnal Turnover of Primary Metabolites Is Maintained in Low Night Temperature Regimes
The diurnal turnover of C-containing metabolites (i.e., the difference between levels at ED and EN) is a measure for the amount of photoassimilate that is stored during the day to support metabolism and growth at night. Diurnal turnover was calculated for starch and for total C. Total C was defined as the sum of C in starch, sugars, organic acids, and amino acids, which collectively represent the main part of the stored C that is available for metabolism and growth at night. Diurnal turnover is presented on a DW basis (Figure 2; original data inSupplemental Data Set 2 online).
When the temperature was decreased for the entire light/dark cycle, diurnal starch turnover (Figure 2A) remained unaltered or increased slightly in the 16°C/16°C thermocycle, and decreased slightly in the 12°C/12°C thermocycle compared with the 24°C/24°C control treatment. Although starch levels at ED are slightly lower in the 12°C/12°C thermocycle, this was partly compensated because starch is also lower at EN. Total C turnover (Figure 2E) was also largely unaltered for all thermocycles. A decrease of the daytime temperature led to a similar response. A decrease of the night temperature also had no effect on the diurnal turnover of starch (Figure 2A) or total C (Figure 2E). This is remarkable, as low night temperature would be expected to inhibit use of C during the night.
Dark Respiration and Photosynthesis
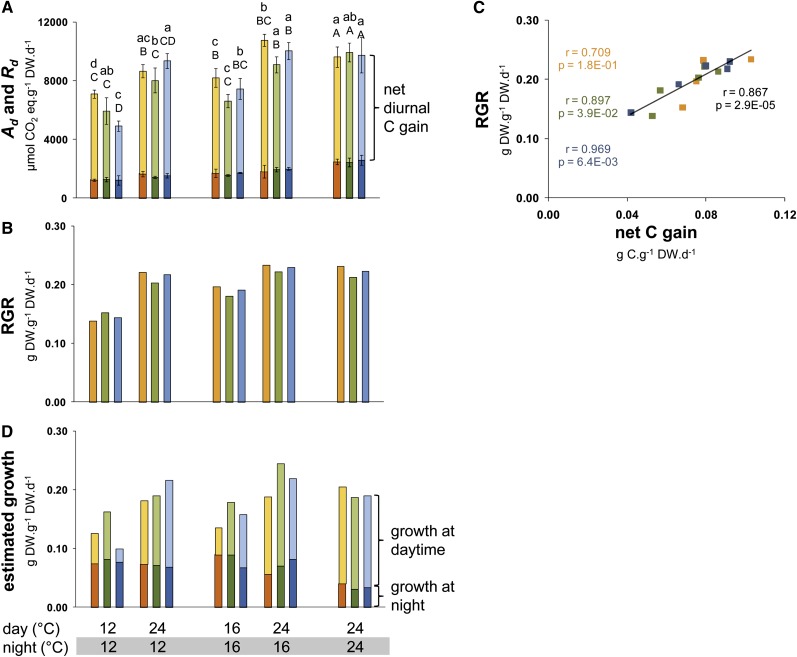
To place the information about stored C in a broader context, we measured photosynthesis (A) and respiration (R). A was measured at the growth daytime temperature, and R was measured at the growth night temperature. The cumulative amount of C that was assimilated in the light (Ad) and respiration in the night (Rd) was calculated from A and R, taking the length of the light period (8 h) and night (16 h) into account. Ad and Rd are shown on a DW basis in Figure 3A.
Figure 3.
Photosynthesis, Respiration, and Modeled Growth Rate in the Daytime and the Night on a DW Basis.
(A) Photosynthesis (A, pale color) and respiration (R, dark color) were measured at the growth temperature in five different thermocycles. The rates are shown on a per day basis, after correcting for the length of the light period (8 h) and the night (16 h). The net diurnal C gain is the difference between A and R.
(B) The RGR was estimated from the difference between biomass at harvest (Figure 2B) and biomass at transfer to the thermocycle treatments at 21 d.
(C) Correlation coefficient between the net diurnal C gain and RGR. Note that C accounts for ∼42% of the DW, so the numbers on the y axis must be multiplied by 0.42 to allow a comparison of the absolute rate of use of C for growth.
(D) Estimated rate of growth in the daytime and the night. The rate of growth in the day is estimated as A minus the sum of C accumulated in starch, sugars, organic acids, and amino acids (Figure 2E), divided by 0.42 (the proportion of C in DW). The rate of growth at night is estimated as the sum of C accumulated in starch, sugars, organic acids, and amino acids minus R, divided by 0.42.
Data represents the mean ± sd (n = 4), with one replicate comprising five pooled rosette plants. Orange, Bu-2; green, Col-0; blue, Lip-0. For details of the calculations, see Figure 2A; seeTable 1 andSupplemental Data Set 3 online. One-way ANOVA was used to identify potential candidates for a statistically significant difference in A and R between treatments separately for each of the three accessions and two time points. After ANOVA P value correction using Holm’s method (P < 0.05), individual contrasts were then identified in a post-hoc Tukey HSD test (P < 0.05). They are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). Error bars and significance tests are absent for (B) and (D), where the calculations are based on average values for A, R, and summed C.
A decreased by 15 to 33% and 26 to 50% in the 16°C/16°C and 12°C/12°C thermocycles, respectively, compared with the 24°C/24°C thermocycle. A decreased to a similar extent when only the daytime temperature was decreased (24 to 27% in 16°C/16°C versus 24°C/16°C and 18 to 47% in 12°C/12°C versus 24°C/12°C). The increase in total protein (see above) allows photosynthesis to be maintained at a relatively high rate, despite a lower daytime temperature. This is even more so on a leaf FW basis or area basis (seeSupplemental Data Set 2 online). When only the night temperature was decreased, A was stable except for Bu-2 in the 24°C/16°C thermocycle, where it increased slightly.
R decreased significantly in all three accessions when temperature was decreased across the entire light/dark cycle (by 31 to 36% and 48 to 53% at 16°C/16°C and 12°C/12°C, respectively, compared with 24°C/24°C). This is slightly more than the decrease in photosynthesis (see above). Earlier studies also found differences in the temperature dependency of respiration and photosynthesis (ap Rees et al., 1988; Artuso et al., 2000; Atkin et al., 2005; Campbell et al., 2007). When only the daytime temperature was decreased, R decreased slightly (by 6 to 19% and 10 to 25% in the 16°C/16°C versus 24°C/16°C and 12°C/12°C versus 24°C/12°C comparison, respectively; only significant for Col-0 in the former and Bu-2 in the latter comparison). When only the night temperature was decreased, R decreased significantly in all accessions (21 to 26% and 33 to 40% in the 24°C/16°C and 24°C/12°C thermocycles compared 24°C/24°C). This is less than the decrease expected from typical Q10 values of metabolic processes (see Introduction).
Comparison of Diurnal C Gain and the Relative Growth Rate
We used information about Ad and Rd to model overall biomass formation in the different thermocycles. The C available per day for growth is equivalent to the difference between Ad and Rd (net diurnal C gain; Figure 3A). The differences in net C gain between the various thermocycles were relatively small (averaged across the accessions, 6.79, 5.50, and 4.52 mmol CO2/g DW per 24 h in the 24°C/24°C, 16°C/16°C, and 12°C/12°C regimes and 7.60 and 6.85 in the 24°C/16°C and 24°C/12°C regimes).
The rate of growth is given by the relative growth rate (RGR; defined as the amount of DW accumulated in a day per unit DW at the start of the day and recorded as g DW g DW−1 day−1; Evans, 1972; Hoffmann and Poorter, 2002). As RGR is linearly related to the log of biomass, RGR can be estimated from the biomass gain between transfer to the thermocycle (at 21 d) and harvest (at 35 d). Our approach assumes constant growth rates between transfer and harvest. This assumption is supported by sequential harvests of plants growing in similar growth conditions (Tschoep et al., 2009). RGR at the day of harvest would be overestimated if growth rates were to decline with time. It should be noted that the large (three- to fourfold) differences in final biomass (Figure 1B) are the result of relatively small (<30%) differences in fluxes and the momentary growth rate. This is because biomass accumulation is exponential with time. Transformation of biomass into RGR reveals that there is good agreement between the net diurnal C gain (Figure 3A) and RGR (Figure 3B). Net C gain correlates strongly and linearly with RGR (r = 0.87; Figure 3C), the variation in RGR (<30%) between the various treatments (Figure 3B) resembles the variation in net C gain, and the absolute values of net C gain and RGR are of a similar magnitude (Figure 3C). C accounts for ∼45% of the DW in an Arabidopsis rosette (Smith, 1943). The agreement between net C gain and RGR is even closer when both parameters are expressed in g C g DW−1 day−1 (seeSupplemental Data Set 3 online; C-RGR).
Modeled Growth Rate in the Daytime and the Night
We next combined the measured values of A and R with information about metabolite turnover to model the rates of biomass formation in the daytime and the night. The use of C for growth in the daytime is equivalent to the Ad minus the C that accumulates in the light in the major C pools (starch, sugars, amino acids, fumarate, and malate). The use of C for growth in the night is equivalent to the C that is remobilized out of the major C pools during the night minus Rd. This approach requires the simplifying assumption that all of the C that moves out of these central C pools in a given time interval is used for growth. The input data, calculations, and predictions are provided inSupplemental Data Set 3 online, and selected aspects are summarized in Figure 3D and Table 1.
Table 1. Influence of Daytime and Night Temperatures on C Allocation, C Utilization, and the Diurnal Timing of Growth.
| Line | Thermocycle | Diurnal C Turnover as % of A | Growth Rate at Night as % of That in the Daytime | R as % of Diurnal C Turnover | Estimated Rg (g C/g DW per Night) | Estimated Maintenance Respiration (g C/DW per Night) |
|---|---|---|---|---|---|---|
| Col-0 | 12°C/12°C | 70 | 73 | 37 | 0.0062 | 0.0123 |
| Bu-2 | 12°C/12°C | 60 | 51 | 33 | 0.0069 | 0.0101 |
| Lip-0 | 12°C/12°C | 84 | 160 | 35 | 0.0064 | 0.0106 |
| Col-0 | 24°C/12°C | 53 | 34 | 40 | 0.0061 | 0.0140 |
| Bu-2 | 24°C/12°C | 52 | 30 | 45 | 0.0060 | 0.0182 |
| Lip-0 | 24°C/12°C | 45 | 23 | 43 | 0.0058 | 0.0156 |
| Col-0 | 16°C/16°C | 76 | 99 | 38 | 0.0075 | 0.0151 |
| Bu-2 | 16°C/16°C | 62 | 49 | 38 | 0.0075 | 0.0157 |
| Lip-0 | 16°C/16°C | 58 | 37 | 45 | 0.0057 | 0.0175 |
| Col-0 | 24°C/16°C | 49 | 21 | 56 | 0.0047 | 0.0252 |
| Bu-2 | 24°C/16°C | 43 | 20 | 47 | 0.0060 | 0.0204 |
| Lip-0 | 24°C/16°C | 52 | 30 | 45 | 0.0068 | 0.0215 |
| Col-0 | 24°C/24°C | 42 | 12 | 66 | 0.0034 | 0.0297 |
| Bu-2 | 24°C/24°C | 43 | 10 | 74 | 0.0026 | 0.0342 |
| Lip-0 | 24°C/24°C | 44 | 11 | 72 | 0.0028 | 0.0343 |
Growth in daytime was estimated as the difference between photosynthesis (A) and C turnover, defined as the sum of the C in starch, sugars, organic acids, and amino acids turned over during the night. Growth at night was estimated as the difference between C turnover and respiration (R). The growth rates represent the growth per hour. Rg was estimated as growth rate × 0.2. Maintenance respiration was estimated as R – Rg. The original data and more information are available inSupplemental Data Set 3 online.
The modeled rate of growth in the light period was strongly dependent on daytime temperature (Figure 3D, Table 1). It decreased more than twofold when the temperature was decreased from 24 to 16°C or 12°C across the entire 24-h cycle or when the temperature was only decreased in the daytime. This decrease was partly due to a lower rate of photosynthesis (Figure 3A) and partly because the same amount and hence (due to the lower rate of photosynthesis) a larger proportion of the fixed C was allocated to transient C storage pools (Table 1; cf. Figures 2E and 3A). The percentage of fixed C that accumulated in starch and other C metabolites (Table 1; for original data, compare Figures 3A with 2A and 2E; seeSupplemental Data Set 2 online) increased from 42 to 44% at 24°C/24°C to 58 to 76% and 60 to 84% at 16°C/16°C and 12°C/12°C, respectively. A similar increase occurred when only the daytime temperature was decreased (from 43 to 52% to 58 to 76% and from 45 to 53% to 60 to 84% in the 24°C/16°C versus 16°C/16°C and 24°C/12°C versus 12°C/12°C comparisons, respectively). Importantly, low daytime temperature results in a larger decrease of daytime growth than of A. For example, daytime growth and A decreased by 53 and 26%, respectively, in the 24°C/12°C versus 12°C/12°C comparison and by 65 and 28% in the 24°C/16°C versus 16°C/16°C comparison in Col-0. The corresponding values were 32 and 18%, and 48 and 24% in Bu-2, 84 and 47%, and 35 and 26% in Lip-0 (seeSupplemental Data Set 3 online for the original data).
The rate of growth at night was not decreased by low night temperature in the treatments used in this study. The modeled rates of growth at night actually increased slightly between 24 and 16°C and remained stable between 16 and 12°C (Figure 3D).
The absence (or even inverse) effect of low night temperature on nighttime growth partly reflected the fact that night temperature had almost no effect on the amount of C that was accumulated in the light period and remobilized at night (Figures 2A to 2E, Table 1). Another contributing factor was the decrease in R in low night temperature regimes (Figure 3A). R accounted for 67 to 73% of the stored C in the 24°C/24°C regime, compared with 37 to 45% and 33 to 37% in 16°C/16°C and 12°C/12°C thermocycles and 45 to 56% and 40 to 45% when only the night temperature was decreased to 16 and 12°C (Table 1; seeSupplemental Data Set 3 online for the original data).
R is often subdivided into Rg and Rm (see Introduction). These components were estimated (Table 1; seeSupplemental Data Set 3 online) using a typical value of 0.2 C released in growth-related respiration per unit C deposited in biomass (Penning de Vries et al., 1979) and assuming that the stoichiometry between C deposition in biomass and growth-related CO2 release is temperature independent. We may underestimate Rg at lower daytime temperature, when protein increases. When these estimates for Rg are added to the biomass increment, growth consumed 31 to 40% of the stored C in the 24°C/24°C thermocycle, rising to 66 to 75% and 75 to 80% in the 16°C/16°C and 12°C/12°C thermocycles and 53 to 4% and 66 to 72% of the stored C in the 24°C/16°C and 24°C/12°C thermocycles (Table 1). Rm accounted for 90 to 93% of the total respiration in the 24°C/24°C regime, falling to 67 to 75% and 69 to 72% in the 16°C/16°C and 12°C/12°C thermocycles and 76 to 84% and 69 to 72% in the 24°C/16°C and 24°C/12°C thermocycles (Table 1).
This simple model highlights two important ways in which temperature influences the diurnal C utilization. First, the timing of growth is highly dependent on the temperature regime (Figure 3D). While 75 to 80% of the daily growth occurred in the daytime in the 24°C/24°C thermocycle, growth was more or less equally distributed between the daytime and night in the 16°C/16°C thermocycle, and more growth occurred at night than in the daytime in a 12°C/12°C thermocycle. A decrease of only the daytime temperature also resulted in a larger proportion of the growth occurring at night. Rather unexpectedly, when only the night temperature was decreased, there was also an increase in the proportion of the growth that occurred at night. Secondly, at a given temperature the absolute rate of growth is usually faster in the daytime than the night (Table 1). Compared with the rate in the daytime, the absolute rate of growth at night was almost 90% lower in the 24°C/24°C regime, 50 to 60% lower (Lip-0 and Bu-2) or similar (Col-0) in the 16°C/16°C regime, and 30 to 50% lower in the 12°C/12°C regime (except for Lip-0) (Table 1). This implies that the machinery that converts C into biomass is not used as fully in the night as it is in the daytime. There is an especially large excess capacity when the night temperature is high (Table 1; note that the plants were growing in an 8-h-light/16-h-dark cycle, so the ratio of the hourly growth rates in the daytime and night is double and half, respectively, the ratio for the distribution of growth between the daytime and night; Figure 3D).
Activities of Representative Primary Metabolism Enzymes, When Expressed on a Protein Basis, Do Not Show Major Changes between the Different Growth Thermocycles
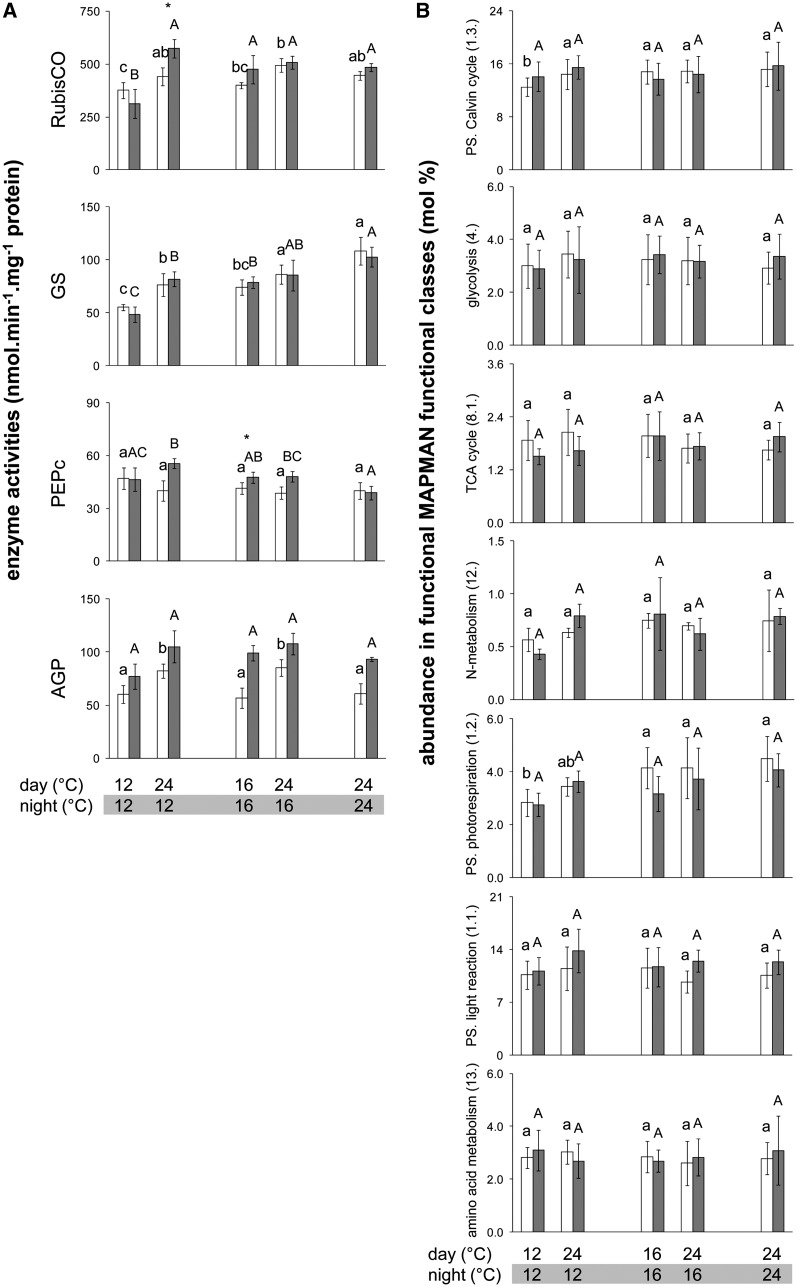
We next asked if the changes in overall protein content in different thermocycles are accompanied by qualitative modifications in protein composition. We first measured the maximum catalytic activities of 17 enzymes involved in Calvin-Benson cycle, tricarboxylic acid (TCA) cycle, glycolysis, amino acid, starch, and Suc metabolism in the three accessions (Figure 4A; seeSupplemental Figure 3 and Supplemental Data Set 2 online).
Figure 4.
Enzyme Activities and Protein Levels in Col-0 Grown in Different Thermocycles.
(A) Activities of four representative primary metabolism enzymes, determined at ED (open bars) and EN (closed bars). Activities are given on a protein basis (mean ± sd, n = 4). Significant differences between treatments were identified by ANOVA, followed by P value correction using Holm’s method (P < 0.05) and selection in a post-hoc Tukey HSD test (P < 0.05), and are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). Comparisons between ED and EN using a paired t test at a given thermocycle are indicated by an asterisk if significant (P < 0.05). The complete data set containing 17 enzymes is available in theSupplemental Figure 3 online and all data are available inSupplemental Data Set 2 online.
(B) Qualitative variation in proteins comprised in seven major MapMan functional classes in rosettes of Col-0 plants grown in five different thermocycles. Data for each time point (ED, open bars; EN, closed bars) and thermocycle are expressed in mol % of proteins detected by LC-MS/MS. To get the relative abundance of each functional class at each time point and thermocycle, the relative abundance of each protein was summed. To calculate the standard deviations for each protein within a category, the global average across all thermocycles and harvest time points was calculated and all molar fractions were then expressed relative to this global average. By averaging these relative abundances (mol %) of all proteins belonging to a functional class, the standard deviation across all proteins within this functional class was determined and used to test for significant differences between all thermocycles and time points. Significant changes were identified as in (A) and are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). Comparisons between ED and EN using a paired t test at a given thermocycle gave no significant differences. Graphs of three additional functional classes are shown in theSupplemental Figure 4 online. All raw data and information about the proteins analyzed are available inSupplemental Data Set 4 online. PS, photosynthesis.
Almost all the enzymes showed a trend to higher activity on a FW basis when the thermocycle included a lower daytime temperature (seeSupplemental Data Set 2 online). This extends earlier studies that have shown a marked increase of Calvin-Benson cycle enzyme activities on a FW basis in plants grown at 4°C (Strand et al., 1999; Stitt and Hurry, 2002) by showing that the increase affects a larger set of enzymes and occurs in response to small changes in the temperature.
This increase in enzyme activities per unit FW broadly reflects the ∼30 and 50% increase in overall protein content at 16 and 12°C (Figure 1C). The increase was largely abolished when enzyme activities were expressed on a protein basis (seeSupplemental Figure 2 online). This resembles the response during acclimation to 4°C (Strand et al., 1999; Stitt and Hurry, 2002). Representative enzymes are shown in Figure 4A (only results for Col-0 are presented as Bu-2 and Lip-0 showed very similar trends). Four enzymes showed a small but significant decrease in activity per unit protein in lower daytime temperatures: ribulose-1,5-bis-phosphate carboxylase/oxygenase (Rubisco; Figure 4A), transketolase (TK; seeSupplemental Figure 3 online; significant for all three accessions); Gln synthetase (GS; Figure 4A), and NAD-Glu dehydrogenase (NAD-GDH; seeSupplemental Figure 3 online; significant for all three accessions). There was a slight upwards trend for phosphoenolpyruvate carboxylase (PEPc) (Figure 4A; seeSupplemental Figure 3 online; significant for Lip-0). No significant differences were observed between ED and EN, except for ADP-Glc pyrophosphorylase (AGPase) that was higher at EN than ED (Figure 4A) as previously described (Gibon et al., 2004a).
Under constant daytime and varying night temperature, none of the enzymes showed a significant change of activity (Figure 4A; seeSupplemental Figure 3 andSupplemental Data Set 2 online) except for GS and NAD-GDH (significant in Bu-2 and Col-0) and PEPc (significant in Col-0), which decreased and increased with lower night growth temperatures, respectively.
No Significant Changes in Relative Protein Abundances in Major Functional Classes
We next performed a proteomics study to investigate temperature-dependent changes in a wider range of proteins. As the three accessions showed similar responses for the measured enzyme activities, we focused the analysis on Col-0. This allowed us to avoid problems due to possible changes in peptide sequence between accessions that complicates peptide identification.
Protein composition was investigated by estimating relative protein abundances using the emPAI index derived from liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based protein quantification (Ishihama et al., 2005). This method yields abundances in mol % of all detected proteins. It is linear over three orders of magnitude in protein concentration (10 nM to 10 µM). There is good agreement between protein abundance estimated in this way, and protein abundance calculated from measured enzyme activities and published values for the specific activity (Piques et al., 2009). However, the emPAI index underestimates highly abundant proteins. For example, Rubisco was only ∼6% of all identified proteins (seeSupplemental Data Set 4 online), which is clearly an underestimate. In total, 1176 proteins were detected over all the samples, and 268 were detected in >50% of the samples. They are listed inSupplemental Data Set 4 online, together with their relative abundances. These 268 proteins were assigned to 14 functional classes (seeSupplemental Data Set 4 online) using MapMan ontology (Thimm et al., 2004; Usadel et al., 2005). Proteins with photosynthetic functions contributed 29.7% ± 2.0% (Calvin-Benson cycle alone 14.5% ± 0.9%, as noted above this is an underestimate). Redox regulation was the second largest functional group with 9.2% ± 0.9%. Proteins with functions in protein synthesis, TCA cycle/organic acid transformations, glycolysis, and amino acid metabolism contributed 7.4% ± 0.7%, 6.3% ± 0.3%, 3.2% ± 0.2%, and 2.8% ± 0.2%, respectively.
To compare thermocycles for changes in major functional protein classes, the relative protein abundances of all the proteins in a given functional class were summed separately at ED and EN. When daytime and night temperature were both decreased or only daytime temperature was decreased, two of the 10 functional protein classes showed a significant decrease: Calvin-Benson cycle and photorespiration (Figure 4B; seeSupplemental Figure 4 online). This confirms the decrease in activities per unit protein of enzymes from these pathways (Figure 4A). When only the night temperature was modified, no functional class showed a significant change. We inspected the responses of protein classes that are required for growth, focusing on protein synthesis. There was an apparent slight but nonsignificant upwards trend for cytosolic ribosomal proteins at ED and EN and protein elongation at ED when the temperature was decreased in the daytime but not when it was decreased only at night (seeSupplemental Figure 4 online). Taken together, these results show that major protein classes were not subject to strong changes in abundance, except for photosynthesis-related classes, which showed a slight decrease.
Although the applied growth temperature had only little effect on average abundances of proteins in major functional classes, the variation within a single class was sometimes quite high (i.e., in glycolysis, TCA cycle, and ribosomal proteins) (seeSupplemental Data Set 4 online). This indicates that some quantitative changes in individual protein levels are present, but not significant at a broader level. A weak positive correlation (R2 ∼0.09) was obtained when the ratio of protein abundances between the 12°C/12°C treatment and 24°C/24°C treatments for 252 well-covered proteins was plotted against the change in the corresponding transcript level 3 d after transfer to 12°C from 20°C in a published data set (Usadel et al., 2008b) (seeSupplemental Figure 4B online). The 28 individual proteins that increased by 50% or more in 12°C/12°C compared with 24°C/24°C included PEPC2, four ribosomal proteins, three chaperones, and three classic cold-responsive proteins from the CBF regulon (COR15a, COR15b, and COR6.6; Vogel et al., 2005). Proteins encoded by a further two members of the CBF regulon (cold and ABA inducible protein/At5g15960 and Low-Temperature-Induced78/At5g52310) were not detectable in 24°C/24°C samples but were reproducibly detectable at lower temperatures (seeSupplemental Data Set 4 online). No members of the CBF regulon showed unaltered or decreased protein level in the low temperature growth regimes.
Transcripts for Genes Involved in Starch Synthesis and Degradation
As already mentioned, it was rather surprising that temperature did not decrease starch accumulation or degradation. The proteomics analysis did not provide good coverage of enzymes for starch metabolism. The 268 proteins that remained after filtering for proteins with <50% missing values included only two polypeptides involved in starch synthesis (APS1/At5g48300 and APL1/At5g19220; the two subunits of AGPase, the key regulatory enzyme for starch synthesis) and two proteins involved in starch breakdown (At3g46970 and At3g29320, corresponding to cytosolic and plastidic α-glucan phosphorylase). No clear response was seen for APS1 and APL1. Plastidic α-glucan phosphorylase showed a trend to decreased abundance when the night temperature was decreased (seeSupplemental Data Set 4 online).
To provide more information about possible adjustments in starch metabolism, we used quantitative RT-PCR (qRT-PCR) to analyze transcript levels for 14 enzymes involved in starch synthesis and 11 involved in starch breakdown (see reviews of these pathways in Zeeman et al., 2007, 2010). To avoid assumptions and possible errors associated with the use of housekeeping genes, we added seven external RNA species at known concentrations before extracting the RNA. This provides a calibration curve, which allows the CT values to be converted into an absolute value (copy number⋅g−1 DW) (Piques et al., 2009). A summary is provided in Table 2, selected examples are shown in Figure 5, and the full data set is provided inSupplemental Figure 5 online.
Table 2. Ratios of the Transcript Levels of Genes Involved in Starch Synthesis and Degradation of Col-0 Plants Grown in 24/24°C, 24/16°C, 24/12°C, and 16/16°C Thermocycles.
| Gene |
ED Ratio to 24/24°C |
Ratio to 24/16°C |
EN Ratio to 24/24°C |
Ratio to 24/16°C |
||
|---|---|---|---|---|---|---|
| 24/12 |
24/16 | 16/16 | 24/12 | 24/16 | 16/16 | |
| Starch degradation | ||||||
| GWD1 | 1.84 | 1.26 | 1.39 | 1.77 | 1.33 | 1.17 |
| GWD2 | 2.31 | 1.56 | 1.23 | 1.58 | 1.25 | 1.30 |
| GWD3 | 1.62 | 1.36 | 1.16 | 0.83 | 1.21 | 1.34 |
| DPE1 | 1.85 | 1.27 | 1.10 | 1.65 | 1.15 | 1.40 |
| DPE2 | 1.26 | 0.97 | 1.85 | 1.33 | 1.41 | 1.06 |
| BAM1 | 1.21 | 0.93 | 1.24 | 0.91 | 1.28 | 1.19 |
| BAM2 | 1.77 | 1.37 | 2.26 | 2.53 | 1.78 | 1.61 |
| BAM3 | 2.34 | 1.53 | 2.70 | 3.29 | 2.16 | 0.93 |
| BAM4 | 1.28 | 0.93 | 2.21 | 0.74 | 1.18 | 1.41 |
| SEX4 | 1.95 | 1.23 | 1.43 | 2.03 | 1.17 | 1.23 |
| PTPKIS2 | 1.31 | 1.07 | 1.18 | 1.17 | 1.06 | 1.36 |
| Starch synthesis | ||||||
| STS1 | 1.51 | 1.05 | 1.76 | 1.79 | 1.61 | 1.20 |
| STS2 | 0.91 | 0.69 | 2.02 | 1.78 | 1.70 | 1.36 |
| STS3 | 1.11 | 0.93 | 1.60 | 1.00 | 1.35 | 1.57 |
| STS4 | 1.43 | 1.05 | 1.45 | 2.26 | 1.77 | 1.53 |
| APS1 | 1.79 | 1.18 | 1.69 | 2.00 | 1.74 | 1.63 |
| APS2 | 1.09 | 1.17 | 1.50 | 1.92 | 1.72 | 1.73 |
| APL1 | 1.43 | 0.86 | 2.05 | 1.84 | 1.73 | 1.57 |
| APL2 | 1.56 | 1.12 | 2.52 | 1.89 | 2.04 | 1.17 |
| APL3 | 1.48 | 1.13 | 2.20 | 2.42 | 1.72 | 1.40 |
| APL4 | 1.38 | 0.96 | 2.16 | 1.43 | 1.88 | 1.13 |
| SBE1 | 1.49 | 1.05 | 3.61 | 7.07 | 8.08 | 0.50 |
| SBE2 | 1.09 | 0.79 | 1.39 | 1.19 | 1.12 | 1.48 |
| SBE3 | 1.41 | 1.20 | 1.84 | 2.09 | 1.62 | 1.09 |
| ISA1 | 1.25 | 0.89 | 1.90 | 0.94 | 1.27 | 1.32 |
One-way ANOVA was used to identify potential candidates for a statistically significant difference between treatments. After ANOVA P value correction using Holm’s method (P < 0.05), significant ratios were then identified in a post-hoc Tukey HSD test (P < 0.05) and are highlighted in bold. Complete data set is available inSupplemental Data Set 5 online.
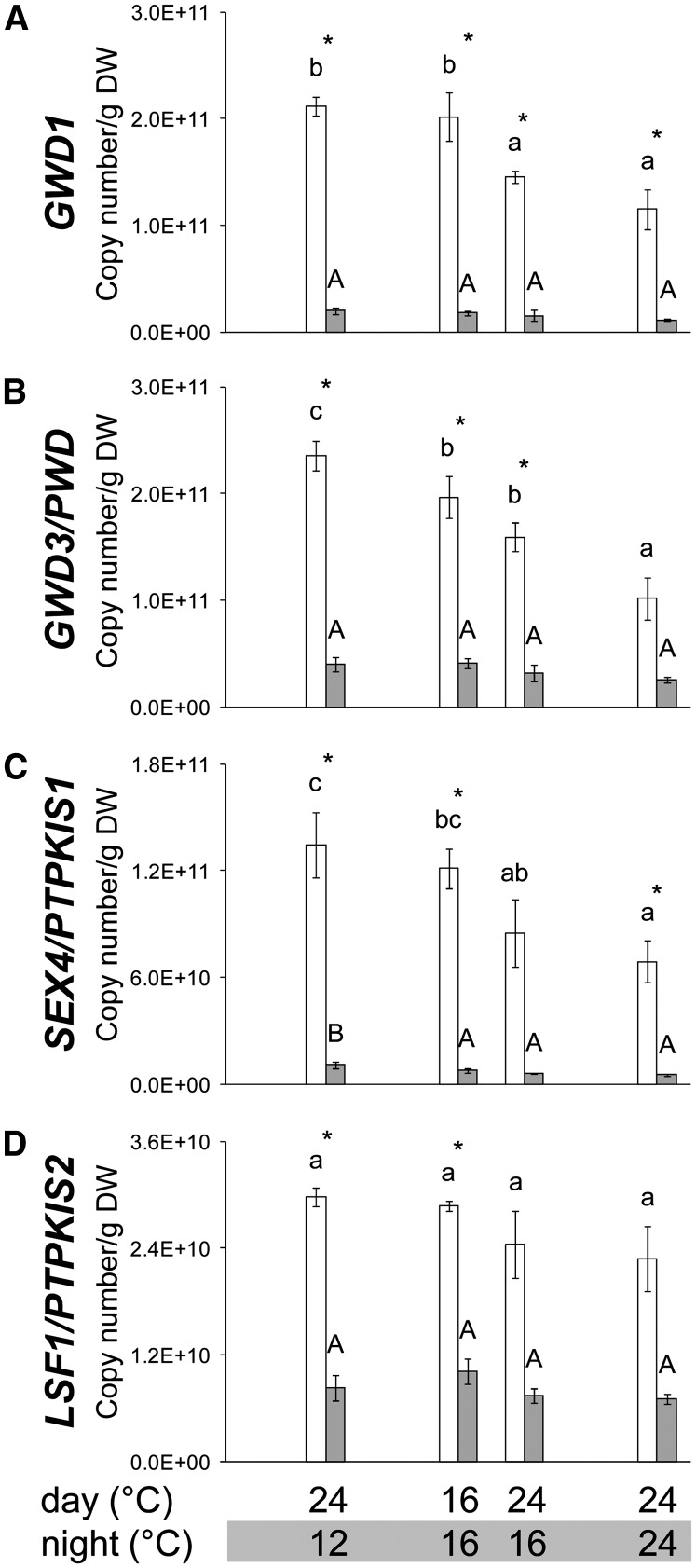
Figure 5.
Response of Transcripts Encoding Enzymes Involved in the Cycle of Glucan Phosphorylation and Dephosphorylation to a Decrease of the Night Temperature.
GWD1 (A), GWD3/PWD (B), SEX4/PTPKIS1 (C), and LSF1/PTPKIS2 (D). Transcripts were measured by qRT-PCR in samples harvested at ED (open bars) and EN (closed bars) from plants growing in a 24°C/12°C, 16°C/16°C, 24°C/16°C, and 24°C/24°C thermocycle. Absolute quantification of transcripts was achieved by adding seven artificial RNA species at different concentrations before RNA purification. The results are the mean ± sd (n = 3) of the determined copy number per g DW. One-way ANOVA was used to identify potential candidates for a statistically significant difference between treatments separately for each of the two time points. After ANOVA P value correction using Holm’s method (P < 0.05), individual contrasts were then identified in a post-hoc Tukey HSD test (P < 0.05). They are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). Comparisons between ED and EN using a paired t test at a given thermocycle are indicated by an asterisk if significant (P < 0.05). More data for further transcripts involved in starch metabolism is shown inSupplemental Figure 5 online.
Decreasing the night temperature from 24 to 16°C and 12°C led to a progressive 30 to 140% increase in the levels of the transcripts for several starch synthesis enzymes (APS1, APL2, STS1, STS4, and SBE3). There was also a widespread increase in transcripts for starch degradation, including five that encode proteins involved in a cycle of glucan phosphorylation and dephosphorylation that is the first step in starch degradation (GWD1, GWD2, GWD3, SEX4, and PTPKIS2; Figure 5; Kötting et al., 2009; Comparot-Moss et al., 2010; Hejazi et al., 2010; Zeeman et al., 2010) and two plastidial β-amylases (BAM2 and BAM3; Fulton et al., 2008) that are thought to be involved in the initial attack on the starch granule. As previously seen (Smith et al., 2004; Usadel et al., 2008a), most transcripts for starch degradation (with the exception of the BAM family) showed large diurnal changes, with very low levels at the EN and high levels at the end of the light period. The increase in transcript levels for these genes was especially marked at the end of the light period, even though only the night temperature was decreased (Table 2). This pattern is especially marked for GWD1, GWD2, DPE1, and SEX4 (Figure 5, Table 2).
For comparison, we investigated the effect of decreasing the daytime temperature from 24 to 16°C. This led to an increase in transcripts for all starch synthesis enzymes except SBS2, with especially large increases for APS1 (twofold) and APL1-4 (two- to threefold) (Table 2; seeSupplemental Figure 5 online). A lower daytime temperature also led to ∼50% increase of transcripts for proteins involved in the cycle of glucan phosphorylation and dephosphorylation (Figure 5, Table 2) and other proteins for starch degradation, including BAM2 and BAM3 (Table 2; seeSupplemental Figure 5 online).
Summarizing, these results show lower temperatures result in a large increase of transcripts for enzyme involved in starch synthesis and degradation. This includes effects of the night temperature on genes encoding proteins that are required for starch synthesis in the light and effects of the daytime temperature on genes encoding proteins that are involved in starch mobilization at night.
Response of Starch Turnover to a Sudden Change in the Night Temperature
We next asked how quickly starch degradation adjusts to a decrease in the night temperature. Col-0, Bu-2, and Lip-0 were grown in a 24°C/24°C thermocycle and then shifted to a 12°C/12°C thermocycle at the start of the night. Plants were harvested at EN and ED the 2 d before the temperature shift, and after the shift at the end of the first night, at ED and EN on the following day and on days 3 and 7 (Figure 6). Starch was almost completely exhausted at the end of the first night at 12°C. The pattern of starch turnover was maintained in the following days (Figure 6A) except for a slight decrease in the level at ED (see also Figure 2A). By contrast, there was a marked accumulation of Suc at ED (Figure 6B), an increase of amino acids at ED and EN, and a gradual rise of malate and fumarate at ED and EN for several days after the shift (seeSupplemental Figure 6 online). Total protein remained unaltered on the first day after the shift and then rose gradually (Figure 6C; Usadel et al., 2008b) as did the activities of Rubisco and other photosynthetic enzymes (seeSupplemental Figure 6 online). Thus, diurnal starch turnover retains the same pattern throughout the temperature shift, while other metabolites and protein show marked transients and/or slow adjustments.
Figure 6.
Starch Metabolism after a Sudden Change in the Night Temperature.
(A) to (C) Changes of starch and other metabolic parameters at EN and ED on successive days after transfer from 24°C/24°C to 12°C/12°C. Black and white bars at the top indicate light and dark periods, respectively. Col-0 (orange bars), Lip-0 (green bars), and Bu-2 (blue bars) growing in a 24°C/24°C thermocycle were transferred at the ED to a 12°C/12°C thermocycle. Samples were harvested at EN and ED on the days before the transfer, at EN on the first night after the transfer, and at ED and EN on the first, third, and seventh day after transfer and analyzed for starch (A), Suc (B), and total protein (C). SeeSupplemental Figure 6 online for displays of changes in other metabolites and enzyme activities andSupplemental Data Set 6 online for the original data. The results are given as mean ± sd (n = 3, each sample contains five individual plants).
(D) and (E) Kinetics of starch depletion in Col-0 plants in the first night after reciprocal transfer between 24°C/24°C and 24°C/12°C. Plants were grown in a 24°C/24°C thermocycle and some transferred to 12°C at the ED (D). Other Col-0 plants were grown in a 24°C/12°C thermocycle (E) and some were transferred to 24°C at the ED (E). Plants were harvested from the control (nonshifted, shown as closed symbols) and shifted plants (open symbols) at various times during the night to determine the starch level. The results are on a DW basis given as the mean ± sd (n = 4, each sample contains five individual plants).
To provide more detailed information about how quickly starch degradation responds to a change in temperature, Col-0 was grown in a 24°C/24°C thermocycle and transferred to 12°C at ED, and samples were taken throughout the following night. Compared with control plants left at 24°C, the rate of starch degradation was marginally slower during the first 4 h after the switch to 12°C (Figure 6D) and identical in the remainder of the night. In a complementary experiment, Col-0 was grown in a 24°C/12°C regime and the temperature then maintained at 24°C for one night. Compared with controls, where the temperature was decreased to 12°C, the rate of starch degradation was marginally faster in the first 4 h of the night and slightly slower in the remainder of the night (Figure 6E). We conclude that there is almost immediate temperature compensation of starch degradation.
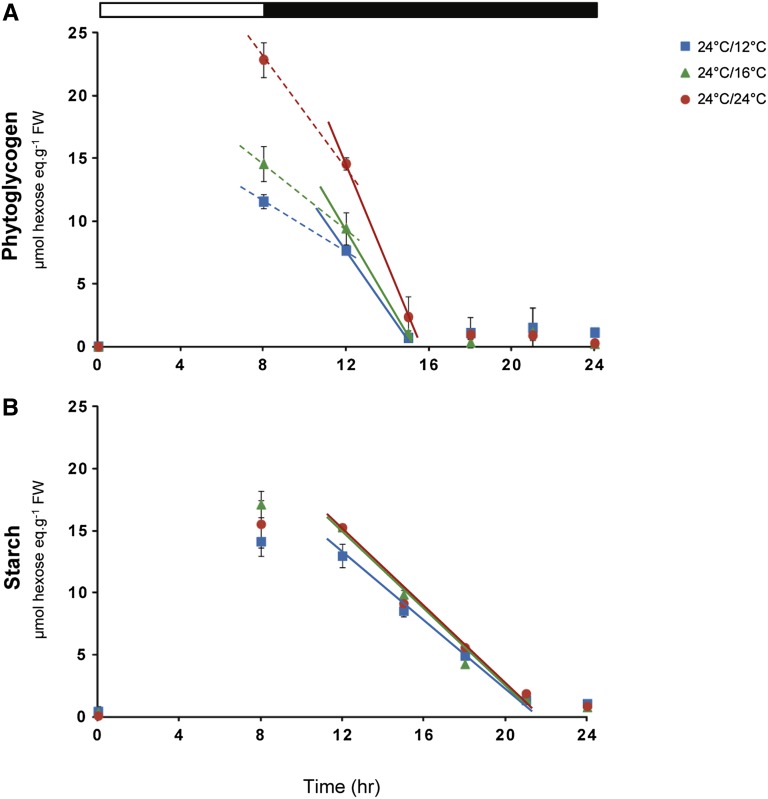
Use of the isa1 Mutant to Distinguish the Temperature Compensation of Degradation of the Starch Granule and Phytoglycogen
Starch accumulates as a crystalline granule (Zeeman et al., 2007, 2010). We took advantage of the isa1 mutant to investigate if the temperature compensation of starch degradation depends on particular features of the starch granule. This mutant lacks isoamylase 1, which is involved in the trimming of preamylopectin chains. It has lower levels of starch and accumulates water-soluble polysaccharides whose structure is similar to glycogen (Zeeman et al., 1998; Delatte et al., 2005; Wattebled et al., 2008). This provides the opportunity to compare the temperature sensitivity of the degradation of starch and a chemically related glucan that also accumulates in the chloroplast. As seen previously (Zeeman et al., 1998; Delatte et al., 2005; Wattebled et al., 2008), phytoglycogen is degraded rapidly in the first part of the night. The rate of degradation was twofold higher at 24°C than 12°C, with an intermediate rate at 16°C (Figure 7A). Starch degradation started after a short lag. Identical rates of starch degradation were found at 24 and 16°C and only marginally slower rates at 12°C (Figure 7B).
Figure 7.
Starch and Phytoglycogen Breakdown in the isa1 Mutant.
The isa1 mutant was grown in a 24°C/24°C, 24°C/16°C, or 24°C/12°C thermocycle and harvested at various times during the night to determine the level of phytoglycogen (A) and starch (B). The results are given as the mean ± sd (n = 4, each sample contains three individual plants). Black and white bars at the top indicate light and dark periods, respectively.
Effect of Low Night Temperatures on Polysome Loading
As already mentioned, lower night temperatures do not decrease, and may even increase, the rate of growth at night (Figure 3D, Table 1). We investigated the effect of temperature on protein synthesis, a representative and major cellular growth process. In warm night regimes, most ribosomes are not loaded into polysomes at night (Piques et al., 2009). Low temperature will decrease the rate of progression of individual ribosomes along mRNA (Takanashi et al., 1987). We investigated whether maintenance of protein synthesis at a lower temperature is associated with increased loading of ribosomes into polysomes.
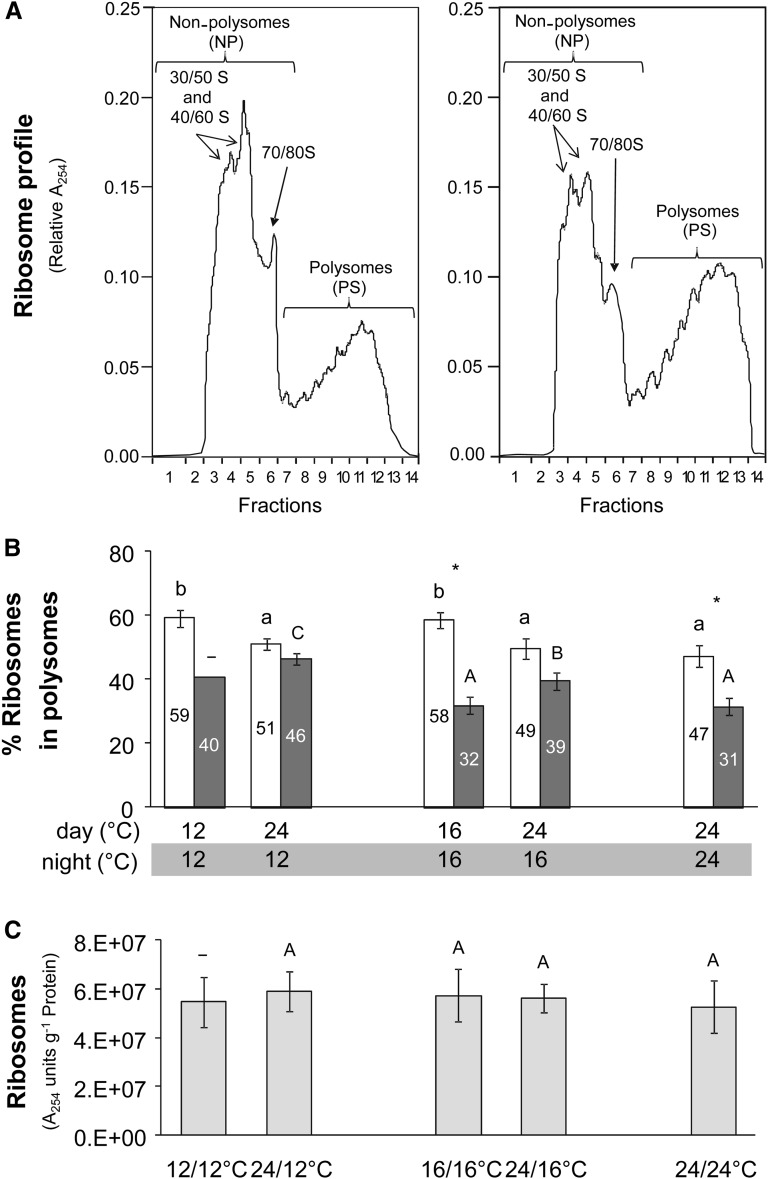
Polysome density gradients were performed using material harvested at ED and EN. Figure 8A shows typical examples for material harvested at EN from plants growing in a 24°C/24°C and a 24°C/12°C thermocycle. As ribosomes represent ∼90% of the total RNA in a cell (Warner, 1999), the distribution of RNA as monitored by A254 reflects the distribution of ribosomes in the gradient. Free ribosome subunits and monosomes remain at the top of the gradient (nonpolysome fraction), while polysomes move into the gradient in a mass-dependent manner, with the larger polysomes moving further down the gradient (polysome fraction). Visual inspection of Figure 8A reveals more polysomes at EN in the 24°C/12°C than the 24°C/24°C thermocycle.
Figure 8.
Polysome Loading Analysis.
Polysome gradients were performed for Col-0 plants grown in five different thermocycles, harvested at ED (white bars) and EN (dark-gray bars).
(A) Examples of the distribution of ribosomes in fractions collected from a density gradient obtained from plants harvested at EN and grown in a 24°C/24°C (left panel) and 24°C/12°C (right panel) thermocycle. RNA was measured as absorbance at 254 nm (A254). Free ribosomes and monosomes are on the left-hand side and increasingly large polysomes toward the right-hand side of the display.
(B) Estimated proportion of ribosomes in polysomes. This is calculated as (PS)/(NP + PS). The percentage is given as numbers in the figure panel. The results are the mean ± sd (n = 3), except in the case of 12°C/12°C at EN, where n = 1.
(C) Ribosome content, estimated from the sum of the ribosome profile at A254. The average of the ribosome number at ED and EN was calculated for each treatment. The results are given as mean ± sd (n = 4). One-way ANOVA was used to identify potential candidates for a statistically significant difference between treatments. After ANOVA P value correction using Holm’s method (P < 0.05), individual contrasts were then identified in a post-hoc Tukey HSD test (P < 0.05). They are indicated by different letters within the same time point (ED, lowercase; EN, uppercase). In the case of 12°C/12°C at EN, this analysis was not possible due to lack of replication (n = 1). Comparisons between ED and EN using a paired t test at a given thermocycle are indicated by an asterisk if significant (P < 0.05).
Similar analyses for all samples are summarized in Figure 8B. When the temperature was decreased from 24 to 16°C and 12°C across the entire light/dark cycle, there was a significant increase in polysome loading at ED (from 47 to 58% and 59%) and an apparently small but not significant increase at EN (from 31 to 32% and 40%). When only the daytime temperature was decreased, there was a small significant increase in polysome loading at ED (from 49 to 58% and from 51 to 59% in the 24°C/16°C versus 16°C/16°C and 24°C/12°C versus 12°C/12°C comparisons) but not at EN, when polysome loading appeared to decrease slightly but nonsignificantly (from 39 to 32% and from 46 to 40%, respectively). When only the night temperature was decreased, there was a significant and progressive increase in polysome loading at EN (from 31 to 39% and 46% at 24°C, 16°C, and 12°C, respectively), whereas the marginal increase in polysome loading at ED was not significant (from 47 to 49% and 51%, respectively).
As rRNA accounts for the vast majority of the total RNA, ribosome number is roughly proportional to total A254 in the gradient (Figure 8C). As the estimates did not vary significantly between ED and EN, we calculated the average across both time points. When the temperature was decreased from 24 to 16°C and 12°C across the entire light/dark cycle, ribosome content rose strongly and significantly by 38 and 71% on a FW basis (seeSupplemental Data Set 2 online), showed an apparent but nonsignificant increase (28 and 27%) on a DW basis, and did not change on a protein basis (Figure 8C). When the temperature was decreased from 24 to 16°C or 12°C in the daytime only, estimated ribosome content again rose significantly on a FW and DW basis (seeSupplemental Data Set 2 online) but not on a protein basis. When the temperature was decreased in the night only, ribosome content did not change significantly, even on a FW basis.
DISCUSSION
Biomass Accumulation Is Dependent on Daytime, and Not Night, Growth Temperature
The diurnal cycle provides a good experimental system to investigate how C metabolism and growth adjust to a fluctuating temperature regime. In the light, C is assimilated and is either used for growth or temporarily stored. At night, the stored C is remobilized to support metabolism and growth. Rapid growth depends on regulation of diurnal C fluxes to ensure that almost all the C is used for growth, while avoiding premature exhaustion of C before the EN. We asked how the reference species Arabidopsis achieves this balance in the face of changes in the ambient temperature. We restricted our study to plants growing in short-day conditions and moderately limiting light to focus on the response in C-limited plants and on temperatures between 12 and 24°C to avoid possible complications due to low- or high-temperature stress. In this temperature range, there was a more than threefold variation in biomass, which was almost exclusively dependent on the daytime temperature (Figure 1A). Thus, in this range of growth regimes, the overall growth rate of Arabidopsis is (1) set by the daytime temperature and (2) is completely compensated against low night temperature. This resembles earlier studies with other species, in which biomass was often largely determined by the daytime temperature (see Introduction; Rajan and Blackman, 1975; Gent and Enoch, 1983; Frantz et al., 2004).
Daytime and Nighttime Temperatures Have Different Effects on Metabolism, Leaf Morphology, and Protein Content
To understand why the overall growth rate responds differently to daytime and night temperature, we investigated A, R, the diurnal turnover of major C pools, protein content, and the levels of many individual proteins. We will first outline how these parameters respond to a change in the daytime or the night temperature and then discuss to what extent this explains the response of the growth rate.
Lower daytime temperatures resulted in lower A, while growth in lower night temperatures resulted in lower R. This resembles earlier studies, in which acclimation allowed only partial maintenance of A and R in low-temperature growth regimes (Lambers et al., 1998; Campbell et al., 2007; see Introduction). Some newly fixed C accumulates as starch and other C metabolites in the light and is remobilized to support metabolism and growth at night (Smith and Stitt, 2007; Graf and Smith, 2011). Despite the differences in A, R, and growth, the diurnal turnover of starch was similar in all thermocycles investigated (Figure 2A). This finding has two important implications. First, starch accumulation is largely buffered against changes in the daytime temperature and the resulting changes in A. Second, degradation must be regulated such that starch is almost completely exhausted at the EN in all thermocycles, including those with a cool night. This is unexpected because low temperature would be expected to slow down biochemical processes like starch synthesis and degradation. This conserved response is consistent with the idea that the amplitude and timing of starch turnover are system properties, which are maintained across a wide range of environmental conditions (Geiger and Servaites, 1994; Geiger et al., 2000; Smith and Stitt, 2007; Graf et al., 2010; Graf and Smith, 2011).
Acclimation to low temperature involves changes in leaf architecture and composition, including an increase in DWC (DW per unit FW), overall protein content, and leaf thickness (Larcher, 1995; Strand et al., 1999; Ercoli et al., 2004). Our results show that these changes in leaf architecture and composition, and especially the increase in protein, are a response to the temperature experienced in the light period rather than a response to low temperature per se. The 30% increase in DWC and the 50% increase in protein between 24 and 12°C were almost entirely driven by the daytime temperature (Figures 1C and 1D). The increase in DWC is partly explained by the increase in protein content. The remainder is presumably due to increased production of cell wall and other structural components. The twofold increase in thickness (or decrease in SLA; leaf area per unit DW) between 24 and 12°C was mainly driven by the daytime temperature (Figure 1E), although there was a small effect of night temperature, as seen earlier (Rajan and Blackman, 1975; Hurd and Enoch, 1976).
Functional Significance of the Increased Protein Content under Low Daytime Temperatures
Earlier studies interpreted the increase in overall protein content in terms of the acclimation of photosynthesis and respiration (see Introduction). During the acclimation of photosynthesis, there is an increase in the activities of many Calvin-Benson cycle enzymes on a FW basis. As photosynthesis proteins represent a major part of the total protein in a mature leaf, it was assumed that this was a major reason for the increase in the total protein content (Strand et al., 1999; Stitt and Hurry 2002). We have now used robotized enzyme activity analyses and LC-MS/MS to investigate how a wider range of enzymes and proteins respond to a decrease in growth temperature. Unexpectedly, there was a small decrease in representation of the Calvin-Benson cycle and photorespiration in low daytime temperature regimes. The activities of Rubisco, TK, NAD-GDH, and GS also decreased slightly when they were related to total protein (Figures 5A and 5B). A decrease in GLDH, GS, and GOGAT activity at 8 to 12°C was also reported by Usadel et al. (2008b). Many of the protein classes that remain stable or increase in abundance in lower daytime temperature are involved in growth, including amino acid metabolism, protein synthesis, nucleotide metabolism, and the cell cycle (Figure 5B; seeSupplemental Data Set 4 online). This implies that the general increase in protein also serves to compensate for a temperature-related decrease in the activities of proteins that utilize C for growth.
This increase in protein content will require upregulation of protein synthesis in growth regimes with a low daytime temperature. Transfer of Arabidopsis from 20°C to 12 to 14°C leads to a coordinated induction of genes for RNA processing, amino acid activation, cytosolic and mitochondrial ribosomal proteins, ribosome assembly, and translation initiation and elongation (Usadel et al., 2008b). Many low-temperature responses are orchestrated by the CBF family of transcription factors (Thomashow, 1999). They are transiently induced at temperatures as high as 14°C (Zarka et al., 2003) and contribute to adaptation to small changes in the temperature (Vogel et al., 2005; Usadel et al., 2008b). In agreement with this, several of the proteins whose abundance increased markedly at low temperature in our study are encoded by genes that are induced by the CBF program (Vogel et al., 2005). Furthermore, CBF expression is gated by the clock, such that low temperature leads to a stronger induction earlier in the 24 h cycle, at Zeitgeber time 4, than later (at Zeitgeber time 16) (Fowler et al., 2005; Dong et al., 2011). This would potentially provide an explanation as to why aspects of low temperature acclimation, like changes in leaf morphology and protein content, occur in response to low daytime temperature. However, many genes that are induced at low temperature, including genes for amino acid activation and protein synthesis, are not part of the core CBF regulon (Vogel et al., 2005). It is an open question if the CBF program interacts in some circumstances with further signaling programs to regulate a wider range of genes. An attractive hypothesis would be that signals generated by an imbalance between photosynthesis and growth and the resulting increase in C metabolites (see below) trigger a regulatory response that leads to an increase in the protein content. The decrease in GLDH activity at low temperature may also be related to an increase in C (Gibon et al., 2004a, 2009).
The slight decrease in Calvin-Benson cycle, photorespiration, and GOGAT pathway enzymes in low daytime temperature regimes might reflect a temperature-dependent shift in the relative rates of CO2 fixation and photorespiration. Decreasing temperature leads to an increase in Rubisco selectivity for CO2 compared with O2 (Sage and Kubien, 2007) and may favorably alter the relation between CO2 entry and fixation, decrease the rate of photorespiration, and allow a given net assimilation rate to be maintained with lower enzyme activities. The proportional decrease in GOGAT pathway enzyme activities may also reflect this decrease in photorespiration. In leaves of C3 plants, up to 90% of flux through this pathway involves reassimilation of NH4 that is released in photorespiration (Temple et al., 1998). Irrespective of these detailed considerations, the main conclusion from the enzyme and protein profiling is that the increase in protein content is due to increases in proteins involved in biosynthesis and growth in addition to those associated with photosynthesis.
Biomass Accumulation Is Decreased by a Lower Daytime Temperature Due to a Lower Rate of Photosynthesis and because Acclimation Involves a Trade-Off with Growth Efficiency
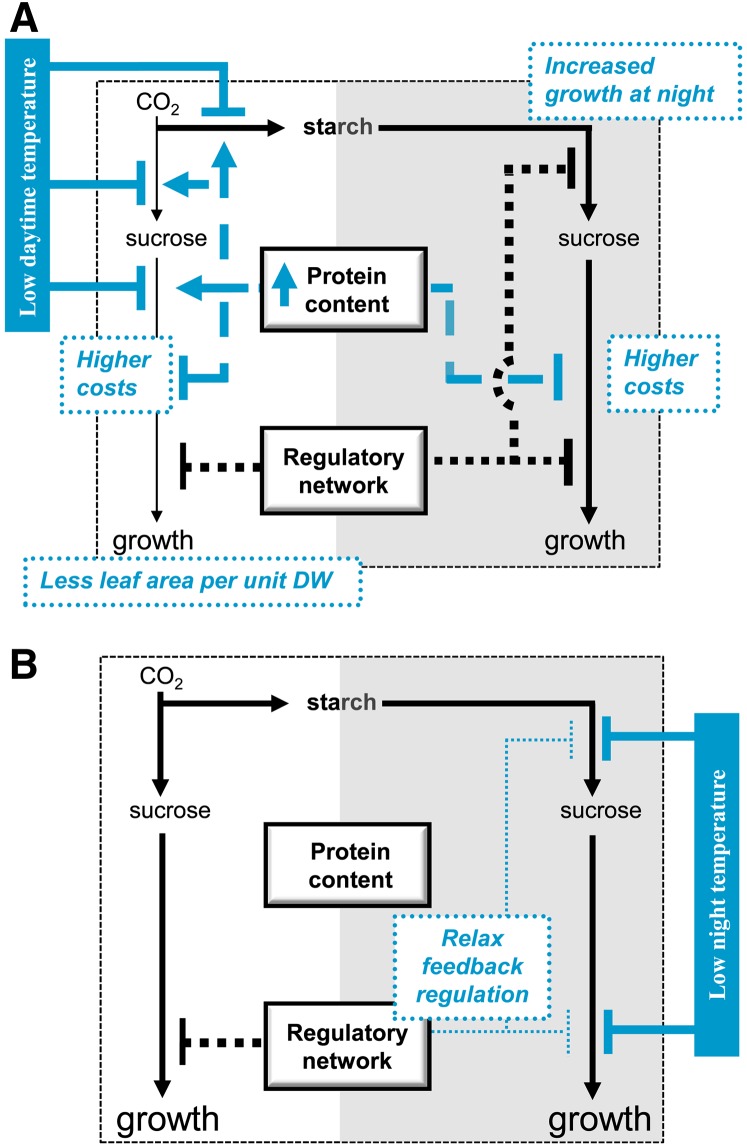
Figure 9A summarizes how low daytime temperature influences photosynthesis and C utilization and how these direct effects are modified by acclimation. Briefly, low daytime temperature decreases A. This is partially ameliorated by changes in leaf architecture and composition that result in a higher enzymatic capacity per unit FW or leaf area. Low daytime temperature also restricts metabolic pathways and cellular processes that use the fixed C for growth. This is also partly ameliorated by the general increase in protein content and partly by storing a larger proportion of the photosynthate and using it later for growth during the night. However, these acclimatory changes involve a trade-off. The decrease in leaf area and, hence, light interception per unit DW, results in a decrease in whole plant photosynthesis, while the increase in DWC and protein content increases growth costs. This multilayered response is discussed in the following paragraphs.
Figure 9.
Schematic Representation of the Effect of Growth Temperature on the Metabolism and Growth of Arabidopsis.
Pathways for C flow are indicated with solid lines, processes affected by a general increase in the protein content are indicated by dashed lines, and processes by clock-dependent regulation networks are indicated by dotted lines. Processes affected when the daytime (A) or the night (B) temperature is decreased are depicted in blue. External arrows indicate the direct effect of the decrease in temperature, and internal arrows the effect of acclimation of the protein content and adjustment of regulatory networks. The thickness of the lines, or the size of the letters, qualitatively depict the intensities. For simplicity, the effect of decreased maintenance respiration is not shown.
As outlined in the Introduction, while physical or photochemical processes like light absorption and CO2 entry are largely temperature independent, photosynthetic electron transport and downstream metabolic processes like CO2 fixation and end-product synthesis are slowed down by low temperatures. During acclimation, this is partly compensated for by the general increase in protein, which supports an increase in the capacity of many enzymes on a leaf area basis. Our results extend earlier studies at 4°C (Strand et al., 1997, 1999; Stitt and Hurry, 2002) by showing that this increase occurs at much more moderate temperatures, is exclusively driven by the daytime temperature and (see below) encompasses proteins involved in biosynthesis and growth.
The increases in DWC, leaf thickness, and protein content mean that less leaf area is produced per unit investment of DW; leaves growing in a daytime temperature of 16 and 12°C generate 11 to 33% and 48 to 53% less leaf area per unit DW than plants growing at 24°C. This will decrease light interception and, hence, whole-plant photosynthesis. The decrease in light absorption cannot be quantified because we did not measure how much light penetrated the leaf. However, at the light intensities used in our experiment, it is likely that most of the intercepted light is absorbed.
However, a central and novel feature of our results is that low daytime temperature has a larger effect on C utilization than on photosynthesis. In low daytime growth regimes, an increased proportion of the newly fixed C accumulates in starch (cf. Figures 2E and 3A, Table 1). This is accompanied by an increase in the levels of soluble C metabolites like sugars and organic acids (Figures 2B and 2D), showing that the increase in starch is due to decreased use of photosynthate for growth, rather than vice versa. Our model of diurnal C use reveals that, at a given temperature, growth is faster in the daytime than at night (Table 1). This implies that the machinery that converts C into biomass is used more intensively in the daytime than in the night and is consistent with the finding that utilization of C for growth is very sensitive to a decrease in the daytime temperature and rather insensitive to a decrease in the night temperature.
The direct inhibitory effect of low daytime temperature on C utilization may be offset partially by an increase in the levels of proteins involved in biosynthesis and growth. Low daytime temperatures also lead to an increase in polysome loading, indicating that an increased proportion of the protein synthesis capacity is being used (Figure 8B). However, protein synthesis is itself a large component of the cellular budget (Penning de Vries, 1975; Warner, 1999; Hachiya et al., 2007; Pace and Manahan, 2007; Piques et al., 2009). A higher protein content will contribute to a decrease in growth in low daytime temperature regimes by increasing the cost of newly formed tissue and, probably, increasing maintenance costs. An increase of other DW components may add further to the costs of growth. This points to a trade-off between temperature acclimation and growth costs. A reciprocal relationship between protein content and biomass accumulation has already been observed in a large panel of Arabidopsis accessions grown in short days (Sulpice et al., 2009, 2010). We now report a similar relationship in response to changes in the daytime temperature.
In summary, low daytime temperature has an effect on photosynthesis and, even more strongly, on the utilization of C for growth. The reduction in daytime C utilization is moderated by an increase in the levels of proteins that are involved in cellular growth processes and by increased use of this capacity. However, these adjustments are not fully compensated for and proportionally more C is accumulated for use at night. These studies were performed in limiting light intensities. It appears likely that a similar pattern will be retained at higher light intensities, but with additional complications at saturating light intensities, when additional factors related to high light stress and oxidative stress may lead to photoinhibition and inhibition of photosynthesis (Niyogi, 1999; Nishiyama et al., 2006; Lawlor and Tezara, 2009).
Starch Degradation and Biomass Accumulation Are Largely Independent of Night Temperature
The overall rate of biomass accumulation in Arabidopsis is largely independent of the night temperature, as previously seen for other species (see above). This is surprising from a mechanistic point of view. Growth requires the operation of enzymes that have Q10 values of 2 to 3 (Haschemeyer et al., 1979; Atkin et al., 2005; Lewis and Driedzic, 2007), and low night temperatures would be expected to have an adverse effect on biomass accumulation, especially in short-day conditions like those used in this study. Our study uncovers night-specific mechanisms that buffer metabolism and growth against moderate changes in temperature (Figure 9B).
One potential explanation for the insensitivity of growth to low night temperatures reported would be that there is a compensatory increase in growth in the daytime. Our detailed measurements of photosynthesis, respiration, and C metabolites allow us to model the rate of C utilization for growth in the light period and the night. Most unexpectedly, growth rates during the night are unaltered or even increase when the night temperature is decreased in the range between 24 and 12°C.
It has previously been discussed that temperature-dependent changes in R will influence growth at night. In our experiments, R decreased twofold between 24 and 16°C and fell marginally between 16 and 12°C, while the modeled rate of growth at night rose between 24 and 16°C and remained stable down to 12°C. The stoichiometry between growth and Rg is thought to be largely independent of temperature (Penning de Vries et al., 1979). Our results therefore indicate that maintenance respiration (Rm) rises sharply between 16 and 24°C, as seen in earlier studies (Gent and Enoch, 1983; Amthor, 2000). High Rm will compete with growth for stored C in warm nights: The percentage of the C stores that was respired increased from 35 to 45% at 12°C and 16°C to ∼70% at 24°C. However, on their own, changes in Rm do not explain how starch mobilization and growth are buffered against low night temperature.
Decreasing the night temperature from 24 to 12°C had no effect on the rate of starch degradation, while the modeled rate of nocturnal growth increased slightly (Figure 3D). In contrast with the response to low daytime temperature, low night temperature did not lead to an increase in total protein (Figure 1C), the abundance of proteins involved in respiratory metabolism, biosynthesis, and cellular growth (Figure 4), or ribosome abundance (Figure 8C). Our model of diurnal C use reveals why this is not necessary. At a given temperature, the rate of growth is lower in the night than in the daytime. This is especially so in warm regimes (Table 1). However, levels of proteins that are required for growth are not subject to marked diurnal changes (Figures 5 and 8; seeSupplemental Figure 3 andSupplemental Data Set 4 online; Piques et al., 2009). These results imply that there is unused capacity in the biosynthetic pathways and growth processes at night. We propose that feedback regulation restricts the mobilization of stored C and utilization of C for growth in warm nights and that this feedback regulation is relaxed when the night temperature is decreased (Figure 9B). This provides a simple conceptual model to understand how direct inhibitory effects of temperature on a plethora of proteins and processes can be counteracted at a system level.
This model is supported by our analysis of protein synthesis, a major and energetically costly component of cellular growth. In warm nights, most ribosomes are not loaded into polysomes (Piques et al., 2009), and in cool nights, there is an increase in polysome loading that partially compensates for the temperature-dependent inhibition of protein synthesis (Figure 8). While more studies are needed to uncover the mechanisms that allow continued translation initiation at lower temperature, this increase in polysome loading provides a flexible posttranslational mechanism to allow automatic buffering of a major component of C consumption and growth (Piques et al., 2009) against changes in the night temperature.
This model can also be integrated with recent advances in our understanding of the regulation of starch turnover. The rate of starch degradation is typically regulated to avoid periods of C depletion during the night (Smith and Stitt, 2007). Recently, Graf et al. (2010) showed that clock-dependent feedback inhibition sets the momentary rate of degradation such that starch is almost exhausted at dawn, as anticipated by the circadian clock. We propose that when the night temperature is decreased, this higher level feedback regulation is relaxed to compensate for the temperature-dependent decrease in the momentary activity of the individual enzymes and proteins that are involved in starch degradation. Our finding that there is almost immediate temperature compensation of starch degradation after increasing or decreasing the night temperature is consistent with this hypothesis. This rapid response also highlights how starch degradation is regulated by a highly flexible mechanism, which has the capacity to cope with the fluctuations in night temperatures that plants encounter in their natural environment.
More studies are needed to understand how starch degradation is regulated. Using the isa1 mutant, we showed that while phytoglycogen is degraded in a temperature-dependent manner, starch is degraded in a temperature-compensated manner in the same plants and indeed cells and chloroplasts. We conclude that temperature compensation requires a specific feature of the starch granule or, more likely, of the proteins that are involved in degradation of the starch granule. Related to this, the observation that phytoglycogen is rapidly degraded at the start of the night indicates that clock-dependent feedback inhibition of starch degradation (Graf et al., 2010; Graf and Smith, 2011; Yazdanbakhsh et al., 2011) also acts on processes involved in the mobilization of the starch granule.
A cycle of glucan phosphorylation (Kötting et al., 2009; Comparot-Moss et al., 2010) and dephosphorylation (Zeeman et al., 2007, 2010) is required for the initial attack on the starch granule, possibly by facilitating a phase transition that makes the surface of the crystalline starch granule accessible to attack by β-amylases (Hejazi et al., 2010). Low night temperatures led to an increase in transcripts for proteins involved in starch degradation, in particular in this cycle. Strikingly these transcripts increased in the light period, even when the temperature was decreased only in the night. This implies that the increase in transcripts is the result of an integrated response that operates across the entire 24-h cycle. While diurnal changes in transcripts often have little immediate effect on the level of the encoded proteins (Gibon et al., 2004b; Smith et al., 2004), it is likely that sustained changes over several days will lead to an increase of protein that partly compensates for the temperature-dependent decrease in enzyme activity. However, in view of the rapidity of the temperature compensation, our results imply that further, and at this time unknown, posttranslational mechanisms or inhibitory effectors regulate the rate of starch degradation.
Contrasting Effect of Daytime and Night Temperature on Respiration
Plant respiration releases half or more of the C that is assimilated by plants. At a global scale, this release into the atmosphere is equivalent ∼60 gigaton C per year, which is approximately sevenfold more CO2 than is released at this time by burning fossil fuels (Amthor, 1995; Friedlingstein et al., 2010). Gaining a better and predictive understanding of the temperature-dependence of respiration is important for modeling plant growth, crop yields, and the global C balance (Leakey et al., 2009; Mahecha et al., 2010; Reich, 2010). There has been considerable research into the temperature acclimation of respiration (see Introduction). One proposed mechanism relates to the higher protein content that is found in plants from many different functional groups when they are grown at low temperatures (Atkin et al., 2005; Campbell et al., 2007). Our finding that this increase is dependent on the daytime temperature but independent of the night temperature shows that the protein content–related increase in respiration is likely to be largely an indirect effect due to the added costs of synthesizing and maintaining protein that is required for photosynthesis and growth when at low daytime temperatures. Our and earlier (Rajan and Blackman, 1975) results also show that high night temperatures lead to changes in respiration that are independent of changes in the total protein content. These results imply that the effect of daytime and night temperature on respiration should be separately parameterized.
Concluding Remarks
When Arabidopsis is grown in a short photoperiod and limiting irradiance, growth is under almost exclusive control of the daytime temperature, while the night temperature has remarkably little effect on the rate at which stored C is remobilized and used for growth. To explain this remarkable temperature compensation, we propose that starch mobilization and growth are restricted by feedback regulation during warm nights and that this inhibition is alleviated when the night temperature is decreased. More generally, the strategy employed by C-limited Arabidopsis to regulate C allocation and growth during the diurnal cycle exhibit a remarkable combination of robustness and flexibility. Changes in the photoperiod, the light intensity, or the temperature will lead to marked, and usually uncontrollable, changes in the rate of photosynthesis and the amount of C fixed per day. These fluctuations are countered by regulating starch turnover to ensure that starch is almost, but not totally, exhausted at the EN. This pattern of starch turnover is maintained across a wide range of different photoperiods and light intensities (Geiger and Servaites, 1994; Smith and Stitt, 2007; Stitt et al., 2010; Graf and Smith, 2011) and different temperature regimes. Most importantly, it can be maintained in the face of sudden changes in the photoperiod (Graf et al., 2010) and night temperature. This robust system response ensures that C is immediately invested in growth, while minimizing the risk of deleterious periods of C starvation during the night. Further downstream, there is a considerable degree of flexibility in the timing of growth. In warm regimes, most growth occurs in the daytime, whereas in cooler thermocycles, a very considerable part of the growth occurs at night. Studies with mutants that are impaired in C export from the chloroplast in the light have also revealed a large flexibility in the timing of growth (Schneider et al., 2002; Walters et al., 2004). Temperature is an important, and often unpredictable, environmental variable. The ability to maintain a fixed pattern of starch turnover while allowing flexibility in the timing of growth may play an important role in optimizing growth in fluctuating temperature regimes. This study focused on Arabidopsis growing in C-limited conditions and a moderate temperature range. Further studies are needed to investigate how these responses are modified in long-day and high irradiance conditions and in more extreme temperature regimes and to investigate to what extent our findings can be extended to other species and life forms.
METHODS
Selection of Accessions
Arabidopsis thaliana accessions were selected from the set described by Sulpice et al. (2009) by choosing nine genotypes with low (Ang-0, Bla-11, Bu-2, Je-54, Mh-1, Petergof, RRS-7, Van-0, and Rubezhnoe-1) and nine genotypes with high (Bsch-2, Cl-0, Da-0, Ei-2, Kl-0, Kn-0, Lip-0, Old-1, and Wei-1) biomass in the applied short-day conditions, plus two reference accessions (Col-0 and C-24). To ensure homogeneity, single-seed propagation and bulk amplification was performed (Törjék et al., 2003).
Growth Conditions and Harvest
Growth conditions were exactly as described by Cross et al. (2006). Briefly, seeds were germinated and grown for the first 7 d with a daylength of 16 h, a temperature of 6°C at night and 20°C during day, a humidity of 75%, and a luminosity of 145 µmol m−2 s−1. After 7 d, seedlings were transferred to a phytotron. Growth was continued in an 8-h-light/16-h-dark regime at temperatures and humidity of 16°C and 75% at night and of 20°C and 60% during the day. Illumination was 145 µmol m−2 s−1. At 2 weeks, plants of average sizes were transferred to 10-cm-diameter pots (five plants per pot) filled with the same soil as for germination and grown for one further week in short-day conditions. Plant FW at this stage was 5.90, 9.33, and 9.77 mg/plant for Bu-2, Col-0, and Lip-0, respectively. Plants were then transferred to small growth cabinets with an 8-h-light/16-h-dark regime (160 µmol m−2 s−1) and temperature corresponding to the thermocycle tested. Pots were totally randomized to decrease positional effects. Plants were harvested 30 d after germination for the experiment using 20 accessions grown at constant 16 or 20°C, and 35 d after germination for the experiment using Bu-2, Col-0, and Lip-0 accessions grown in five thermocycles. Five independent samples per accession/treatment, each a pool of five whole rosettes, were harvested and immediately frozen in liquid N. Sampling was performed in the last hour of the day, or night, and completed within 1 h. Samples were powdered at −70°C using an automated grinding robot (Stitt et al., 2007) and stored at −80°C until use.
Respiration and Photosynthesis Measurements
Gas exchange measurements were made on full rosettes using the LI-6400XT portable photosynthesis system (LI-COR Biosciences). Plants were exposed to a CO2 concentration of 375 ppm for photosynthesis and 300 ppm for respiration measurements using the built-in LI-6400XT CO2 controller. Relative humidity was 60%. Photosynthesis was measured in the first hour of the day at a light intensity of 160 μmol m−2 s−1 at the day growth temperature of the plants. Dark respiration was measured in the last hour of the night at the respective night growth temperature of the plants. Five determinations were made per genotype/growth condition. Determinations were made after the plants have been in the growth thermocycle for 14 d (24°C/24°C), 16 d (24°C/16°C), 18 d (24°C/12°C), 23 d (16°C/16°C), or 29 d (12°C/12°C). Leaf area was determined using the MRI cell image analyzer (Montpellier RIO Imaging) to allow calculation of respiration and photosynthesis rates on a leaf area basis.
Metabolite and Enzyme Assays
Chemicals were purchased as described by Gibon et al. (2004b). Total protein was assayed using the Bradford method (Bradford, 1976). Starch, Glc, Fru, Suc, and total amino acids were determined by enzymatic assays in ethanolic extracts of 20 mg frozen plant material as described by Cross et al. (2006) and malate and fumarate as described by Nunes-Nesi et al. (2007). Assays were performed in 96-well microplates using a Janus pipetting robot (Perkin-Elmer). Absorbances were determined using a Synergy, an ELX-800, or an ELX-808 microplate reader (Bio-Tek). For all the assays, two technical replicates were determined per biological replicate. Average standard deviations calculated based on all determinations performed in this study were 3.54, 4.97, 4.67, 4.84, 3.17, 7.91, 8.51, and 12.5% for amino acids, malate, fumarate, protein, starch, Glc, Fru, and Suc, respectively.
For enzyme measurements, 20 mg of powdered frozen material were extracted by mixing with extraction buffer (Nunes-Nesi et al., 2007). AGPase, NAD-GAPDH, NADP-GAPDH, PK, SPS, TK, AlaAT, fumarase, NAD-GLDH, PEPc, NR, and GS were determined as described by Gibon et al. (2004a), NAD-MDH as described by Jenner et al. (2001), Rubisco as described by Sulpice et al. (2007), cytosolic phosphoglucose isomerase and plastidic phosphoglucose isomerase as described by Weeden and Gottlieb (1982), and PFK as described by Keurentjes et al. (2008). Enzyme activities were expressed on a protein basis by dividing enzyme activity (nmol min−1 g−1 FW) of an individual sample by the average total protein concentration (mg g−1 FW) of the corresponding thermocycle and time point.
Determination of Starch and Phytoglycogen in the isa1 Mutant
Starch and phytoglycogen were determined in isa1 mutants (line N542704; Nottingham Arabidopsis Stock Centre) by a modification of the perchloric acid method (Delatte et al., 2005; Wattebled et al., 2008). The levels of starch and phytoglycogen obtained in this study show much less biological variation than in previous studies because methods were optimized to allow full recovery of both starch and phytoglycogen. On ice, 20 mg of powdered frozen plant material were extracted by mixing with 500 μL of precooled 1.5 M perchloric acid. After 30 min incubation at 4°C, soluble and insoluble fractions were separated by centrifugation (3500g, 15 min, 4°C). The pellet was washed once with 250 μL of water and three times with 80% (v/v) of ethanol (centrifugation at 2000g, 5 min, room temperature), and finally resuspended in 200 μL of 0.05 M acetate/NaOH, pH 4.9. Of the supernatant, which contained the phytoglycogen, 400 μL was adjusted to pH 5.0 by adding 2 M KOH, 0.4 M MES, and 0.4 M KCl. In contrast with Delatte et al. (2005), the precipitated potassium perchlorate was not removed after centrifugation (2000g, 5 min, room temperature) as this was found to result in overall lower amounts of phytoglycogen being measured, probably because a significant fraction of the phytoglycogen was trapped in the salt. Sugars were measured (Cross et al., 2006) in the clear supernatant. Subsequently, the remaining supernatant and the resuspended pellet were heated at 120°C for 30 min to solubilize phytoglycogen and starch, respectively. Both were incubated overnight on a plate shaker (180 rpm) with α-amylase and amyloglucosidase at 37°C. After centrifugation (2000g, 5 min, room temperature), Glc was determined as described by Cross et al. (2006). Amounts of phytoglycogen were corrected for the amounts of Glc present in the supernatant prior to the digest.
Protein Quantification by LC-MS/MS
Whole rosette protein identification and quantification by LC-MS/MS was as described by Piques et al. (2009). Database search by Mascot algorithm (version 2.2.0; Matrix Science) was used to obtain emPAI protein abundance index values (Ishihama et al., 2005). In total, emPAI values were obtained for 1176 proteins in at least one of the conditions/time points. To estimate the abundance of proteins in the rosette, all 1176 emPAI index values were normalized against the total sum of emPAI values of all 1176 proteins identified in the leaf extract to calculate the molar fraction of each protein in the sample (mol %). In general, as only a small proportion of all proteins of the leaf were identified, abundance values calculated from emPAI will result in overestimation of the true amounts. The full list of identified peptides is available inSupplemental Data Set 4 online. Biological replicates were averaged for each thermocycle and time point. Proteins that could not be detected in >50% of the measurements were excluded, resulting in a reduced set of 268 proteins that was further analyzed. These 268 proteins were grouped into major MapMan functional classes (Thimm et al., 2004; Usadel et al., 2005). To obtain the relative abundance of each functional class at each time point and thermocycle, the relative abundance of each protein was summed. To calculate the standard deviations, for each protein within a category, the global average across all thermocycles and harvest time points was calculated and all molar fractions were then expressed relative to this global average. By averaging these relative abundances (mol %) of all proteins belonging to a functional class, the standard deviation across all proteins within this functional class was determined and used to test for significant differences between all thermocycles and time points.
Quantification of Transcript Levels by qRT-PCR
Total RNA was isolated from 20 mg of homogenized plant material using the RNeasy Plant mini Kit (Qiagen) according to the manufacturer’s protocol. For normalization and absolute quantification of the transcript levels, a mix of seven artificial poly(A)+ RNAs (ArrayControl RNA spikes; Applied Biosystems/Ambion) were spiked into the samples after lysis of the material. Concentrations of the spike-in controls were adjusted to generate a calibration curve from 3.0E+11 to 1.3E+08 copy number per gram FW (copy number⋅g−1 FW) (seeSupplemental Data Set 5 online). Before and after DNaseI digestion (Turbo DNA-free DNase I; Applied Biosystems/Ambion), RNA concentration and integrity were measured using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies) and an Agilent-2100 Bioanalyzer using RNA 6000 NanoChips (Agilent Technologies). cDNA was synthesized from 250 ng total RNA and quality checked as described by Piques et al. (2009). Primers were designed using the PRIME program of GCG Wisconsin Package, version 10.2, and synthesized at MWG Biotech. qRT-PCR reactions were performed and data analysis was performed as described by Piques et al. (2009). All standard curves, derived from the seven spike-in controls, had R2 values higher than 0.99 and were used to calculate the concentration of the mRNA as copy number⋅g−1 FW.
Statistics
Standard procedures were performed using functions of the Microsoft Excel program. To partition the variance into genetic and environmental variance separately for each trait k, measured in the 20 accessions grown at constant day/night temperatures of 16°C/16°C or 20°C/20°C, ANOVA was performed using the R software (R Development Core Team, 2008) with the following alternative models tested:
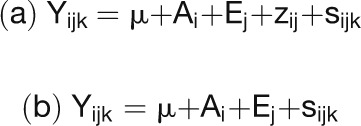 |
where Yijk are the experimental responses, µ is the general mean, Ai is the effect of the ith accession genotype, Ej is the effect of the jth environmental condition, zij is the interaction term between the ith accession genotype and the jth environmental condition, and sjik is the error of Yijk. Both alternative models were tested, and the Akaike’s information criterion, which was extracted as implemented in R (Venables and Ripley, 2002), was used to select the better performing model by having the lowest Akaike’s information criterion.
To identify protein functional classes with statistically significant differences in protein abundances (Y) between thermocycles at a given time point, the two-way ANOVA procedure was used. The individual protein and the environmental variance were partitioned separately for each protein functional class k, determined in Col-0 grown in five different thermocycles. The following model was tested:
where Yijk are the experimental responses, µ is the general mean, Ai is the effect of the ith thermocycle, Ej is the effect of the jth protein within the functional class, zij is the interaction term between the ith thermocycle and the jth protein within the functional class, and sjik is the error of Yijk. Significant changes in functional classes were identified by correcting ANOVA P values using Holm’s method (Holm, 1979) (P < 0.05). For significant functional classes, significant contrasts of thermocycles were subsequently identified in a post-hoc Tukey honestly significant difference (HSD) test (P < 0.05). For all protein functional classes, comparisons between ED and EN were done using a paired t test for all thermocycles.
To identify statistically significant differences of enzyme activities between thermocycles at a given time point, one-way ANOVAs with the following model were used:
where Yijk are the experimental responses (activity of the enzyme in j replicates), µ is the general mean, Ai is the effect of the ith thermocycle, and sji is the error of Yij. For each accession, 17 ANOVA models were built accordingly. Enzymes showing significant changes in activities were identified by correcting ANOVA P values using Holm’s method (P < 0.05). For enzymes with significant activity changes, significant contrasts of thermocycles were subsequently identified in a post-hoc Tukey HSD test (P < 0.05). For all 17 enzymes, comparisons between ED and EN were done using a paired t test for all thermocycles.
The same procedure as for the enzyme activities was used to find statistically significant differences in the qRT-PCR transcript data of the 14 and 11 enzymes involved in starch synthesis and breakdown, respectively. The ANOVA model is as above, with j then being the replicates of transcript copy number⋅g−1 DW and Yijk being the experimental responses (copy number⋅g−1 DW of the enzyme transcript). For metabolites, FW, DW, respiration, photosynthesis, and the percentage of ribosomes in the polysomes, the one-way ANOVA model was adapted accordingly.
The statistical comparison of the ribosome content between the thermocycles at a given time point was performed applying a Student’s two-tailed heteroscedastic t test using the Microsoft Excel program. The comparisons of the percentage of ribosomes in the polysomes between ED and EN for all thermocycles were done using a two-tailed Student’s paired t test. The P values were corrected using Bonferroni’s method and were considered statistically significant when <0.05.
Ribosome Loading Analysis
Polysomes were fractionated from crude leaf extracts as described by Piques et al. (2009) but using 50 mg FW. The gradients were fractionated using a Programmable Density Gradient Fractionation System (Teledyne Isco) to continuously record absorbance at 254 nm (ribosome profile). Polysome levels were determined from the area under the polysome profile after subtracting gradient baseline absorbance. The area of each polysome profile was normalized to an equal value to account for differences in sample loading. Levels of nonpolysomes (gradient region containing mRNA-protein-complexes complexes, 40/60S and 30/50S ribosome subunits, and 70/80S monosomes) and polysomes (gradient region containing more than two ribosomes per mRNA were determined by calculating corresponding peak areas of gradient regions). Areas corresponding to nonpolysome and polysome fractions were reported as a percentage of the total area under the profile. Total ribosome content was estimated from the total absorbance at 254 nm of the ribosome profiles after subtracting gradient baseline absorbance.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Box Plots of Fresh Weight, Protein, Starch, Soluble Sugars, and Amino Acids in 20 Arabidopsis Accessions and Correlations between Protein and Rosette Fresh Weight.
Supplemental Figure 2. Correlation Plots between the Protein Amount and the DW for the Three Accessions Grown in the Five Thermocycles.
Supplemental Figure 3. Variation of 17 Representative Primary Metabolism Enzyme Activities Determined in Rosettes of Three Accessions Grown in Five Different Thermocycles.
Supplemental Figure 4. Changes in Protein Levels.
Supplemental Figure 5. Response of Transcripts for Proteins Involved in Starch Synthesis and Breakdown.
Supplemental Figure 6. Responses of Metabolites and Protein and Enzyme Activities to a Sudden Change in the Temperature.
Supplemental Data Set 1. Metabolite Levels and Passport Data of the 20 Arabidopsis Accessions Grown in 16 or 20°C Daily Constant Temperatures and Two-Way ANOVA to Distinguish Genetic and Environmental Influences on Metabolic Traits.
Supplemental Data Set 2. Phenotypic Data, Metabolite Amounts, Ribosome Levels and Loading, and Enzyme Activities of the Three Accessions Col-0, Bu-2, and Lip-0 Grown in Five Thermocycles for ED and EN.
Supplemental Data Set 3. Estimation of Carbon Allocation in the Daytime and the Night.
Supplemental Data Set 4.Molar Fractions (mol %) of Proteins in Col-0 Samples Grown in the Five Thermocycles.
Supplemental Data Set 5. Absolute Transcript Levels of a Set of Genes Involved in Starch Metabolism.
Supplemental Data Set 6. Starch Metabolism after a Sudden Change in the Temperature.
Acknowledgments
We thank Beatrice Encke and Nicole Krohn for assays of metabolites and enzyme activities, Melanie Hoehne for qRT-PCR assays, and Christin Abel for excellent care of the plants. We also thank Bjorn Usadel for advice on statistics. This research was supported by the Max Planck Society and the German Ministry for Education and Research (GABI-GNADE 0315060E, GoFORSYS). We acknowledge financial support of the European Commission FP7 collaborative project TiMet under Contract 245143.
AUTHOR CONTRIBUTIONS
R.S., E.-T.P., and M.S. designed the research. E.-T.P., M.P., A.I., W.S., and H.I. performed research. E.-T.P., M.P., A.I., W.S., H.I., M.S., and R.S. analyzed data. R.S. and M.S. wrote the article.
Glossary
- FW
fresh weight
- ANOVA
analysis of variance
- Col-0
Columbia-0
- Lip-0
to be defined
- Bu-2
to be defined
- ED
end of the day
- EN
end of the night
- DW
dry weight
- DWC
dry weight content
- SLA
specific leaf area
- RGR
relative growth rate
- TCA
to be defined
- Rubisco
ribulose-1,5-bis-phosphate carboxylase/oxygenase
- TK
transketolase
- GS
Gln synthetase
- NAD-GDH
NAD-Glu dehydrogenase
- PEPc
to be defined
- AGPase
ADP-Glc pyrophosphorylase
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- qRT-PCR
quantitative RT-PCR
- Rg
growth respiration
- Rg
growth respiration
- Rm
maintenance respiration
- Rm
maintenance respiration
- HSD
to be defined
Footnotes
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Amthor J.S. (1995). Terrestrial higher-plant response to increasing atmospheric [Co2] in relation to the global carbon-cycle. Glob. Change Biol. 1: 243–274 [Google Scholar]
- Amthor J.S. (2000). The McCree-de Wit-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann. Bot. (Lond.) 86: 1–20 [Google Scholar]
- ap Rees T., Burrell M.M., Entwistle T.G., Hammond J.B., Kirk D., Kruger N.J. (1988). Effects of low temperature on the respiratory metabolism of carbohydrates by plants. Symp. Soc. Exp. Biol. 42: 377–393 [PubMed] [Google Scholar]
- Armstrong A.F., Wardlaw K.D., Atkin O.K. (2007). Assessing the relationship between respiratory acclimation to the cold and photosystem II redox poise in Arabidopsis thaliana. Plant Cell Environ. 30: 1513–1522 [DOI] [PubMed] [Google Scholar]
- Artuso A., Guidi L., Soldatini G.F., Pardossi A., Tognoni F. (2000). The influence of chilling on photosynthesis and activities of some enzymes of sucrose metabolism in Lycopersicon esculentum Mill. Acta Physiol. Plant. 22: 95–101 [Google Scholar]
- Atkin O.K., Atkinson L.J., Fisher R.A., Campbell C.D., Zaragoza-Castells J., Pitchford J.W., Woodward F.I., Hurry V. (2008). Using temperature-dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climate-vegetation model. Glob. Change Biol. Bioenergy 14: 2709–2726 [Google Scholar]
- Atkin O.K., Bruhn D., Hurry V.M., Tjoelker M.G. (2005). The hot and the cold: Unravelling the variable response of plant respiration to temperature. Funct. Plant Biol. 32: 87–105 [DOI] [PubMed] [Google Scholar]
- Atkin O.K., Loveys B.R., Atkinson L.J., Pons T.L. (2006). Phenotypic plasticity and growth temperature: understanding interspecific variability. J. Exp. Bot. 57: 267–281 [DOI] [PubMed] [Google Scholar]
- Atkin O.K., Tjoelker M.G. (2003). Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 8: 343–351 [DOI] [PubMed] [Google Scholar]
- Atkinson L.J., Campbell C.D., Zaragoza-Castells J., Hurry V., Atkin O.K. (2010). Impact of growth temperature on scaling relationships linking photosynthetic metabolism to leaf functional traits. Funct. Ecol. 24: 1181–1191 [Google Scholar]
- Atkinson L.J., Hellicar M.A., Fitter A.H., Atkin O.K. (2007). Impact of temperature on the relationship between respiration and nitrogen concentration in roots: an analysis of scaling relationships, Q10 values and thermal acclimation ratios. New Phytol. 173: 110–120 [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Campbell C., Atkinson L., Zaragoza-Castells J., Lundmark M., Atkin O., Hurry V. (2007). Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. New Phytol. 176: 375–389 [DOI] [PubMed] [Google Scholar]
- Chia D.W., Yoder T.J., Reiter W.D., Gibson S.I. (2000). Fumaric acid: An overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751 [DOI] [PubMed] [Google Scholar]
- Comparot-Moss S., et al. (2010). A putative phosphatase, LSF1, is required for normal starch turnover in Arabidopsis leaves. Plant Physiol. 152: 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J.M., von Korff M., Altmann T., Bartzetko L., Sulpice R., Gibon Y., Palacios N., Stitt M. (2006). Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol. 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J.E. (1965). Leaf growth in Phaseolus vulgaris. 2. Temperature Effects and Light Factor. Ann. Bot. (Lond.) 29: 293–308 [Google Scholar]
- Delatte T., Trevisan M., Parker M.L., Zeeman S.C. (2005). Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J. 41: 815–830 [DOI] [PubMed] [Google Scholar]
- Dong M.A., Farré E.M., Thomashow M.F. (2011). Circadian clock-associated 1 and late elongated hypocotyl regulate expression of the C-repeat binding factor (CBF) pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 7241–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercoli L., Mariotti M., Masoni A., Arduini I. (2004). Growth responses of sorghum plants to chilling temperature and duration of exposure. Eur. J. Agric. 21: 93–103 [Google Scholar]
- Evans G.C. (1972). The Quantitative Analysis of Plant Growth. (Los Angeles, CA: Oxford Blackwell Scientific; ). [Google Scholar]
- Fowler S.G., Cook D., Thomashow M.F. (2005). Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol. 137: 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J.M., Cometti N.N., Bugbee B. (2004). Night temperature has a minimal effect on respiration and growth in rapidly growing plants. Ann. Bot. (Lond.) 94: 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlingstein P., Houghton R.A., Marland G., Hackler J., Boden T.A., Conway T.J., Canadell J.G., Raupach M.R., Ciais P., Le Quere C. (2010). Update on CO2 emissions. Nat. Geosci. 3: 811–812 [Google Scholar]
- Fulton D.C., et al. (2008). Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell 20: 1040–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D.R., Servaites J.C. (1994). Diurnal regulation of photosynthetic carbon metabolism in C-3 plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 235–256 [Google Scholar]
- Geiger D.R., Servaites J.C., Fuchs M.A. (2000). Role of starch in carbon translocation and partitioning at the plant level. Aust. J. Plant Physiol. 27: 571–582 [Google Scholar]
- Gent M.P.N., Enoch H.Z. (1983). Temperature dependence of vegetative growth and dark respiration: A mathematical model. Plant Physiol. 71: 562–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Blaesing O.E., Hannemann J., Carillo P., Höhne M., Hendriks J.H.M., Palacios N., Cross J., Selbig J., Stitt M. (2004a). A Robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: Comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y., Bläsing O.E., Palacios-Rojas N., Pankovic D., Hendriks J.H., Fisahn J., Höhne M., Günther M., Stitt M. (2004b). Adjustment of diurnal starch turnover to short days: Depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. Plant J. 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Gibon Y., Pyl E.T., Sulpice R., Lunn J.E., Höhne M., Günther M., Stitt M. (2009). Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ. 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Gibon Y., Usadel B., Blaesing O.E., Kamlage B., Hoehne M., Trethewey R., Stitt M. (2006). Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol. 7: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A., Schlereth A., Stitt M., Smith A.M. (2010). Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc. Natl. Acad. Sci. USA 107: 9458–9463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf A., Smith A.M. (2011). Starch and the clock: The dark side of plant productivity. Trends Plant Sci. 16: 169–175 [DOI] [PubMed] [Google Scholar]
- Griffith M., Huner N.P.A., Espelie K.E., Kolattukudy P.E. (1985). Lipid polymers accumulate in the epidermis and mestome sheath cell walls during low-temperature development of winter rye leaves. Protoplasma 125: 53–64 [Google Scholar]
- Hachiya T., Terashima I., Noguchi K. (2007). Increase in respiratory cost at high growth temperature is attributed to high protein turnover cost in Petunia x hybrida petals. Plant Cell Environ. 30: 1269–1283 [DOI] [PubMed] [Google Scholar]
- Haschemeyer A.E.V., Persell R., Smith M.A.K. (1979). Effect of temperature on protein synthesis in fish of the Galapagos and Perlas Islands. Comp. Biochem. Physiol. B 64: 91–95 [DOI] [PubMed] [Google Scholar]
- Hejazi M., Fettke J., Kötting O., Zeeman S.C., Steup M. (2010). The Laforin-like dual-specificity phosphatase SEX4 from Arabidopsis hydrolyzes both C6- and C3-phosphate esters introduced by starch-related dikinases and thereby affects phase transition of alpha-glucans. Plant Physiol. 152: 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W.A., Poorter H. (2002). Avoiding bias in calculations of relative growth rate. Ann. Bot. (Lond.) 90: 37–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. (1979). A simple sequentially rejective multiple test procedure. Scand. J. Statist. 6: 65–70 [Google Scholar]
- Huner N.P.A., Palta J.P., Li P.H., Carter J.V. (1981). Anatomical changes in leaves of puma rye in response to growth at cold-hardening temperatures. Bot. Gaz. 142: 55–62 [Google Scholar]
- Hurd R.G., Enoch H.Z. (1976). Effect of night temperature on photosynthesis, transpiration, and growth of spray carnations. J. Exp. Bot. 27: 695–703 [Google Scholar]
- Hussey G. (1965). Growth and development in young tomato. 3. Effect of night and day temperatures on vegetative growth. J. Exp. Bot. 16: 373–385 [Google Scholar]
- Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. (2005). Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4: 1265–1272 [DOI] [PubMed] [Google Scholar]
- Jenner H.L., Winning B.M., Millar A.H., Tomlinson K.L., Leaver C.J., Hill S.A. (2001). NAD malic enzyme and the control of carbohydrate metabolism in potato tubers. Plant Physiol. 126: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keurentjes J.J.B., Sulpice R., Gibon Y., Steinhauser M.C., Fu J.Y., Koornneef M., Stitt M., Vreugdenhil D. (2008). Integrative analyses of genetic variation in enzyme activities of primary carbohydrate metabolism reveal distinct modes of regulation in Arabidopsis thaliana. Genome Biol. 9: R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. (2005). Climate change: The science and the policy. J. Appl. Ecol. 42: 779–783 [Google Scholar]
- Kötting O., Santelia D., Edner C., Eicke S., Marthaler T., Gentry M.S., Comparot-Moss S., Chen J., Smith A.M., Steup M., Ritte G., Zeeman S.C. (2009). STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 21: 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H., Chapin F.S.I., Pons T.L. (1998). Plant Physiological Ecology. (New York: Springer; ). [Google Scholar]
- Larcher W. (1995). Physiological Plant Ecology. (Berlin: Springer; ). [Google Scholar]
- Lawlor D.W. (2005). Plant responses to climate change: Impacts and adaptation. In Plant Responses to Air Pollution and Global Change, K. Omasa, I. Nouchi, and L.J. De Kock, eds (New York: Springer), pp. 81–88
- Lawlor D.W., Tezara W. (2009). Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes. Ann. Bot. (Lond.) 103: 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey A.D.B., Ainsworth E.A., Bernacchi C.J., Rogers A., Long S.P., Ort D.R. (2009). Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 60: 2859–2876 [DOI] [PubMed] [Google Scholar]
- Lewis J.M., Driedzic W.R. (2007). Tissue-specific changes in protein synthesis associated with seasonal metabolic depression and recovery in the north temperate labrid, Tautogolabrus adspersus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R474–R481 [DOI] [PubMed] [Google Scholar]
- Loveys B.R., Atkinson L.J., Sherlock D.J., Roberts R.L., Fitter A.H., Atkin O.K. (2003). Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob. Change Biol. Bioenergy 9: 895–910 [Google Scholar]
- Loveys B.R., Scheurwater I., Pons T.L., Fitter A.H., Atkin O.K. (2002). Growth temperature influences the underlying components of relative growth rate: An investigation using inherently fast- and slow-growing plant species. Plant Cell Environ. 25: 975–987 [Google Scholar]
- Mahecha M.D., et al. (2010). Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 329: 838–840 [DOI] [PubMed] [Google Scholar]
- Murray D.R. (1995). Plant-responses to carbon-dioxide. Am. J. Bot. 82: 690–697 [Google Scholar]
- Nishiyama Y., Allakhverdiev S.I., Murata N. (2006). A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim. Biophys. Acta. 1757: 742–749 [DOI] [PubMed] [Google Scholar]
- Niyogi K.K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A., Carrari F., Gibon Y., Sulpice R., Lytovchenko A., Fisahn J., Graham J., Ratcliffe R.G., Sweetlove L.J., Fernie A.R. (2007). Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Pace D.A., Manahan D.T. (2007). Cost of protein synthesis and energy allocation during development of antarctic sea urchin embryos and larvae. Biol. Bull. 212: 115–129 [DOI] [PubMed] [Google Scholar]
- Pantin F., Simonneau T., Rolland G., Dauzat M., Muller B. (2011). Control of leaf expansion: A developmental switch from metabolics to hydraulics. Plant Physiol. 156: 803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning de Vries F.W., Brunsting A.H., van Laar H.H. (1974). Products, requirements and efficiency of biosynthesis: a quantitative approach. J. Theor. Biol. 45: 339–377 [DOI] [PubMed] [Google Scholar]
- Penning de Vries F.W.T. (1975). Cost of maintenance processes in plant cells. Ann. Bot. (Lond.) 39: 77–92 [Google Scholar]
- Penning de Vries F.W.T., Witlage J.M., Kremer D. (1979). Rates of respiration and of increase in structural dry matter in young wheat, ryegrass and maize plants in relation to temperature, to water stress and to their sugar content. Ann. Bot. (Lond.) 44: 595–609 [Google Scholar]
- Piques M., Schulze W.X., Höhne M., Usadel B., Gibon Y., Rohwer J., Stitt M. (2009). Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Mol. Syst. Biol. 5: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiré R., Wiese-Klinkenberg A., Parent B., Mielewczik M., Schurr U., Tardieu F., Walter A. (2010). Diel time-courses of leaf growth in monocot and dicot species: Endogenous rhythms and temperature effects. J. Exp. Bot. 61: 1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pracharoenwattana I., Zhou W., Keech O., Francisco P.B., Udomchalothorn T., Tschoep H., Stitt M., Gibon Y., Smith S.M. (2010). Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J. 62: 785–795 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008). R: A Language and Environment for Statistical Computing. (Vienna, Austria: R Foundation for Statistical Computing; ). [Google Scholar]
- Rajan A.K., Blackman G.E. (1975). Interacting effects of light and day and night temperatures on growth of 4 species in vegetative phase. Ann. Bot. (Lond.) 39: 733–743 [Google Scholar]
- Reich P.B. (2010). Climate Change. The carbon dioxide exchange. Science 329: 774–775 [DOI] [PubMed] [Google Scholar]
- Sage R.F., Kubien D.S. (2007). The temperature response of C(3) and C(4) photosynthesis. Plant Cell Environ. 30: 1086–1106 [DOI] [PubMed] [Google Scholar]
- Schneider A., Häusler R.E., Kolukisaoglu U., Kunze R., van der Graaff E., Schwacke R., Catoni E., Desimone M., Flügge U.I. (2002). An Arabidopsis thaliana knock-out mutant of the chloroplast triose phosphate/phosphate translocator is severely compromised only when starch synthesis, but not starch mobilisation is abolished. Plant J. 32: 685–699 [DOI] [PubMed] [Google Scholar]
- Sharkey T.D., Laporte M.M., Micallef B.J., Shewmaker C.K., Oakes J.V. (1995). Sucrose synthesis, temperature, and plant yield. In Photosynthesis: From Light to Biosphere, Vol. 5, P. Mathis, ed (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 635–640 [Google Scholar]
- Smith A.M., Stitt M. (2007). Coordination of carbon supply and plant growth. Plant Cell Environ. 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Smith J.H.C. (1943). Molecular equivalence of carbohydrates to carbon dioxide in photosynthesis. Plant Physiol. 18: 207–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fulton D.C., Chia T., Thorneycroft D., Chapple A., Dunstan H., Hylton C., Zeeman S.C., Smith A.M. (2004). Diurnal changes in the transcriptome encoding enzymes of starch metabolism provide evidence for both transcriptional and posttranscriptional regulation of starch metabolism in Arabidopsis leaves. Plant Physiol. 136: 2687–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Grosse H. (1988). Interactions between sucrose synthesis and Co2 fixation. 4. Temperature-dependent adjustment of the relation between sucrose synthesis and Co2 fixation. J. Plant Physiol. 133: 392–400 [Google Scholar]
- Stitt M., Hurry V. (2002). A plant for all seasons: Alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr. Opin. Plant Biol. 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Stitt M., Lunn J., Usadel B. (2010). Arabidopsis and primary photosynthetic metabolism - More than the icing on the cake. Plant J. 61: 1067–1091 [DOI] [PubMed] [Google Scholar]
- Stitt M., Sulpice R., Gibon Y., Whitwell A., Skilbeck R., Parker S., Ellison R.(2007). Cryogenic grinder system. Patent number 0402-3754 ZBC, Germany [Google Scholar]
- Strand A., Foyer C.H., Gustafsson P., Gardestrom P., Hurry V. (2003). Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ. 26: 523–535 [Google Scholar]
- Strand A., Hurry V., Gustafsson P., Gardeström P. (1997). Development of Arabidopsis thaliana leaves at low temperatures releases the suppression of photosynthesis and photosynthetic gene expression despite the accumulation of soluble carbohydrates. Plant J. 12: 605–614 [DOI] [PubMed] [Google Scholar]
- Strand A., Hurry V., Henkes S., Huner N., Gustafsson P., Gardeström P., Stitt M. (1999). Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol. 119: 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R., et al. (2009). Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 106: 10348–10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R., et al. (2010). Network analysis of enzyme activities and metabolite levels and their relationship to biomass in a large panel of Arabidopsis accessions. Plant Cell 22: 2872–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R., Tschoep H., VON Korff M., Büssis D., Usadel B., Höhne M., Witucka-Wall H., Altmann T., Stitt M., Gibon Y. (2007). Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ. 30: 1163–1175 [DOI] [PubMed] [Google Scholar]
- Takanashi J., Maruyama S., Kabaki N., Tajima K. (1987). Temperature dependency of protein synthesis by cell-free system constructed with polysomes from rice radicle. Jpn. J. Crop. Sci. 56: 44–50 [Google Scholar]
- Temple S.J., Vance C.P., Gantt J.S. (1998). Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 3: 51–56 [Google Scholar]
- Thimm O., Bläsing O., Gibon Y., Nagel A., Meyer S., Krüger P., Selbig J., Müller L.A., Rhee S.Y., Stitt M. (2004). MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Thomashow M.F. (1999). Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 571–599 [DOI] [PubMed] [Google Scholar]
- Tjoelker M.G., Oleksyn J., Reich P.B. (1999). Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob. Change Biol. Bioenergy 5: 679–691 [Google Scholar]
- Tjoelker M.G., Oleksyn J., Reich P.B., Zytkowiak R. (2008). Coupling of respiration, nitrogen, and sugars underlies convergent temperature acclimation in Pinus banksiana across wide-ranging sites and populations. Glob. Change Biol. Bioenergy 14: 782–797 [Google Scholar]
- Törjék O., Berger D., Meyer R.C., Müssig C., Schmid K.J., Rosleff Sörensen T., Weisshaar B., Mitchell-Olds T., Altmann T. (2003). Establishment of a high-efficiency SNP-based framework marker set for Arabidopsis. Plant J. 36: 122–140 [DOI] [PubMed] [Google Scholar]
- Tschoep H., Gibon Y., Carillo P., Armengaud P., Szecowka M., Nunes-Nesi A., Fernie A.R., Koehl K., Stitt M. (2009). Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ. 32: 300–318 [DOI] [PubMed] [Google Scholar]
- Usadel B., Bläsing O.E., Gibon Y., Poree F., Höhne M., Günter M., Trethewey R., Kamlage B., Poorter H., Stitt M. (2008b). Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant Cell Environ. 31: 518–547 [DOI] [PubMed] [Google Scholar]
- Usadel B., Bläsing O.E., Gibon Y., Retzlaff K., Höhne M., Günther M., Stitt M. (2008a). Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B., et al. (2005). Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol. 138: 1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D., et al. (2002). Modern Applied Statistics with S. (New York: Springer-Verlag; ). [Google Scholar]
- Vogel J.T., Zarka D.G., Van Buskirk H.A., Fowler S.G., Thomashow M.F. (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41: 195–211 [DOI] [PubMed] [Google Scholar]
- Walters R.G., Ibrahim D.G., Horton P., Kruger N.J. (2004). A mutant of Arabidopsis lacking the triose-phosphate/phosphate translocator reveals metabolic regulation of starch breakdown in the light. Plant Physiol. 135: 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J.R. (1999). The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24: 437–440 [DOI] [PubMed] [Google Scholar]
- Wattebled F., Planchot V., Dong Y., Szydlowski N., Pontoire B., Devin A., Ball S., D’Hulst C. (2008). Further evidence for the mandatory nature of polysaccharide debranching for the aggregation of semicrystalline starch and for overlapping functions of debranching enzymes in Arabidopsis leaves. Plant Physiol. 148: 1309–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden N.F., Gottlieb L.D. (1982). Dissociation, reassociation, and purification of plastid and cytosolic phosphoglucose isomerase isozymes. Plant Physiol. 69: 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese A., Christ M.M., Virnich O., Schurr U., Walter A. (2007). Spatio-temporal leaf growth patterns of Arabidopsis thaliana and evidence for sugar control of the diel leaf growth cycle. New Phytol. 174: 752–761 [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh N., Sulpice R., Graf A., Stitt M., Fisahn J. (2011). Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 34: 877–894 [DOI] [PubMed] [Google Scholar]
- Zarka D.G., Vogel J.T., Cook D., Thomashow M.F. (2003). Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol. 133: 910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman S.C., Delatte T., Messerli G., Umhang M., Stettler M., Mettler T., Streb S., Reinhold H., Kotting O. (2007). Starch breakdown: Recent discoveries suggest distinct pathways and novel mechanisms. Funct. Plant Biol. 34: 465–473 [DOI] [PubMed] [Google Scholar]
- Zeeman S.C., Kossmann J., Smith A.M. (2010). Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 61: 209–234 [DOI] [PubMed] [Google Scholar]
- Zeeman S.C., Northrop F., Smith A.M., Rees T. (1998). A starch-accumulating mutant of Arabidopsis thaliana deficient in a chloroplastic starch-hydrolysing enzyme. Plant J. 15: 357–365 [DOI] [PubMed] [Google Scholar]
- Zell M.B., Fahnenstich H., Maier A., Saigo M., Voznesenskaya E.V., Edwards G.E., Andreo C., Schleifenbaum F., Zell C., Drincovich M.F., Maurino V.G. (2010). Analysis of Arabidopsis with highly reduced levels of malate and fumarate sheds light on the role of these organic acids as storage carbon molecules. Plant Physiol. 152: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]