Whole genome duplication is typically followed by considerable gene loss, during which the gene dosage hypothesis predicts the preferential retention of highly networked or dose-sensitive genes. Here, circadian clock genes are found to conform to the gene dosage hypothesis in Brassica rapa, consistent with the current model of the circadian clock as a key regulator of multiple cellular pathways.
Abstract
Much has been learned about the architecture and function of the circadian clock of Arabidopsis thaliana, a model for plant circadian rhythms. Circadian rhythms contribute to evolutionary fitness, suggesting that circadian rhythmicity may also contribute to agricultural productivity. Therefore, we extend our study of the plant circadian clock to Brassica rapa, an agricultural crop. Since its separation from Arabidopsis, B. rapa has undergone whole genome triplication and subsequent diploidization that has involved considerable gene loss. We find that circadian clock genes are preferentially retained relative to comparison groups of their neighboring genes, a set of randomly chosen genes, and a set of housekeeping genes broadly conserved in eukaryotes. The preferential retention of clock genes is consistent with the gene dosage hypothesis, which predicts preferential retention of highly networked or dose-sensitive genes. Two gene families encoding transcription factors that play important roles in the plant core oscillator—the PSEUDO-RESPONSE REGULATORS, including TIMING OF CAB EXPRESSION1, and the REVEILLE family, including CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL—exhibit preferential retention consistent with the gene dosage hypothesis, but a third gene family, including ZEITLUPE, that encodes F-Box proteins that regulate posttranslational protein stability offers an exception.
INTRODUCTION
The evolution of angiosperm genomes has been characterized by polyploidization through whole genome duplication (WGD) followed by diploidization that is typically accompanied by considerable homoeologous gene loss. Cytogenetic analyses suggested widespread polyploidization among angiosperms (Stebbins, 1950), and molecular genetic analysis of divergence among paralogous gene pairs has suggested that virtually all angiosperms are ancient polyploids (Blanc and Wolfe, 2004; Cui et al., 2006), a conclusion supported by recent analyses of genome sequences (Soltis et al., 2009; Paterson et al., 2010). For example, Arabidopsis thaliana has undergone three paleopolyploidy events, a paleohexaploidy (γ) duplication shared with most dicots (asterids and rosids) and two more recent tetraploidy events (α and β) shared with other members of the order Brassicales (Bowers et al., 2003). Diploid Brassica species, including Brassica rapa (A genome) and Brassica oleracea (C genome), not only share this polyploidization history with Arabidopsis, but have undergone a further whole genome triplication since their divergence from Arabidopsis 13 to 17 (Yang et al., 1999; Town et al., 2006) or perhaps as much as 43 (Beilstein et al., 2010) million years ago. B. rapa and B. oleracea are estimated to have diverged ∼3.7 million years ago (Inaba and Nishio, 2002). More recently, probably during human cultivation (<10,000 years ago), B. rapa and B. oleracea have hybridized to form the allotetraploid Brassica napus (canola or oilseed rape) (U, 1935). Thus, Brassica species afford an excellent system in which to study the evolution of genome structure.
The recent determination of the genome sequence of B. rapa (Wang et al., 2011) now permits a detailed analysis of gene fate after multiple genome duplications. Although B. rapa has triploidized since its divergence from Arabidopsis, the ∼42,000 genes in the B. rapa genome are considerably fewer than simple triplication of the ∼30,000 genes in the Arabidopsis genome would predict. Thus, there must have been considerable gene loss (fractionation) after triploidization (Wang et al., 2011), which is consistent with the widespread gene loss seen after other eukaryotic WGD events (Sankoff et al., 2010). In B. rapa, the extent of gene loss among the three subgenomes is not equal. One subgenome, the Least Fractionated (LF) genome, retains ∼70% of the genes during fractionation after triploidization, whereas the other two copies, termed Medium Fractionated (MF1) and Most Fractionated (MF2), retain roughly 46 and 36%, respectively (Wang et al., 2011). It has been hypothesized that this reflects fractionation of subgenomes MF1 and MF2 in a tetraploid nucleus, with fractionation of the LF genome commencing only after its more recent introduction during formation of the hexaploid (Wang et al., 2011; Cheng et al., 2012; Tang et al., 2012). However, differential gene loss after tetraploidy in Arabidopsis and Zea mays (maize) has been attributed to different epigenetic marking of the two parental genomes followed by differential silencing and subsequent gene loss (Thomas et al., 2006; Woodhouse et al., 2010; Schnable et al., 2011). In maize, the mechanism of gene loss involves multiple short deletions resulting from nonhomologous recombination (Woodhouse et al., 2010).
Does gene function contribute to the frequency of gene loss during fractionation after WGD? The gene dosage hypothesis posits that genes whose products interact with one another or in networks in a dose-sensitive manner should be overretained, because both products are required in stoichiometric balance (Thomas et al., 2006; Birchler and Veitia, 2007; Freeling, 2009). Consistent with this, B. rapa genes encoding cytoplasmic ribosomal components, or proteasomal components, or transcription factors, are retained at higher frequency (Wang et al., 2011). We wished to examine the relative retention of B. rapa components of the circadian clock. The architecture of the circadian clock is that of a network of multiple interconnected negative feedback loops (McClung and Gutiérrez, 2010). Many clock genes exhibit dose sensitivity (Millar et al., 1995; Somers et al., 2004). In addition, many clock components form complexes with other clock components (Lu et al., 2009; Pruneda-Paz et al., 2009; Nusinow et al., 2011; Herrero et al., 2012). Therefore, we hypothesized that genes encoding components of the circadian clock should be retained at higher frequency than other categories of genes and tested this hypothesis with a set of 60 circadian clock and clock-associated genes. Indeed, this set of 60 clock genes was retained at a higher frequency than many comparator gene sets, including a set of randomly chosen genes, the set of neighboring genes flanking the clock genes, and a set of core eukaryotic genes.
RESULTS
Copy Number Variation of Circadian Clock Genes in B. rapa
Sixty genes involved in the Arabidopsis circadian clock network (see Supplemental Table 1 online) (Harmer, 2009; McClung and Gutiérrez, 2010; Pruneda-Paz and Kay, 2010) were used as seed sequences to identify homoeologs in B. rapa using a combination of BLAST searches and syntenic analysis. Because of the well-conserved microsynteny between Arabidopsis and Brassica (Parkin et al., 2005), analysis of a 21-gene window including both the Arabidopsis clock gene and the 10 genes on either side allowed the identification of microsyntenic regions in B. rapa. The analysis of Parkin et al. (2005) predicts that the B. rapa genome should include 157 microsyntenic blocks corresponding to the 60 Arabidopsis clock genes. The higher resolution afforded by the whole genome assembly allowed the identification of an additional 17 syntenic regions for a total of 174 B. rapa syntenic regions for the Arabidopsis clock genes. Fifty-one Arabidopsis clock genes were in regions present in B. rapa in three syntenic blocks, eight were in two syntenic blocks, and one was in four syntenic blocks.
Whole genome analysis of the B. rapa genome has established that the three subgenomes can be distinguished by the degree of fractionation (gene loss) (Wang et al., 2011; Cheng et al., 2012; Tang et al., 2012). We assigned each of our syntenic blocks to one of the three subgenomes LF, MF1, and MF2 (see Supplemental Table 2 online).
In total, 114 clock gene homoeologs were identified in B. rapa (http://brassicadb.org/brad/). Among these 114 copies, 106 (93%) were located in the 174 syntenic regions and eight clock-gene homologs (7%) were identified at nonsyntenic sites. We also identified two clock homoeologs that had undergone tandem duplication and another clock homoeolog that had undergone tandem triplication in B. rapa (see Supplemental Figure 1 online).
Differential Retention of Clock Genes
The gene dosage hypothesis predicts that genes whose products are dose-sensitive, interacting either with other proteins or in networks, should be overretained (Thomas et al., 2006; Birchler and Veitia, 2007). We compared the retention of circadian clock genes relative to the retention of three other gene sets: the set of 1200 neighboring genes (10 on either side) flanking the 60 clock genes, a set of 458 core eukaryotic genes, and a third set of 458 randomly selected genes from the microsyntenic regions corresponding to the clock genes. Circadian clock genes have been retained after triplication and fractionation in B. rapa at a significantly higher rate than each of the three comparison groups (Figure 1). Overall, 58% of the clock genes have been retained in the syntenic regions, relative to 42% of neighboring genes, 51.3% of the core eukaryotic genes, and 44.2% of randomly selected genes (chi square = 33.6, P < 0.001). Most (∼65%) clock genes were retained in two or three copies, which is significantly greater than the retention of any of the other gene sets (Figure 1A). Similar numbers (∼15%) of clock and core eukaryotic genes were retained in three copies. Significantly fewer (only 4/60 = 6.7%) of the clock genes were completely lost than for any of the other gene sets (Figure 1A).
Figure 1.
Retention of Circadian Clock Genes and of Neighboring Genes (10 Flanking Genes on Either Side of the Clock Gene), 458 Randomly Selected Genes, and 458 Core Eukaryotic Genes in the Syntenic Region after Genome Triplication and Fractionation in B. rapa.
(A) Retention by number of homoeologous copies in the syntenic region.
(B) Retention of homoeologs among the three subgenomes of B. rapa. Different letters indicate statistical significance (P < 0.05) as determined by Scheffé’s Test.
(C) Retention of homoeologs among the clock genes (stars) and their immediate neighbors in the three subgenomes of B. rapa. Filled circles indicate LF, open squares indicate MF1, and open circles indicate MF2.
The proportion of homoeologs retained varied among the three subgenomes (Figures 1B and 1C). In the LF subgenome, significantly more clock-gene homoeologs were retained than for each of the other gene sets. In the MF1 and MF2 subgenomes, clock genes showed similar retention rates compared with the core eukaryotic genes, and both gene sets were retained in significantly greater proportion than either the neighboring or randomly selected gene sets (Figures 1B and 1C). The distribution of retained clock-gene homoeologs among the subgenomes differed from the distributions of the other gene sets—approximately one-half of the retained clock-gene homoeologs were in the LF, which is a greater proportion than for the other three gene sets (see Supplemental Figure 2 online).
Footprint of a Myb Domain Gene Family in Eudicots
Two single myeloblastosis (Myb) domain transcription factors, encoded by CIRCADIAN CLOCK ASSOCIATED1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY), play critical roles in the central loop of the Arabidopsis clock (Harmer, 2009; McClung, 2011). CCA1 and LHY are the founding members of a CCA1/LHY/REVEILLE (RVE) gene family of 10 members. In addition to CCA1 and LHY, many other members of this family, including RVE1 (Rawat et al., 2009), RVE2 (Zhang et al., 2007), RVE7 (Kuno et al., 2003), and RVE8 (Farinas and Mas, 2011; Rawat et al., 2011), have been shown to play roles in clock function or regulation of clock output pathways in Arabidopsis. We therefore characterized this gene family in B. rapa and identified 18 RVE homoeologs. We also identified five RVEs in each of Vitis vinifera and Carica papaya and 12 RVEs in Populus trichocarpa and used these sequences to construct a phylogenetic tree by maximum likelihood using MEGA5 (Tamura et al., 2011) (Figure 2; see Supplemental Figure 3 online). The RVE family is divided into two clades, which we will refer to as the LHY and RVE8 clades. We included V. vinifera, P. trichocarpa, and C. papaya in our analysis, because they did not undergo the α and β duplications and therefore allow the identification of the RVE genes that predated those duplication events as those common to all five species: LHY, RVE7, and RVE1 in the LHY clade and RVE3, RVE6, and RVE8 in the RVE8 clade. RVE7 is missing from V. vinifera and C. papaya but is present in P. trichocarpa, Arabidopsis, and B. rapa. This suggests that RVE7 was lost from C. papaya after the divergence of the Brassicales. V. vinifera either lost RVE7 after its divergence from P. trichocarpa or RVE7 originated by duplication from RVE1 and RVE2 after that divergence; these data do not permit us to distinguish between these two possibilities. CCA1, RVE2, RVE4, and RVE5 are restricted to the Brassicales and therefore probably originated in the α and β duplications. We also included Amborella trichopoda, a basal angiosperm that is the earliest diverging species on the flowering plant tree of life and that did not undergo the γ duplication event (Jiao et al., 2011). A. trichopoda has three RVE family members, LHY, RVE1, and RVE6, indicating that these three RVE genes originated with duplication events preceding the γ event. This conclusion is consistent with evidence supporting duplications in a number of gene families, including PHYTOCHROME (Mathews and Sharrock, 1997; Mathews, 2006), the TIR1/AFB genes encoding auxin receptors (Parry et al., 2009), and many transcription factor gene families (Prigge and Clark, 2006; Hu et al., 2008).
Figure 2.
Copy Number Variation in the CCA1/LHY/RVE Family in Eudicots.
CCA1/LHY/RVE phylogenetic tree is shown on the left, and the species tree is shown on the top. The α, β, γ, and salicoid duplications and the Brassica-specific triplication are indicated on the branches of the trees. The CCA1/LHY/RVE phylogenetic tree was constructed from protein sequences using maximum likelihood (MEGA 5), and the species tree is modified from Beilstein et al. (2010).
Syntenic Analysis of the RVE Family in Eudicots
To refine our understanding of the timing of the duplications that resulted in Brassicales-specific RVEs, we more closely investigated microsynteny in the regions flanking these RVEs. Both C. papaya and V. vinifera have single LHY/CCA1 loci, whereas Arabidopsis has four LHY/CCA1 loci as a result of the α and β duplications. We identified these four regions of microsynteny by comparison of Arabidopsis with V. vinifera and C. papaya using CoGE (short for comparative genomics) (Lyons et al., 2008; Lyons and Freeling, 2008) (Figure 3A; see Supplemental Table 3 online). This indicates the LHY/CCA1 locus duplicated in the β duplication, yielding LHY on Arabidopsis chromosome 1 and CCA1 on Arabidopsis chromosome 2. After the α duplication, only single copies of each of LHY and CCA1 were retained (Figure 3A). Most of the Arabidopsis genome is represented in three syntenic blocks in B. rapa, because of the Brassica-specific genome triplication. After fractionation in B. rapa, LHY has been retained in two copies, and CCA1 has been retained in a single copy. There are also two copies of the LHY/CCA1 locus in P. trichocarpa because of the salicoid duplication, which occurred independently of the duplications in the Brassicales. Two RVEs, RVE2 and RVE4, are restricted to the Brassicales and therefore probably originated in the β and α duplications, respectively (Figure 2; see Supplemental Figure 3 online).
Figure 3.
Microsynteny Analysis of Members of the LHY/CCA1/RVE Gene Family in Eudicots.
(A) Microsynteny analysis, derived with the CoGe comparative genomic tool (Lyons et al., 2008; Lyons and Freeling, 2008), indicates that LHY is the ancestral gene and that CCA1 arose during the β duplication in the Brassicales. LHY and CCA1 duplicates that originated during the α duplication have been lost during fractionation.
(B) Microsynteny analysis indicates that Arabidopsis RVE5 arose from RVE3 during the α duplication and that other copies have been lost during fractionation. The species tree (Beilstein et al., 2010) is indicated at the top. Chromosomes are indicated by vertical lines, and genes are indicated by orange triangles (clock genes) and blue circles (flanking genes). Genes with no syntenic matches in the selected regions are not plotted. At, Arabidopsis; Br, B. rapa; Cp, C. papaya; Pt, P. trichocarpa; Vv, V. vinifera.
We also investigated RVE3 and RVE5 in the same way as LHY/CCA1. This analysis suggests that the RVE5 copy retained in Arabidopsis arose during the α duplication (Figure 3B). Moreover, this microsynteny analysis also clarifies relationships among the RVE genes in V. vinifera. In V. vinifera, the RVE ortholog (GSVIVT01026956001) is most similar to At-RVE6 on the basis of sequence similarity, but because of microsynteny of the flanking genes, it is clear that this is in the RVE3/RVE5 syntenic position and therefore represents the V. vinifera RVE3 ortholog.
Evolutionary History of the LHY/CCA1/RVE Family in Eudicots
Based on phylogenetic and syntenic analysis, we have constructed a possible evolutionary history of the LHY/CCA1/RVE family (see Supplemental Figure 3 online). Before the divergence of the Brassicales, the family included LHY and five RVE genes (RVE1, RVE7, RVE3, RVE6, and RVE8). (Figure 2; see Supplemental Figure 3 online). The gene family further expanded in the Brassicaceae. In the current Arabidopsis genome, two new family members, CCA1 and RVE2, are descended from LHY and RVE1, respectively, in the β duplication, and two additional family members (RVE4 and RVE5) arose from RVE8 and RVE3, respectively, during the α duplication (see Supplemental Figure 3 online). Thus, in the Brassicaceae, the size of the LHY/CCA1/RVE family increased by one-third after the α and β duplications and doubled in the B. rapa genome after triplication and fractionation.
Triplication and Fractionation of PRR Genes in B. rapa
Phylogenetic analysis indicates that there are three clades of PSEUDO-RESPONSE REGULATOR (PRR) genes in angiosperms (Matsushika et al., 2000; Takata et al., 2010), and recent research in a basal land plant, the moss Physcomitrella patens, also identified four PRR genes (Satbhai et al., 2011), suggesting a very early origin in plant evolution. Here we focus on the PRR gene family in B. rapa after its divergence from Arabidopsis. Despite the two genome duplications in Arabidopsis after its divergence from C. papaya, only single copies of each PRR gene are retained in Arabidopsis, indicating substantial gene loss during fractionation. However, there has not been similar gene loss after the genome triplication in B. rapa. Earlier work, based on partial genome sequence and DNA gel blot hybridization analyses, indicated an expansion of the PRR gene family in B. rapa relative to Arabidopsis (Kim et al., 2007; Kim et al., 2012). We again used microsynteny analysis to determine the retention status of each PRR gene in blocks of 21 genes (10 genes on either side) flanking each PRR in B. rapa. PRR genes have been retained at a significantly higher rate than their neighboring genes (73.3 versus 41.3%; P < 0.05 as determined by chi-square analysis) (Figure 4). All five PRR genes have been retained in the LF subgenome, but only a single intact PRR gene, PRR5c, has been retained in the MF2 subgenome. Each PRR gene has been retained in the MF1 subgenome, although two of those copies, PRR3b and PRR9b, are predicted to be nonfunctional because of rearrangements and partial deletions. In total, we identified 11 PRR genes in the B. rapa genome, including one partially deleted copy, PRR9b, and one possible gene fusion involving PRR3b.
Figure 4.
Fractionation of PRR Genes after Triplication in B. rapa.
Filled circles indicate individual genes in regions of microsynteny flanking each PRR gene (black triangles). Empty circles and triangles indicate genes lost during fractionation, and half-filled black triangles indicate partially deleted or rearranged genes. Double or triple circles indicate local tandem duplication or triplication, respectively. Block indicates the three B. rapa subgenomes: LF, MF1, and MF2. Retained indicates the number of B. rapa genes retained relative to Arabidopsis.
Partial Deletion and Gene Fusion of PRR genes in B. rapa
In Arabidopsis, PRR9 exhibits partial functional redundancy with PRR7 and PRR5 (Farré et al., 2005; Nakamichi et al., 2005; Salomé and McClung, 2005; Nakamichi et al., 2010; Salomé et al., 2010). In Arabidopsis, PRR9 (At2g44790), which shows a greater rate of substitution than other PRR family members (Takata et al., 2010), has duplicated to yield a second tightly linked copy (At2g46670) that is nonfunctional because of the deletion of the Pseudo-Receiver (PsR) domain (Takata et al., 2010). We identified two copies of PRR9 in the B. rapa genome, one in the LF subgenome, and a second, Br-PRR9b, in the MF1 subgenome. Br-PRR9b is probably nonfunctional, because of a partial deletion of PsR domain, as in Arabidopsis. However, two pieces of evidence argue that Br-PRR9b is derived from At-PRR9 (At2g44790) through WGD and is not derived from the At-PRR9 pseudogene (At2g46670). First, the truncations are different between the two pseudogenes. Second, the gene order of flanking genes in this microsyntenic region is consistent with this B. rapa pseudogene being derived from At-PRR9 (At2g44790) during WGD followed by partial gene loss. We find no evidence for copies of the At-PRR9b pseudogene (At2g46670) in the B. rapa, C. papaya, P. trichocarpa, or V. vinifera genomes (Figure 3A), which suggests that At-PRR9b (At2g46670) originated in an Arabidopsis-specific event that occurred after the divergence of Arabidopsis from B. rapa. The tight linkage of PRR9 and CCA1 in Arabidopsis and B. rapa is conserved in P. trichocarpa and V. vinifera, suggesting that the PRR9 homoeolog linked to LHY was probably lost in the Arabidopsis-Brassica lineage after the β duplication.
A second rearrangement has affected Br-PRR3b, the MF1 subgenome copy of PRR3. In both Arabidopsis and B. rapa, PRR3 lies upstream of two protein kinase C genes that apparently resulted from a tandem duplication that occurred before the divergence of Arabidopsis and B. rapa. Some gene prediction programs (e.g., FGENESG [http://linux1.softberry.com/berry.phtml?topic=fgeneshandgroup=programsandsubgroup=gfind]) suggest that Br-PRR3b (Bra020263) and its downstream neighbor, Bra020264, represent a gene fusion capable of expressing a fusion protein consisting of two domains, an N-terminal CCT domain derived from a partially deleted Br-PRR3b and a protein kinase C domain derived from Bra020264, the ortholog of At5g60090 (Figure 5). This would offer the potential for acquisition of novel function. However, to date we have been unable to detect RT-PCR evidence for expression of this chimeric gene, Bra020263/Bra020264, although we have detected transcripts from each individual gene. In Arabidopsis, the PRR3 gene offers an example of subfunctionalization in that its expression is restricted to the vasculature (Para et al., 2007), in contrast with other PRRs, which exhibit widespread expression. In addition, PRR3 also has acquired the novel function of regulation of TIMING OF CAB EXPRESSION1 (TOC1) stability through direct protein–protein interaction (Para et al., 2007). This contrasts with the roles of TOC1 and PRR5, PRR7, and PRR9 as DNA-binding regulators of gene expression (Pruneda-Paz et al., 2009; Nakamichi et al., 2010; Gendron et al., 2012).
Figure 5.
A Model for a Possible Gene Fusion between PRR3b and an Adjacent Protein Kinase C (PKC) Ortholog in the MF1 Subgenome of B. rapa.
(A) Genome region flanking PRR3 in Arabidopsis.
(B) B. rapa subgenome MF1 region of synteny to At-PRR3. Exons are indicated by filled boxes, and introns are indicated by horizontal lines. Direction of transcription is indicated by arrowheads. ? indicates possible gene fusion between Bra020263 (PRR3) and Bra020264 (Protein kinase).
Proposed Evolutionary History of the ZTL/LKP2/FKF Family in B. rapa
Regulated protein degradation is essential for oscillatory gene expression in the circadian network (Somers and Fujiwara, 2009; van Ooijen et al., 2011). In Arabidopsis, ZEITLUPE (ZTL) and its close relatives LOV KELCH PROTEIN2 (LKP2) and FLAVIN BINDING, KELCH REPEAT, F-BOX1 (FKF1) encode F-box proteins with an N-terminal LOV domain, a central F-box, and a series of Kelch repeats at the C terminus. These three proteins contribute to the determination of circadian period length through the regulated degradation of TOC1 via recruitment to an SCF ubiquitin E3 ligase that targets TOC1 for proteasomal degradation, although ZTL is the predominant determinant of period length among the three (Somers et al., 2000; Baudry et al., 2010). Although FKF1 has an apparently minor and largely redundant role in circadian period determination, loss of FKF1 function results in a dramatic delay in flowering (Nelson et al., 2000).
We used a combination of phylogenetic and syntenic analyses to evaluate the evolutionary history of the ZTL/LKP2/FKF family in B. rapa. On the basis of sequence similarity, ZTL and LKP2 are most closely related; with FKF1 more distantly related (see Supplemental Figure 4 online). Our analysis suggests that ZTL and LKP2 arose in the Brassicaceae lineage in the β duplication after divergence from C. papaya, which has ZTL but not LKP2 (Figure 6A). However, neither ZTL nor FKF1 are retained in the B. rapa genome (Figure 6A). This result is surprising, given the importance of ZTL and FKF1 to circadian period determination and flowering time, respectively. Despite loss of both genes, B. rapa accessions retain a circadian (∼24 h) period, and some accessions flower very early (Xu et al., 2010; Lou et al., 2011). We first confirmed that loss of ZTL and FKF1 has also occurred in B. oleracea (see Supplemental Table 4 online), which also retains a ∼24-h circadian period (Salathia et al., 2007) and exhibits a range of flowering times (Camargo and Osborn, 1996). Thus, we conclude that ZTL and FKF1 were both lost in the Brassica lineage after its divergence from Arabidopsis but before the separation of B. rapa and B. oleracea. Apparently, LKP2, which has been retained in both B. rapa and B. oleracea, or some other gene(s) fulfills the functions of ZTL and FKF1 in these two Brassica species (as well as in B. napus, which is a hybrid between B. rapa and B. oleracea). This gene family clearly does not exhibit preferential retention after the Brassica WGDs and is thus distinguished from the larger set of circadian clock genes.
Figure 6.
Evolution of the ZTL/LKP2/FKF1 Gene Family in Dicots.
(A) Phylogenetic analysis of the ZTL/LKP2 clade in dicots. The salicoid duplication in poplar is indicated with a circle, the β duplication is indicated with an oval, the α duplication is indicated with a diamond, and the Brassica-specific triplication is indicated with a star. Gene losses are indicated by an X.
(B) Genomic organization of the LF subgenome of B. rapa at the triplicated LKP2 locus.
(C) Domain structures of the ZTL/LKP2/FKF1 genes of Arabidopsis and B. rapa.
We also observed that LKP2 has been retained in only one of the three regions of microsynteny of B. rapa to Arabidopsis but that three tightly linked copies of LKP2 are found in the predicted LF subgenomic region, indicating a local triplication (Figures 6A and 6B). BrLKP2a and BrLKP2c are in the same gene orientation, but BrLKP2b is in the inverted orientation (Figure 6B). Additionally, a gene orthologous to At4G24990 encoding a geranylgeranylated protein of unknown function has been inserted between Br-LKP2a and Br-LKP2b. Thus, the events associated with this triplication were likely complex. The genomic organization of the retained LKP2 region in B. rapa is similar in B. oleracea (see Supplemental Table 4 online), indicating that this triplication and rearrangement occurred after separation from Arabidopsis but before separation of B. rapa and B. oleracea.
As discussed above, Arabidopsis ZTL, LKP2, and FKF1 have overlapping roles in circadian period determination, with that function predominantly specified by ZTL, whereas FKF1 has the major role in flowering time determination (Nelson et al., 2000; Somers et al., 2000; Baudry et al., 2010). It will be very interesting to determine whether the three copies of LKP2 in B. rapa and B. oleracea have undergone functional specialization. Analysis of the domain structure suggests that this may be the case. Although At-ZTL, At-LKP2, and At-FKF1 all have multiple Kelch repeats at the C terminus, At-FKF1 has one fewer repeat (four) than do At-ZTL and At-LKP2 (five each). In B. rapa and B. oleracea, LKP2c has one fewer repeat (four) than LKP2a and LKP2b (five each). RT-PCR analysis indicates that all three genes are expressed. It will be important to assess function through mutational analysis. Although a robust TILLING population exists for B. rapa (Stephenson et al., 2010), the tight linkage among the three LKP2 copies precludes the easy construction of mutant strains with defects in multiple LKP2 genes. Alternate strategies, such as the use of artificial microRNAs (Schwab et al., 2006) or transgenic rescue of Arabidopsis mutants with Brassica genes, should allow the functional analysis of Brassica LKP2 genes.
DISCUSSION
WGDs have been proposed to be important in the evolution of complexity in multicellular eukaryotes (Ohno, 1970; Edger and Pires, 2009). There is evidence for ancient WGD in basal angiosperm lineages, and a paleohexaploid event is believed to have occurred close to the eudicot divergence (Soltis et al., 2009). Although there is no evidence for WGD in Amborella and gymnosperms, effectively all extant angiosperms are believed to have undergone at least one and often multiple WGD events (Soltis et al., 2009; Jiao et al., 2011). WGD may have led to dramatic increases in species richness in several angiosperm lineages, including Poaceae, Solanaceae, Fabaceae, and of particular relevance to our study, Brassicaceae. Most families in the Brassicales are small, but lineages for which there is strong evidence for recent genome triplication (e.g., the Cleomaceae and Brassicaceae) are species-rich, with 300 species in the Cleomaceae, and 3710 species in the Brassicaceae (Soltis et al., 2009).
WGD is typically followed by substantial gene loss (fractionation), However, the gene balance hypothesis predicts that genes whose products participate in macromolecular complexes or in transcriptional or signaling networks are more likely to be retained, because the imbalance associated with loss of one member of a complex or network is likely to decrease fitness (Thomas et al., 2006; Birchler and Veitia, 2007; Edger and Pires, 2009; Freeling, 2009). Consistent with this hypothesis, some gene functional categories, including genes encoding ribosomal proteins, transcription factors, photosystem components, and signal transduction components, have been preferentially retained after WGD in Arabidopsis (Maere et al., 2005; Freeling, 2009) and other taxa (Edger and Pires, 2009; Coate et al., 2011). Similarly, genes that are highly connected within metabolic networks exhibit preferential retention in Arabidopsis (Bekaert et al., 2011). Many clock genes exhibit dose sensitivity; many encode transcription factors and constituents of multiprotein complexes (Harmer, 2009; McClung, 2011; Nusinow et al., 2011; Herrero et al., 2012). Others, including CCA1, are highly networked (Gutiérrez et al., 2008; McClung and Gutiérrez, 2010). Consistent with the predictions of the gene dosage hypothesis, we observe preferential retention of B. rapa genes encoding clock components relative to comparison gene sets. This preferential retention was statistically significant for clock genes as a whole and was especially evident for the CCA1/LHY/RVE and PRR gene families, but was not observed in the small ZTL/FKF1/LKP2 gene family. The former two families encode transcription factors, which typically display preferential retention (Maere et al., 2005; Edger and Pires, 2009; Freeling, 2009; Coate et al., 2011). The latter ZTL/FKF1/LKP2 proteins participate in protein (SCF) complexes that serve as an E3 ubiquitin ligases that target proteins for proteasomal degradation, with ZTL, LKP2, and FKF1 providing substrate specification (Somers et al., 2000; Baudry et al., 2010). Beyond noting that these genes are associated with posttranslational regulation of substrate protein stability, rather than with transcriptional networks, we cannot offer any molecular explanation of why the genes encoding these proteins offer exceptions to the gene dosage hypothesis.
Consistent with the observations of others, we find that gene loss from the three subgenomes in B. rapa is biased, and the three subgenomes show preferential retention of clock genes relative to neighboring genes and other comparison gene sets. In other species, this nonequivalence among duplicated subgenomes has been extended to gene expression. For example, in recently resynthesized Arabidopsis suecica–like allotetraploids, gene expression is biased in favor of the Arabidopsis arenosa over the Arabidopsis genome (Wang et al., 2006), and this bias extends to the clock gene CCA1 (Ni et al., 2009). Similar results have been observed in the recent natural allotetraploid Tragopogon miscellus (Buggs et al., 2010) as well as in much older cotton allotetraploids (Flagel and Wendel, 2010). Similar subgenome dominance has recently been reported for the maize subgenomes that resulted from an ancient duplication (Schnable et al., 2011). At this time, we have no data directly addressing relative expression levels among the different subgenomes in B. rapa, although such experiments clearly are of interest.
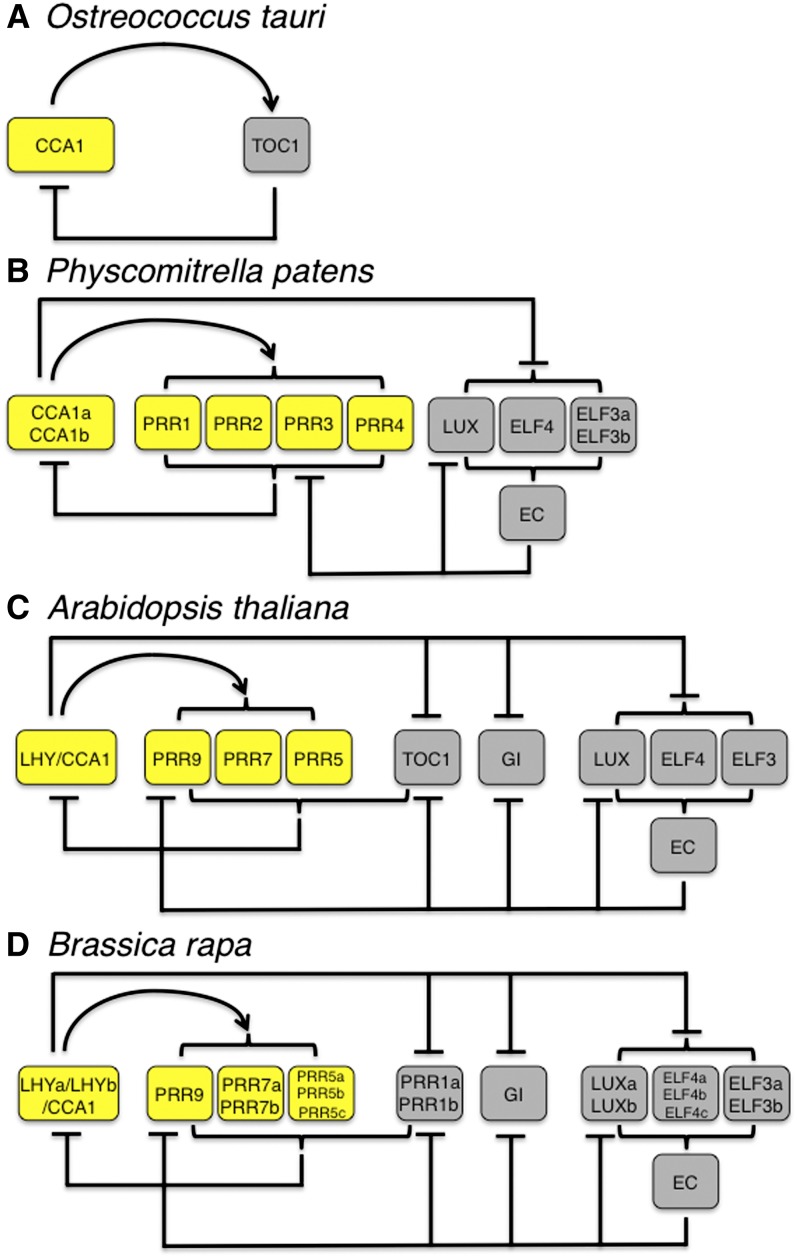
Eukaryotic evolution has been characterized by increasing morphological complexity, and it has been argued that WGD coupled with the retention of duplicated genes because of the necessity of genome balance provide impetus toward increasing morphological complexity (Freeling and Thomas, 2006). We postulate that WGD duplication followed by retention of dose-sensitive circadian clock genes has permitted the evolution of increasingly complex circadian clock mechanisms. The model of the circadian clock developed in Arabidopsis shows multiple interlocked feedback loops (Figure 7) (Harmer, 2009; McClung, 2011). However, in more basal taxa, only a single loop has been described. The simplest eukaryotic example described to date is the clock of the unicellular green alga, Ostreococcus tauri, which apparently consists of a single loop of CCA1 and TOC1 (Corellou et al., 2009; Troein et al., 2011). The moss clock of P. patens includes two CCA1 genes, four PRR genes, ELF3, ELF4, and LUX, but lacks orthologs of TOC1, GI, and ZTL/LKP2/FKF1, and it has been suggested that this reduced cast is organized into a single loop (Holm et al., 2010). Whether the P. patens clock includes a single or multiple loops is not firmly established; P. patens does have the constituents of the evening complex, which, in Arabidopsis, includes LUX, ELF3, and ELF4 (Nusinow et al., 2011; Herrero et al., 2012), and LUX is a negative regulator of PRR9 (Helfer et al., 2011). Nonetheless, P. patens lacks TOC1 and GI, two of the key evening loop components of the angiosperm clock, and therefore has a simpler architecture. During the evolution of the green lineage, the circadian clock has become increasingly complex, and the common angiosperm ancestor of monocots and eudicots had components sufficient to construct a circadian clock consisting of multiple interlocked feedback loops (Takata et al., 2010). It seems reasonable that repeated WGD events facilitated that increase in complexity, which raises the question of the consequences for clock architecture and function of more recent polyploidization events, such as those in poplar and Brassica.
Figure 7.
Evolution of Complexity of Circadian Clock Architecture in the Green Lineage.
Clock networks in O. tauri (A), P. patens (B), Arabidopsis (C), and B. rapa (D). The so-called morning and evening loop components are shown in yellow and gray, respectively. The evening complex, composed of LUX, ELF3, and ELF4, is shown as EC. The Arabidopsis clock network is simplified from that of Pokhilko et al. (2012), and the other networks are based on that of Arabidopsis. Regulatory relationships shown in the other networks are largely extrapolated from the Arabidopsis network rather than based on experimental analysis. Arrowheads indicate positive regulatory relationships, and T-shaped arrows indicate negative regulatory relationships.
Explanations for the architectural complexity of the plant circadian clock have included enhanced robustness and increased flexibility, particularly in light of environmental noise and seasonally changing photoperiod (Troein et al., 2009; Edwards et al., 2010). However, the O. tauri clock is architecturally simple yet both robust and flexible (Troein et al., 2011). An alternate view is that the evolution of complexity in the angiosperm circadian clock was not necessary for clocks to be flexible, but rather has been exploited for that purpose. In that sense, the architectural complexity of the plant circadian clock may recall the spandrel metaphor (Gould and Lewontin, 1979), in which complexity arose as a necessary byproduct of WGD but subsequently has been exploited to the end of increased flexibility, both in terms of entrainment (responsiveness to environmental signals) and in terms of fine-grained temporal regulation of output pathways.
METHODS
Identification of Clock Genes and Comparison Gene Sets
The coding sequences of the 60 Arabidopsis thaliana circadian clock genes that constituted the set of clock genes used in this study (see Supplemental Table 1 online) were retrieved from the Arabidopsis TAIR9 database (http://www.Arabidopsis.org/tools/bulk/sequences/index.jsp). Brassica rapa homologous regions of Arabidopsis clock genes were retrieved from the BRAD database (http://brassicadb.org/brad/) based on BLASTn search (E-value threshold 1e-10) (Altschul et al., 1990). FGENESH (http://mendel.cs.rhul.ac.uk/mendel.php?topic=fgen-file) and Genescan (Burge and Karlin, 1997) were used to predict protein coding open reading frames with parameters optimized for Arabidopsis. Mispredicted genes were manually modified based on the intron-exon structure of known Arabidopsis genes. For predicted genes lacking a conserved domain (like PRR3b) or for partially deleted genes, we searched the Brassica database for corresponding expressed sequence tags and reannotated the gene.
Homologs of Arabidopsis clock genes in Vitis vinifera, Carica papaya, and Populus trichocarpa were retrieved from Phytozome v7.0 (http://www.phytozome.net/).
The core eukaryotic genes and random genes were downloaded from CEGMA (Parra et al., 2007) (http://korflab.ucdavis.edu/Datasets/cegma) and used to search the Brassica database using BLAST. BLAST results were parsed with BioPerl’s Bio::Tools::BPlite module (Stajich et al., 2002).
Phylogenetic Analysis of Clock Genes
Predicted protein sequences were deduced from FGENESH analysis, and then the CCA1/LHY/RVE, PRR, and ZTL/LKP2/FKF families were aligned by CLUSTALW (Larkin et al., 2007). The alignments (see Supplemental Data Sets 1 and 2 online) were further corrected based on the exon-intron structure from Arabidopsis. A phylogenetic tree was then constructed by the maximum likelihood method, and bootstrap values were calculated with 1000 replications using MEGA5 (Tamura et al., 2011).
Chromosomal Synteny Analysis of Clock Genes
Conservation of chromosomal synteny around the circadian clock genes in V. vinifera, C. papaya, P. trichocarpa, and Arabidopsis are derived with CoGe (Lyons et al., 2008; Lyons and Freeling, 2008) from the data sets at (http://synteny.cnr.berkeley.edu/wiki/index.php/Syntenic_gene_sets). Chromosomal synteny between Arabidopsis and Brassica are extracted from the Brassica database (BRAD; http://brassicadb.org/brad/).
Accession Numbers
Sequence data for the article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL or Brassica (BRAD; http://brassicadb.org/brad/) databases under the accession numbers given in Supplemental Tables 1, 3, and 4 online.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Genomic Position of Circadian Clock Genes in B. rapa.
Supplemental Figure 2. Retention of Clock Gene Homoeologs, as Well as Homoeologs of Three Comparator Sets, Neighboring Genes, Random Genes, and Core Eukaryotic Genes, among the Three Subgenomes of B. rapa.
Supplemental Figure 3. Phylogenetic Analysis of the CCA1/LHY/RVE Gene Family in Angiosperms.
Supplemental Figure 4. Phylogenetic Analysis of the ZTL/LKP2/FKF1 Families of F-Box Proteins in Arabidopsis, B. rapa, P. trichocarpa, V. vinifera, and A. trichopoda.
Supplemental Table 1. B. rapa Homoeologs of Arabidopsis Clock Genes.
Supplemental Table 2. Fractionation of Circadian Clock Gene Homeologs after Triplication in B. rapa.
Supplemental Table 3. Assessment of Synteny between B. rapa and Arabidopsis Clock Genes.
Supplemental Table 4. B. oleracea Homoeologs of Arabidopsis Clock Genes.
Supplemental Data Set 1. Text File of the CCA1/LHY/RVE Family Alignment Used for the Phylogenetic Analysis Shown in Supplemental Figure 3 online.
Supplemental Data Set 2. Text File of the ZTL/LKP2/FKF Family Alignment Used for the Phylogenetic Analysis Shown in Supplemental Figure 4 online.
Acknowledgments
This study was supported by grants to C.R.M. from the National Science Foundation (IOS-0605736, IOS-0923752, and IOS-1025965).
AUTHOR CONTRIBUTIONS
P.L., X.W., and C.R.M. designed the experiments. P.L., J.W., F.C., and L.G.C. performed the analyses. P.L. and C.R.M. wrote the article, with advice from X.W.
Glossary
- WGD
whole genome duplication
- LF
Least Fractionated
- MF1
Medium Fractionated
- MF2
Most Fractionated
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Baudry A., Ito S., Song Y.H., Strait A.A., Kiba T., Lu S., Henriques R., Pruneda-Paz J.L., Chua N.-H., Tobin E.M., Kay S.A., Imaizumi T. (2010). F-box proteins FKF1 and LKP2 act in concert with ZEITLUPE to control Arabidopsis clock progression. Plant Cell 22: 606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilstein M.A., Nagalingum N.S., Clements M.D., Manchester S.R., Mathews S. (2010). Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107: 18724–18728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekaert M., Edger P.P., Pires J.C., Conant G.C. (2011). Two-phase resolution of polyploidy in the Arabidopsis metabolic network gives rise to relative and absolute dosage constraints. Plant Cell 23: 1719–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler J.A., Veitia R.A. (2007). The gene balance hypothesis: From classical genetics to modern genomics. Plant Cell 19: 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc G., Wolfe K.H. (2004). Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 16: 1667–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers J.E., Chapman B.A., Rong J., Paterson A.H. (2003). Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 422: 433–438 [DOI] [PubMed] [Google Scholar]
- Buggs R.J.A., Chamala S., Wu W.E.I., Gao L.U., May G.D., Schnable P.S., Soltis D.E., Soltis P.S., Barbazuk W.B. (2010). Characterization of duplicate gene evolution in the recent natural allopolyploid Tragopogon miscellus by next-generation sequencing and Sequenom iPLEX MassARRAY genotyping. Mol. Ecol. 19 (Suppl 1): 132–146 [DOI] [PubMed] [Google Scholar]
- Burge C., Karlin S. (1997). Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268: 78–94 [DOI] [PubMed] [Google Scholar]
- Camargo L.E.A., Osborn T.C. (1996). Mapping loci controlling flowering time in Brassica oleracea. Theor. Appl. Genet. 92: 610–616 [DOI] [PubMed] [Google Scholar]
- Cheng F., Wu J., Fang L., Sun S., Liu B., Lin K., Bonnema G., Wang X. (2012). Biased gene fractionation and dominant gene expression among the subgenomes of Brassica rapa. PLoS ONE 7: e36442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coate J.E., Schlueter J.A., Whaley A.M., Doyle J.J. (2011). Comparative evolution of photosynthetic genes in response to polyploid and nonpolyploid duplication. Plant Physiol. 155: 2081–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corellou F., Schwartz C., Motta J.-P., Djouani-Tahri B., Sanchez F., Bouget F.-Y. (2009). Clocks in the green lineage: Comparative functional analysis of the circadian architecture of the picoeukaryote ostreococcus. Plant Cell 21: 3436–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., et al. (2006). Widespread genome duplications throughout the history of flowering plants. Genome Res. 16: 738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edger P.P., Pires J.C. (2009). Gene and genome duplications: The impact of dosage-sensitivity on the fate of nuclear genes. Chromosome Res. 17: 699–717 [DOI] [PubMed] [Google Scholar]
- Edwards K.D., Akman O.E., Knox K., Lumsden P.J., Thomson A.W., Brown P.E., Pokhilko A., Kozma-Bognar L., Nagy F., Rand D.A., Millar A.J. (2010). Quantitative analysis of regulatory flexibility under changing environmental conditions. Mol. Syst. Biol. 6: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas B., Mas P. (2011). Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant J. 66: 318–329 [DOI] [PubMed] [Google Scholar]
- Farré E.M., Harmer S.L., Harmon F.G., Yanovsky M.J., Kay S.A. (2005). Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 15: 47–54 [DOI] [PubMed] [Google Scholar]
- Flagel L.E., Wendel J.F. (2010). Evolutionary rate variation, genomic dominance and duplicate gene expression evolution during allotetraploid cotton speciation. New Phytol. 186: 184–193 [DOI] [PubMed] [Google Scholar]
- Freeling M. (2009). Bias in plant gene content following different sorts of duplication: Tandem, whole-genome, segmental, or by transposition. Annu. Rev. Plant Biol. 60: 433–453 [DOI] [PubMed] [Google Scholar]
- Freeling M., Thomas B.C. (2006). Gene-balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Res. 16: 805–814 [DOI] [PubMed] [Google Scholar]
- Gendron J.M., Pruneda-Paz J.L., Doherty C.J., Gross A.M., Kang S.E., Kay S.A. (2012). Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc. Natl. Acad. Sci. USA 109: 3167–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S.J., Lewontin R.C. (1979). The spandrels of San Marco and the Panglossian paradigm: A critique of the adaptationist programme. Proc. R. Soc. Lond. B Biol. Sci. 205: 581–598 [DOI] [PubMed] [Google Scholar]
- Gutiérrez R.A., Stokes T.L., Thum K., Xu X., Obertello M., Katari M.S., Tanurdzic M., Dean A., Nero D.C., McClung C.R., Coruzzi G.M. (2008). Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc. Natl. Acad. Sci. USA 105: 4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer S.L. (2009). The circadian system in higher plants. Annu. Rev. Plant Biol. 60: 357–377 [DOI] [PubMed] [Google Scholar]
- Helfer A., Nusinow D.A., Chow B.Y., Gehrke A.R., Bulyk M.L., Kay S.A. (2011). LUX ARRHYTHMO encodes a nighttime repressor of circadian gene expression in the Arabidopsis core clock. Curr. Biol. 21: 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E., et al. (2012). EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 24: 428–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm K., Källman T., Gyllenstrand N., Hedman H., Lagercrantz U. (2010). Does the core circadian clock in the moss Physcomitrella patens (Bryophyta) comprise a single loop? BMC Plant Biol. 10: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., dePamphilis C.W., Ma H. (2008). Phylogenetic analysis of the plant-specific zinc finger-homeobox and mini zinc finger gene families. J. Integr. Plant Biol. 50: 1031–1045 [DOI] [PubMed] [Google Scholar]
- Inaba R., Nishio T. (2002). Phylogenetic analysis of Brassiceae based on the nucleotide sequences of the S-locus related gene, SLR1. Theor. Appl. Genet. 105: 1159–1165 [DOI] [PubMed] [Google Scholar]
- Jiao Y., et al. (2011). Ancestral polyploidy in seed plants and angiosperms. Nature 473: 97–1165 [DOI] [PubMed] [Google Scholar]
- Kim J.A., Kim J.S., Hong J.K., Lee Y.-H., Choi B.-S., Seol Y.-J., Jeon C.H. (2012). Comparative mapping, genomic structure, and expression analysis of eight pseudo-response regulator genes in Brassica rapa. Mol. Genet. Genomics 287: 373–388 [DOI] [PubMed] [Google Scholar]
- Kim J.A., et al. (2007). Isolation of circadian-associated genes in Brassica rapa by comparative genomics with Arabidopsis thaliana. Mol. Cells 23: 145–153 [PubMed] [Google Scholar]
- Kuno N., Møller S.G., Shinomura T., Xu X.M., Chua N.-H., Furuya M. (2003). The novel MYB protein EARLY-PHYTOCHROME-RESPONSIVE1 is a component of a slave circadian oscillator in Arabidopsis. Plant Cell 15: 2476–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lou P., Xie Q., Xu X., Edwards C.E., Brock M.T., Weinig C., McClung C.R. (2011). Genetic architecture of the circadian clock and flowering time in Brassica rapa. Theor. Appl. Genet. 123: 397–409 [DOI] [PubMed] [Google Scholar]
- Lu S.X., Knowles S.M., Andronis C., Ong M.S., Tobin E.M. (2009). CIRCADIAN CLOCK ASSOCIATED1 and LATE ELONGATED HYPOCOTYL function synergistically in the circadian clock of Arabidopsis. Plant Physiol. 150: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons E., Freeling M. (2008). How to usefully compare homologous plant genes and chromosomes as DNA sequences. Plant J. 53: 661–673 [DOI] [PubMed] [Google Scholar]
- Lyons E., Pedersen B., Kane J., Alam M., Ming R., Tang H., Wang X., Bowers J., Paterson A., Lisch D., Freeling M. (2008). Finding and comparing syntenic regions among Arabidopsis and the outgroups papaya, poplar, and grape: CoGe with rosids. Plant Physiol. 148: 1772–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere S., De Bodt S., Raes J., Casneuf T., Van Montagu M., Kuiper M., Van de Peer Y. (2005). Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. USA 102: 5454–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews S. (2006). Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 15: 3483–3503 [DOI] [PubMed] [Google Scholar]
- Mathews S., Sharrock R.A. (1997). Phytochrome gene diversity. Plant Cell Environ. 20: 666–671 [Google Scholar]
- McClung C.R. (2011). The genetics of plant clocks. Adv. Genet. 74: 105–139 [DOI] [PubMed] [Google Scholar]
- McClung C.R., Gutiérrez R.A. (2010). Network news: Prime time for systems biology of the plant circadian clock. Curr. Opin. Genet. Dev. 20: 588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A.J., Carré I.A., Strayer C.A., Chua N.-H., Kay S.A. (1995). Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267: 1161–1163 [DOI] [PubMed] [Google Scholar]
- Nakamichi N., Kiba T., Henriques R., Mizuno T., Chua N.-H., Sakakibara H. (2010). PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 22: 594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi N., Kita M., Ito S., Yamashino T., Mizuno T. (2005). PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, together play essential roles close to the circadian clock of Arabidopsis thaliana. Plant Cell Physiol. 46: 686–698 [DOI] [PubMed] [Google Scholar]
- Nelson D.C., Lasswell J., Rogg L.E., Cohen M.A., Bartel B. (2000). FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101: 331–340 [DOI] [PubMed] [Google Scholar]
- Ni Z., Kim E.-D., Ha M., Lackey E., Liu J., Zhang Y., Sun Q., Chen Z.J. (2009). Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature 457: 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinow D.A., Helfer A., Hamilton E.E., King J.J., Imaizumi T., Schultz T.F., Farré E.M., Kay S.A. (2011). The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475: 398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. (1970). Evolution by Gene Duplication. (London: Allen & Unwin/Springer-Verlag; ). [Google Scholar]
- Para A., Farré E.M., Imaizumi T., Pruneda-Paz J.L., Harmon F.G., Kay S.A. (2007). PRR3 is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 19: 3462–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin I.A.P., Gulden S.M., Sharpe A.G., Lukens L., Trick M., Osborn T.C., Lydiate D.J. (2005). Segmental structure of the Brassica napus genome based on comparative analysis with Arabidopsis thaliana. Genetics 171: 765–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra G., Bradnam K., Korf I. (2007). CEGMA: A pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 23: 1061–1067 [DOI] [PubMed] [Google Scholar]
- Parry G., Calderon-Villalobos L.I., Prigge M., Peret B., Dharmasiri S., Itoh H., Lechner E., Gray W.M., Bennett M., Estelle M. (2009). Complex regulation of the TIR1/AFB family of auxin receptors. Proc. Natl. Acad. Sci. USA 106: 22540–22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A.H., Freeling M., Tang H., Wang X. (2010). Insights from the comparison of plant genome sequences. Annu. Rev. Plant Biol. 61: 349–372 [DOI] [PubMed] [Google Scholar]
- Pokhilko A., Fernández A.P., Edwards K.D., Southern M.M., Halliday K.J., Millar A.J. (2012). The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Mol. Syst. Biol. 8: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M.J., Clark S.E. (2006). Evolution of the class III HD-Zip gene family in land plants. Evol. Dev. 8: 350–361 [DOI] [PubMed] [Google Scholar]
- Pruneda-Paz J.L., Breton G., Para A., Kay S.A. (2009). A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science 323: 1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda-Paz J.L., Kay S.A. (2010). An expanding universe of circadian networks in higher plants. Trends Plant Sci. 15: 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R., Schwartz J., Jones M.A., Sairanen I., Cheng Y., Andersson C.R., Zhao Y., Ljung K., Harmer S.L. (2009). REVEILLE1, a Myb-like transcription factor, integrates the circadian clock and auxin pathways. Proc. Natl. Acad. Sci. USA 106: 16883–16888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat R., Takahashi N., Hsu P.Y., Jones M.A., Schwartz J., Salemi M.R., Phinney B.S., Harmer S.L. (2011). REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock. PLoS Genet. 7: e1001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathia N., Lynn J.R., Millar A.J., King G.J. (2007). Detection and resolution of genetic loci affecting circadian period in Brassica oleracea. Theor. Appl. Genet. 114: 683–692 [DOI] [PubMed] [Google Scholar]
- Salomé P.A., McClung C.R. (2005). PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes essential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell 17: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé P.A., Weigel D., McClung C.R. (2010). The role of the Arabidopsis morning loop components CCA1, LHY, PRR7, and PRR9 in temperature compensation. Plant Cell 22: 3650–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoff D., Zheng C., Zhu Q. (2010). The collapse of gene complement following whole genome duplication. BMC Genomics 11: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satbhai S.B., Yamashino T., Mizuno T., Aoki S. (2011). Heterologous expression and functional characterization of a Physcomitrella Pseudo response regulator homolog, PpPRR2, in Arabidopsis. Biosci. Biotechnol. Biochem. 75: 786–789 [DOI] [PubMed] [Google Scholar]
- Schnable J.C., Springer N.M., Freeling M. (2011). Differentiation of the maize subgenomes by genome dominance and both ancient and ongoing gene loss. Proc. Natl. Acad. Sci. USA 108: 4069–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. (2006). Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis D.E., Albert V.A., Leebens-Mack J., Bell C.D., Paterson A.H., Zheng C., Sankoff D., Depamphilis C.W., Wall P.K., Soltis P.S. (2009). Polyploidy and angiosperm diversification. Am. J. Bot. 96: 336–348 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Fujiwara S. (2009). Thinking outside the F-box: Novel ligands for novel receptors. Trends Plant Sci. 14: 206–213 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Kim W.Y., Geng R. (2004). The F-box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell 16: 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D.E., Schultz T.F., Milnamow M., Kay S.A. (2000). ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101: 319–329 [DOI] [PubMed] [Google Scholar]
- Stajich J.E., et al. (2002). The Bioperl toolkit: Perl modules for the life sciences. Genome Res. 12: 1611–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins G.L. (1950). Variation and Evolution in Plants. (New York: Columbia University Press; ). [Google Scholar]
- Stephenson P., Baker D., Girin T., Perez A., Amoah S., King G.J., Østergaard L. (2010). A rich TILLING resource for studying gene function in Brassica rapa. BMC Plant Biol. 10: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N., Saito S., Saito C.T., Uemura M. (2010). Phylogenetic footprint of the plant clock system in angiosperms: Evolutionary processes of pseudo-response regulators. BMC Evol. Biol. 10: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Woodhouse M.R., Cheng F., Schnable J.C., Pedersen B.S., Conant G., Wang X., Freeling M., Pires J.C. (2012). Altered patterns of fractionation and exon deletions in Brassica rapa support a two-step model of paleohexaploidy. Genetics 190: 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B.C., Pedersen B., Freeling M. (2006). Following tetraploidy in an Arabidopsis ancestor, genes were removed preferentially from one homeolog leaving clusters enriched in dose-sensitive genes. Genome Res. 16: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town C.D., et al. (2006). Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. Plant Cell 18: 1348–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troein C., Corellou F., Dixon L.E., van Ooijen G., O’Neill J.S., Bouget F.-Y., Millar A.J. (2011). Multiple light inputs to a simple clock circuit allow complex biological rhythms. Plant J. 66: 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troein C., Locke J.C.W., Turner M.S., Millar A.J. (2009). Weather and seasons together demand complex biological clocks. Curr. Biol. 19: 1961–1964 [DOI] [PubMed] [Google Scholar]
- van Ooijen G., Dixon L.E., Troein C., Millar A.J. (2011). Proteasome function is required for biological timing throughout the twenty-four hour cycle. Curr. Biol. 21: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tian L., Lee H.-S., Wei N.E., Jiang H., Watson B., Madlung A., Osborn T.C., Doerge R.W., Comai L., Chen Z.J. (2006). Genomewide nonadditive gene regulation in Arabidopsis allotetraploids. Genetics 172: 507–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., et al. , Brassica rapa Genome Sequencing Project Consortium (2011). The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 43: 1035–1039 [DOI] [PubMed] [Google Scholar]
- Woodhouse M.R., Schnable J.C., Pedersen B.S., Lyons E., Lisch D., Subramaniam S., Freeling M. (2010). Following tetraploidy in maize, a short deletion mechanism removed genes preferentially from one of the two homologs. PLoS Biol. 8: e1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Xie Q., McClung C.R. (2010). Robust circadian rhythms of gene expression in Brassica rapa tissue culture. Plant Physiol. 153: 841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.-W., Lai K.-N., Tai P.-Y., Li W.-H. (1999). Rates of nucleotide substitution in angiosperm mitochondrial DNA sequences and dates of divergence between Brassica and other angiosperm lineages. J. Mol. Evol. 48: 597–604 [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen Y., Wang Z.-Y., Chen Z., Gu H., Qu L.-J. (2007). Constitutive expression of CIR1 (RVE2) affects several circadian-regulated processes and seed germination in Arabidopsis. Plant J. 51: 512–525 [DOI] [PubMed] [Google Scholar]