Abstract
In the face of an increasing world population and climate instability, the demands for food and fuel will continue to rise. Plant science will be crucial to help meet these exponentially increasing requirements for food and fuel supplies. Fundamental plant research will play a major role in providing key advances in our understanding of basic plant processes that can then flow into practical advances through knowledge sharing and collaborations. The model plant Arabidopsis thaliana has played a major role in our understanding of plant biology, and the Arabidopsis community has developed many tools and resources to continue building on this knowledge. Drawing from previous experience of internationally coordinated projects, The international Arabidopsis community, represented by the Multinational Arabidopsis Steering Committee (MASC), has drawn up a road map for the next decade of Arabidopsis research to inform scientists and decision makers on the future foci of Arabidopsis research within the wider plant science landscape. This article provides a summary of the MASC road map.
INTRODUCTION: GLOBAL CHALLENGES AND THE SMALL PLANT WITH BIG IDEAS
Humans depend on plants for almost every aspect of life, from the food we eat to the medicine we rely on. However, this interdependent relationship is being stretched to its limits as a result of an ever-increasing world population and the effects of climate instability. The world population is estimated to increase from the current 7 billion to 9 billion by 2050 (Ronald, 2011), and emerging economies with wealthier populations will require increasing amounts of fuel to run vehicles, operate factories, and produce consumer goods at a pace that until recently only occurred in a few countries. Expanding our knowledge of plant processes will therefore be crucial to meet the pressing need for food, feed, fuel, and shelter and to mitigate the negative effects of climate change (Weckwerth, 2011a). This is a very exciting time for the international plant science community, because recognition and public awareness of the importance of plant research to our future has increased significantly in the past few years. This presents the community with both opportunity and challenge. To help solve these difficult global problems, we must increase our commitment to the fundamental research that provides key advances in understanding while simultaneously guaranteeing that this augments practical advances through efficient knowledge sharing and beneficial collaborations.
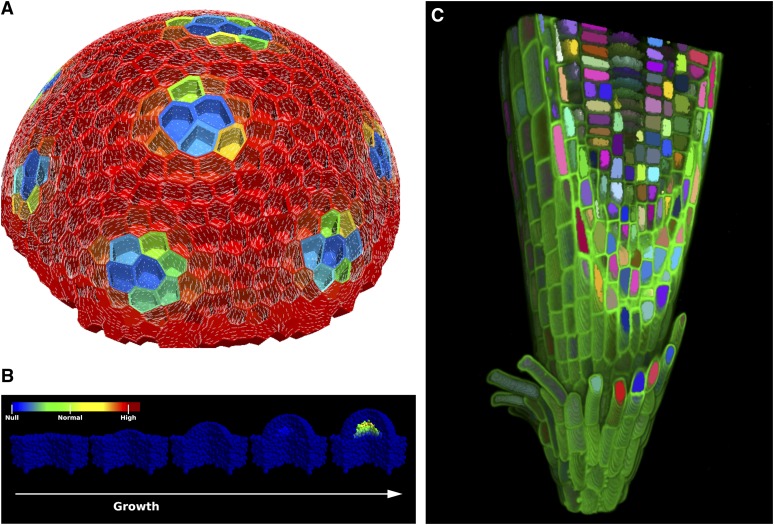
Arabidopsis thaliana is a member of the Brassicaceae family, which includes mustard, cabbage, and oilseed rape. Its small size, rapid generation time, small genome, and ability to generate a large amount of seed by self-pollination have contributed to making Arabidopsis the reference plant for plant biology. Significant investments in Arabidopsis research during past decades have led to the generation of a fully annotated genome (Arabidopsis Genome Initiative, 2000), myriad tools and resources, and a flourishing international community of researchers. Arabidopsis is the most studied flowering plant and a reference organism for all biology; in 2011 alone, 3768 peer-reviewed journal articles were published on Arabidopsis (based on a PubMed search on March 12, 2012). This compares very favorably to the 3637 and 2267 articles published on rice (Oryza sativa) and maize (Zea mays), respectively. By focusing on a single tractable system, the international community has made dramatic advances in our understanding of plants and basic biology, including the identification of the major plant hormone receptors (Dharmasiri et al., 2005a; Dharmasiri et al., 2005b; Kepinski and Leyser, 2005; Ueguchi-Tanaka et al., 2005; Chini et al., 2007; Thines et al., 2007; Melotto et al., 2008; Ma et al., 2009; Park et al., 2009), the elucidation of florigen (Corbesier et al., 2007; Tamaki et al., 2007), and the identification of small RNAs (Hamilton and Baulcombe, 1999; Baulcombe, 2004). In parallel, the resources required for systems approaches in Arabidopsis have developed at an unforeseen speed: from genome-wide mutagenesis, genotyping of hundreds of accessions, epigenomics, transcriptomics, proteomics, protein interactions, metabolomics, to genome-scale metabolic reconstruction and computational modeling. Arabidopsis serves as a unique plant model that will inform attempts to establish similar platforms for crop and ecological research (Figure 1) (Fiehn et al., 2000; Alonso et al., 2003; Heazlewood et al., 2008; Lister et al., 2008; Weckwerth et al., 2008; Poolman et al., 2009; Hancock et al., 2011). As a result of this outstanding progress, the Arabidopsis research community is now ideally placed to help address current and future global challenges.
Figure 1.
Arabidopsis Serves as a Unique Plant Model for Predictive Modeling Approaches That Will Inform Attempts to Establish Similar Platforms for Crop and Ecological Research.
(A) Virtual Shoot Apical Meristem (SAM). Example of a model of the Arabidopsis SAM combining biochemical and mechanical interactions. Auxin transport model is simulated on the three-dimensional (3D) cellular template of the L1 layer of meristem. Resulting peaks of auxin are visualized as a color map from red (low) to blue (high). High auxin concentration is assumed to loosen the walls of the cells, which is simulated in a Finite Element model as lowering the elastic modulus of those walls. This leads to the pattern of the maximal principal stress direction (pictured as thin white lines) circumventing the auxin maxima, which coincide with microtubule organization during primordia formation. (Image courtesy of Henrik Jönsson, Lund University, Sweden and Elliot Meyerowitz, The Sainsbury Laboratory University of Cambridge, United Kingdom.)
(B) SAM signaling model displaying possible WUSCHEL activation by tissue geometry modifications. From left to right, a bulge grows on top of a flat tissue section. The geometrical changes are sufficient to activate the expression of WUSCHEL important for a functional shoot meristem. (Image courtesy of Henrik Jönsson, Lund University, Sweden and Elliot Meyerowitz, The Sainsbury Laboratory University of Cambridge, United Kingdom.)
(C) Virtual root. 3D visualization of an Arabidopsis root apex produced using automated image analysis and visualization techniques. Automated image analysis techniques are used to extract the 3D geometry from confocal microscope images of an Arabidopsis root apex. The extracted geometry can then be used in modeling applications to provide a foundation for realistic 3D models. (Image courtesy of the Centre for Plant Integrative Biology, University of Nottingham, United Kingdom.)
As many major projects and funding programs involving Arabidopsis research came to a close in 2010, the international Arabidopsis community, represented by the Multinational Arabidopsis Steering Committee (MASC), have drawn up a new road map for Arabidopsis research, entitled “From Bench to Bountiful Harvests,” that will be made available in full in the annual MASC report (Multinational Arabidopsis Steering Committee, 2012). The purpose of the road map is to inform scientists, funding bodies, and decisions makers on the future foci of Arabidopsis research. Drawing from several reports, discussions, and recommendations that have emerged in recent years, including the more recent “2020 European Vision for Plant Science” (Beynon et al., 2008) and “An International Model for the Future of Plant Science” (Dennis et al., 2011), the road map details the future direction of an internationally coordinated plan for Arabidopsis research within the wider plant science landscape.
Here we provide a summary of the road map, in which we seek to inform the wider scientific community of the research objectives and goals that are to be pursued by the international Arabidopsis community over the next 10 years. For Arabidopsis scientists, it provides an international framework for their research in the absence of a dedicated funding program. We hope this will be useful not only to Arabidopsis scientists but also to the rest of the plant science community and beyond.
THE NEXT 10 YEARS: FROM BENCH TO FIELDS
In the past 20 years, Arabidopsis and plant biology research have largely focused on a reductionist approach to answering biological questions by studying a single gene or limited group of genes in specific pathways or processes. Although this approach has been and will continue to be tremendously successful, plant research is moving into a new era in which studies of complex biological organization and processes will consider all the relevant molecular constituents. This type of holistic approach has revealed that biological processes are much more complex than previously believed; the simple linear relationship between a gene and its biological output (i.e., the relationship between a genotype and phenotype) is no longer adequate. Mutations do not always generate distinct phenotypes, and a phenotype is often dependent on environmental conditions. A major challenge for the future is therefore to understand this complexity so that genomic sequences can be linked to physiological outcomes. This will provide the fundamental knowledge base required for agricultural improvement, to generate the vast array of plant-based products, and for efforts to improve our environment.
The new decadal vision for international Arabidopsis research, “From Bench to Bountiful Harvests,” has been proposed by MASC in an effort to understand the fundamental processes of plant biology in sufficient detail to accurately predict both outputs of the system and the effect of perturbations. By obtaining in-depth knowledge of how the genome is translated into a continuum of processes, from the single molecule to cells and tissues, the whole plant, plant populations, and fields of plants, it will be possible to build in silico models with the predictive power needed to drive plant science forward (Weckwerth 2011a).
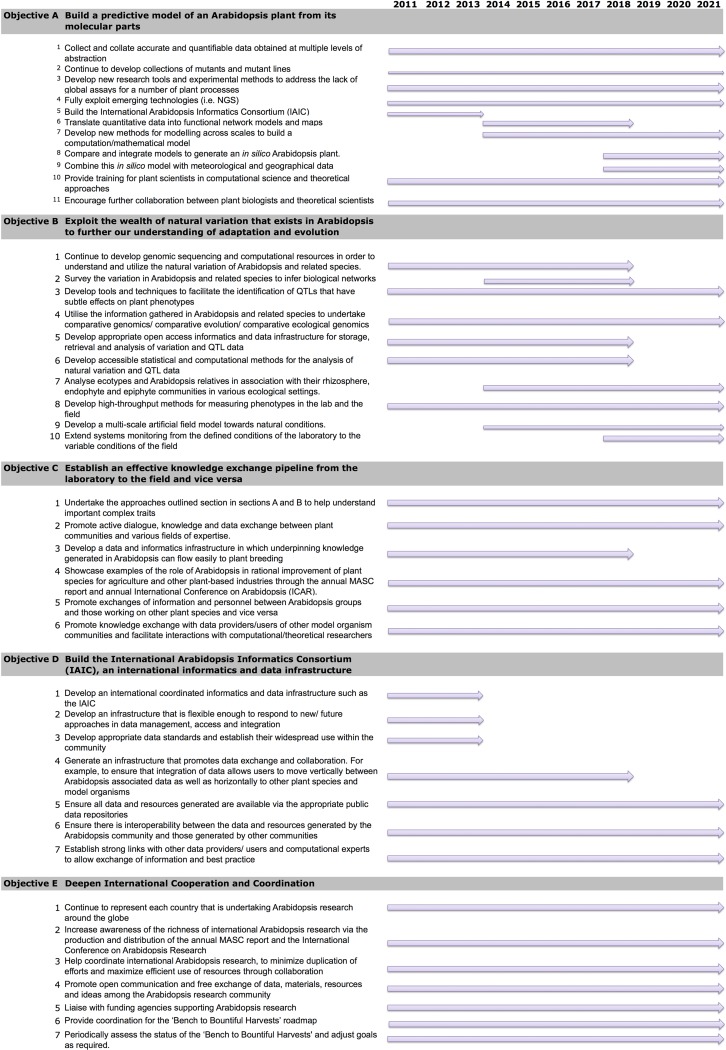
To achieve this vision, the road map outlines the following five objectives (Figure 2).
Figure 2.
Timeline of the Objectives and Goals of the “Bench to Bountiful Harvests” MASC Road Map.
1. Build a Predictive Model of an Arabidopsis Plant from Its Molecular Parts
Functional genome annotation of Arabidopsis has been a major concern of the Arabidopsis and wider plant science communities over the past decade. This has led to one of the most comprehensively annotated genomes available. Consequently, the Arabidopsis genome provides one of the richest resources for the establishment of predictive models. For example, recently the first steps toward a genome-scale metabolic reconstruction were undertaken (Poolman et al., 2009; de Oliveira Dal'Molin et al., 2010). These first drafts of predictive metabolic models are fragmentary and require refinement. However, they provide the backbone for continuous improvement of a genome-scale understanding of metabolism and physiology (Weckwerth, 2011b). The inventory of metabolites, proteins, and transcripts in Arabidopsis are described qualitatively and to some extent quantitatively, but the dynamic interactions between the plant and the environment that lead to specific phenotypes and phenotypic plasticity are poorly understood, and only rare examples exist (Atwell et al., 2010). In the next decade, the road map therefore proposes not only large-scale molecular studies in Arabidopsis, but more importantly linkage of these data with modeling and predictive approaches. This implies a strong focus on the integration of different types of experimental and environmental data with the metabolic reconstructions of Arabidopsis to build computational models with predictive value. Only by integrating all the available information will it be possible to build models that enhance our understanding of these interactions, enabling us to make accurate predictions and exploit this knowledge to optimize plant growth. Accurate and quantifiable data obtained at many levels (including transcripts, methylation patterns, chromatin patterns, comparative genomics, proteomics, metabolomics, protein interactions, protein location, and temporal data) will be required. The data will then need to be translated into functional networks at various scales linked to mathematical models compared across thousands of accessions and associated with meteorological and geographical information. Although this is an ambitious goal, work to integrate these different types of data has begun (Hancock et al., 2011).
2. Build the International Arabidopsis Informatics Consortium, an International Informatics and Data Infrastructure
The recent explosion in data means that careful consideration of data management, data analysis, and data access will be key to the success of plant science and biology in general. Bioinformatics is therefore a key component of the road map. An internationally coordinated data infrastructure to collect, store, visualize, and interpret the diverse array of biological and theoretical data will be required. To address these issues, the Arabidopsis community has recently proposed the initiation of the International Arabidopsis Informatics Consortium (IAIC). The IAIC will consist of a globally distributed system of data and resources that would be managed by a single international consortium capable of leveraging expertise and funding on a global scale (International Arabidopsis Informatics Consortium, 2010). A model for the establishment of this new Arabidopsis Information Portal (AIP) is proposed in this issue (International Arabidopsis Informatics Consortium, 2012). Key data, tools, and resources, such as the databases for genomic and key molecular stocks, the gold standard genome, and annotation and curation of functional data, will provide the core of the information platform on to which many different international modules, including those driven by the MASC subcommittees, can be added. Good examples of integrated and user-friendly structures that could function as modules of the AIP included the MASCP-Gator (http://www.masc-proteomics.org/mascp/index.php/Gator)–––a Web interface integrating world-wide distributed proteomics databases and providing Arabidopsis researchers with first hints regarding functional interpretation of proteins (Joshi et al., 2011). A similar structure for a metabolomics Web interface is currently in development, driven by the international MASC metabolomics subcommittee.
The construction and implementation of the AIP as described by the IAIC offers the opportunity to establish Arabidopsis not only as the primary plant for predictive modeling, but also to serve as a mechanism for enhancing collaboration with other model systems (such as yeast, worm, and mouse), for interaction and comparisons with other plants species, and to visualize the importance of plant sciences for our society.
3. Exploit the Wealth of Natural Variation That Exists in Arabidopsis to Further Our Understanding of Adaptation and Evolution
In another approach to understand how genotype relates to phenotype, new technologies, such as Next Generation Sequencing and phenotyping platforms, will be exploited to study Arabidopsis natural variation and develop an efficient method to predict phenotype from genotype information. Linking the phenotypic variations that can be observed in nature to the underlying genotypic variation will enable us to exploit this variation to engineer beneficial traits into plants (Atwell et al., 2010). For example, a genome-wide association study by Filiault and Maloof (2012) assessed the shade avoidance response across a range of Arabidopsis accessions and revealed that this trait is controlled by several small to moderate effect loci, which include those involved in auxin biosynthesis and signaling as well as gibberellin biosynthesis and signaling. The results of this study provide a platform to understand the molecular nature of this physical response, which is of great relevance to commercially cultivated crops, where high planting densities can trigger the shade avoidance response and decrease yield.
The wealth of genomic and genetic information available in Arabidopsis (Atwell et al., 2010) provides a means to address fundamental questions of quantitative genetics, such as biomass production, water and nitrogen use efficiency, or drought resistance, that become too expensive to study in crops initially, because of labor costs and field space requirements. However, the information obtained through primary studies in Arabidopsis will need to feed into and be linked up with the activities of crop researchers and international consortia (see objective 4 and 5).
4. Establish an Effective Knowledge Exchange Pipeline from the Laboratory to the Field and Vice Versa
By establishing an efficient two-way knowledge exchange pipeline between Arabidopsis scientists and other plant scientists, fundamental advances generated by basic research will flow into practical programs, such as the breeding of plants of agricultural and commercial importance. The unprecedented genome-scale technologies combined with theoretical and computational approaches are allowing research to be conducted in previously intractable plant species, and this is predicted to increase exponentially during the next decade. At the same time, the wealth of data and resources accrued in Arabidopsis is an essential resource for plant scientists working on the development of tools and techniques in other species. By establishing an efficient two-way information exchange, and by responding to the requests and needs from the field, Arabidopsis research can deliver cutting-edge technologies and underpinning knowledge that can then be translated and adapted to other plant species.
To facilitate this information exchange, we will plan to partner with groups focused on key crop species, such as the International Rice Research Institute, the International Research Initiative for Wheat Improvement, the International Maize and Wheat Improvement Center, and other centers of the Consultative Group on International Agricultural Research, to organize workshops at the annual International Conference on Arabidopsis Research (ICAR). We expect these workshops to focus on key traits and issues, such as water use efficiency, disease resistance, and the sustainable intensification of agriculture. The workshops will build on and learn from current efforts, such as the workshop between the International Plant Proteomics Organization and the MASC proteomics subcommittee that will be held this year at the ICAR 2012 in Vienna. The International Plant Proteomics Organization was recently founded to extend proteomics studies from model systems to applied systems and to amplify the interest in applied research in agriculturally important plant systems (http://www.inppo.com/). We hope that these proposed workshops will help to foster communication across the plant science sector, with the aim of understanding and outlining where the Arabidopsis community can best place its efforts to help crop researchers provide answers to global problems. Similar workshops could also be organized at other meetings, such as the American Society of Plant Biology and European Plant Science Organization (EPSO) meetings as well as the International Congress of Plant Molecular Biology.
5. Deepen International Cooperation and Coordination
The challenges that plant biologists must address over the next decade are too large for any single country to address alone. International coordination of research and investment is critical for the future success of plant science research. The Arabidopsis community has a long tradition of international collaboration. This was amply illustrated by the efficient data and resource sharing that occurred during the sequencing of the Arabidopsis genome (Arabidopsis Genome Initiative, 2000) and in the Multinational Coordinated Arabidopsis thaliana Functional Genomics Project (Multinational Arabidopsis Steering Committee, 2010, 2011). In each of these initiatives, MASC helped to ensure success by promoting coordination among the international community. More recently, MASC has been central to the establishment of the IAIC, which aims to facilitate the construction of a single core informatics resource for Arabidopsis (International Arabidopsis Informatics Consortium, 2010).
Originally established in 1990 to promote international cooperation and assist in the free exchange of ideas and information, MASC has continued to provide a unifying voice for the international community in subsequent decades. In the current period of financial uncertainty, an internationally coordinated research plan is more important than ever to leverage national investments, prevent duplication of effort, and synergize the outcomes assisting decision makers and funding agencies as they develop priorities.
In addition, we plan to promote international communication and coordination outside the Arabidopsis community with organizations such as Global Plant Council (GPC) and EPSO. EPSO is an independent academic organization that represents more than 226 research institutes, departments, and universities from 30 countries in Europe and beyond. EPSO's mission is to improve the effect and visibility of plant science in Europe (http://www.epsoweb.org/). The GPC was recently formed representing 23 plant societies from around the world and aims to “brings plant scientists together to work synergistically toward solving the pressing problems facing humankind” (http://www.globalplantcouncil.org/index.htm). To initiate a dialog between MASC, EPSO, and the GPC, and to determine how the Arabidopsis community might best help the EPSO and GPC achieve their goals, we will invite their representatives to attend the annual MASC meetings at the ICAR. This communication, alongside the activities of objective 4, will foster a better understanding among Arabidopsis researchers of global problems in agriculture and help to ensure that the goals of MASC are aligned with those of the wider plant science sector.
CONCLUSION
Arabidopsis research played a central role in enabling the revolutionary changes that occurred in plant biology during the past 20 years. In the decade after the publication of the Arabidopsis genome, publically available shared genomic tools, resources (http://www.Arabidopsis.org; http://Arabidopsis.info), and collections of genome-wide insertion mutations (Alonso et al., 2003) have allowed researchers to make major advances in plant and general biology. This vast toolbox, in addition to the inherent strengths that led to its initial adoption as a reference system, make it likely that Arabidopsis will continue to be the plant species in which novel forms of data are first accrued and exploited and in which new techniques are tested and perfected. Moreover, functional genome annotation is still lagging behind the speed of genome sequencing. Here, Arabidopsis serves as a primary model system for continuous genome annotation improvement and comparative genome annotation exploiting natural genetic variation. Eventually, the catalog of functionally characterized genes in Arabidopsis accessions will serve as a template for newly sequenced plant species and translational research in crop species.
Research in commercially important species, such as wheat, is beginning to blossom, but technological challenges remain. It is therefore of fundamental importance that Arabidopsis research continue to provide fundamental understanding of basic plant biology. Arabidopsis research has also made surprising contributions in some very unexpected areas. For example, despite not being a halophyte, a clever screen for salt hypersensitivity in Arabidopsis (Zhu, 2000) provided key information on a signaling pathway that is central to salt tolerance and sodium homeostasis in Arabidopsis, crops, and halophytes (Li et al., 2006; Remans et al., 2006; Xu et al., 2006; Amtmann et al., 2008; Ho et al., 2009; Weinl and Kudla, 2009). There are many other examples of how Arabidopsis research has affected surprising areas of plant and animal biology (Multinational Arabidopsis Steering Committee, 2010, 2011). It will therefore be essential that Arabidopsis, along with a small number of other key plant species, continue to be used for focused studies of plant biology.
The next decade will present many challenges for plant science, including the sustainable production of food and energy. By fulfilling the objectives of the road map, we can build on the success of the past and continue to make rapid progress toward meeting these challenges. An in-depth understanding of the interaction of plant processes that were traditionally studied in isolation will be obtained. Computational models with predictive value will be generated. A deeper understanding of the interaction between plant and environment will be obtained through the integration of climatological and environmental data with experimental data, enabling the development of predictive models. The road map will help to build and maintain a cohesive and productive Arabidopsis community by providing vision and goals for the next 10 years. By doing so, the Arabidopsis research community will continue to provide the fundamental advances in understanding that will be needed to meet future global challenges.
Acknowledgments
The MASC Coordinator position is supported by a United Kingdom Biotechnology and Biological Science Research Council grant (BBG0214811). The authors thank the members of the current MASC (www.arabidopsis.org/portals/masc/MASC_members.jsp) for their input on the road map. We thank anonymous reviewers for their useful and thoughtful comments.
AUTHOR CONTRIBUTIONS
All authors contributed to writing the article.
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Amtmann A., Troufflard S., Armengaud P. (2008). The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 133: 682–691 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Atwell S., et al. (2010). Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. (2004). RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Beynon J., Coupland G., Graham I., Harter K. (2008). 2020 European Vision for Plant Science, 2–3 June 2008, Bonn, Germany. http://www.arabidopsis.org/portals/masc/2020_European_Vision.pdf. (Accessed June 26, 2012.)
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- Corbesier L., Vincent C., Jang S., Fornara F., Fan Q., Searle I., Giakountis A., Farrona S., Gissot L., Turnbull C., Coupland G. (2007). FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- de Oliveira Dal'Molin C.G., Quek L.E., Palfreyman R.W., Brumbley S.M., Nielsen L.K. (2010). AraGEM, a genome-scale reconstruction of the primary metabolic network in Arabidopsis. Plant Physiol. 152: 579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis L., Ecker J., Gale M., Harter K., Kersey P., Lee S., Matsui M., Millar A., Parkin I., van Sluys M.A., Traas J. (2011). An International Model for the Future of Plant Science, March 2009, Banbury Center, USA. http://www.uni-tuebingen.de/plantphys/AFGN/InternatModelFuturePlantSci.pdf. (Accessed June 26, 2012.)
- Dharmasiri N., Dharmasiri S., Estelle M. (2005a). The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Weijers D., Lechner E., Yamada M., Hobbie L., Ehrismann J.S., Jürgens G., Estelle M. (2005b). Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Fiehn O., Kopka J., Dörmann P., Altmann T., Trethewey R.N., Willmitzer L. (2000). Metabolite profiling for plant functional genomics. Nat. Biotechnol. 18: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Filiault D.L., Maloof J.N. (2012). A genome-wide association study identifies variants underlying the Arabidopsis thaliana shade avoidance response. PLoS Genet. 8: e1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A.J., Baulcombe D.C. (1999). A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286: 950–952 [DOI] [PubMed] [Google Scholar]
- Hancock A.M., Brachi B., Faure N., Horton M.W., Jarymowycz L.B., Sperone F.G., Toomajian C., Roux F., Bergelson J. (2011). Adaptation to climate across the Arabidopsis thaliana genome. Science 334: 83–86 [DOI] [PubMed] [Google Scholar]
- Heazlewood J.L., Durek P., Hummel J., Selbig J., Weckwerth W., Walther D., Schulze W.X. (2008). PhosPhAt: A database of phosphorylation sites in Arabidopsis thaliana and a plant-specific phosphorylation site predictor. Nucleic Acids Res. 36 (Database issue): D1015–D1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- International Arabidopsis Informatics Consortium (2010). An international bioinformatics infrastructure to underpin the Arabidopsis community. Plant Cell 22: 2530–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Arabidopsis Informatics Consortium (2012). Taking the next step: Building an Arabidopsis Information Portal.Plant Cell 24: 2248–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H.J., et al. (2011). MASCP Gator: An aggregation portal for the visualization of Arabidopsis proteomics data. Plant Physiol. 155: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S., Leyser O. (2005). The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451 [DOI] [PubMed] [Google Scholar]
- Li L., Kim B.G., Cheong Y.H., Pandey G.K., Luan S. (2006). A Ca(2)+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R., O'Malley R.C., Tonti-Filippini J., Gregory B.D., Berry C.C., Millar A.H., Ecker J.R. (2008). Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133: 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Melotto M., Mecey C., Niu Y., Chung H.S., Katsir L., Yao J., Zeng W., Thines B., Staswick P., Browse J., Howe G.A., He S.Y. (2008). A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 55: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multinational Arabidopsis Steering Committee (2010). The Multinational Coordinated Arabidopsis thaliana Functional Genomics Project. http://www.arabidopsis.org/portals/masc/2010_MASC_Report.pdf. (Accessed June 26, 2012.)
- Multinational Arabidopsis Steering Committee (2011). The Multinational Coordinated Arabidopsis thaliana Functional Genomics Project. http://www.arabidopsis.org/portals/masc/2011_MASC_Report.pdf. (Accessed June 26, 2012.)
- Multinational Arabidopsis Steering Committee (2012). The Multinational Coordinated Arabidopsis thaliana Functional Genomics Project, in press
- Park S.Y., et al. (2009). Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman M.G., Miguet L., Sweetlove L.J., Fell D.A. (2009). A genome-scale metabolic model of Arabidopsis and some of its properties. Plant Physiol. 151: 1570–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Tillard P., Forde B.G., Gojon A. (2006). The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl. Acad. Sci. USA 103: 19206–19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald P. (2011). Plant genetics, sustainable agriculture and global food security. Genetics 188: 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuo S., Wong H.L., Yokoi S., Shimamoto K. (2007). Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M., Ashikari M., Nakajima M., Itoh H., Katoh E., Kobayashi M., Chow T.Y., Hsing Y.I., Kitano H., Yamaguchi I., Matsuoka M. (2005). GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437: 693–698 [DOI] [PubMed] [Google Scholar]
- Weckwerth W. (2011a). Green systems biology—From single genomes, proteomes and metabolomes to ecosystems research and biotechnology. J. Proteomics 75: 284–305 [DOI] [PubMed] [Google Scholar]
- Weckwerth W. (2011b). Unpredictability of metabolism—the key role of metabolomics science in combination with next-generation genome sequencing. Anal. Bioanal. Chem. 400: 1967–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckwerth W., Baginsky S., van Wijk K., Heazlewood J.L., Millar H. (2008). The multinational Arabidopsis steering subcommittee for proteomics assembles the largest proteome database resource for plant systems biology. J. Proteome Res. 7: 4209–4210 [DOI] [PubMed] [Google Scholar]
- Weinl S., Kudla J. (2009). The CBL-CIPK Ca(2+)-decoding signaling network: Function and perspectives. New Phytol. 184: 517–528 [DOI] [PubMed] [Google Scholar]
- Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Zhu J.K. (2000). Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 124: 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]