This work examines the function of ethylene in freezing in Arabidopsis thaliana, finding that ethylene negatively regulates freezing stress signaling through the direct repression of cold-regulated CBF and type-A Arabidopsis response regulator genes targeted by EIN3.
Abstract
The phytohormone ethylene regulates multiple aspects of plant growth and development and responses to environmental stress. However, the exact role of ethylene in freezing stress remains unclear. Here, we report that ethylene negatively regulates plant responses to freezing stress in Arabidopsis thaliana. Freezing tolerance was decreased in ethylene overproducer1 and by the application of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid but increased by the addition of the ethylene biosynthesis inhibitor aminoethoxyvinyl glycine or the perception antagonist Ag+. Furthermore, ethylene-insensitive mutants, including etr1-1, ein4-1, ein2-5, ein3-1, and ein3 eil1, displayed enhanced freezing tolerance. By contrast, the constitutive ethylene response mutant ctr1-1 and EIN3-overexpressing plants exhibited reduced freezing tolerance. Genetic and biochemical analyses revealed that EIN3 negatively regulates the expression of CBFs and type-A Arabidopsis response regulator5 (ARR5), ARR7, and ARR15 by binding to specific elements in their promoters. Overexpression of these ARR genes enhanced the freezing tolerance of plants. Thus, our study demonstrates that ethylene negatively regulates cold signaling at least partially through the direct transcriptional control of cold-regulated CBFs and type-A ARR genes by EIN3. Our study also provides evidence that type-A ARRs function as key nodes to integrate ethylene and cytokinin signaling in regulation of plant responses to environmental stress.
INTRODUCTION
Low temperature is a major environmental factor that limits plant growth, productivity, and geographical distribution. Plants must adjust various physiological and biochemical processes in response to cold stress. Currently, the most well understood cold signaling pathway is the C-repeat Binding Factor/DRE Binding Factor (CBF/DREB) transcriptional regulatory cascade (Thomashow, 1999). In Arabidopsis thaliana, three CBF transcription factors (CBF1/DREB1b, CBF2/DREB1c, and CBF3/DREB1a) that belong to the APETALA2/ETHYLENE-RESPONSIVE FACTOR (ERF) superfamily bind to the C-repeat/dehydration-responsive element DNA regulatory elements found in the promoters of COLD-REGULATED (COR) genes (Stockinger et al., 1997; Liu et al., 1998). Cold stress activates CBF expression (Gilmour et al., 1998; Liu et al., 1998) and induces the expression of a large subset of COR genes (Maruyama et al., 2004; Vogel et al., 2005), and the overexpression of CBFs leads to constitutively enhanced freezing tolerance (Jaglo-Ottosen et al., 1998; Liu et al., 1998; Gilmour et al., 2000). Moreover, several components, including INDUCER OF CBF EXPRESSION1 (ICE1), HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES1, SIZ1 (for SAP and Miz), and CALMODULIN BINDING TRANSCRIPTION ACTIVATOR3 (CAMTA3), have been shown to function in the CBF pathway, acting upstream of CBFs (Chinnusamy et al., 2003; Dong et al., 2006; Miura et al., 2007; Doherty et al., 2009).
In addition to the CBF regulatory cascade, phytohormones have been implicated in plant responses to cold stress. The plant stress hormone abscisic acid has been shown to be involved in plant responses to cold stress (Gilmour and Thomashow, 1991; Yamaguchi-Shinozaki and Shinozaki, 1994; Liu et al., 1998; Llorente et al., 2000; Tamminen et al., 2001). It has been suggested that the cytokinin receptors Arabidopsis histidine kinase2/3 and type-A Arabidopsis response regulators (ARRs) act as negative regulators in cold stress signaling through the inhibition of the abscisic acid–dependent pathway (Jeon et al., 2010).
Ethylene is a gaseous plant hormone that plays important roles in a wide range of cellular and developmental processes and during abiotic and biotic stress (Abeles et al., 1992; Bleecker and Kende, 2000; Kim et al., 2003; Zhao and Schaller, 2004; Achard et al., 2006; Cao et al., 2007; Wang et al., 2007; Chen et al., 2009). In Arabidopsis, ethylene is perceived by a family of receptors (ETHYLENE RESPONSE1 [ETR1], ETR2, ETHYLENE RESPONSE SENSOR1 [ERS1], ERS2, and ETHYLENE INSENSITIVE4 [EIN4]) that act negatively and redundantly in ethylene signaling (Chang et al., 1993; Hua et al., 1995, 1998; Sakai et al., 1998). In the absence of ethylene, ethylene receptors interact with CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), a Raf-like Ser/Thr protein kinase, and positively regulate its activity (Kieber et al., 1993; Gao et al., 2003). The negative regulator CTR1 in turn directly or indirectly inhibits EIN2, which is an essential positive regulator of ethylene signaling (Alonso et al., 1999). The EIN3/EIN3-Like1 (EIL1) transcription factors function downstream of EIN2 in ethylene signaling (Chao et al., 1997; Solano et al., 1998). It has been shown that ethylene-induced EIN3/EIL1 stability is mediated by the proteasomal degradation of two F-box proteins, EIN3 Binding F-box1 (EBF1) and EBF2 (Guo and Ecker, 2003; Potuschak et al., 2003; An et al., 2010). EIN3/EIL1 activate or repress the expression of ethylene response target genes by specifically binding to their promoters, thereby modulating the ethylene-related responses of plants (Alonso et al., 2003; Chen et al., 2009; Zhong et al., 2009; Boutrot et al., 2010; Zhang et al., 2011).
Although ethylene has been implicated in the cold stress response (Harber and Fuchigami, 1989; Ciardi et al., 1997; Yu et al., 2001; Zhang and Huang, 2010), the exact role that ethylene plays in the regulation of freezing stress remains unclear. Here, we undertook a molecular and genetic approach to investigate the role of ethylene biosynthesis and signaling in plant response to freezing stress. We showed that increased ethylene levels led to decreased tolerance of freezing, whereas blocking ethylene biosynthesis and ethylene signaling enhanced freezing tolerance. Moreover, a set of ethylene-insensitive mutants, including etr1-1, ein4-1, ein2-5, and ein3 eil1, displayed enhanced tolerance to freezing, whereas the ctr1-1 mutant and EIN3-overexpressing plants were greatly impaired in their responses to freezing stress. We further showed that the EIN3 protein was capable of directly binding to the promoters of the CBF1-CBF3 and type-A ARR5, ARR7, and ARR15 genes to repress their expression. Consistent with this, the overexpression of these ARR genes promoted freezing tolerance. These results indicate that ethylene biosynthesis and signaling negatively regulate plant freezing tolerance by repressing the cold-inducible CBFs and type-A ARR genes in Arabidopsis.
RESULTS
Effect of Exogenous 1-Aminocyclopropane-1-Carboxylic Acid and Aminoethoxyvinyl Gly on Plant Responses to Freezing Tolerance
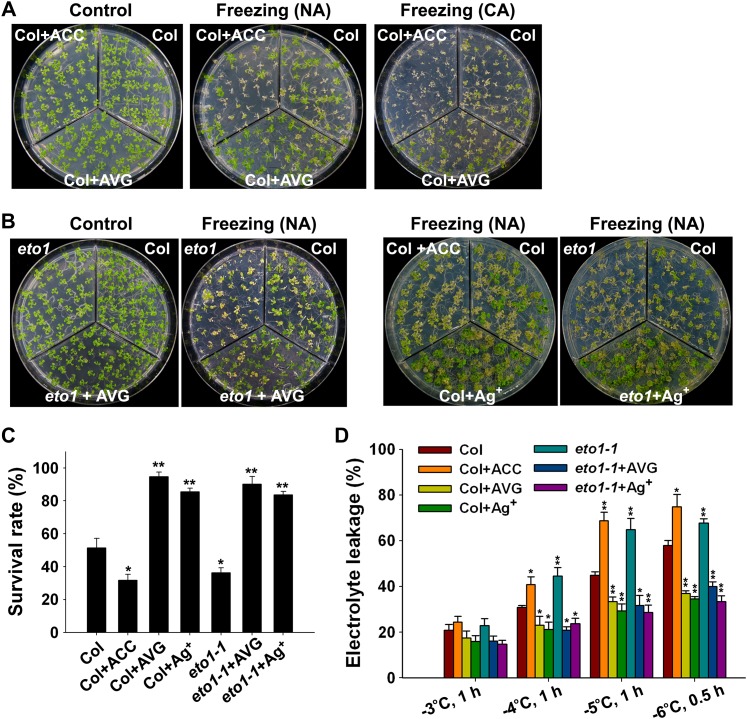
Ethylene is thought to regulate the responses of plants to various abiotic stresses, including salt, drought, and cold stresses (Zhao and Schaller, 2004; Achard et al., 2006; Cao et al., 2007; Wang et al., 2007). To explore the exact role of ethylene in cold stress in Arabidopsis, we first tested whether the freezing tolerance of wild-type plants was affected by altered ethylene biosynthesis under nonacclimated and cold-acclimated (7 d at 4°C) conditions. With or without cold acclimation, the wild-type plants treated with the ethylene biosynthetic precursor 1-aminocyclopropane-1-carboxylic acid (ACC) displayed decreased tolerance to freezing (Figure 1A). In the absence of cold acclimation, ∼50% of the wild-type Columbia (Col) plants were still alive, whereas only 31% of the ACC-treated plants survived after exposure to a temperature of −4°C (Figure 1C). Consistent with this, the ethylene overproduction mutant eto1 also showed reduced freezing tolerance compared with the wild-type plants (Figures 1B and 1C). By contrast, application of the ethylene biosynthesis inhibitor aminoethoxyvinyl glycine (AVG) dramatically enhanced the freezing tolerance of both wild-type and eto1 plants (Figures 1A and 1B). AVG-treated Col and eto1 plants exhibited significantly higher survival rates after exposure to −4°C (95 and 90% survival, respectively) compared with the untreated Col and eto1 plants (50 and 36% survival, respectively) (Figure 1C). The relative electrolyte leakage serves as an indicator of cell membrane integrity damage caused by cold stress (Lyons, 1973); therefore, we measured changes in the electrolyte leakage of wild-type Col, eto1, and ACC- and AVG-treated plants. The eto1 and ACC-treated Col plants had higher electrolyte leakage, whereas the AVG-treated Col and eto1 plants had lower electrolyte leakage than the wild-type Col plants in response to freezing temperatures of −4, −5, and −6°C (Figure 1D). Taken together, these results indicate that ethylene has a negative effect on freezing tolerance in Arabidopsis.
Figure 1.
The Effect of ACC, AVG, and Ag+ on the Freezing Tolerance of Wild-Type and eto1 Mutant Plants.
(A) Freezing phenotypes of 2-week-old wild-type seedlings grown on MS medium or on MS medium supplemented with 10 μM ACC or 2 μM AVG. The plants were subjected to a freezing treatment at −4°C for 1 h for nonacclimated (NA) plants and −6°C for 1 h for cold-acclimated plants (CA; 7 d at 4°C), followed by recovery at 22°C for 4 d.
(B) Freezing phenotypes of nonacclimated wild-type and eto1 seedlings (2 weeks old) grown on MS medium with or without 2 μM AVG or 20 μM AgNO3 at −6°C for 1 h.
(C) The survival rates of plants in (A) and (B). The data are the mean values of three replicates ± sd. *P < 0.05, **P < 0.01 (t test), indicating a significant difference between the treated seedlings (or mutants) and nontreated wild-type Col.
(D) Ion leakage assay of the plants in (A) and (B) at the indicated freezing temperatures. The data are the mean values of three replicates ± sd. *P < 0.05, **P < 0.01 (t test), indicating a significant difference between the treated seedlings (or mutants) and nontreated wild-type Col.
Mutations in Key Components in the Ethylene Signaling Pathway Alter Freezing Tolerance
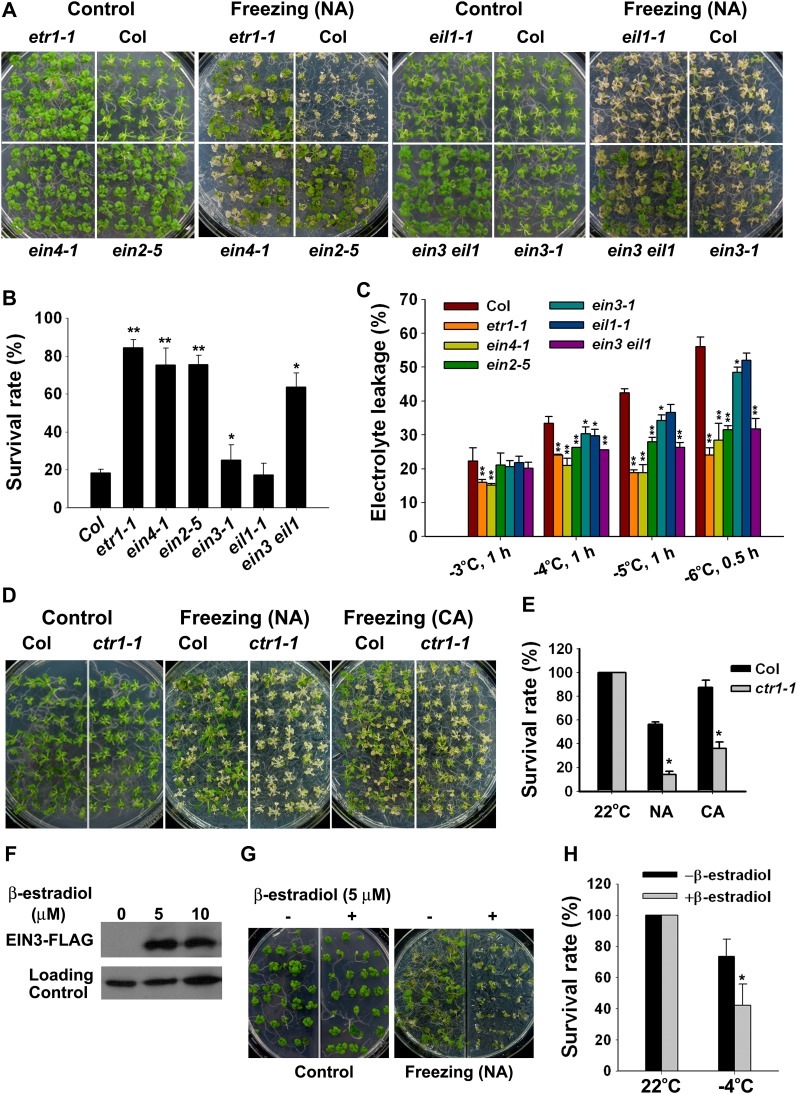
We next examined whether ethylene signaling is involved in plant responses to freezing stress. In the presence of the ethylene receptor antagonist Ag+, the survival rates of both wild-type Col and eto1 plants were significantly increased when the plants were exposed to freezing stress in the absence of cold acclimation (Figures 1B and 1C), and the levels of electrolyte leakage in both the Ag+-treated Col and eto1 plants were much lower than in untreated Col and eto1 (Figure 1D). This result indicates that the blockage of ethylene signaling improves the tolerance of plants to freezing temperatures. To determine the role that ethylene signaling plays during cold stress further, we tested the freezing tolerance of a set of mutants in the ethylene signaling pathway: etr1-1, ein4-1, ctr1-1, ein2-5, ein3-1, eil1-1, and ein3 eil1. etr1-1 and ein4-1 mutants are the gain-of-function ethylene receptor mutants; they exhibited a substantially increased tolerance to freezing under nonacclimated and acclimated conditions (Figures 2A and 2B; see Supplemental Figure 1 online). EIN2 and EIN3 are key positive regulators in the ethylene signaling. The loss-of-function mutant ein2-5 was more tolerant to freezing stress than the wild-type plants. The survival rate of the loss-of-function mutant ein3-1 was slightly higher than that of the wild-type plants, and the eil1-1 mutant behaved similarly to the wild-type plants after freezing treatment. The ein3 eil1 double mutant displayed a constitutively enhanced freezing tolerance (Figures 2A and 2B; see Supplemental Figure 1 online). Electrolyte leakage was dramatically less in etr1-1, ein4-1, ein2-5, and ein3 eil1 double mutant than in the wild-type plants following the freezing treatment, and the ein3-1 and eil1-1 plants exhibited slightly lower electrolyte leakage than the wild-type plants (Figure 2C). Conversely, the constitutive ethylene response mutant ctr1-1 showed decreased freezing tolerance compared with the wild-type plants with or without acclimation (Figures 2D and 2E). Based on the freezing tolerance of these mutants, we conclude that ethylene receptors and CTR1 are positive regulators, whereas EIN2 and EIN3/EIL1 are negative regulators of plant response to freezing stress.
Figure 2.
Freezing Tolerance Assay of Mutants in the Ethylene Signaling Pathway.
(A) and (B) Freezing phenotypes (A) and survival rate (B) of the ein4-1, etr1-1, ein2-5, ein3-1, eil1-1, and ein3 eil1 mutants without cold acclimation. Two-week-old plants grown at 22°C were treated at −5°C for 1 h, followed by recovery at 22°C for 4 d. The data represent the mean values of three replicates ± sd. *P < 0.05, **P < 0.01 (t test), indicating a significant difference between the mutants and wild-type Col. NA, nonacclimated.
(C) Ion leakage assay of the mutants in (A) treated at freezing temperatures. The data represent the mean values of three replicates ± sd. *P < 0.05, **P < 0.01 (t test), indicating a significant difference between the mutants and wild-type Col.
(D) and (E) Freezing phenotypes (D) and survival rate (E) of the ctr1-1 mutant. Two-week-old plants grown at 22°C were treated at −4°C for 1 h (NA) or −6°C for 1 h (cold acclimated [CA]), followed by recovery at 22°C for 4 d. The data represent the mean values of three replicates ± sd. *P < 0.01 (t test), indicating a significant difference between the ctr1-1 mutant and wild-type Col.
(F) EIN3 protein analysis of EIN3-FLAG transgenic plants expressing estradiol-inducible EIN3 in the ein3 eil1 ebf1 ebf2 quadruple mutant background (iE/qm). Two-week-old plants grown on MS medium supplemented with 0, 5, and 10 μM β-estradiol were harvested for an immunoblot assay using an anti-FLAG antibody. A nonspecific band was used as the loading control.
(G) and (H) Freezing phenotypes (G) and survival rate (H) of the iE/qm plants without cold acclimation. Two-week-old plants iE/qm grown on MS with or without 5 μM β-estradiol were subjected to a freezing treatment at −4°C for 1 h, followed by recovery at 22°C for 4 d. The data represent the mean values of three replicates ± sd. *P < 0.01 (t test), indicating a significant difference between the estradiol-treated and nontreated iE/qm seedlings.
Overexpression of EIN3 Confers a Reduced Freezing Tolerance
To dissect further the role of EIN3 in freezing tolerance, we performed a freezing tolerance assay using EIN3-FLAG transgenic plants that express estradiol-inducible EIN3 in the ein3 eil1 ebf1 ebf2 quadruple mutant background (iE/qm) (An et al., 2010), with or without β-estradiol treatment. The EIN3-FLAG protein was strongly expressed in the iE/qm plants after the application of 5 or 10 μM β-estradiol for 4 h (Figure 2F). In the absence or presence of 5 μM β-estradiol, 2-week-old iE/qm plants grown on plates were exposed to freezing temperatures without cold acclimation; strikingly, the β-estradiol-treated iE/qm plants displayed a lower survival rate (42%) than the nontreated iE/qm plants (73%) at the freezing temperature of −4°C (Figures 2G and 2H). There was no obvious difference in freezing tolerance of control wild-type plants untreated or treated with β-estradiol (see Supplemental Figure 2 online). These results indicate that the overexpression of EIN3 leads to hypersensitivity to freezing stress.
Effect of Cold Stress on Ethylene Production and the Expression of Genes in the Ethylene Signaling Pathway
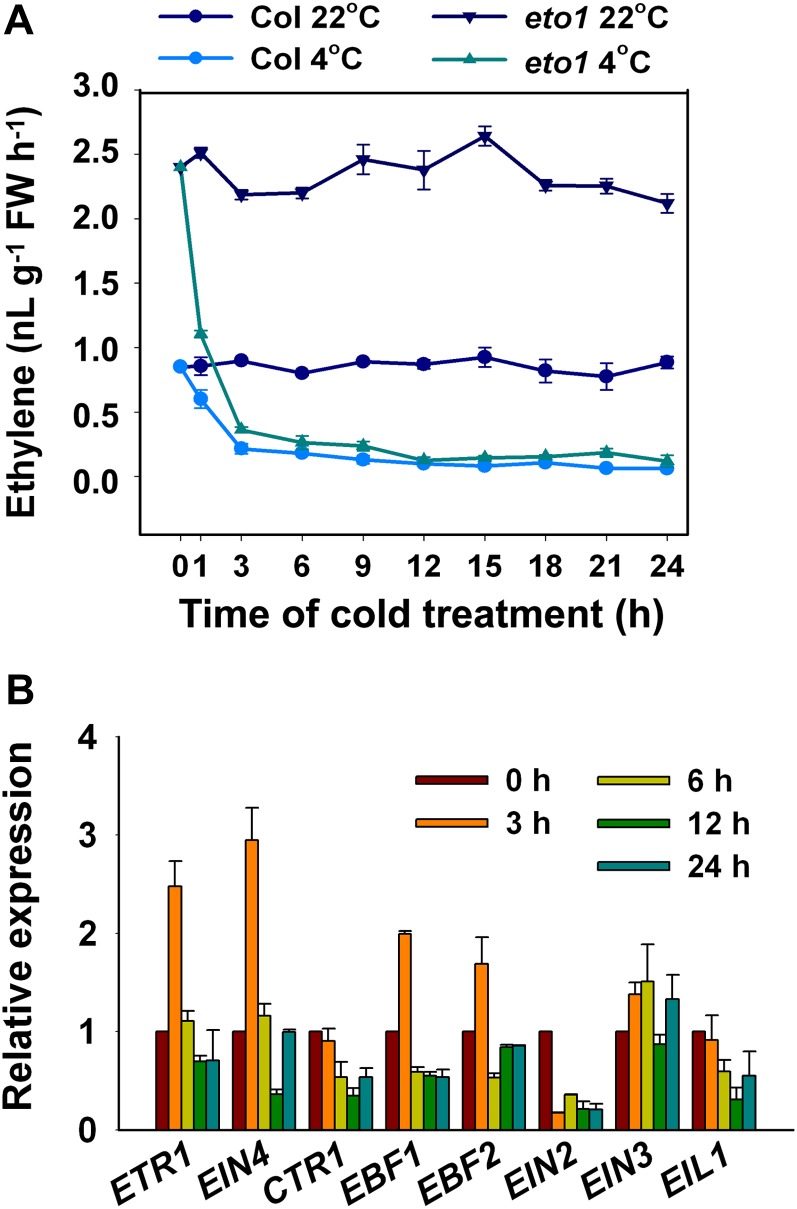
Cold stress has been shown to promote ethylene production in several plant species (Yu et al., 2001; Hershkovitz et al., 2009; Zhang and Huang, 2010). To test whether this is the case in Arabidopsis, we measured ethylene production in wild-type Col and eto1 seedlings following cold treatment. As shown in Figure 3A, the eto1 mutant accumulated threefold more ethylene than the wild-type plants at 22°C, which is consistent with results from a previous study (Guzmán and Ecker, 1990). After cold treatment, however, we unexpectedly found that ethylene production was rapidly reduced after the exposure to cold stress for 1 h in both wild-type Col and eto1 seedlings, reaching the lowest level after 3 h of cold treatment and remaining at this low level over time (Figure 3A). No obvious change in ethylene production was observed in wild-type Col or eto1 mutant kept at 22°C. These results indicate that cold stress inhibits ethylene production in Arabidopsis.
Figure 3.
Ethylene Production and Expression of Genes in the Ethylene Signaling Pathway under Cold Stress.
(A) Ethylene production in wild-type Col and the eto1 mutant under cold stress. Two-week-old seedlings grown in 15-mL gas chromatography vials were grown at 22°C and treated at 4°C or kept at 22°C. The ethylene levels in the headspace were measured at various time points. FW, fresh weight.
(B) Expression of genes in the ethylene signaling pathway under cold stress. Two-week-old wild-type Col seedlings grown at 22°C were treated at 4°C for the indicated periods of time. The transcription levels were determined by qRT-PCR. The data represent the mean values of three replicates ± sd.
We further examined the expression of ETR1, EIN4, CTR1, EBF1, EBF2, EIN2, EIN3, and EIL1 in plants exposed to cold stress. Quantitative real-time PCR (qRT-PCR) analysis showed that ETR1, EIN4, EBF1, and EBF2 were induced at 3 h of cold treatment and then decreased to the basal level after 6 h of cold treatment. Expression of CTR1 was not altered at 3 h of cold and slightly reduced afterwards. EIN2 expression was dramatically decreased after 3 h of cold treatment and remained at a low level under the continuous exposure to cold stress. EIL1 expression was also decreased by cold stress, whereas EIN3 expression was not obviously affected by the cold treatment (Figure 3B). These results indicate that the transcripts of ETR1, EIN4, EBF1, and EBF2 are rapidly upregulated by cold stress, whereas EIN2 and EIL1, but not EIN3, are negatively regulated by cold stress.
Cold Induces EIN3 Protein Accumulation
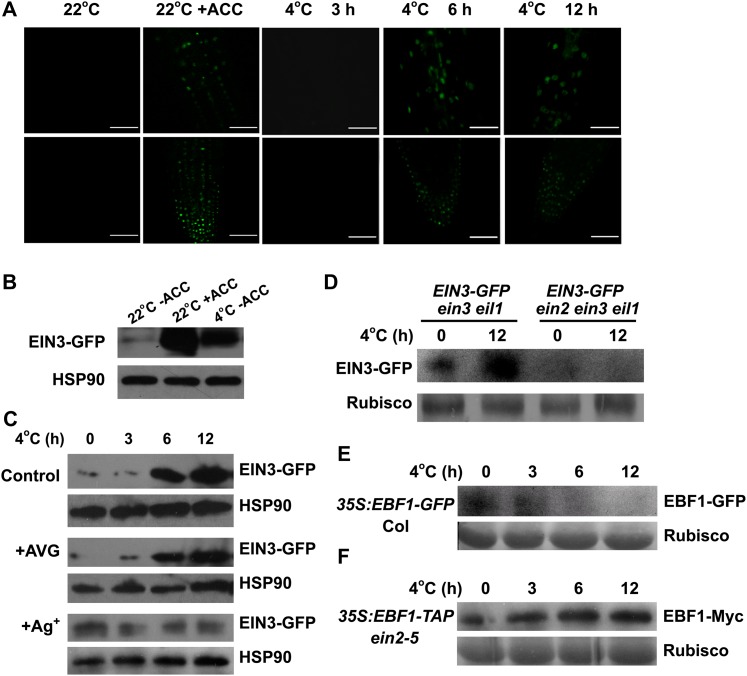
We then asked whether the EIN3 protein accumulation was affected by cold stress. To this end, we treated 35S:EIN3-GFP (for green fluorescent protein) transgenic seedlings in the ein3 eil1 background (He et al., 2011) with the exposure to 4°C for different periods of time. Interestingly, we found that the EIN3 protein accumulated after 6 h of cold treatment (Figures 4A and 4B). The addition of AVG, which effectively suppressed ACC-induced EIN3 accumulation (Guo and Ecker, 2003), barely inhibited the cold-induced EIN3 accumulation (Figure 4C). These results imply that cold stress enhances the stability of the EIN3 protein independently of ethylene biosynthesis.
Figure 4.
EIN3 Protein Is Stabilized by Cold Stress.
(A) Cold treatment stabilizes the EIN3-GFP protein. 35S:EIN3-GFP seedlings (7 d old) in the ein3 eil1 mutant background (EIN3-OE/dm) were treated at 4°C for 0, 3, 6, and 12 h before the tissues were used for detecting GFP fluorescence. Treatment with ACC (10 μM) for 6 h was used as a positive control. Bars = 50 μm.
(B) Immunoblot analysis of EIN3-GFP under cold treatment. EIN3-OE/dm seedlings (7 d old) were treated with ACC (10 μM) or cold for 6 h, and the proteins were extracted and subjected to immunoblot assays using an anti-GFP antibody.
(C) Immunoblot analysis of EIN3-GFP under cold stress in the presence of AVG and Ag+. EIN3-OE/dm seedlings (7 d old) grown on MS medium with AVG (2 μM) or AgNO3 (20 μM) were treated at 4°C for 0, 3, 6, and 12 h.
(D) Immunoblot analysis of EIN3-GFP protein in 7-d-old 35S:EIN3-GFP seedlings in the ein3 eil1 and ein2 ein3 eil1 backgrounds after cold stress. Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase.
(E) Immunoblot analysis of EBF1-GFP protein in 7-d-old 35S:EBF1-GFP seedlings after cold treatment.
(F) Immunoblot analysis of EBF1-TAP protein (detected by anti-Myc antibody) in 7-d-old 35S:EBF1-TAP ein2 seedlings after cold treatment.
To dissect whether cold-induced EIN3 accumulation was dependent on ethylene signaling, we examined the EIN3 protein level in 35S:EIN3-GFP transgenic plants in the ein2-5 background. We found that the EIN3 protein level was not detectable before or after cold treatment in 35S:EIN3-GFP ein2-5 plants (Figure 4D; see Supplemental Figure 3A online). Furthermore, application of Ag+ inhibited cold-induced EIN3 accumulation (Figure 4C). These results suggest that EIN2 is required for cold-induced EIN3 accumulation.
As ethylene-induced EIN3 accumulation is mediated by proteasomal degradation of EBF1/2, we then explored whether the cold effect on EIN3 is also via this pathway. The protein level of EBF1 decreased in 35S:EBF1-GFP seedlings after 3 h of cold treatment, whereas no obvious change of EBF1 protein level was detected in 35S:EBF1-TAP1 ein2-5 seedlings (Figures 4E and 4F; see Supplemental Figure 3B online). These results suggest that EBF1 protein is degraded by cold stress in an EIN2-dependent manner. Therefore, EIN2 appears to be required for EBF1-mediated EIN3 stabilization at cold stress.
Expression of Stress-Responsive Genes in the ein3 eil1 Mutant
To explore how the EIN3/EIL1 genes negatively regulate plant responses to freezing stress, we analyzed data from public microarray experiments to identify cold-regulated genes that are differentially expressed in the ein3 eil1 double mutant (http://www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-18631). A total of 1264 genes were upregulated in the ein3 eil1 double mutant in comparison to Col-0 plants grown at 22°C (more than twofold change, Q < 0.05). We classified these genes according to their functional categories and Gene Ontology annotations and found a subset of genes (98) involved in the responses of plants to environmental stress (from Munich Information Center for Protein Sequences Arabidopsis thaliana functional distribution) (see Supplemental Table 1 online). This subset included cold-regulated genes, such as CBF1, CBF3, COR15a, COR15b, and COR414, a result that is consistent with our qRT-PCR results (Figure 5A). Intriguingly, the upregulated genes in the ein3 eil1 mutant included two type-A response regulator genes, ARR7 and ARR15, that are induced by cold and implicated in cold stress responses (Vogel et al., 2005; Kilian et al., 2007; Jeon et al., 2010) (see Supplemental Table 1 online). Therefore, EIN3/EIL1 may mediate plant responses to freezing stress by regulating the expression of various stress-related genes.
Figure 5.
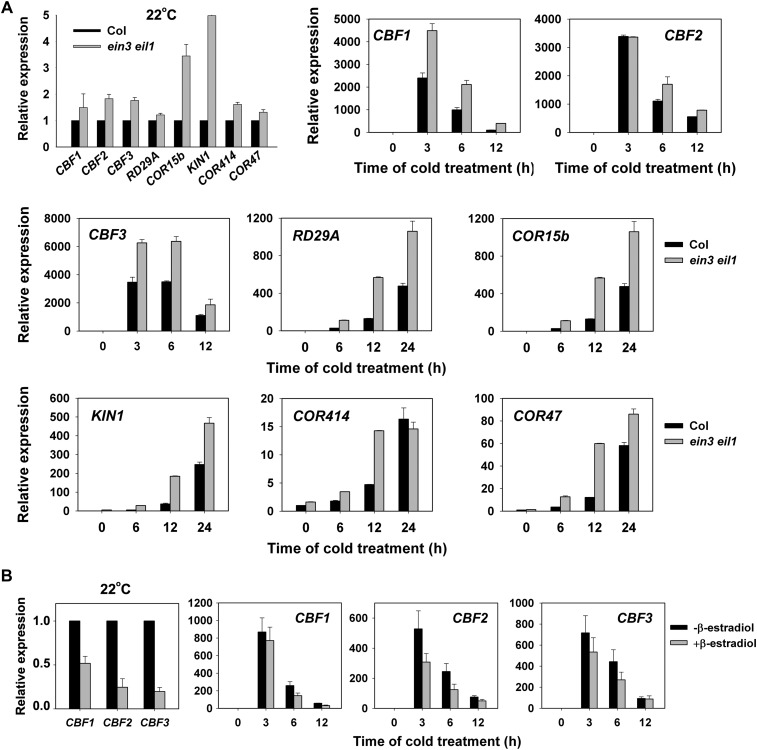
EIN3 Negatively Regulates the Expression of Cold-Regulated Genes in the CBF Pathway.
(A) Expression of CBFs and their regulons in wild-type and ein3 eil1 seedlings at 22 and 4°C. Two-week-old seedlings grown at 22°C were treated at 4°C for the indicated periods of time. The data are the mean values of three replicates ± sd.
(B) Expression of CBF genes in the iE/qm transgenic plants with or without 5 μM β-estradiol treatment for 4 h, followed by cold treatment at 4°C for the indicated period of time. The data represent the mean values of three replicates ± sd.
Expression of CBFs and Their Target Genes in the ein3 eil1 Mutant and in EIN3-Overexpressing Plants
Because the expression of some genes in the CBF pathway was shown by microarray data to be upregulated in ein3 eil1 at 22°C, we next verified the expression of these genes using qRT-PCR analysis. Consistent with the microarray data (see Supplemental Table 1 online), the ein3 eil1 mutant showed higher expression levels of all of the genes tested, including CBF1-3, RD29A, COR15b, KIN1, COR414, and COR47, compared with the expression of these genes in wild-type plants grown at 22°C. Moreover, the cold induction of these genes was consistently increased in the ein3 eil1 mutant compared with the wild-type plants (Figure 5A). The expression of the CBF genes was then examined in the iE/qm transgenic plants: CBF1, CBF2, and CBF3 were dramatically decreased after β-estradiol treatment at 22°C, and the application of β-estradiol also significantly inhibited the cold-induced expression of the CBF1-3 genes (Figure 5B). Therefore, EIN3/EIL1 might be involved in cold signaling by negatively regulating the expression of CBF1-3 and their target genes.
Expression of ARR5, -7, and -15 Is Negatively Regulated by EIN3
ARR7 and ARR15 belong to the type-A ARR family, members of which rapidly respond to cytokinin and low temperatures at the mRNA level (Vogel et al., 2005). To explore the possible connection between type-A ARRs and the ethylene signaling pathway, we first verified the expression of ARR7 and ARR15 in the ein3 eil1 double mutant using qRT-PCR. The transcription levels of these genes were upregulated in the ein3 eil1 mutant without cold treatment (Figure 6A), which coincides with the data from the microarray analysis (see Supplemental Table 1 online), and the cold induction of both genes was consistently higher in the ein3 eil1 mutant than in the wild-type plants (Figure 6A). Similar to ARR7 and ARR15, ARR5 and ARR6 were also reported to be induced by cold (Vogel et al., 2005), and we found that ARR5, but not ARR6, was highly expressed in the ein3 eil1 mutant compared with the wild type grown under conditions of cold stress (Figure 6A). Next, the expression levels of the ARR5, ARR7, and ARR15 genes were evaluated in the iE/qm transgenic plants. Without β-estradiol treatment, the expression of the ARR5, ARR7, and ARR15 genes in the transgenic plants was rapidly induced by cold; however, the induction of these ARR genes by cold was markedly inhibited in the presence of β-estradiol (Figure 6B). These results suggest that the expression of type-A ARR genes is repressed by EIN3/EIL1.
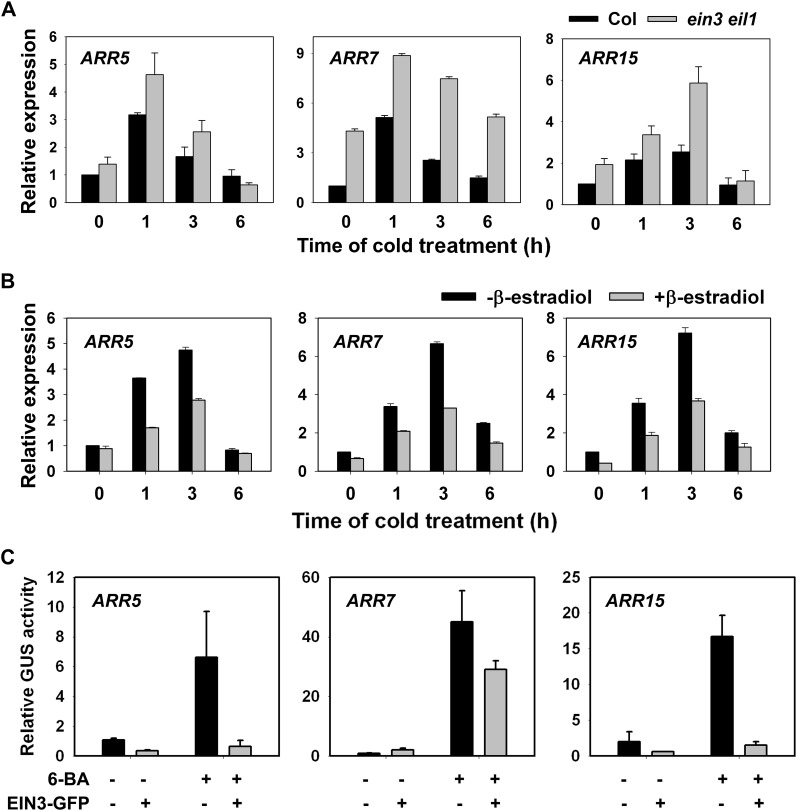
Figure 6.
EIN3 Negatively Regulates the Expression of ARR5, ARR7, and ARR15 Genes.
(A) Expression of ARR5, ARR7, and ARR15 genes in two-week-old wild-type and ein3 eil1 seedlings at 4°C. The data represent the mean values of three replicates ± sd.
(B) Expression of ARR5, ARR7, and ARR15 in iE/qm transgenic plants with or without 5 μM β-estradiol treatment for 4 h, followed by cold treatment at 4°C for the indicated time. The data represent the mean values of three replicates ± sd.
(C) Transient expression of ARR:GUS with Super:EIN3-GFP in N. benthamiana leaves. Cotransformation of ARR:GUS with the Super1300 vector was used as the control. The data are the mean values of three replicates ± sd.
To confirm this idea, we generated ARR5:GUS (for β-glucuronidase) iE/qm plants by crossing ARR5:GUS plants (D’Agostino et al., 2000) with the iE/qm mutant and tested the GUS activity with and without β-estradiol treatment. In the absence of β-estradiol, the GUS activity in the iE/qm plants was induced by benzylamino adenine (BA) and cold; however, this induction was dramatically decreased after β-estradiol treatment (see Supplemental Figure 4 online). These results demonstrate that the transcription of the ARR5, ARR7, and ARR15 genes was repressed by the overexpression of EIN3.
To dissect further the regulation of the ARR5, ARR7, and ARR15 genes by EIN3, we performed transient expression assays in Nicotiana benthamiana leaves cotransformed with pARR:GUS and 35S:EIN3-GFP. A vector construct containing the luciferase gene was used to monitor the transformation efficiencies. To achieve high levels of ARR transcripts, we infiltrated the leaves with exogenous cytokinin (BA), which is reported to induce type-A ARR expression (D’Agostino et al., 2000). Without BA treatment, the effect of coexpression on the activities of ARR5:GUS, ARR7:GUS, or ARR15:GUS cannot be easily determined due to their low expression levels. BA treatment significantly induced the expression of the ARR5, ARR7, and ARR15 genes, and cotransformation of 35S:EIN3-GFP dramatically inhibited the BA-mediated induction of the ARR5, ARR7, and ARR15 genes (Figure 6C). These results indicate that EIN3 acts as a repressor of ARR5, ARR7, and ARR15 gene expression.
CBF1-CBF3 and Three Type-A ARR Genes Are Direct Target Genes of EIN3
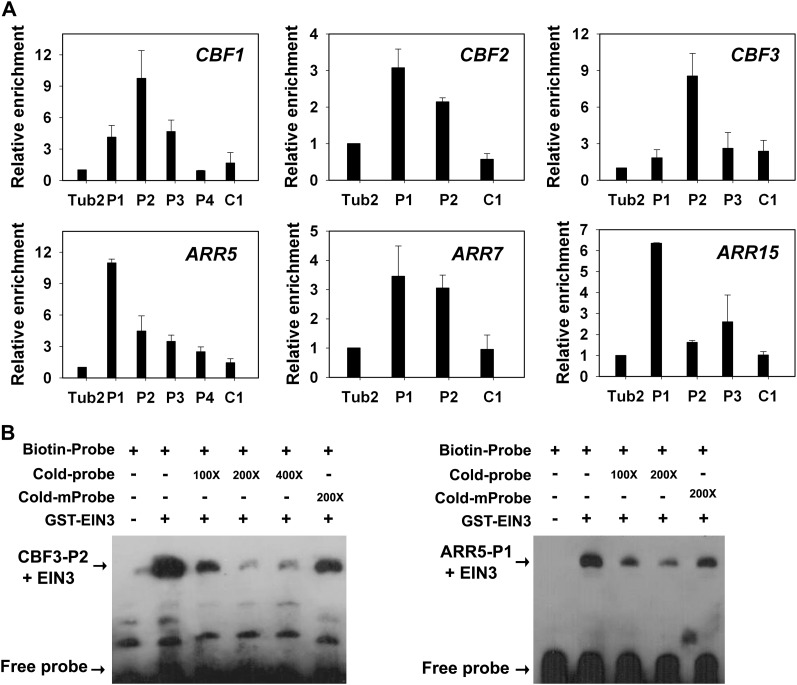
EIN3/EIL1 act as redundant nuclear transcription factors in the ethylene signaling pathway and are able to bind to EIN3 binding sites (EBSs) in the promoters of target genes (Kosugi and Ohashi, 2000; Chen et al., 2009; Zhong et al., 2009; Boutrot et al., 2010; Zhang et al., 2011). Sequence analysis revealed that the promoter regions of the CBF1-CBF3 and type-A ARR genes (ARR5, -7, and -15) contain several putative EBSs (see Supplemental Figure 5 online). To test whether EIN3 protein binds directly to the promoters of these genes, chromatin immunoprecipitation (ChIP) was performed, and qRT-PCR was used to determine whether the promoter fragments (P1, P2, P3, and P4) of the CBFs and type-A ARRs were enriched by ChIP using anti-EIN3 antibody (Zhong et al., 2009). Chromatin immunoprecipitated with the anti-EIN3 antibody was obviously enriched in fragments P2 in CBF1, P1 in CBF2, P2 in CBF3, P1 in ARR5, P1 and P2 in ARR7, and P1 in ARR15 in Col seedlings (Figure 7A). We also detected a weak binding of EIN3 with fragments of P1 and P3 in CBF1, P2 in CBF2, P2 in ARR5, and P3 in ARR15 in Col seedlings. By contrast, fragments from exons or 3′-untranslated region (C1) of these genes and a tubulin promoter fragment did not show any detectable binding to EIN3 (Figure 7A). Therefore, EIN3 can specifically bind to the promoters of the CBF1-CBF3 and ARR5, ARR7, and ARR15 genes in vivo.
Figure 7.
EIN3 Binds to the Promoters of CBFs and Type-A ARR Genes.
(A) ChIP-qRT-PCR assay of EIN3 binding to the promoters of CBF and ARR genes. Chromatin from wild-type Col seedlings was immunoprecipitated with an anti-EIN3 antibody, and the amount of the indicated DNA in the immune complex was determined by qRT-PCR. The EIN3 binding sites are indicated in Supplemental Figure 5 online. A Tubulin2 fragment and a fragment from the coding region (C1) were amplified as controls. At least three independent experiments were performed with similar results. The data are the mean values of three replicates ± sd from one experiment.
(B) EMSA assay for EIN3 binding to the promoters of CBF3 and ARR5. Each biotin-labeled DNA fragment was incubated with the GST-EIN3 protein. Competition for the labeled promoter sequences were performed by adding an excess of unlabeled wild-type or mutated probes.
Next, electrophoretic mobility shift assays (EMSAs) were performed to test whether EIN3 binds directly to the promoters of these genes in vitro. The recombinant EIN3 protein (amino acids 141 to 352) containing DNA binding domain (Chen et al., 2009) was expressed as a glutathione S-transferase (GST) fusion protein in Escherichia coli and purified. The EIN3 protein bound specifically to the EBSs of CBF1-CBF3, ARR5, ARR7, and ARR15, whereas no binding was observed in the control. Furthermore, the binding was abolished by the addition of increasing amounts of unlabeled competitors with the same sequences and was reduced to a lesser degree using mutated DNA probes (Figure 7B; see Supplemental Figure 6 online). These results demonstrate that EIN3 binds directly to the promoters of the CBF1-CBF3, ARR5, ARR7, and ARR15 genes in vitro. These results, together with in vivo ChIP assay, suggest that CBF1-CBF3 and ARR5, ARR7, and ARR15 are direct target genes of EIN3.
Overexpression of ARR5, -7, and -15 and the Application of Cytokinin Confer Enhanced Freezing Tolerance
Elevated expression levels of ARR5, ARR7, and ARR15 were observed in the ein3 eil1 double mutant. To elucidate the function of the overexpressed ARRs in freezing tolerance, we examined the freezing tolerance of ARR-Myc–overexpressing transgenic plants (ARR5-OE, ARR7-OE, and ARR15-OE) (Ren et al., 2009). The ARR5, ARR7, and ARR15 proteins were indeed overexpressed in these transgenic plants (see Supplemental Figure 7 online). After the freezing treatment, the ARR-OE plants showed higher survival rates than the wild-type plants with or without cold acclimation, indicating that the plants overexpressing the ARR5, ARR7, and ARR15 genes possessed increased capacities to withstand freezing temperatures (Figures 8A and 8B). Consistent with this, we found that the ARR5, ARR7, and ARR15 protein levels were dramatically induced after 3 h of cold treatment (Figure 8C).
Figure 8.
Freezing Tolerance Is Enhanced by the Overexpression of ARR5, ARR7, and ARR15 Genes and Exogenous Cytokinin.
(A) and (B) Survival rates (A) and freezing phenotypes (B) of the wild type and ARR-overexpressing transgenic lines (ARR5-OE, ARR7-OE, and ARR15-OE).
(A) Two-week-old plants grown at 22°C were treated at −4, −5, and −6°C for 1 h, followed by recovery at 22°C for 4 d. The data are the mean values of three replicates ± sd. *P < 0.05 (t test), indicating a significant difference between the transgenic plants and wild-type Col. At least three independent experiments were performed, with similar results.
(B) Plants were treated at −5°C for 1 h, followed by recovery at 22°C for 4 d. Representative pictures are shown. NA, nonacclimated.
(C) The ARR5, ARR7, and ARR15 proteins are stabilized by cold treatment in ARR-overexpressing transgenic lines (ARR5-OE, ARR7-OE, and ARR15-OE). Two-week-old seedlings grown on MS medium were treated with BA (1 μM) for 3 h or at 4°C for 0, 3, 6, and 12 h before the tissues were harvested for immunoblot assays using anti-Myc antibody. HSP90 was used as a loading control.
(D) Survival rates of wild type and ARR-overexpressing transgenic lines in the presence of BA without cold acclimation. Two-week-old plants grown on MS supplemented with 50 nM BA were treated at freezing temperatures for 1 h, followed by recovery at 22°C for 4 d. The data are the mean values of three replicates ± sd. *P < 0.05 (t test), indicating a significant difference between the transgenic and wild-type plants. At least three independent experiments were performed with similar results.
To examine the correlation between freezing tolerance and the levels of ARR proteins further, we tested the freezing tolerance of wild-type and ARR-OE plants treated with exogenous BA, which is reported to stabilize type-A ARR proteins (Ren et al., 2009). As expected, BA enhanced the tolerance of both the wild-type and ARR-OE transgenic plants under freezing conditions. Without cold acclimation, the LT50 of the wild-type seedlings grown on Murashige and Skoog (MS) medium was −4°C; however, all of the wild-type seedlings survived when grown on medium supplemented with 10 nM or 50 nM BA at this temperature (see Supplemental Figure 8 online). Moreover, the ARR-overexpressing plants treated with BA displayed more tolerance to freezing than the wild type grown at −5 and −6°C (Figure 8D). To exclude the possibility that this effect is BA specific, we examined the freezing tolerance of ARR5-OE plants grown on MS medium supplemented with t-zeatin and observed a similar increase in the freezing tolerance of these ARR5-OE plants (see Supplemental Figure 9 online). These results further confirmed that ARR5, ARR7, and ARR15 are positive regulatory factors involved in the response to freezing stress.
Next, we examined whether these ARR-OE transgenic plants had altered expression of cold-regulated genes in the CBF pathway. No obvious differences were detected in the expression of CBF1-3, RD29A, COR15b, and COR47 between ARR-OE plants and the wild type before or after cold treatment (see Supplemental Figure 10 online). These results suggest that ARR5, ARR7, and ARR15 are involved in freezing tolerance uncoupled with the CBF pathway.
Induction of ARR5, -7, and -15 by Cold and BA Is Antagonistically Inhibited by ACC
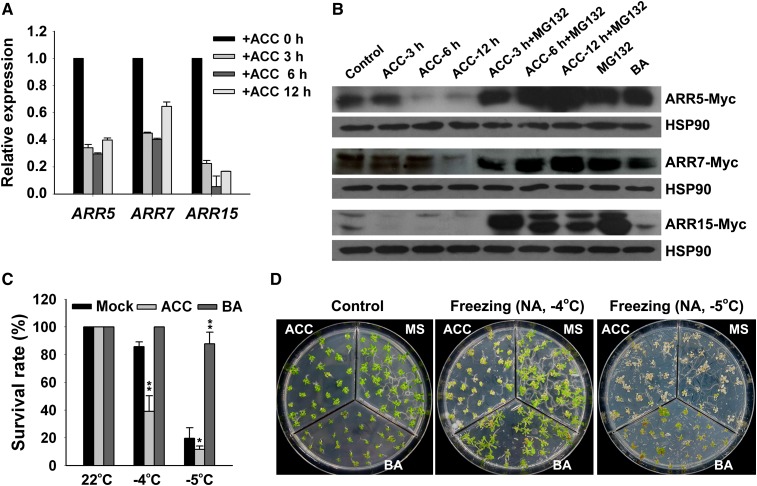
Because the overaccumulation of type-A ARR contributes to the freezing tolerance of ethylene-insensitive mutants (Figure 8) and the ACC-treated seedlings exhibited a hypersensitivity to freezing tolerance (Figure 1), we reasoned that the activation of ethylene biosynthesis should disturb the accumulation of type-A ARR proteins. To test this hypothesis, we first analyzed the transcript levels of the ARR5, ARR7, and ARR15 genes in plants treated with ACC. Strikingly, the levels of ARR5, ARR7, and ARR15 expression were drastically reduced after ACC treatment (Figure 9A). Next, we examined the effect of ACC on the GUS activity in the transgenic plants expressing ARR5:GUS under cold stress and BA treatment. In agreement with previous studies (D’Agostino et al., 2000; Fowler and Thomashow, 2002), ARR5 was induced by cold stress and BA treatment. However, the induction of ARR5 by cold and BA was significantly blocked by exogenous ACC (see Supplemental Figure 11 online), further indicating the negative regulation of ARR5 expression by ethylene.
Figure 9.
Transcription and Protein Levels of ARR5, ARR7, and ARR15 Are Downregulated by ACC.
(A) Analysis of the expression of ARR5, ARR7, and ARR15 by qRT-PCR in 2-week-old seedlings without or with 10 μM ACC treatment for the indicated period of time.
(B) Protein levels of ARR5, ARR7, and ARR15 determined by immunoblot analysis. Two-week-old seedlings were treated with ACC or/and MG132 for the indicated period of time in half-strength MS liquid medium. The total proteins were subjected to immunoblotting assays using an anti-Myc monoclonal antibody. HSP90 was used as a loading control. ACC, 10 μM; MG132, 50 μM; BA, 1 μM.
(C) and (D) Survival rates (C) and freezing phenotypes (D) of ARR5-OE transgenic plants in the presence of BA and ACC. Two-week-old wild-type and ARR5-OE transgenic plants grown on MS supplemented with 50 nM BA or 10 μM ACC were treated at −4 and −5°C for 1 h followed by recovery at 22°C for 4 d. The data are the mean values of three replicates ± sd (C). *P < 0.05, **P < 0.01 (t test), indicating a significant difference between the transgenic and wild-type plants. NA, nonacclimated.
Next, we found that the decrease in the ARR5, ARR7, and ARR15 protein levels in response to ACC treatment was similar to the pattern of expression of the corresponding genes (Figure 9B). The type-A ARR proteins could be stabilized by MG132, a specific proteasomal inhibitor, which is consistent with the results from a previous report (Ren et al., 2009). Furthermore, the ACC-induced reduction of the ARR5, ARR7, and ARR15 protein levels was also inhibited by MG132 (Figure 9B), indicating that ACC-induced type-A ARR degradation is dependent on the proteasome pathway.
We also compared the freezing tolerance of ARR5-OE transgenic plants grown on MS medium supplemented with ACC and BA. The survival rates of the ARR5-OE transgenic plants treated with BA were dramatically increased under freezing stress, whereas the application of ACC significantly decreased the freezing tolerance of ARR5-OE transgenic plants (Figures 9C and 9D). Therefore, the freezing sensitivity of ACC-treated seedlings appears at least partially to be a consequence of the downregulation of type-A ARRs by ACC.
DISCUSSION
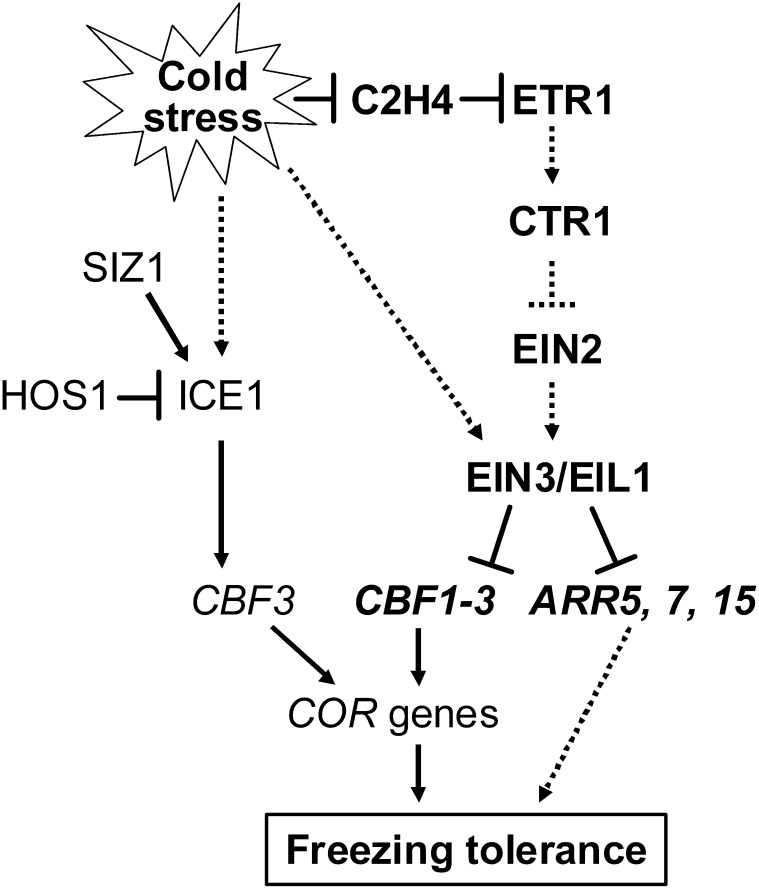
In this study, we explored the function of ethylene in cold stress in Arabidopsis by investigating the consequences of exogenous and endogenous ethylene level changes on plant responses to freezing, the responses of ethylene signaling genes to cold, and the effect of impaired ethylene signaling on freezing stress. We demonstrated that the essential components of ethylene signaling ETR1 and EIN4 play positive roles, while EIN2 and EIN3/EIL1 play negative roles in modulating the adaptations of plants in response to freezing stress. By examining the target genes of the master transcription factor EIN3 involved in cold signaling, we revealed that EIN3 negatively regulates freezing stress by directly regulating the expression of CBF and type-A ARR genes. Our findings suggest crosstalk between ethylene and cytokinin signaling to modulate their responses to cold stress (Figure 10).
Figure 10.
A Model of Ethylene Signaling Regulation of the Freezing Stress Response in Arabidopsis.
Cold stress rapidly inhibits ethylene production and downregulates EIN2 and EIN3/EIL1. The EIN3/EIL1 proteins, in turn, suppress the expression of CBF1-CBF3 genes and type-A ARR genes (ARR5, -7, and -15) by directly binding to their promoters. Therefore, type-A ARRs serve as mediators in the responses to ethylene and cytokinin signals to positively regulate freezing stress responses. On the other hand, cold slowly promotes EIN3 protein accumulation. Solid and dotted lines indicate direct and indirect regulation, respectively.
Ethylene Signaling Plays an Important Role in Cold Stress Signaling in Arabidopsis
In this study, we provided several lines of evidence supporting the notion that ethylene signaling plays a negative role in the adaptation of Arabidopsis to freezing stress. First, ethylene production in wild-type and eto1 mutant plants showed a dramatic decrease after 1 to 3 h of cold treatment and remained at a low level when the cold stress was continued for up to 24 h. Second, increased ethylene biosynthesis, produced by either treating wild-type plants with ACC or using the ethylene overproduction mutant eto1, resulted in decreased tolerance to freezing. By contrast, treating wild-type or eto1 plants with AVG or Ag+ enhanced freezing tolerance. Third, ethylene insensitive mutants, such as etr1-1, ein4-1, ein2-5, ein3-1, and ein3 eil1, are more tolerant to freezing stresses than wild-type plants, whereas the overexpression of EIN3 led to a decrease in freezing tolerance. Moreover, a loss-of-function mutation of a negative regulator in ethylene signaling, ctr1-1, results in reduced freezing tolerance. Therefore, ethylene biosynthesis and signaling negatively regulate freezing tolerance in Arabidopsis.
Our findings are in agreement with the previous studies in maize (Zea mays) showing that an increase in the ACC level has a high positive linear correlation with the chilling sensitivities of different maize genotypes, and the ACC content is thought to be an indicator of cold damage (Janowiak and Dorffling, 1995; Janowiak and Dörffling, 1996). In addition, ethylene production decreased sharply at low temperatures and rose rapidly (within 1 d) after a return to normal temperatures in winter wheat (Triticum aestivum) and dwarf bean (Phaseolus vulgaris) (Field, 1984; Macháčcková et al., 1989). Another study from mung bean (Vigna radiata) seedlings showed that improved tolerance was associated with suppression of ethylene biosynthesis (Collins et al., 1995). However, the opposite conclusion was reached in other studies in several plant species, including tomato (Solanum lycopersicum), cucumber (Cucumis sativus), and tobacco (Nicotiana tabacum cv NC89) (Wang and Adams, 1982; Ciardi et al., 1997; Zhang and Huang, 2010). Therefore, differing views exist concerning the function of ethylene in the cold response. This may reflect that the cold sensitivity in different species might display different ethylene effects on cold responses. Arabidopsis Col and mung bean are relatively cold-insensitive species, whereas tomato and cucumber are cold-sensitive species. It is possible that additional ethylene production in cold-sensitive species is only a symptom of injury. It is noteworthy that relative humidity is another factor that affects ethylene production during cold stress (Guye et al., 1987; Janowiak and Dorffling, 1995). The function of ethylene in cold stress in different species needs further investigation.
EIN3 Functions As a Negative Regulator in the CBF-Dependent Pathway
The CBF genes are considered to play important roles in cold acclimation (Thomashow, 1999). Our microarray and qRT-PCR analyses showed that the CBF genes were constitutively upregulated in the ein3 eil1 double mutant, with or without cold treatment. Moreover, the expression of the CBF genes was negatively correlated with the level of EIN3 protein in inducible EIN3-FLAG transgenic plants. Further studies revealed that the key transcription factor EIN3 directly targets cold-regulated CBF genes, as the EIN3 protein could specifically bind to the promoters of the CBF1-3 genes in vitro and in vivo. Consistent with our finding of increased freezing tolerance of the ein3 eil1 mutant, the overexpression of CBF genes results in enhanced freezing tolerance in different plant species (Yamaguchi-Shinozaki and Shinozaki, 2006; Chinnusamy et al., 2007). Collectively, these data indicate that the ethylene signaling–mediated cold response of plants is mediated at least partially through the transcriptional repression of the CBF genes by EIN3.
The CBF genes are shown to be positively regulated by ICE1 and CAMTA3 (Chinnusamy et al., 2003; Doherty et al., 2009) and negatively regulated by MYB15 (Agarwal et al., 2006). These results, combined with our finding concerning the transcriptional repression of the CBF genes by EIN3, suggest that the CBF genes are controlled by multiple transcription factors to fine-tune their expression, thus orchestrating the transcription level of the COR genes and the cold stress response.
Type-A ARRs Serve as Key Mediators of the Crosstalk in Ethylene and Cytokinin Signaling to Regulate Response to Cold Stress
The complex interactions between ethylene and cytokinin have been studied extensively. Cytokinin has been shown to stabilize the ethylene biosynthesis enzyme ACS5, resulting in the increased production of ethylene (Vogel et al., 1998; Chae et al., 2003; Hansen et al., 2009). Several cytokinin-related responses are thought to be mediated by ethylene (Cary et al., 1995; Golan et al., 1996; Tanaka et al., 2006). Type-B ARR2 is shown to act as a signaling component, functioning downstream of ETR1 in ethylene signal transduction (Hass et al., 2004). Using bioinformatics in this study, we identified type-A ARR genes, including ARR5, ARR7, and ARR15, as the downstream targets of EIN3. Although the function of type-A ARRs in Arabidopsis is not well understood, our phenotypic analysis showed that the overexpression of ARR5, ARR7, and ARR15 enhances freezing tolerance, similar to ein3 and ein3 eil1 mutants. Exogenous cytokinin treatment strongly stabilized the ARR proteins and enhanced the freezing phenotypes of both wild-type and type-A ARR-overexpressing transgenic plants. We found that arr5, arr7, and arr15 mutants resembled wild-type plants in nonacclimated conditions and displayed a weak increase in freezing tolerance under acclimated conditions (see Supplemental Figure 12 online). This finding is in agreement with the results in a previous study (Jeon et al., 2010). As ARR-OE plants and arr mutants showed similar enhanced freezing phenotypes after acclimation, it is possible that feedback regulation may exist among type-A ARR genes, which awaits in-depth study. Therefore, these results suggest that the type-A ARR5, ARR7, and ARR15 genes are positive regulators of freezing stress. Along with the finding of the negative regulation of cytokinin receptors during cold stress (Jeon et al., 2010), our findings are consistent with the notion that cytokinin receptors promote the cytokinin response, but type-A ARRs are negative response regulators in cytokinin signaling (Kiba et al., 2003; To et al., 2004; To et al., 2007). Furthermore, ARR5, ARR7, and ARR15 are strongly expressed in the ein3 eil1 double mutant, whereas their transcription and protein stability were dramatically inhibited in the wild-type plants treated with ACC and in the EIN3-overexpressing transgenic plants. Therefore, EIN3 exerts negative regulation by direct repression of type-A ARR gene expression. Thus, we uncovered a new antagonistic link between ethylene and cytokinin signaling in the cold stress response.
EIN3 has been shown to activate transcription of ethylene response genes, such as ERF1, PORA/B, FLS2, EBF2, and ESE1 (Solano et al., 1998; Konishi and Yanagisawa, 2008; Zhong et al., 2009; Boutrot et al., 2010; Zhang et al., 2011). It also possesses transcription repressor activity to repress SID2 expression to negatively regulate plant innate immunity (Chen et al., 2009). In this study, we provide another example that EIN3 acts as a transcriptional repressor to repress the expression of CBF genes and type-A ARR genes in cold stress.
The interactions between ethylene and cold stress are seemingly complicated. On the one hand, ethylene production decreases rapidly when plants are exposed to cold stress; on the other hand, a long duration of cold stress promotes the accumulation of EIN3 protein. Our finding that type-A ARR5, ARR7, and ARR15 are downstream targets of EIN3 may reveal an inherent connection between cold stress and ethylene. To enhance the freezing tolerance of plants, cold stress activates type-A ARR expression and decreases ethylene biosynthesis to stabilize ARR protein. In addition, type-A ARR expression is rapidly activated after 1 to 3 h of cold treatment, followed by a rapid decrease, and reaches the lowest levels after 6 h of cold exposure. This quick induction of type-A ARR genes may act as an early response to cold stress. Because the induction of type-A ARR genes is transient, it is likely that some important transcription repressors that are induced by cold mediate the reduction of type-A ARR expression. We provide strong evidence that EIN3 is one such transcriptional repressor that functions to ensure that the expression of type-A ARRs is transient and tightly regulated. Although the mechanisms by which type-A ARRs modulate the cold response remain unclear and need to be further investigated, these findings provide a new perspective on the genetic control of freezing tolerance and further our understanding of the molecular basis of the effects of ethylene and cytokinin on plant responses to adverse environments.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Col plants were grown on MS plates at 22 ± 2°C under a 16-h-light/8-h-dark photoperiod at 100 μmol m–2 s–1. Mutants eto1 (Guzmán and Ecker, 1990), etr1-1 (Hua and Meyerowitz, 1998), ein4 (Roman et al., 1995), ctr1-1 (Kieber et al., 1993), ein2-5 (Alonso et al., 1999), ein3-1 (Chao et al., 1997), eil1-1 (Alonso et al., 2003), and ein3 eil1 (Alonso et al., 2003) and 35S:EIN3-GFP transgenic plants in ein3 eil1 (EIN3-OE/dm) and ein2 ein3 eil1 backgrounds (He et al., 2011), 35S:EBF1-GFP transgenic plants, 35S:EBF1-TAP transgenic plants ein2-5, pER8-EIN3-3FLAG transgenic plants in the ein3 eil1 ebf1 ebf2 quadruple mutant background (iE/qm) (An et al., 2010), ARR5-OE, ARR7-OE, and ARR15-OE transgenic plants (Ren et al., 2009), arr5 (To et al., 2004), arr7 (Jeon et al., 2010), arr15 (Müller and Sheen, 2008), and ARR5:GUS transgenic plants (To et al., 2004) were used in this study. ARR5:GUS iE/qm plants were generated by crossing ARR5:GUS plants with iE/qm plants.
Unless otherwise indicated, concentrations of 10 μM ACC, 2 μM AVG, 20 μM AgNO3, and 50 μM MG132 were used for the seedling treatments.
Freezing Tolerance Assay
The freezing tolerance assay was performed as described (Yang et al., 2010), with some modifications. Briefly, 2-week-old plants grown at 22°C on 0.8% agar plates were treated with or without cold acclimation at 4°C for 7 d and were then placed in a freezing chamber (RuMED4001) set to −1°C and programmed to cool at −1°C per hour. Petri dishes of plants were removed when the desired temperatures were reached. After the freezing treatment, the plants were incubated at 4°C in the dark for 12 h and then transferred to light at 22°C. The survival rates of the seedlings were scored visually after 4 d.
Measurement of Electrolyte Leakage and Ethylene Content
Two-week-old plants grown at 22°C were treated at freezing temperatures for the indicated time periods. The amount of electrolyte leakage was measured as previously described (Yang et al., 2010). Two-week-old seedlings of wild-type Col and eto1 mutants grown in 15-mL gas chromatography vials were grown at 22°C and treated at 4°C or kept at 22°C. The ethylene levels in the headspace of the gas chromatography vials were determined at various time points by gas chromatography.
RNA Extraction and RT-PCR
The total RNA from 2-week-old plants was extracted using TRIzol reagent (Invitrogen) and reverse transcribed using M-MLV reverse transcriptase (Promega). The quantitative real-time PCR was performed using a SYBR Green PCR Master Mix kit (Takara). The relative expression levels were calculated as described (Huang et al., 2010), and the specific primers used are listed in Supplemental Table 2 online.
Plasmid Construction, Plant Transformation, and Histochemical GUS Assays
The ARR promoters were amplified by PCR from Arabidopsis genomic DNA and cloned into the pCAMBIA1381 vector to generate ARR:GUS constructs. All of the primer sequences used for the vector construction are listed in Supplemental Table 2 online. To construct Super:EIN3-GFP, the EIN3 cDNA fragment was amplified by PCR using the primers EIN3-F1 and EIN3-R1 and subsequently cloned into the pSuper1300 vector (Yang et al., 2010) containing a GFP tag.
The plasmids were infiltrated into Nicotiana benthamiana according to a described protocol (Walter et al., 2004). GUS staining was performed as previously described (Huang et al., 2010). The GUS activity was assayed using methyl umbelliferyl glucuronide (Sigma-Aldrich), and the luciferase activity was used as an internal control. The GUS/luciferase ratio was used to determine the promoter activity.
Purification of Recombinant Protein and EMSA
The truncated EIN3 protein (amino acids 141 to 352) containing the DNA binding domain was fused in frame with GST (Chen et al., 2009) and expressed in Escherichia coli. The recombinant protein was purified by GST-agarose affinity chromatography. The EMSA was performed using the LightShift Chemiluminescent EMSA kit (Pierce) according to the manufacturer’s instructions. The biotin-labeled DNA fragments listed in Supplemental Table 3 online were synthesized and used as probes, and biotin-unlabeled DNA fragments of the same sequences were used as the competitors in this assay.
ChIP Assay
ChIP was performed as previously described (Gendrel et al., 2005). Twelve-day-old wild-type Col seedlings grown at 22°C on MS medium were fixed in 1% formaldehyde for 15 min under a vacuum and neutralized with 0.125 M Gly under a vacuum for an additional 5 min. After washing twice with cold sterilized water, the tissues were homogenized. The chromatin was isolated and sonicated. The EIN3 protein was immunoprecipitated using anti-EIN3 antibody (Zhong et al., 2009), and the enriched DNA fragments were determined by qRT-PCR using the primers listed in Supplemental Table 2 online. All ChIP assays were repeated three times with similar results, and representative data are presented in Figure 7.
Immunoblot Assay
For EIN3-FLAG immunoblots, 2-week-old iE/qm transgenic plants were transferred to liquid MS medium with different concentrations of β-estradiol for 4 h. The EIN3-3FLAG fusion proteins were visualized on immunoblots using an anti-FLAG antibody (Sigma-Aldrich).
For the ARR-Myc immunoblots, 2-week-old ARR-OE transgenic seedlings (Ren et al., 2009) were treated with 10 μM ACC at 4°C or treated with ACC plus MG132 for the indicated period of time. The ARR-Myc fusion proteins were visualized on immunoblots using an anti-Myc antibody (Sigma-Aldrich).
For the EIN3-GFP immunoblots, 7-d-old 35S:EIN3-GFP transgenic plants were treated at 4°C for the indicated period of time, or they were treated with ACC or MG132 for 6 h. The EIN3-GFP fusion proteins were visualized on immunoblots using an anti-GFP antibody (Sigma-Aldrich).
For the EBF1-GFP and EBF1-Myc immunoblots, 7-d-old 35S:EIN3-GFP and 35S:EBF1-TAP ein2 plants were treated at 4°C for the indicated period of time. The EIN3-GFP and EBF1-Myc fusion proteins were visualized on immunoblots using an anti-GFP or anti-Myc antibody (Sigma-Aldrich).
As controls, HSP90 protein was visualized on immunoblots using an anti-HSP90 antibody (Sigma-Aldrich), and ribulose-1,5-bisphosphate carboxylase/oxygenase protein was stained by Ponceau S.
Confocal Laser Microscopy
The fluorescence of GFP in the roots of 7-d-old transgenic plants expressing 35S:EIN3-GFP or 35S:EBF1-GFP was imaged using a confocal laser scanning microscope (LSM510; Carl Zeiss) at 0, 3, 6, and 12 h of cold treatment or at 6 h of ACC treatment.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ETR1 (At1g66340), EIN4 (At3g04580), CTR1 (At5g03730), EIN2 (At5g03280), EIN3 (At3g20770), EIL1 (At2g27050), ETO1 (At3g51770), EBF1 (At2g25490), EBF2 (At5g25350), CBF1 (At4g25490), CBF2 (At4g25470), CBF3 (At4g25480), RD29A (At5g52310), COR15B (At2g42530), KIN1 (At5g15960), COR414 (At1g29395), COR47 (At1g20440), ARR5 (At3g48100), ARR7 (At1g19050), ARR15 (At1g74890), ACTIN2/8 (At3g18780/At1g49240), and TUBULIN2 (At5g62690).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Freezing Tolerance Assay of Mutants in the Ethylene Signaling Pathway after Cold Acclimation.
Supplemental Figure 2. Freezing Tolerance of Col Plants Is Not Affected by β-Estradiol Application.
Supplemental Figure 3. Levels of EIN3 and EBF1 Proteins after Cold Stress.
Supplemental Figure 4. GUS Activity of ARR5:GUS in iE/qm Transgenic Plants.
Supplemental Figure 5. Schematic Diagrams Showing the Promoter Structures of the CBF1-CBF3, ARR5, ARR7, and ARR15 Genes.
Supplemental Figure 6. EMSA Assays for EIN3 Binding to the Promoters of CBF1, CBF2, ARR7, and ARR15.
Supplemental Figure 7. Immunoblot Analysis of the ARR5, ARR7, and ARR15 Proteins in ARR-Overexpressing Plants.
Supplemental Figure 8. The Effect of BA on the Freezing Tolerance of Wild-Type Plants.
Supplemental Figure 9. The Effect of t-Zeatin on the Freezing Tolerance of ARR5-OE Plants.
Supplemental Figure 10. Expression of Cold-Regulated Genes in ARR-OE Plants under Cold Stress.
Supplemental Figure 11. GUS Expression of ARR5:GUS Plants under Cold, ACC, and BA Treatments.
Supplemental Figure 12. Freezing Tolerance Assay of arr5, arr7, and arr15 Mutants under Nonacclimated and Acclimated Conditions.
Supplemental Table 1. Significantly Upregulated Genes Involved in Environmental Stress Responses in the ein3 eil1 Mutant.
Supplemental Table 2. Primer Sequences Used for qRT-PCR and Cloning.
Supplemental Table 3. Primer Sequences Used for ChIP and EMSA.
Acknowledgments
We thank Jianru Zuo for providing the ARR-OE transgenic seeds and Junping Gao for support with ethylene measurement. We thank Jian Hua and Zhizhong Gong for critical reading of this article. This work was supported by grants from the National Key Basic Research Program of China (2009CB119100 and 2011CB915401), from the National Natural Science Foundation of China (31121002), from the Ministry of Agricultural of China for transgenic research (2011ZX08009), and by the Chinese Universities Scientific Fund.
AUTHOR CONTRIBUTIONS
S.Y. directed the project. S.Y., Y.S., S.T., and H.G. designed the research and analyzed data. Y.S., S.T., L.H., X.H., and X.Z. performed the research. S.Y. and Y.S. wrote the article.
Glossary
- ARR
Arabidopsis response regulator
- ACC
1-aminocyclopropane-1-carboxylic acid
- AVG
aminoethoxyvinyl glycine
- qRT-PCR
quantitative real-time PCR
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- BA
to be defined
- EBS
EIN3 binding site
- ChIP
chromatin immunoprecipitation
- EMSA
electrophoretic mobility shift assay
- GST
glutathione S-transferase
- MS
Murashige and Skoog
References
- Abeles F., Morgen P., Saltveit J. (1992). Ethylene in Plant Biology. (San Diego, CA: Academic Press)
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Agarwal M., Hao Y., Kapoor A., Dong C.H., Fujii H., Zheng X., Zhu J.K. (2006). A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 281: 37636–37645 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Stepanova A.N., Solano R., Wisman E., Ferrari S., Ausubel F.M., Ecker J.R. (2003). Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc. Natl. Acad. Sci. USA 100: 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F., et al. (2010). Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A.B., Kende H. (2000). Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Boutrot F., Segonzac C., Chang K.N., Qiao H., Ecker J.R., Zipfel C., Rathjen J.P. (2010). Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc. Natl. Acad. Sci. USA 107: 14502–14507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W.H., Liu J., He X.J., Mu R.L., Zhou H.L., Chen S.Y., Zhang J.S. (2007). Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 143: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary A.J., Liu W., Howell S.H. (1995). Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 107: 1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae H.S., Faure F., Kieber J.J. (2003). The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. Plant Cell 15: 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Kwok S.F., Bleecker A.B., Meyerowitz E.M. (1993). Arabidopsis ethylene-response gene ETR1: Similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chao Q., Rothenberg M., Solano R., Roman G., Terzaghi W., Ecker J.R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen H., Xue L., Chintamanani S., Germain H., Lin H., Cui H., Cai R., Zuo J., Tang X., Li X., Guo H., Zhou J.M. (2009). ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21: 2527–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Ohta M., Kanrar S., Lee B.H., Hong X., Agarwal M., Zhu J.K. (2003). ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V., Zhu J., Zhu J.K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12: 444–451 [DOI] [PubMed] [Google Scholar]
- Ciardi J.A., Deikman J., Orzolek M.D. (1997). Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol. Plant. 101: 333–340 [Google Scholar]
- Collins G.G., Nie X., Saltveit M.E. (1995). Heat shock increases chilling tolerance of mung bean hypocotyls tissues. Physiol. Plant. 89: 117–124 [Google Scholar]
- D’Agostino I.B., Deruère J., Kieber J.J. (2000). Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 124: 1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty C.J., Van Buskirk H.A., Myers S.J., Thomashow M.F. (2009). Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 21: 972–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C.H., Agarwal M., Zhang Y., Xie Q., Zhu J.K. (2006). The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 103: 8281–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field R.J. (1984). The role of 1-aminocyclopropane-1-carboxylic acid in the control of low temperature induced ethylene production in leaf tissue of Phaseolus vulgaris L. Ann. Bot. (Lond.) 54: 61–67 [Google Scholar]
- Fowler S., Thomashow M.F. (2002). Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell 14: 1675–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Chen Y.F., Randlett M.D., Zhao X.C., Findell J.L., Kieber J.J., Schaller G.E. (2003). Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J. Biol. Chem. 278: 34725–34732 [DOI] [PubMed] [Google Scholar]
- Gendrel A.V., Lippman Z., Martienssen R., Colot V. (2005). Profiling histone modification patterns in plants using genomic tiling microarrays. Nat. Methods 2: 213–218 [DOI] [PubMed] [Google Scholar]
- Gilmour S.J., Sebolt A.M., Salazar M.P., Everard J.D., Thomashow M.F. (2000). Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. Plant Physiol. 124: 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour S.J., Thomashow M.F. (1991). Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol. Biol. 17: 1233–1240 [DOI] [PubMed] [Google Scholar]
- Gilmour S.J., Zarka D.G., Stockinger E.J., Salazar M.P., Houghton J.M., Thomashow M.F. (1998). Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 16: 433–442 [DOI] [PubMed] [Google Scholar]
- Golan A., Tepper M., Soudry E., Horwitz B.A., Gepstein S. (1996). Cytokinin, acting through ethylene, restores gravitropism to Arabidopsis seedlings grown under red light. Plant Physiol. 112: 901–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Ecker J.R. (2003). Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Guye M.G., Vigh L., Wilson J.M. (1987). Chilling-induced ethylene production in relation to chill-sensitivity in Phaseolus spp. J. Exp. Bot. 38: 680–690 [Google Scholar]
- Guzmán P., Ecker J.R. (1990). Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2: 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M., Chae H.S., Kieber J.J. (2009). Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 57: 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber R., Fuchigami L. (1989). Low Temperature Stress Physiology in Crops. (Boca Raton, FL: CRC Press)
- Hass C., Lohrmann J., Albrecht V., Sweere U., Hummel F., Yoo S.D., Hwang I., Zhu T., Schäfer E., Kudla J., Harter K. (2004). The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis. EMBO J. 23: 3290–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., et al. (2011). A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershkovitz V., Friedman H., Goldschmidt E.E., Feygenberg O., Pesis E. (2009). Induction of ethylene in avocado fruit in response to chilling stress on tree. J. Plant Physiol. 166: 1855–1862 [DOI] [PubMed] [Google Scholar]
- Hua J., Chang C., Sun Q., Meyerowitz E.M. (1995). Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J., Meyerowitz E.M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J., Sakai H., Nourizadeh S., Chen Q.G., Bleecker A.B., Ecker J.R., Meyerowitz E.M. (1998). EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Li J., Bao F., Zhang X., Yang S. (2010). A gain-of-function mutation in the Arabidopsis disease resistance gene RPP4 confers sensitivity to low temperature. Plant Physiol. 154: 796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. (1998). Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280: 104–106 [DOI] [PubMed] [Google Scholar]
- Janowiak F., Dorffling K. (1995). Chilling-induced changes in the contents of 1-aminocyclopropane-1-carboxylic acid (ACC) and its N-malonyl conjugate (MACC) in seedlings of two maize inbreds differing in chilling tolerance. J. Plant Physiol. 147: 257–262 [Google Scholar]
- Janowiak F., Dörffling K. (1996). Chilling tolerance of 10 maize genotypes as related to chilling-induced changes in ACC and MACC contents. J. Agron. Crop Sci. 177: 175–184 [Google Scholar]
- Jeon J., Kim N.Y., Kim S., Kang N.Y., Novák O., Ku S.J., Cho C., Lee D.J., Lee E.J., Strnad M., Kim J. (2010). A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. J. Biol. Chem. 285: 23371–23386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T., Yamada H., Sato S., Kato T., Tabata S., Yamashino T., Mizuno T. (2003). The type-A response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 44: 868–874 [DOI] [PubMed] [Google Scholar]
- Kieber J.J., Rothenberg M., Roman G., Feldmann K.A., Ecker J.R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Kilian J., Whitehead D., Horak J., Wanke D., Weinl S., Batistic O., D’Angelo C., Bornberg-Bauer E., Kudla J., Harter K. (2007). The AtGenExpress global stress expression data set: Protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Kim C.Y., Liu Y., Thorne E.T., Yang H., Fukushige H., Gassmann W., Hildebrand D., Sharp R.E., Zhang S. (2003). Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell 15: 2707–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Yanagisawa S. (2008). Ethylene signaling in Arabidopsis involves feedback regulation via the elaborate control of EBF2 expression by EIN3. Plant J. 55: 821–831 [DOI] [PubMed] [Google Scholar]
- Kosugi S., Ohashi Y. (2000). Cloning and DNA-binding properties of a tobacco Ethylene-Insensitive3 (EIN3) homolog. Nucleic Acids Res. 28: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. (1998). Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente F., Oliveros J.C., Martínez-Zapater J.M., Salinas J. (2000). A freezing-sensitive mutant of Arabidopsis, frs1, is a new aba3 allele. Planta 211: 648–655 [DOI] [PubMed] [Google Scholar]
- Lyons J.M. (1973). Chilling injury in plants. Annu. Rev. Plant Physiol. 24: 445–466 [Google Scholar]
- Macháčcková I., Hanišová A., Krekule J. (1989). Levels of ethylene, ACC, MACC, ABA and proline as indicators of cold hardening and frost resistance in winter wheat. Physiol. Plant. 76: 603–607 [Google Scholar]
- Maruyama K., Sakuma Y., Kasuga M., Ito Y., Seki M., Goda H., Shimada Y., Yoshida S., Shinozaki K., Yamaguchi-Shinozaki K. (2004). Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 38: 982–993 [DOI] [PubMed] [Google Scholar]
- Miura K., Jin J.B., Lee J., Yoo C.Y., Stirm V., Miura T., Ashworth E.N., Bressan R.A., Yun D.J., Hasegawa P.M. (2007). SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1A expression and freezing tolerance in Arabidopsis. Plant Cell 19: 1403–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Sheen J. (2008). Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potuschak T., Lechner E., Parmentier Y., Yanagisawa S., Grava S., Koncz C., Genschik P. (2003). EIN3-dependent regulation of plant ethylene hormone signaling by two arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Ren B., Liang Y., Deng Y., Chen Q., Zhang J., Yang X., Zuo J. (2009). Genome-wide comparative analysis of type-A Arabidopsis response regulator genes by overexpression studies reveals their diverse roles and regulatory mechanisms in cytokinin signaling. Cell Res. 19: 1178–1190 [DOI] [PubMed] [Google Scholar]
- Roman G., Lubarsky B., Kieber J.J., Rothenberg M., Ecker J.R. (1995). Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Hua J., Chen Q.G., Chang C., Medrano L.J., Bleecker A.B., Meyerowitz E.M. (1998). ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc. Natl. Acad. Sci. USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R., Stepanova A., Chao Q., Ecker J.R. (1998). Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger E.J., Gilmour S.J., Thomashow M.F. (1997). Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen I., Mäkelä P., Heino P., Palva E.T. (2001). Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J. 25: 1–8 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sano T., Tamaoki M., Nakajima N., Kondo N., Hasezawa S. (2006). Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. J. Exp. Bot. 57: 2259–2266 [DOI] [PubMed] [Google Scholar]
- Thomashow M.F. (1999). PLANT COLD ACCLIMATION: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 571–599 [DOI] [PubMed] [Google Scholar]
- To J.P., Deruère J., Maxwell B.B., Morris V.F., Hutchison C.E., Ferreira F.J., Schaller G.E., Kieber J.J. (2007). Cytokinin regulates type-A Arabidopsis response regulator activity and protein stability via two-component phosphorelay. Plant Cell 19: 3901–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To J.P., Haberer G., Ferreira F.J., Deruère J., Mason M.G., Schaller G.E., Alonso J.M., Ecker J.R., Kieber J.J. (2004). Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16: 658–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.P., Woeste K.E., Theologis A., Kieber J.J. (1998). Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc. Natl. Acad. Sci. USA 95: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.T., Zarka D.G., Van Buskirk H.A., Fowler S.G., Thomashow M.F. (2005). Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 41: 195–211 [DOI] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schutze K., Batistic O., Weckermann K., Nake C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Adams D.O. (1982). Chilling-induced ethylene production in cucumbers (Cucumis sativus L.). Plant Physiol. 69: 424–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu C., Li K., Sun F., Hu H., Li X., Zhao Y., Han C., Zhang W., Duan Y., Liu M., Li X. (2007). Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol. Biol. 64: 633–644 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (1994). A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57: 781–803 [DOI] [PubMed] [Google Scholar]
- Yang H., Shi Y., Liu J., Guo L., Zhang X., Yang S. (2010). A mutant CHS3 protein with TIR-NB-LRR-LIM domains modulates growth, cell death and freezing tolerance in a temperature-dependent manner in Arabidopsis. Plant J. 63: 283–296 [DOI] [PubMed] [Google Scholar]
- Yu X.M., Griffith M., Wiseman S.B. (2001). Ethylene induces antifreeze activity in winter rye leaves. Plant Physiol. 126: 1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li Z., Quan R., Li G., Wang R., Huang R. (2011). An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol. 157: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Huang R. (2010). Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 73: 241–249 [DOI] [PubMed] [Google Scholar]
- Zhao X.C., Schaller G.E. (2004). Effect of salt and osmotic stress upon expression of the ethylene receptor ETR1 in Arabidopsis thaliana. FEBS Lett. 562: 189–192 [DOI] [PubMed] [Google Scholar]
- Zhong S., Zhao M., Shi T., Shi H., An F., Zhao Q., Guo H. (2009). EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc. Natl. Acad. Sci. USA 106: 21431–21436 [DOI] [PMC free article] [PubMed] [Google Scholar]