The ability of secondary cell walls to provide mechanical support to plant stems is well known. This work shows that primary cell walls also provide support. PECTIN METHYLESTERASE35 strengthens Arabidopsis thaliana shoots by demethylesterifying homogalacturonan in primary cell walls, including the middle lamella.
Abstract
Secondary cell walls, which contain lignin, have traditionally been considered essential for the mechanical strength of the shoot of land plants, whereas pectin, which is a characteristic component of the primary wall, is not considered to be involved in the mechanical support of the plant. Contradicting this conventional knowledge, loss-of-function mutant alleles of Arabidopsis thaliana PECTIN METHYLESTERASE35 (PME35), which encodes a pectin methylesterase, showed a pendant stem phenotype and an increased deformation rate of the stem, indicating that the mechanical strength of the stem was impaired by the mutation. PME35 was expressed specifically in the basal part of the inflorescence stem. Biochemical characterization showed that the activity of pectin methylesterase was significantly reduced in the basal part of the mutant stem. Immunofluorescence microscopy and immunogold electron microscopy analyses using JIM5, JIM7, and LM20 monoclonal antibodies revealed that demethylesterification of methylesterified homogalacturonans in the primary cell wall of the cortex and interfascicular fibers was suppressed in the mutant, but lignified cell walls in the interfascicular and xylary fibers were not affected. These phenotypic analyses indicate that PME35-mediated demethylesterification of the primary cell wall directly regulates the mechanical strength of the supporting tissue.
INTRODUCTION
During the colonization of land, vascular plants acquired the ability to mechanically support the plant body and maintain vigorous growth in the atmosphere. The mechanical strength of the shoot of vascular plants is attributed to the secondary cell wall, characterized by densely deposited cellulose microfibrils impregnated with lignin, which provide the ability to resist compression stress. Typical examples of reinforced secondary cell walls are found in the xylem and interfascicular fibers, which contain primary cell walls that are flexible enough to allow cell expansion during shoot growth, yet can become strong and rigid upon deposition of a secondary cell wall on the inside of the primary cell wall (Raven et al., 2005; Albersheim et al., 2011).
The limited data currently available suggest that pectin, which is exclusively localized to the primary cell wall, can also play a role in the formation of supporting tissue (Willats et al., 2001; Siedlecka et al., 2008). Analysis of transcriptomic data indicates that certain pectin-related genes are preferentially expressed in the supporting tissues or nongrowing parts of plants (Imoto et al., 2005; Ko et al., 2006; Yokoyama and Nishitani, 2006; Minic et al., 2009). In addition, transcriptome coexpression analysis indicates that some pectin-related genes show a high correlation with certain genes involved in secondary wall biosynthesis (Brown et al., 2005; Persson et al., 2005). This line of evidence suggests that cell wall pectin is involved in the regulation of the mechanical support of the shoot. However, the in vivo molecular function of pectin-related genes in relation to the formation of supporting tissue is poorly understood.
Pectin is a highly heterogeneous complex polysaccharide that consists of five structural domains, including homogalacturonan (HG) (Wolf et al., 2009). HG is secreted in a highly methylesterified form and is demethylesterified in muro by pectin methylesterase (PME) (Micheli, 2001). The demethylesterified HG can either form intermolecular Ca2+ bonds, thus forming a rigid gel, or become more susceptible to hydrolysis by pectin-degrading enzymes, such as endopolygalacturonases, thereby affecting both the mechanical properties of the primary cell wall per se and intercellular adhesion via the middle lamella between cell walls (reviewed in Pelloux et al., 2007; Wolf et al., 2009).
Given the tight control of the methylesterification status of HG by PMEs, this class of enzymes could be important in the regulation of the mechanical properties of the cell wall (Ridley et al., 2001; Willats et al., 2001). Accordingly, many studies have examined the correlation between the expression profiles of certain PMEs and stem growth, and PMEs have long been proposed to have putative roles in growth regulation (Ogawa et al., 2003; Wolf et al., 2009). Furthermore, methylesterification status of pectin, which is regulated by certain PMEs in the shoot apical meristem of Arabidopsis thaliana, has been shown to play a critical role in determining phyllotaxis (Peaucelle et al., 2008, 2011). Despite extensive studies, no PME gene responsible for supporting tissue development has been identified in planta.
PME genes belong to a large multigene family in land plants (Markovic and Janecek, 2004; Yokoyama and Nishitani, 2004). Some members possess a characteristic N-terminal extension termed the PRO region, which precedes the well-conserved domain and shows significant similarity to the PME inhibitor domain (Jolie et al., 2010). Depending on the presence of this PRO region, PMEs are classified into two subfamilies: type II/group 1 and type I/group 2. The former lacks the PRO region, and the latter possesses it (Micheli, 2001; Tian et al., 2006).
Arabidopsis PME35, which was classified as a member of the type I/group 2 PME subfamily, was shown to be expressed preferentially in the basal part of the inflorescence stem (Yokoyama and Nishitani, 2006) (see Supplemental Figure 1 online). We also found that this gene was upregulated significantly in response to the application of 50 mg of weight to the stem and, conversely, downregulated when the stem was placed in a horizontal position (Yokoyama and Nishitani, 2006; Koizumi et al., 2009). These findings suggest that PME35 is involved in pectin-mediated strengthening of supporting tissue in the basal part of the Arabidopsis stem. Here, we present evidence that the PME-regulated methylesterification status of pectin in the primary wall plays a critical role in cell wall stiffening and cellular morphology of the supporting tissues of the Arabidopsis inflorescence stem.
RESULTS
Identification of PME35 and Phenotype of Its T-DNA Insertion Mutants
To study the function of PME35, Arabidopsis T-DNA insertion lines were identified in the Columbia-0 (Col-0) background and designated as pme35-1 (SALK_019255), pme35-2 (SALK_012478), pme35-3 (SALK_084661), and pme35-4 (SALK_041794) (Figure 1A). RT-PCR analysis showed that the PME35 transcript was not detected in the mutants (Figure 1B). The pme35-1 mutant showed a pendant stem phenotype (Figure 1C). A similar pendant stem phenotype was observed in the other three mutants. Other organs and tissues showed no apparent morphological differences between the pme35 mutants and wild-type plants under standard growth conditions.
Figure 1.
Structure of PME35 and T-DNA Insertion Sites of pme35 Mutants.
(A) T-DNA insertion sites of four allelic mutants of PME35: pme35-1, SALK_019255; pme35-2, SALK_012478; pme35-3, SALK_084661; and pme35-4, SALK_041794. PME35 consists of two exons (black boxes), one intron (black line), and untranslated sequences (white box).
(B) PME35 transcript levels as detected by RT-PCR in 35-d-old plants of the wild type (WT) and the four allelic mutants of PME35. ACT2 was used as a loading control. Three biologically independent experiments were performed, and representative data are shown.
(C) Forty-day-old plants of the wild type and pme35-1. pme35-1 showed a pendant stem phenotype. Bar = 10 cm.
Expression Profile of ProPME35:GUS
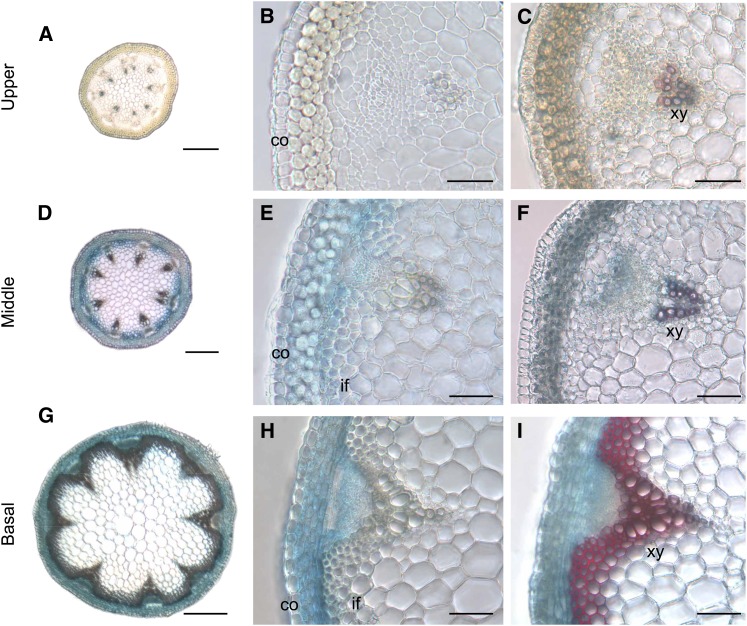
To examine the expression pattern of PME35, the promoter region of PME35 was fused to the β-glucuronidase (GUS) gene, and the construct was expressed in Arabidopsis. This construct, termed ProPME35:GUS was expressed in the middle to basal part of the inflorescence stem of the transgenic plant (Figures 2A, 2B, 2D, 2E, 2G, and 2H). This expression profile was consistent with our previous microarray and real-time RT-PCR results (Imoto et al., 2005; Koizumi et al., 2009). Notably, the GUS reporter gene was predominantly expressed in cortical cell files, the cambial zone, and interfascicular fibers (Figures 2E and 2H). More interestingly, following lignin deposition in interfascicular fibers, GUS staining was primarily limited to the cortical cells in the vicinity of interfascicular fibers (Figures 2B, 2C, 2E, 2F, 2H, and 2I), suggesting that PME35 is not expressed in lignified tissues. ProPME35:GUS was also expressed in other organs and tissues, including the hypocotyl, shoot apical meristem, trichomes, anthers, and the abscission zone of maturing siliques in the shoot. In roots, weak GUS activity was detected in the vascular bundle (see Supplemental Figure 2 online).
Figure 2.
Histochemical Analysis of PME35 Expression and Lignin Deposition Pattern.
(A) and (B) Cross sections derived from the upper part of the inflorescence stem of a ProPME35:GUS plant.
(C) A GUS-stained section of the upper part of the inflorescence stem of a ProPME35:GUS plant was further stained with phloroglucinol-HCl to disclose lignin deposition in red.
(D) and (E) Cross sections derived from the middle part of the inflorescence stem of a ProPME35:GUS plant.
(F) A GUS-stained section of the middle part of the inflorescence stem of a ProPME35:GUS plant was further stained with phloroglucinol-HCl.
(G) and (H) Cross sections derived from the basal part of the inflorescence stem of a ProPME35:GUS plant.
(I) A GUS-stained section of the basal part of the inflorescence stem of a ProPME35:GUS plant was further stained with phloroglucinol-HCl.
co, cortex; if, interfascicular fiber; xy, xylem. Bars = 100 µm in (A), (D), and (G) and 20 µm in (B), (C), (E), (F), (H), and (I).
PME Activity in pme35 Mutants
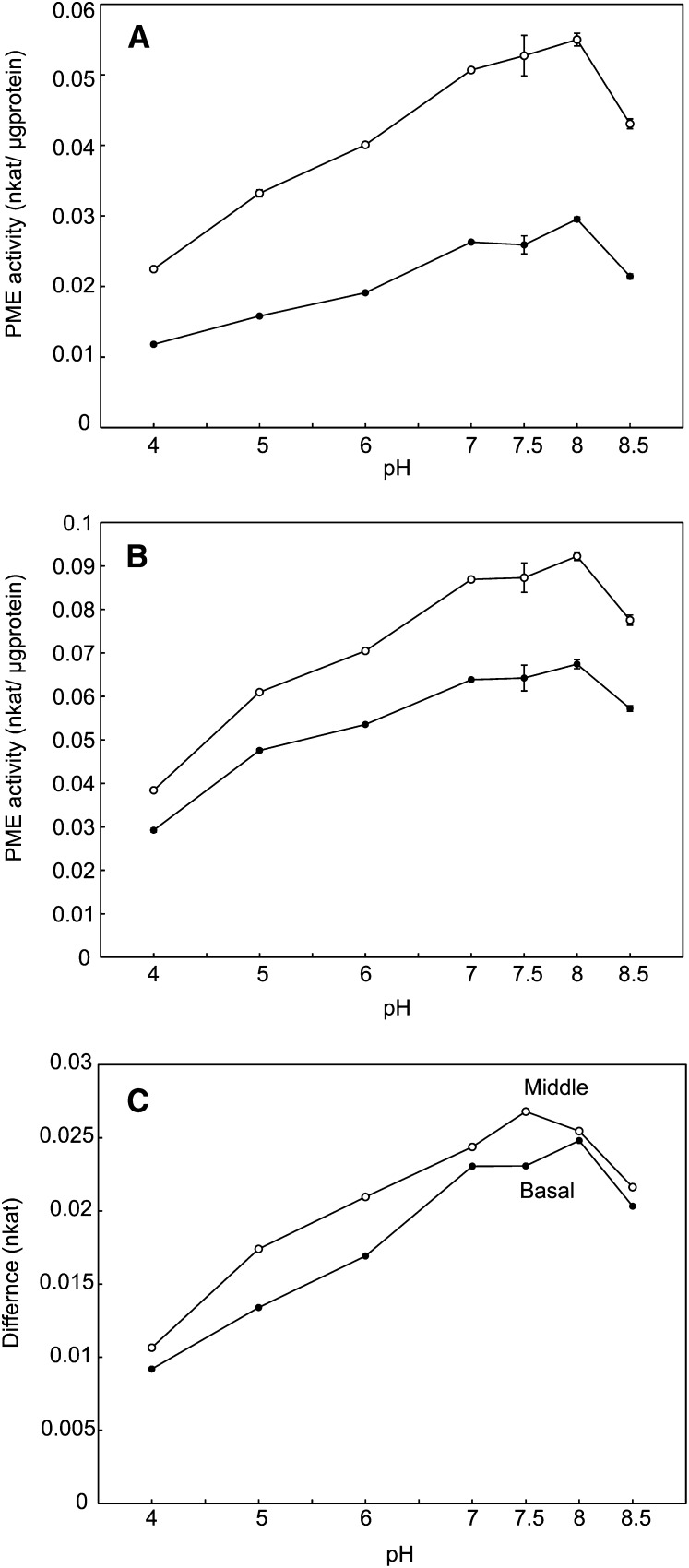
To determine the contribution of PME35 to the demethylesterification of HG in the middle to basal part of the inflorescence stem, total PME activity was assessed in wild-type plants and the pme35-1 mutant under various pH conditions. The maximum difference in activity was observed around pH 7.5 to 8 (Figure 3). At pH 7.5, the activity of PME in the mutant was reduced by 50.8% ± 5.5% in the middle part and 19.7% ± 3.6% in the basal part compared with that of the wild type (Figure 4A). Other PME35 mutant alleles showed similar reductions in enzyme activity in the basal part of the stem (Figure 5). Enzyme activity in the upper part of the stem was not affected by the mutation (Figure 4A). These results indicate that PME35 (Figure 4B) accounts for a significant part of the total PME activity in the middle to basal, but not the upper, part of the inflorescence stem of Arabidopsis. This result is consistent with the RT-PCR (Figure 4C) and the ProPME35:GUS expression results (Figure 2).
Figure 3.
PME Activity Assay at Different pH Levels.
(A) and (B) PME activity in the middle (A) and basal (B) parts of the inflorescence stem of the wild type and pme35-1 at pH levels of 4, 5, 6, 7, 7.5, 8, and 8.5. Means with se (n = 3) are shown. Open circle, the wild type; closed circle, pme35-1.
(C) Differences in PME activity between the wild type and pme35-1 in the middle and basal parts of the inflorescence stem at pH levels of 4, 5, 6, 7, 7.5, 8, and 8.5. Open circle, middle part of the inflorescence stem; closed circle, basal part of inflorescence stem.
Figure 4.
PME Activity and PME35 Transcriptional Level in the Upper, Middle, and Basal Parts of the Inflorescence Stem.
(A) PME activity in the upper, middle, and basal part of the inflorescence stem of the wild type (WT) and pme35-1. Enzyme activity was assayed at pH 7.5. Means with se (n = 3) are shown. Asterisks indicate that the value of pme35-1 was significantly different from that of the wild type at P < 0.05.
(B) PME35 consisted of the pro and mature-PME regions. The pro region includes signal peptide sequences, the PME inhibitor domain, and a processing motif. PMEI domain, PME inhibitor domain (gray box); PME domain, PME catalytic domain (black box). Digits above the rectangle indicate the positions of amino acid residues at the beginning and end of each domain.
(C) PME35 transcriptional levels in the upper, middle, and basal parts of the inflorescence stem of the wild type were detected by RT-PCR, which were repeated three times independently. CT2 was used as a loading control. Three biologically independent experiments were performed, and representative data are shown.
Figure 5.
Reduction of PME Activity in pme35 Mutants.
PME activity was measured in the wild type (WT) and the four allelic mutants of PME35 in the basal part of the inflorescence stem. Enzyme activity was assayed at pH 7.5. Means with se (n = 3) are shown. Asterisks indicate that the value of the four allelic mutants was significantly different from that of the wild type at P < 0.05.
Pattern of Methylesterification of Pectins in the pme35 Mutant
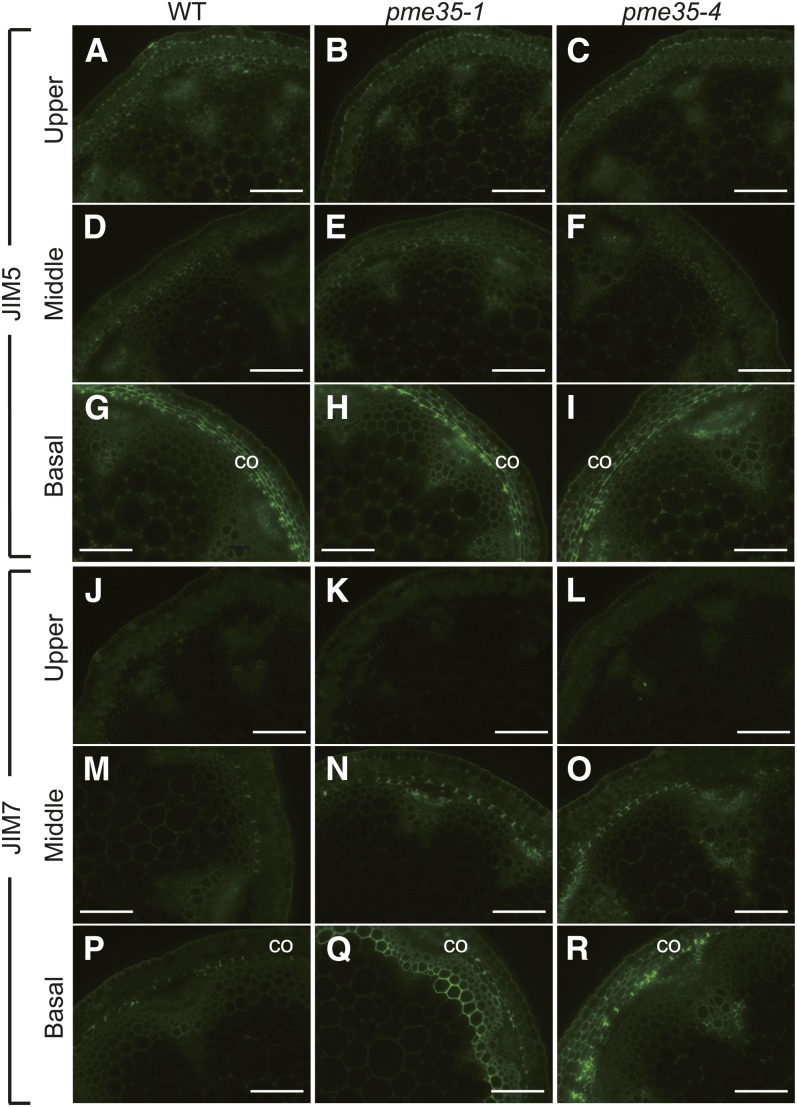
The effect of the PME35 mutation on the methylesterification status of cell wall pectin was examined by immunofluorescence using two monoclonal antibodies, JIM5 (Figures 6A to 6I) and JIM7 (Figures 6J to 6R), for the differential detection of methylesterified HG in cross sections of the upper, middle, and basal parts of the inflorescence stems of wild-type, pme35-1, and pme35-4 plants. JIM5 binds preferentially to less methylesterified pectins, whereas JIM7 recognizes a highly methylesterified pectin epitope (Knox et al., 1990; Clausen et al., 2003).
Figure 6.
Immunohistochemical Analysis of the Upper to the Basal Part of the Inflorescence Stem of the Wild Type and Two pme35 Mutants.
Cross sections derived from the upper ([A] to [C] and [J] to [L]), middle ([D] to [F] and [M] to [O]), and basal ([G] to [I] and [P] to [R]) parts of the inflorescence stem of wild-type (WT; [A], [D], [G], [J], [M], and [P]), pme35-1 ([B], [E], [H], [K], [N], and [Q]), and pme35-4 ([C], [F], [I], [L], [O], and [R]) plants were probed with the JIM5 ([A] to [I]) or JIM7 ([J] to [R]) antibody. co, cortex. Bars = 100 µm.
JIM7 binding was stronger in the middle (Figures 6M to 6O) and basal (Figures 6P to 6R) cortical cells of pme35-1 and pme35-4 plants than in the corresponding sections of wild-type stems. By contrast, JIM5 staining intensity, which was prominent in the cortical cells of the basal part of the stem, was similar between the two mutants and the wild-type stem. The LM20 antibody, which also binds specifically to highly methylesterified HG (Verhertbruggen et al., 2009), showed a similar staining pattern as that of JIM7 (see Supplemental Figure 3 online). These results showed that the demethylesterification of pectin was suppressed in the cortical cells of the basal section of the mutant inflorescence stem and indicate that PME35 is involved in the demethylesterification of pectin in the cortical files.
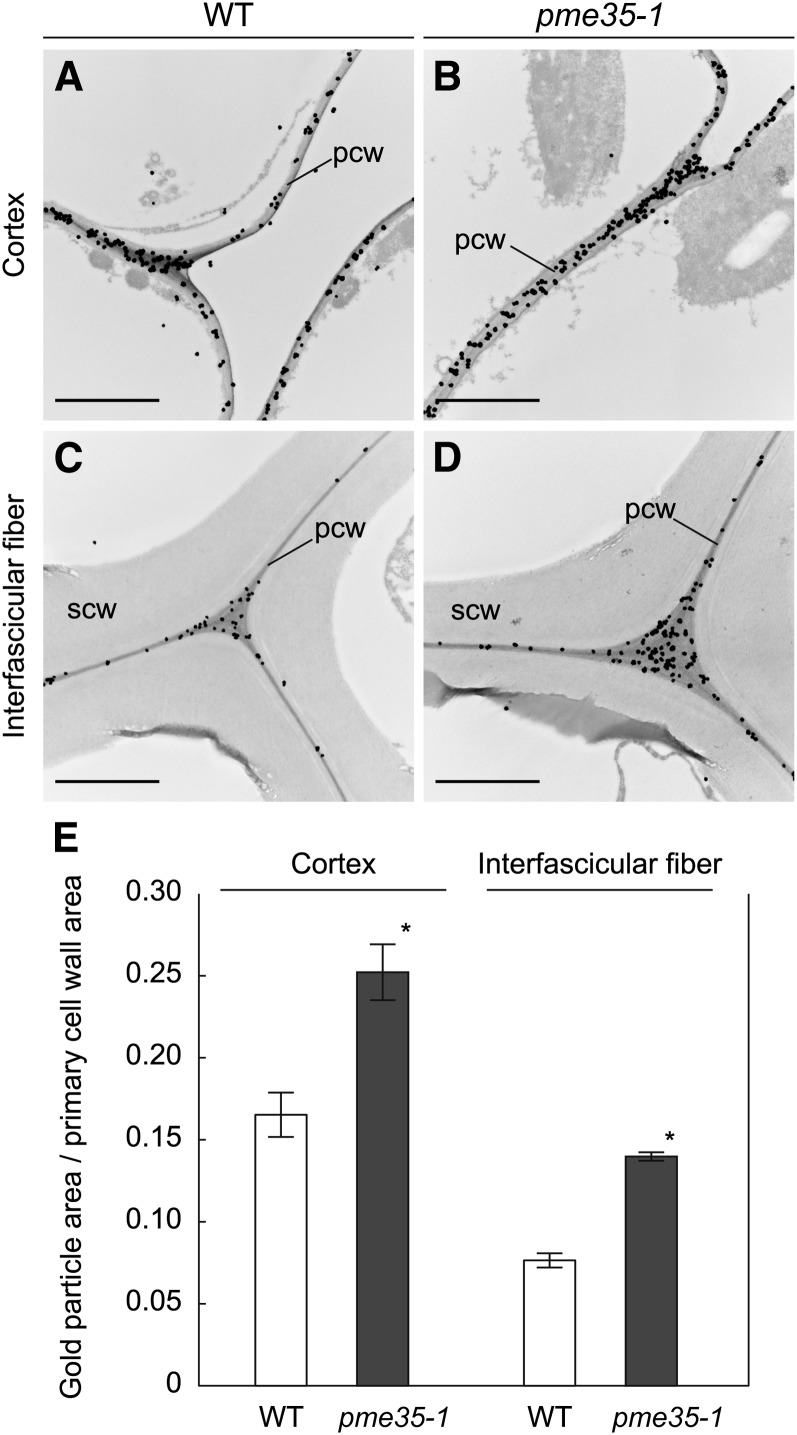
Immunogold electron microscopy using the JIM7 antibody was performed to determine whether the PME35-mediated demethylesterification is localized to the primary wall. JIM7 binding was observed in cortical cells, which lack secondary cell walls, and the outermost cell files of the interfascicular tissue. Notably, JIM7 binding was restricted to the primary cell wall, including the middle lamella (Figures 7A to 7D).
Figure 7.
Immunoelectron microscopy of the Basal Part of the Inflorescence Stem.
Cross sections derived from the basal part of the inflorescence stem of the wild type and pme35-1 were probed with the JIM7 antibody, and cortical or interfascicular fiber cells were observed. pcw, primary cell wall; scw, secondary cell wall. Bars = 2 µm.
(A) Cortical cells of the wild-type (WT) section.
(B) Cortical cells of the pme35-1 section.
(C) Interfascicular fiber cells of the wild-type section.
(D) Interfascicular fiber cells of the pme35-1 section.
(E) The degree of gold particle binding was calculated as the gold particle area per primary cell wall area from electron micrographs of cortical or interfascicular fiber cells. Means with se (the number of independent sections used for the counting was 5, 5, 4, and 3 from left to right) are shown. Asterisks indicate that the value of pme35-1 was significantly different from that of the wild type at P < 0.05.
JIM7 binding to the primary cell wall in wild-type plants and the pme35-1 mutants was quantified by calculating the gold particle area per primary wall area using the immunogold electron micrographs (Figure 7E). The area ratio was 1.5- ± 0.08-fold higher for the cortex and 1.8- ± 0.06-fold higher for the interfascicular fibers in pme35-1 compared with the wild type. Clearly, the intensity of the JIM7 epitope, which represents the intensity of highly methylesterified HG, in the primary cell wall was significantly higher in pme35-1 than in the wild type both for cortical cells and interfascicular fiber cells.
Mechanical Properties of the pme35 Stem
The pendant stem phenotype of pme35-1 shown in Figure 1C implies that the mechanical properties of the inflorescence stem are impaired due to the loss of function of PME35. Because the tensile stress-strain curve is not a good indicator of lodging susceptibility, we performed a compression test and a bending test using a creep meter to examine the mechanical properties of the stem. The results of the compression test showed that the PME35 mutation caused a significant increase in the force deformation rate in the basal part of the inflorescence stem in comparison to the wild type (Figure 8A). The bending test showed that the bending deformation in the pme35-1 mutant (0.34 + 0.01) was significantly higher than in the wild type (0.31 + 0.01, P < 0.05 by Student’s t test). These results demonstrated that lodging susceptibility in the four pme35 mutants was due to a reduction in the mechanical strength of the stem.
Figure 8.
Morphology of the Cortical Cell Files and Mechanical Strength in the Basal Part of the Inflorescence Stem.
(A) Strength of the basal part of the inflorescence stem as assayed by a compression test. Deformation rate was calculated as the load-induced compressed distance with respect to the diameter of the stem when the applied force reached 2 n. Means with se (n = 20) are shown. Asterisks indicate that the value is significantly different from that of the wild type at P < 0.05 according to Student’s t test. WT, the wild type.
(B) Cross sections of the basal part of the inflorescence stem of the wild type were observed by transmission electron microscopy.
(C) Cross sections of the basal part of the inflorescence stem of pme35-1 were observed by transmission electron microscopy.
(D) Enlarged image from (B).
(E) Enlarged images from (C).
Boxes in (B) and (C) indicate the regions enlarged in (D) and (E) respectively. co, cortex; if, interfascicular fiber. Bars = 10 µm in (B) and (C) and 2 µm in (D) and (E).
Morphology and Cell Wall Composition of the Stem Tissues
Transmission electron microscopy was used to analyze the cortical cells surrounding the interfascicular fibers with the aim of gaining insight into the internal anatomy of the pme35-1 mutant stem. The results showed that the radial organization of cell files in the pme35-1 stem and the thickness of the cell wall (see Supplemental Table 1 online) were not distinguishable from those of the wild type. However, certain cortical cells facing the interfascicular fibers showed a severe reduction in the thickness of the cell wall in the mutant and exhibited an aberrant shape and partial collapse (Figures 8B to 8E). In the other tissues of the mutant, no obvious morphological difference was observed.
Because the secondary cell wall contributes to the mechanical strength of the stem, we compared lignified tissues in stem sections stained with phloroglucinol-HCl. The staining intensity and the morphology of the lignified tissues were indistinguishable between the wild type and the four mutants (see Supplemental Figure 4 online).
A quantitative assessment of the effects of the mutation on the chemical composition of the secondary cell wall was performed by fractionation of the basal part of the stem into lignin, cellulose, hemicelluloses, and pectin fractions (see Supplemental Figure 5 online). In addition, the neutral sugar composition of the 2M-trifluoroacetic acid hydrolysable polysaccharide was analyzed (see Supplemental Table 2 online). These analyses indicated that cell wall components, particularly those abundant in the secondary cell walls, such as lignin, cellulose, and xylans, were not affected by the mutation. These findings were further confirmed by immunohistochemical analysis using LM10 and LM15 antibodies, which specifically recognize heteroxylan and xyloglucan, respectively. The immunohistochemistry results showed no significant differences in the staining pattern between the wild type and the mutant (see Supplemental Figure 6 online).
Complementation Analysis
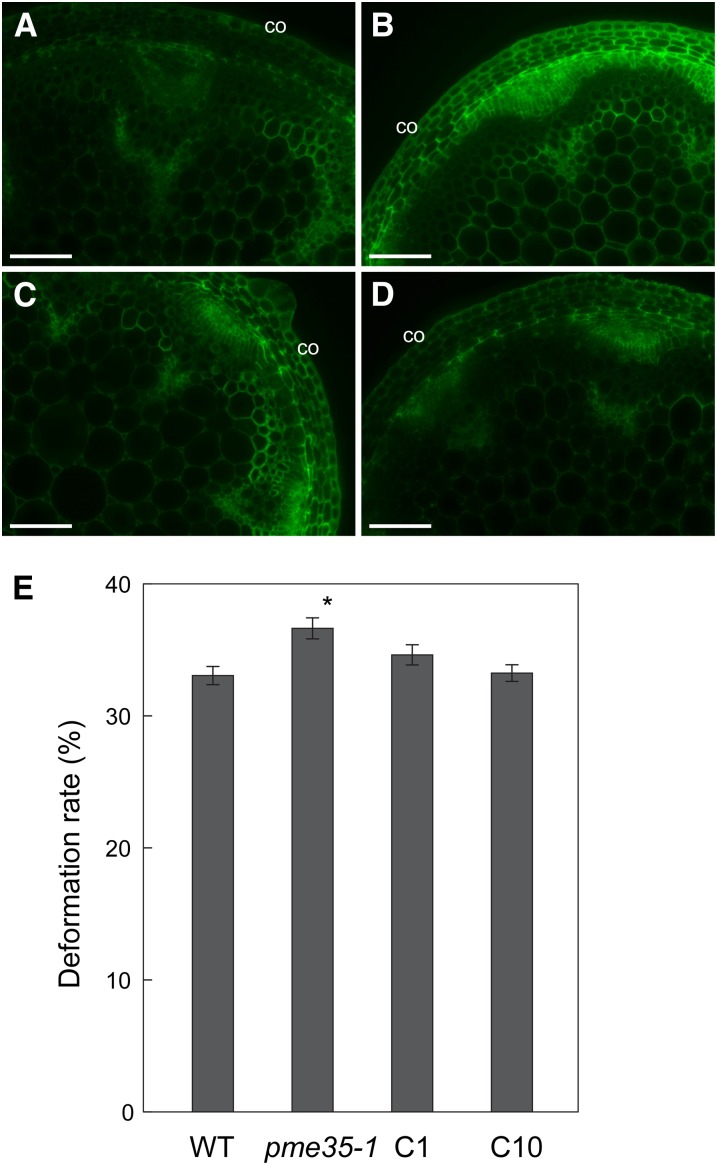
To investigate whether the pme35-1 phenotypes were caused by the loss of function of PME35, the pme35-1 mutant line was transformed with a PME35 construct, and the two complemented lines, C1 and C10, in which expression of PME35 was confirmed by RT-PCR analysis, were analyzed (see Supplemental Figure 7A online). JIM7 binding in cortical cells (Figures 9A to 9D) was reduced in the two complementation lines, indicating the recovery of PME activity by PME35. The two complemented lines also showed relatively upright inflorescence stems (see Supplemental Figure 7B online) and a lower deformation rate (Figure 9E) compared with pem61-1, indicating that the mechanical properties of the stem were partially complemented by PME35. These results suggest that PME35 is responsible for the demethylesterification of cell wall pectin, which reduces the mechanical strength of supporting tissues in the basal part of the stem.
Figure 9.
Complementation of pme35-1 Phenotypes.
Cross sections derived from the basal part of the inflorescence stem of the wild type, pme35-1, and complementation lines of pme35-1 (C1 or C10) were probed with JIM7 antibody and observed under a fluorescence microscope. co, cortex. Bars = 100 µm.
(A) Cross section of the wild type.
(B) Cross section of pme35-1.
(C) Cross section of C1.
(D) Cross section of C10.
(E) Mechanical strength at the basal part of the inflorescence stem of the wild type (WT), pme35-1, and complementation lines of pme35-1 (C1 or C10). Means with se (n = 20) are shown. Asterisk indicates that the value of pme35-1 or complemented lines was significantly different from that of the wild type at P < 0.05 according to a Student’s t test.
Taken together, the data on the mechanical properties and chemical composition of the cell walls of the four pme35 mutants indicated that the pendant phenotype was a result of the loss of mechanical strength caused by reduced demethylesterification of HG in the primary wall and middle lamella of the cortical and interfascicular fiber cells and not related to defects in other components of the lignified secondary cell walls.
DISCUSSION
In this study, PME35 was identified as being necessary for the demethylesterification of HG in the primary cell wall of cortical cells and certain interfascicular fiber cells in the middle to basal part of the inflorescence stem of Arabidopsis. Analyses of the phenotype of the PME35 loss-of-function mutant identified a novel role for the PME protein encoded by PME35 in the regulation of the formation of supporting tissue in the middle to basal section of the stem. We thus show that PME35 is directly involved in providing mechanical support to the plant shoot via demethylesterification of HG in primary cell walls, including the middle lamella.
PME is a ubiquitous enzyme in higher plants and is encoded by at least 66, 59, and 89 genes in Arabidopsis, rice (Oryza sativa), and poplar (Populus trichocarpa), respectively (Geisler-Lee et al., 2006; Louvet et al., 2006; Pelloux et al., 2007). Although the PME family of proteins is considered to play potentially diverse physiological roles, the in planta functions of the individual members of this family largely remain unknown. PME35 was originally identified based on its upregulation in response to a mechanical load and its downregulation in response to a reduction of mechanical stress when the plant was placed in a horizontal position (Yokoyama and Nishitani, 2006; Koizumi et al., 2009). It is therefore likely that the role of PME35 in forming supporting tissues is transcriptionally regulated by mechanical signaling. This was supported by gene coexpression data obtained using the ATTED-II database (Obayashi et al., 2007) (http://atted.jp/top_search.shtml), which demonstrated the coregulation of PME35 with the genes involved in secondary wall formation (see Supplemental Table 3 online). The mechanism by which a mechanical signal alters the transcription of PME35 is not known and should be investigated further.
The PME-mediated demethylesterification of HG can follow two possible patterns, random and linear, which produce two distinct biological consequences (Micheli, 2001; Tucker and Seymour, 2002). The random removal of methyl ester groups from HG results in the activation of endopolygalacturonases, which catalyze the degradation of the main chain of HG and thus the disassembly of the cell wall.
During linear or block-wise demethylesterification, large blocks of demethylesterified galacturonic acid residues on the HG chain are hypothesized to interact with calcium ions (Ca2+) to form a pectate gel, thereby causing cell wall stiffening (Jiang et al., 2005). PMEs with alkaline pH optima are hypothesized to have a general propensity toward linear demethylesterification (Bosch and Hepler, 2005). Our preliminary experiments showed that a crude PME preparation derived from the basal part of the stem of Arabidopsis exhibited optimum activity at around pH 7.0 to 8 (Figure 3), suggesting that PME35 may mediate the linear demethylesterification of HG and may therefore contribute to the mechanical strength of the cell wall.
Recently, Peaucelle et al. (2011) showed that demethylesterification of pectin affects the mechanical properties of the meristematic cell walls using atomic force microscopy and proposed that demethylesterification triggered in subepidermal tissue layers may contribute to an increase in the elasticity of these layers, an opposite effect from our results obtained in the non-meristematic tissue of the stem. This apparently controversial result might be explained by the different actions of PMEs in different cell wall types.
Interfascicular fibers form thick and lignified secondary cell walls in the middle to basal section of the inflorescence stem. Deposition of a secondary wall of interfascicular fiber cells might push the surrounding cortical cells toward the outside, which can result in cell deformation or indirectly affect cell growth and differentiation via a still unknown mechanism. Thus, demethylesterification of pectin, which leads to cell wall stiffening, might provide the cell walls of cortical cells with the mechanical properties necessary to resist the external pressure of interfascicular fibers and allow the cortical cells to undergo changes in morphology. In the cortical cells of the pme35 mutant, cell wall stiffening by demethylesterification of pectins may be impaired, causing the collapse of the cortical cells in the vicinity of interfascicular fibers (Figure 8).
PME35-mediated demethylesterification of pectins was also observed in the middle lamella in certain interfascicular fiber cell layers as well as in the cortex (Figure 7). The attachment of these interfascicular fiber cells to adjoining cortical cells through demethylesterified pectins may provide the entire stem with greater mechanical strength. Recently, Siedlecka et al. (2008) reported that in poplar, a specific PME is involved in the regulation of fiber length by enhancing the cellular adhesion between developing fibers (Siedlecka et al., 2008). This line of evidence supports the idea that demethylesterification of pectin might be of importance for cellular adhesion even during secondary wall formation in vascular plants.
Although pectins are traditionally associated with intercellular adhesion at the middle lamella and regulation of primary cell wall extensibility, this study provides substantial evidence that pectin is directly or indirectly involved in secondary wall thickening in interfascicular fibers. The mechanism by which PME35, which is active in the primary wall, can affect the formation of the secondary cell wall is unknown, and this question merits further investigation. Our findings also imply that mechanical signaling affects cell-to-cell interactions, and these issues will be investigated in future studies.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Col-0 was used as the wild type. T-DNA insertion lines of Arabidopsis (SALK_019255, SALK_012478, SALK_041794, and SALK_084661) were obtained from the ABRC. The pme35-1 mutant line was isolated through three backcrosses of SALK_019255, and the homozygous mutations were confirmed. PCR was performed using GoTaq DNA polymerase (Promega). The genotypes of these T-DNA insertion alleles were determined by PCR using genomic DNA. Primer sets used were a PME35-specific forward primer (5′-TAAGCGCGGCGTTGACTAATC-3′), a T-DNA left border–specific primer (5′-GCGTGGACCGCTTGCTGCAACT-3′), and a PME35-specific reverse primer (5′-ACGTCACTTAAATTATAAAAGGC-3′).
For the expression analysis of PME35, homozygous transgenic lines for the ProPME35:GUS fusion construct, generated as previously described (Koizumi et al., 2009), were used. In all experiments, seeds of the wild type and transgenic/mutant lines were sown on rock-wool blocks (Grodan; Rockwool) moistened with the culture medium described by Tsukaya et al. (1991) in a plastic tray and grown under continuous light (70 to 80 μmol m−2 s−1) at 22°C in a growth chamber as previously described (Yokoyama and Nishitani, 2001).
Complementation Analysis
For complementation analysis, a 5.4-kb genomic fragment of PME35 that contained the 5′ promoter region (3 kb) and 3′ untranslated region (0.4 kb) was amplified from genomic DNA of the Col-0 ecotype. After verifying the nucleotide sequence of the amplified fragment, it was subcloned into a SYN947-D binary vector containing a hygromycin resistance gene (Takara Bio). This construct was transformed into Agrobacterium tumefaciens strain C58 and then introduced into a pme35-1 homozygous mutant line using the floral dip method (Clough and Bent, 1998). To evaluate phenotypic complementation, 12 independent transformants containing a single PME35 genomic fragment were established by hygromycin selection, and two of them were confirmed to be homozygous insertion lines.
RT-PCR
For RT-PCR, the upper, middle, and basal sections of the inflorescence stems (200 mm long) were collected into liquid nitrogen and subjected to total RNA extraction according to the conventional SDS-phenol protocol (Yokoyama and Nishitani, 2001). Total RNA was treated with DNase I (Takara Bio) to eliminate genomic DNA contamination. An aliquot of cDNA was synthesized from total RNA with MultiScribe reverse transcriptase (Applied Biosystems). PCR was performed with GoTaq DNA polymerase (Promega) using cDNA as a template. PCR was performed using a set of specific primers for PME35 (5′-TAAGCGCGGCGTTGACTAATC-3′ and 5′-ACGTCACTTAAATTATAAAAGGC-3′). This primer set was designed to amplify the full-length coding region of PME35 cDNA. The amplification products were separated on an agarose gel and visualized by staining with ethidium bromide.
Histochemical Analysis of GUS Expression
The upper, middle, and basal sections of the inflorescence stem of transgenic plants expressing ProPME35:GUS were cross-sectioned using a Leica VT1200S vibrating blade microtome (Leica Microsystems). Sections 70 μm thick were generated and incubated in a GUS staining solution containing 1 mM 5-bromo-4-chloro-3-indoxyl-β-glucuronide at 37°C for several hours or overnight, followed by washing in 70% ethanol. For lignin staining, cross sections were stained with Wiesner’s solution consisting of 2% (w/v) phloroglucinol in 95% (v/v) ethanol followed by treatment with 50% (v/v) aqueous HCl solution. These sections were cleared according to Matsui et al. (2005) and observed using a microscope equipped with differential interference contrast optics (DMRPX; Leica). Images were recorded using a charge-coupled device camera (Retiga EXi; QImaging).
PME Activity Assay
PME activity was quantified by the gel diffusion assay as described by Downie et al. (1998) with some modifications, as described by Bourgault and Bewley (2002). Protein samples were extracted from each of the upper, middle, and basal parts of inflorescence stems (200 mm long). Stem samples were collected in liquid nitrogen and homogenized with equal volumes (w/v) of extraction buffer (12.5 mM citric acid, 50 mM phosphate buffer, pH 7.0, with 1 M NaCl) and placed at 4°C for 2 h. The sample was centrifuged and the supernatant was desalted and reduced by ultrafiltration to obtain a protein sample. Protein concentrations were measured using the Bradford assay (Bio-Rad). Protein samples and purified PME solution (orange peel PME, P5400; Sigma-Aldrich), used as a standard, were applied to a 5-mm-thick gel containing 2% (w/v) agarose, 500 µg/mL gelatin, and 0.1% (w/v) of high methylesterified pectin (≥85% esterified pectin from citrus fruit, P9561; Sigma-Aldrich) dissolved in McIlvaine buffer adjusted to pHs 4, 5, 6, 7, 7.5, 8, or 8.5. After incubation at 30°C for 16 h, the gel was stained with a 0.05% (w/v) aqueous solution of ruthenium red for 1 h and washed with distilled water. PME activity was calculated by measuring the red-stained area around the well, according to a standard curve derived from standard PME.
Immunofluorescence Localization
The basal part of the inflorescence stems (200 mm long) was cross-sectioned using the vibratome at a thickness of 70 µm and fixed with 4% paraformaldehyde in 20 mM sodium cacodylate buffer, pH 7.4. To enable highly sensitive immunodetection, the tyramide signal amplification method was used according to the manufacturer’s protocol (Tyramide Signal Amplification kits; Invitrogen-Molecular Probes). All the monoclonal antibodies used in this study were supplied by Plantprobes (http://www.plantprobes.net/index.php). The samples were incubated with one of the following monoclonal antibodies: JIM5, JIM7 (Knox et al., 1990), LM10 (McCartney et al., 2005), LM15 (Marcus et al., 2008), and LM20 (Verhertbruggen et al., 2009), at a dilution of 1/10 (v/v) as primary antibodies, followed by incubation with horseradish peroxidase–labeled goat anti-mouse IgG as the secondary antibody. For tyramide labeling, Alexa Fluor 488 was used. Photographs were taken using fluorescein isothiocyanate filters fitted to an epifluorescence microscope (DMRXP; Leica). For each immunohistochemical staining experiment, a minimum of 20 independent tissue sections was analyzed for each treatment condition. The staining experiments were repeated several times for each antibody and typical image sets were selected and shown.
Immunoelectron Microscopy
For immunogold labeling of the JIM5 and JIM7 monoclonal antibodies (described above), the basal part of the inflorescence stem (200 mm long) was fixed for 1 h in 0.1 M phosphate buffer, pH 7.2, containing 4% paraformaldehyde and 0.05% glutaraldehyde, and then embedded in LR White resin (Sigma-Aldrich) and polymerized by heat. Ultrathin sections were obtained and transferred to nickel grids, which were blocked with 3% BSA in PBS, pH 7.4, and incubated overnight in a moist chamber with JIM5 and JIM7 antibodies diluted 1/10 (v/v) in 1% BSA/PBS. After several washes, the sections were incubated for 1 h with a goat anti-rat IgG coupled to 15-nm gold particles (Sigma-Aldrich) in phosphate buffer containing 1% BSA and 1.5% fetal bovine serum. Finally, sections were stained with 2% uranyl acetate for 20 min and observed with a JEM-1200EX transmission electron microscope (JEOL) at 80 kV.
Quantification of Immunogold Labeling
The immunogold label was quantified by calculating the area of gold particles per area of the primary cell wall measured from immunoelectron micrographs of three to five independent plant stem sections of wild-type and pme35-1 mutant plants using ImageJ (National Institutes of Health). For this measurement, same size (2048 × 2048 pixels square) micrographs were used.
Measurement of Mechanical Properties of the Stem
Compression test: To evaluate the mechanical properties of the inflorescence stem, a compression test was performed using a creep meter (RE33005; Yamaden). All samples were freshly collected, and the basal part of the 200-mm-high inflorescence stem was cut into 5-mm sections that were placed horizontally between the plunger and the stage of the creep meter. The stem was compressed in the horizontal axis by raising the stage at a constant speed (0.5 mm/s) until 2 n of force was applied. The force applied to the stem was monitored by the load-cell connected to the plunger, and the force deformation curve was recorded. The deformation rate was calculated when the applied force reached 2 n and represented the deformation distance per the initial stem diameter. The stem diameter was measured using the same creep meter and represented the distance between the surfaces of the plunger and the stage when 0.01 n of force is detected.
Transmission Electron Microscopy
For transmission electron microscopy, the basal sections of the inflorescence stems measuring 200 mm in length were collected and fixed with a 4% paraformaldehyde and 2% glutaraldehyde solution in a 0.2 M sodium cacodylate buffer, pH 7.4, for 1 h at room temperature. Sections were washed in 0.2 M sodium cacodylate buffer, pH 7.4, and postfixed with 1% osmium tetroxide in the same buffer for 2 h. This was followed by dehydration in acetone and embedding in LR White resin. Ultrathin sections were cut with an ultramicrotome, collected on formvar-coated copper grids, and stained with 2% uranyl acetate. Specimens were observed with a JEM-1200EX transmission electron microscope (JEOL) at 80 kV.
Histochemical Analysis of Lignified Tissues
Lignified cell walls were visualized by staining stem sections with phloroglucinol-HCl according to the method described by Jensen (1962).
Cell Wall Fractionation and Analyses
For the analysis of polysaccharides, the cell wall samples were fractionated according to the procedure described by Nishitani and Masuda (1979) and Arai-Sanoh et al. (2011) with modifications. Briefly, 20-mm stem sections excised from the basal part of inflorescence stems measuring 200 mm in length obtained from the wild type or mutant alleles were frozen in liquid nitrogen and lyophilized and then processed by bead-milling using Tissue Lyser II (Qiagen). After measurement of the dry weight, the milled samples were subjected to extraction with 80% ethanol at 80°C and a methanol/chloroform mixture at 25°C. Lysates were digested with protease and α-amylase and fractionated into an ammonium oxalate fraction, a 4 M potassium hydroxide fraction, and a cellulose fraction. Total sugar contents in the three cell wall fractions were measured by the phenol-sulfuric acid method. The uronic acid content in the ammonium oxalate fraction was measured according to Blumenkrantz and Asboe-Hansen (1973).
For analysis of neutral sugar composition, the lyophilized stem sections with lipid and starch removed were hydrolyzed with 2 n trifluoroacetic acid for 1 h at 121°C, and then the neutral sugars liberated were analyzed by high-performance anion exchange chromatography (Dionex ICS 5000) equipped with a CarboPak PA1 column.
The lignin content of the lyophilized bead-milled cell wall sample was measured according to Suzuki et al. (2009).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: PME35, At3g59010; PME16, At2g43050; Lu-PME, AF355056; Vv-PME, Vv05s0062g01160.t01; Os-PME, Os02g0783000; Zm-PME, EU958178; Pt-PME, POPTR 0002s14640.1; and Ps-PME, EF085456.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Schematic Representation of the Structure of the PME35 Protein.
Supplemental Figure 2. Histochemical Analysis of ProPME35:GUS Expression.
Supplemental Figure 3. Immunohistochemical Analysis of the Basal Part of the Inflorescence Stem of the Wild Type and Two Mutant Alleles.
Supplemental Figure 4. Comparison of Lignified Cell Walls in the Interfascicular Fibers and Xylem between the Wild Type and Four Mutant Alleles.
Supplemental Figure 5. Comparison of Chemical Compositions of the Cell Wall in the Basal Part of the Inflorescence Stem between the Wild Type and the Four Mutants, pme35-1, pme35-2, pme35-3, and pme35-4.
Supplemental Figure 6. Immunohistochemical Analysis of the Upper, Middle, and Basal Parts of the Inflorescence Stem of the Wild Type and the pme35-1 Mutant Using LM15 and LM10 Antibodies.
Supplemental Figure 7. Complementation Analysis for pme35-1.
Supplemental Table 1. Wall Thickness of the Cortex, Fibers, and Vessels in the Basal Stems of the Wild Type and pme35-1.
Supplemental Table 2. Neutral Monosaccharide Composition of Cell Walls from Basal Stems of the Wild Type and the Four Mutants, pme35-1, pme35-2, pme35-3, and pme35-4.
Supplemental Table 3. Genes That Are Coexpressed with PME35 According to ATTED-II.
Acknowledgments
We thank Kei Saito and Yuko Noguchi for their excellent experimental assistance. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (23012004) to K.N. and by a Grant-in-Aid for Scientific Research (B) (22370013) to K.N. and (C) to R.Y. from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
AUTHOR CONTRIBUTIONS
All the authors designed research. S.H., K.S., and R.Y. performed experiments. S.H. and R.Y. analyzed the data and contributed to data interpretation and the article preparation. K.N. supervised the study and wrote the article.
Glossary
- HG
homogalacturonan
- PME
pectin methylesterase
- Col-0
Columbia-0
References
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. (2011). Plant Cell Walls. (New York: Garland Science, Taylor & Francis Group). [Google Scholar]
- Arai-Sanoh Y., et al. (2011). Genotypic variations in non-structural carbohydrate and cell-wall components of the stem in rice, sorghum, and sugar vane. Biosci. Biotechnol. Biochem. 75: 1104–1112 [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. (1973). New method for quantitative determination of uronic acids. Anal. Biochem. 54: 484–489 [DOI] [PubMed] [Google Scholar]
- Bosch M., Hepler P.K. (2005). Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell 17: 3219–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault R., Bewley J.D. (2002). Gel diffusion assays for endo-beta-mannanase and pectin methylesterase can underestimate enzyme activity due to proteolytic degradation: A remedy. Anal. Biochem. 300: 87–93 [DOI] [PubMed] [Google Scholar]
- Brown D.M., Zeef L.A., Ellis J., Goodacre R., Turner S.R. (2005). Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen M.H., Willats W.G., Knox J.P. (2003). Synthetic methyl hexagalacturonate hapten inhibitors of anti-homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res. 338: 1797–1800 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Downie B., Dirk L.M., Hadfield K.A., Wilkins T.A., Bennett A.B., Bradford K.J. (1998). A gel diffusion assay for quantification of pectin methylesterase activity. Anal. Biochem. 264: 149–157 [DOI] [PubMed] [Google Scholar]
- Geisler-Lee J., et al. (2006). Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiol. 140: 946–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K., Yokoyama R., Nishitani K. (2005). Comprehensive approach to genes involved in cell wall modifications in Arabidopsis thaliana. Plant Mol. Biol. 58: 177–192 [DOI] [PubMed] [Google Scholar]
- Jensen, W.A. (1962). Botanical Histochemistry. (San Francisco and London: W.H. Freeman & Co.). [Google Scholar]
- Jiang L., Yang S.L., Xie L.F., Puah C.S., Zhang X.Q., Yang W.C., Sundaresan V., Ye D. (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolie R.P., Duvetter T., Van Loey A.M., Hendrickx M.E. (2010). Pectin methylesterase and its proteinaceous inhibitor: A review. Carbohydr. Res. 345: 2583–2595 [DOI] [PubMed] [Google Scholar]
- Knox J.P., Linstead P.J., King J., Cooper C., Roberts K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Ko J.H., Beers E.P., Han K.H. (2006). Global comparative transcriptome analysis identifies gene network regulating secondary xylem development in Arabidopsis thaliana. Mol. Genet. Genomics 276: 517–531 [DOI] [PubMed] [Google Scholar]
- Koizumi K., Yokoyama R., Nishitani K. (2009). Mechanical load induces upregulation of transcripts for a set of genes implicated in secondary wall formation in the supporting tissue of Arabidopsis thaliana. J. Plant Res. 122: 651–659 [DOI] [PubMed] [Google Scholar]
- Louvet R., Cavel E., Gutierrez L., Guénin S., Roger D., Gillet F., Guerineau F., Pelloux J. (2006). Comprehensive expression profiling of the pectin methylesterase gene family during silique development in Arabidopsis thaliana. Planta 224: 782–791 [DOI] [PubMed] [Google Scholar]
- Marcus S.E., Verhertbruggen Y., Hervé C., Ordaz-Ortiz J.J., Farkas V., Pedersen H.L., Willats W.G.T., Knox J.P. (2008). Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic O., Janecek S. (2004). Pectin methylesterases: Sequence-structural features and phylogenetic relationships. Carbohydr. Res. 339: 2281–2295 [DOI] [PubMed] [Google Scholar]
- Matsui A., Yokoyama R., Seki M., Ito T., Shinozaki K., Takahashi T., Komeda Y., Nishitani K. (2005). AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. Plant J. 42: 525–534 [DOI] [PubMed] [Google Scholar]
- McCartney L., Marcus S.E., Knox J.P. (2005). Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 53: 543–546 [DOI] [PubMed] [Google Scholar]
- Micheli F. (2001). Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 6: 414–419 [DOI] [PubMed] [Google Scholar]
- Minic Z., Jamet E., San-Clemente H., Pelletier S., Renou J.P., Rihouey C., Okinyo D.P., Proux C., Lerouge P., Jouanin L. (2009). Transcriptomic analysis of Arabidopsis developing stems: A close-up on cell wall genes. BMC Plant Biol. 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani K., Masuda Y. (1979). Growth and cell wall changes in azuki bean epicotyls I. Changes in wall polysaccharides during intact growth. Plant Cell Physiol. 20: 63–74 [Google Scholar]
- Obayashi T., Kinoshita K., Nakai K., Shibaoka M., Hayashi S., Saeki M., Shibata D., Saito K., Ohta H. (2007). ATTED-II: A database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Res. 35 (Database issue): D863–D869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. (2003). Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15: 1591–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A., Braybrook S.A., Le Guillou L., Bron E., Kuhlemeier C., Höfte H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Peaucelle A., Louvet R., Johansen J.N., Höfte H., Laufs P., Pelloux J., Mouille G. (2008). Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr. Biol. 18: 1943–1948 [DOI] [PubMed] [Google Scholar]
- Pelloux J., Rustérucci C., Mellerowicz E.J. (2007). New insights into pectin methylesterase structure and function. Trends Plant Sci. 12: 267–277 [DOI] [PubMed] [Google Scholar]
- Persson S., Wei H., Milne J., Page G.P., Somerville C.R. (2005). Identification of genes required for cellulose synthesis by regression analysis of public microarray data sets. Proc. Natl. Acad. Sci. USA 102: 8633–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven P.H., Evert R.F., Eichhorn S.E. (2005). Biology of Plants. (San Francisco, CA, and London: W.H. Freeman & Co.)
- Ridley B.L., O’Neill M.A., Mohnen D. (2001). Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57: 929–967 [DOI] [PubMed] [Google Scholar]
- Siedlecka A., Wiklund S., Péronne M.A., Micheli F., Lesniewska J., Sethson I., Edlund U., Richard L., Sundberg B., Mellerowicz E.J. (2008). Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol. 146: 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Suzuki Y., Yamamoto N., Hattori T., Sakamoto M., Umezawa T. (2009). High-throughput determination of thioglycolic acid lignin from rice. Plant Biotechnol. 26: 337–340 [Google Scholar]
- Tian G.W., Chen M.H., Zaltsman A., Citovsky V. (2006). Pollen-specific pectin methylesterase involved in pollen tube growth. Dev. Biol. 294: 83–91 [DOI] [PubMed] [Google Scholar]
- Tsukaya H., Ohshima T., Naito S., Chino M., Komeda Y. (1991). Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 97: 1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker G.A., Seymour G.B. (2002). Modification and degradation of pectins. In Pectins and Their Manipulation, G. Seymour and J.P. Knox, eds (Boca Raton, FL: CRC Press), pp.150–173.
- Verhertbruggen Y., Marcus S.E., Haeger A., Ordaz-Ortiz J. J., Knox J.P. (2009). An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 344: 1858–1862. [DOI] [PubMed]
- Willats W.G., Orfila C., Limberg G., Buchholt H.C., van Alebeek G.J., Voragen A.G., Marcus S.E., Christensen T.M., Mikkelsen J.D., Murray B.S., Knox J.P. (2001). Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 276: 19404–19413 [DOI] [PubMed] [Google Scholar]
- Wolf S., Mouille G., Pelloux J. (2009). Homogalacturonan methyl-esterification and plant development. Mol. Plant 2: 851–860. [DOI] [PubMed]
- Yokoyama R., Nishitani K. (2001). A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol. 42: 1025–1033 [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Nishitani K. (2004). Genomic basis for cell-wall diversity in plants. A comparative approach to gene families in rice and Arabidopsis. Plant Cell Physiol. 45: 1111–1121 [DOI] [PubMed] [Google Scholar]
- Yokoyama R., Nishitani K. (2006). Identification and characterization of Arabidopsis thaliana genes involved in xylem secondary cell walls. J. Plant Res. 119: 189–194 [DOI] [PubMed] [Google Scholar]