Abstract
Essential polyunsaturated fatty acids (PUFAs) are critical nutritional lipids that must be obtained from the diet to sustain homeostasis. Omega-3 and -6 PUFAs are key components of biomembranes and play important roles in cell integrity, development, maintenance, and function. The essential omega-3 fatty acid family member docosahexaenoic acid (DHA) is avidly retained and uniquely concentrated in the nervous system, particularly in photoreceptors and synaptic membranes. DHA plays a key role in vision, neuroprotection, successful aging, memory, and other functions. In addition, DHA displays anti-inflammatory and inflammatory resolving properties in contrast to the proinflammatory actions of several members of the omega-6 PUFAs family. This review discusses DHA signalolipidomics, comprising the cellular/tissue organization of DHA uptake, its distribution among cellular compartments, the organization and function of membrane domains rich in DHA-containing phospholipids, and the cellular and molecular events revealed by the uncovering of signaling pathways regulated by DHA and docosanoids, the DHA-derived bioactive lipids, which include neuroprotectin D1 (NPD1), a novel DHA-derived stereoselective mediator. NPD1 synthesis agonists include neurotrophins and oxidative stress; NPD1 elicits potent anti-inflammatory actions and prohomeostatic bioactivity, is anti-angiogenic, promotes corneal nerve regeneration, and induces cell survival. In the context of DHA signalolipidomics, this review highlights aging and the evolving studies on the significance of DHA in Alzheimer’s disease, macular degeneration, Parkinson’s disease, and other brain disorders. DHA signalolipidomics in the nervous system offers emerging targets for pharmaceutical intervention and clinical translation.
Keywords: omega-3 fatty acids, neuroprotectin D1, photoreceptors, retinal pigment epithelial cells, phagocytosis, liver
INTRODUCTION
The ongoing transformation of biology and medicine has been greatly influenced by the outcomes of systems biology, genomics, proteomics, and metabolomics. Within metabolomics, lipidomics comprises the understanding of the significance and the molecular characteristics of more than 1,000 lipids in health and disease. Here we review the lipidomics of nutrition, function, and diseases that affect the brain and retina from the perspective of the omega-3 fatty acid (FA) family member, docosahexaenoic acid (DHA), which is taken up and tenaciously retained in the central nervous system (CNS). The identification of the bioactive derivative of DHA, neuroprotectin D1 (NPD1; 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid), a docosanoid, allows for the elucidation of DHA-mediated events. The term “DHA signalolipidomics” represents the cellular/tissue organization of DHA uptake, its distribution among molecular species of phospholipids, disposition among cellular compartments, the organization and function of membrane domains rich in DHA-containing phospholipids, and modulation of the synthesis of docosanoids, the DHA bioactive derivatives. Moreover, DHA signalolipidomics encompasses cellular and molecular signaling pathways regulated by DHA, its bioactive derivatives, and receptor-mediated actions. We provide here an assessment of several aspects of DHA signalolipidomics, mainly at the cellular level, and discuss its significance for homeostasis and for the initiation and progression of neurodegenerative diseases. Several of these events are affected by the dietary supply of omega-3 fatty acids.
ESSENTIAL POLYUNSATURATED FATTY ACIDS
Essential polyunsaturated fatty acids (PUFAs) are derived from the diet and are required for membrane organization and function as well as for a myriad of other important roles. The classical lipid bilayer (with embedded proteins) that surrounds and defines cells and organelles in living organisms is abundant in phospholipid (glycerophospholipids) classes composed of a characteristic polar head group at the sn-3 C of glycerol (choline-, ethanolamine-, inositol-, or serine-) and of fatty acyl side chains esterified to the sn-1 and sn-2 positions of glycerol. Thus, the major categories or classes are glycerophospho-cholines, -ethanolamines, -serines, and -inositols. The PUFAs belong to two essential FA families: omega-3 (α-linolenic acid, n-3) and omega-6 (linoleic acid, n-6). Both families are essential because mammalians lack the enzymes that introduce double bonds to critical C-C positions for generating these fatty acids, which are required for cell functions. Here we focus on DHA, the most abundant long-chain polyunsaturated fatty acid of the omega-3 FA family; it is found concentrated in the CNS (21) esterified to phospholipids.
DOCOSANOIDS
Below we describe DHA signalolipidomics, emphasizing the cellular and molecular events leading to the incorporation of DHA into membrane phospholipids, including key loops and pathways involved in the uptake and subsequent fate of DHA and docosanoid derivatives. Docosanoids are derived from 22-C DHA, whereas eicosanoids originate from 20-C arachidonic acid (AA). Strictly speaking, docosanoids include NPD1, maresins (186), neuroprostanes, and related 22-C derivatives. Here we focus on NPD1. Early studies identified the formation of mono-, di-, and trihydroxy derivatives of DHA in the retina. These studies showed that lipoxygenase inhibitors block the synthesis of these derivatives, indicating an enzymatic process of a lipoxygenase nature (28). Although the stereochemistry and bioactivity of DHA-oxygenated derivatives were not defined at the time of these observations, it was suggested that these DHA derivatives might be neuroprotective, and the term “docosanoids” was introduced (26, 29). Liquid chromatography–photodiode array–electrospray ionization tandem mass spectrometry (LC-PDA-ESI-MS/MS)-based lipidomic analysis was used subsequently to identify oxygenation pathways for synthesis of the docosanoid, NPD1, during brain ischemia-reperfusion (132), in the retinal pigment epithelium (145), in the human brain (129), and in other cells (182, 184).
NEUROPROTECTIN D1
NPD1 is derived from the selective oxygenation of DHA by 15-lipoxygenase-1 (15-LOX-1) (44). Photoreceptors, retinal pigment epithelial (RPE) cells, and the brain display an undetectable quantity of unesterified (free) DHA (as is the case with unesterified AA) under basal, nonstimulated conditions (12, 13, 24, 99, 202), even though they all contain phospholipids richly endowed with DHA. This suggests that the pool size of unesterified DHA is tightly regulated by phospholipase A2 (PLA2), reacylation, and, under some conditions, also by peroxidation. DHA, incorporated into membrane phospholipids, first becomes the substrate of docosahexaenoyl-coenzyme A synthetase for channeling through acyltransferases, which incorporate this FA into phospholipids (168-171). RPE cells thus modulate the uptake, conservation, and delivery of DHA to photoreceptors (29). In addition, RPE cells likely utilize a specific DHA-phospholipid pool as a precursor for NPD1 synthesis.
NPD1 induces homeostatic/prosurvival signaling in response to cellular and systemic insults (Figure 1) (26, 129, 132). Specifically, NPD1 up-regulates antiapoptotic proteins (Bcl-2 and Bcl-xL) and down-regulates proapoptotic proteins (Bax and Bad) in response to cellular oxidative stress and cytokine activation leading to an overall prosurvival transcriptome (26, 129, 132, 145). The stereoselective mediator, NPD1, provides a specific mechanism to understand DHA-mediated modulation of neuroinflammation and neuroprotection. Moreover, NPD1 elicits neuroprotection in brain ischemia-reperfusion and in oxidative-stressed retinal cells (132, 144, 145) inhibits retinal ganglion cell death (162), is protective in kidney ischemia-reperfusion (91), and regulates adiponectin (79). DNA microarray profiling shows down-regulation of proinflammatory genes as well as of proapoptotic genes of the Bcl-2 gene family (129). Thus NPD1 is a mediator that executes protective bioactivity of DHA in the CNS. Deficiency of NPD1 and of the enzyme involved in its formation, 15-LOX-1, has been observed in Alzheimer’s disease (AD) brain. Also, NPD1 further influences beta-amyloid precursor protein (βAPP) processing and decreases amyloid-beta (Aβ) 42 release (129). DHA, the precursor of NPD1, elicits an Aβ42-lowering effect both in vitro and in vivo (125, 151, 175). In addition, free radical–mediated DHA peroxidation products accumulate during ischemia and neurodegeneration. These oxidation products in turn may form protein adducts and other cytotoxic molecules that promote further free radical injury (68, 147, 172).
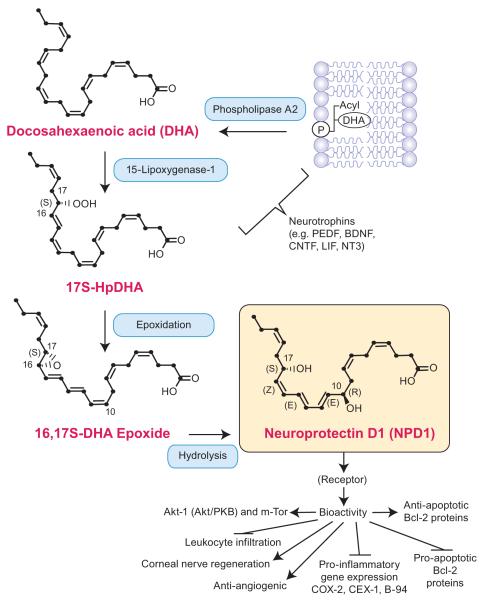
Figure 1.
Biosynthesis of neuroprotectin D1 (NPD1). A membrane phospholipid containing a docosahexaenoyl chain at sn-2 is hydrolyzed by phospholipase A2, generating free (unesterified) docosahexaenoic acid (DHA) (22:6). Lipoxygenation is then followed by epoxidation and hydrolysis, to generate NPD1. Thus far, a binding site for NPD1 has been identified in retinal pigment epithelium cells and polymorphonuclear cells (133). BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; CEX-1, cytokine exodus protein-1; COX-2, cyclooxygenase-2; LIF, leukemia inhibitory factor; NT3, neurotrophin 3; PEDF, pigment epithelium-derived factor.
MOLECULAR SPECIES OF PHOSPHOLIPIDS IN MEMBRANES
Phospholipid classes display a wide diversity of acyl chains in the sn-1 and sn-2 positions, encompassing a variety of molecular species. In most phospholipids, DHA is esterified to the sn-2 position, as is the case for AA. Overall, DHA accounts for approximately 50% of the PUFAs of cell membranes in the CNS; the highest concentrations are in photoreceptor outer segments and synapses. The significance of DHA in CNS function and homeostasis is underscored by the discovery of docosanoids and by the relevance of DHA’s enrichment, physiological role, pathophysiological involvement, and avid retention in the CNS. Docosahexaenoyl-phospholipids in cellular membranes are of interest in neuroscience and medicine due to the biophysical implications of these molecular species of phospholipids for membrane protein organization as well as their role in rhodopsin function, neurotransmission in general and, more specifically, neurotransmitter vesicle release and endocytosis (42). Although lipid-raft microdomains are poor in DHA-containing phospholipids, their formation and function are affected by DHA. G-protein-coupled signal transduction has been implicated in DHA-rich membrane environments, specifically photoreceptor outer segments containing rhodopsin (see below) (9).
DHA-phospholipids support transmembrane receptor function in the CNS, thus resulting in impaired receptor function when DHA bioavailability is decreased. Phosphatidylserine (PS), a DHA-rich phospholipid, modulates AKT signaling by recruiting cytosolic proteins, Raf-1, and protein kinase C to discrete membrane locations, in turn promoting cell survival (2, 90, 112-116, 142). Neuro 2A cells cultured in a DHA- or docosapentaenoic acid (DPA)-enriched medium showed differential susceptibilities to apoptosis as an inverse function of PS membrane concentration. The DPA-treated cells accumulated less than 80% of the PS measured in DHA-enriched cells and displayed increased apoptosis in response to serum starvation (2, 115). In the CNS, preferential loss of PS in response to DHA deprivation illustrates a tight regulation between DHA and PS accumulation in neuronal membranes as well as the specific requirement of neural cells for DHA (112, 114). Rats deprived of DHA and ALA precursors show decreased neuronal PS levels despite normal amounts in the kidney and adrenal tissue, where PS is rich in AA (90). DHA deprivation results in a concomitant increase in DPA, suggesting a physiological compensatory need for very-long-chain PUFAs; however, increased DPA is unable to reconstitute PS levels in the CNS (90, 113, 115, 142). Moreover, the CNS contains very low levels of EPA. Therefore, EPA may compensate DHA roles outside the CNS.
The membrane lipid milieu of photoreceptor membranes in which rhodopsin and other proteins perform their functions is determined mainly by phospholipids rich in DHA and in omega-3 FA derivatives longer than C22 (Figure 1) (25, 65, 179). Photoreceptor membranes contain phospholipids with two omega-3 FAs esterified at both the sn-1 and sn-2 positions of the same glycerol backbone, determining the supraenoic or supraene molecular species (14-18). These early findings, along with the identification of di-docosahexaenoyl di-acyl-glycerides in the retina (10, 11), suggest that supraenoic molecular species of phospholipids are necessary to photoreceptor cell organization and function. DHA is also found enriched in phosphatidic acid in the retina (30), and because of its active incorporation during phosphatidic acid biosynthesis (21), this prompted the suggestion that DHA and highly unsaturated fatty acids may be mainly introduced in phospholipids by this route rather than by acylation-reacylation (22). Supraenoic molecular species of phospholipids represent 31% of phosphatidylcholine, 52% of phosphatidylserine, 20% of phosphatidylethanolamine, and 9% of phosphatidylinositols in photoreceptor discs (17). The supraenoic phosphatidylcholines that contain DHA (at position sn-2) and the 24:6–36:6 elongation products of the omega-3 FA family series (at position sn-1) are tightly bound to rhodopsin (9) and are therefore found in outer segments of photoreceptors (9). These very-long-chain FAs at sn-1 may “curl” and restrict rhodopsin motion, likely conforming to a disk membrane domain not favoring the classical bilayer membrane organization (9). In fact, phospholipids containing DHA provide a favorable environment within which G-protein-coupled events can occur (9).
THE OMEGA-6 AND OMEGA-3 POLYUNSATURATED FATTY ACIDS IN MODERN WESTERN DIETS
DHA metabolism is affected by decreased availability of omega-3 FAs in the diet. We provide here a brief glimpse at the importance of this subject matter in nutrition. The omega-6:omega-3 PUFA ratio in modern Western diets shows a dietary deficiency of DHA. The levels of omega-6 FAs have steadily increased over time, mainly due to the switch from grass-fed livestock (rich in omega-3) to corn-based feed (rich in omega-6) as food production becomes more industrialized in developed nations (189-194, 196). Changes in the formulation of livestock feed have increased AA levels relative to DHA. Moreover, most Western diets are rich in carbohydrates, proteins, and saturated fats, and contain less fish and greens. These dietary modifications have increased the AA:DHA ratio to about 15:1 (51, 150, 189-194, 196). The consequences of this profound dietary change in omega-3 fatty acid availability and as a result in DHA signalolipidomics in the CNS, the cardiovascular system, the immune system, and in other organs are just beginning to be understood.
DIETARY UPTAKE
Plants and certain marine algae possess enzymatic machinery to synthesize omega-3 FA from 2-C precursors. The inability of vertebrates to introduce double bonds distal to the ninth C means that PUFA must originate from the diet (98). The omega-3 precursor α-linolenic acid (ALA) is found in seed oils, such as chia and flax seeds, and in green-leafed plants and algae. The omega-3 FAs, DHA and eicosapentaenoic acid (EPA), predominate in cold-water fatty fish species that feed on marine algae rich in ALA, which these fish convert to DHA. Organisms that lack the ability to convert 18-C precursors to DHA must take up this FA through their diets. PUFAs are released after ingestion via hydrolysis of animal fat or plant oil triglycerides by lingual and pancreatic lipases (48). Free fatty acid (FFA) absorption occurs primarily in the distal small intestine mediated by the specific FA transport protein FATP4, a member of the FA transporter family of proteins. FATP4 is the primary isoform involved in FA absorption across the apical side of the enterocyte (93, 200, 201).
In enterocytes, FFAs (mainly non-PUFAs) are bound either to cytosolic FA-binding proteins for mitochondria beta-oxidation or to the endoplasmic reticulum for reesterification into triglycerides for chylomicron assembly. Chylomicrons, the primary form of lipid transport from the intestines to the liver, are synthesized in the smooth endoplasmic reticulum of enterocytes and secreted into lacteal ducts, which drain into the lymphatics (Figure 2). When lymph reenters the blood-stream at the thoracic duct, chylomicrons circulate and interact with endothelial lipoprotein lipases that facilitate the cleavage of FAs from chylomicrons. As soon as FAs are cleaved from the chylomicron, they bind to albumin for further transport to the liver and other tissues.
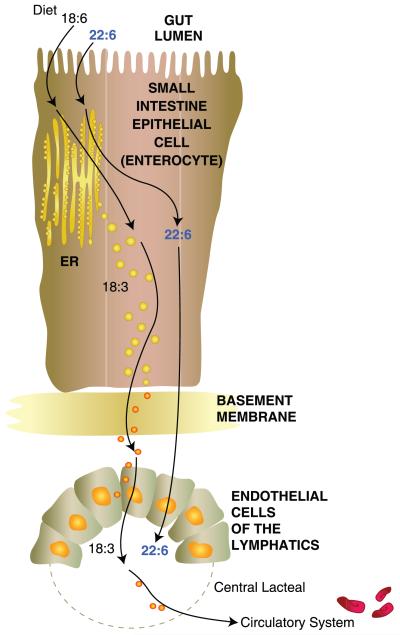
Figure 2.
Flow of the ω-3 fatty acid (FA), docosahexaenoic acid (DHA) (22:6), or its precursor, α-linolenic acid (ALA) (18:3), from the gastrointestinal lumen to the lymphatic system. DHA is required for the synthesis of phospholipids for nervous system membrane biogenesis. If unavailable, DHA can be synthesized from ALA. Enterocytes of the small intestine take up these FAs and package them for delivery to the endothelial cells of the lymphatic system. From there, they are transferred to the central lacteal and delivered to the circulatory system for transport. Detailed molecular events are not well understood as of yet.
THE HEPATOCYTE ENDOPLASMIC RETICULUM, IN CONJUNCTION WITH THE PEROXISOME, ELONGATES AND DESATURATES α-LINOLENIC ACID (18:3) TO DOCOSAHEXAENOIC ACID
Chylomicron remnants are bound by lipoprotein receptors expressed on hepatocytes, where they become endocytosed and are further cleaved of remaining FAs. In the liver, omega-3 FA precursors (mainly ALA), which are taken up from circulation by the hepatocyte, are conveyed to the endoplasmic reticulum. Here a series of desaturation and elongation reactions, carried out by the delta-6 and -5 desaturase (delta-4 desaturase exists in algae) and elongase enzymes, respectively, convert ALA to EPA and tetracosahexaenoic acid (THA). THA is then directed to peroxisomes, where it is converted to DHA by the removal of two carbons through β-degradation before it is transported back to the endoplasmic reticulum. DHA is activated to form DHA- coenzyme A and esterified to sn-2 of phospholipids, mainly phosphatidylcholine (PC). Then DHA-phospholipids are packaged in the form of very-low-density lipoproteins (VLDLs) or other lipoproteins. In this manner, DHA derived from the diet or from hepatic elongation and desaturation is subsequently released into the bloodstream for delivery to the choriocapillaris behind the retina and the neurovascular units within the brain, as well as to other tissues (Figure 3).
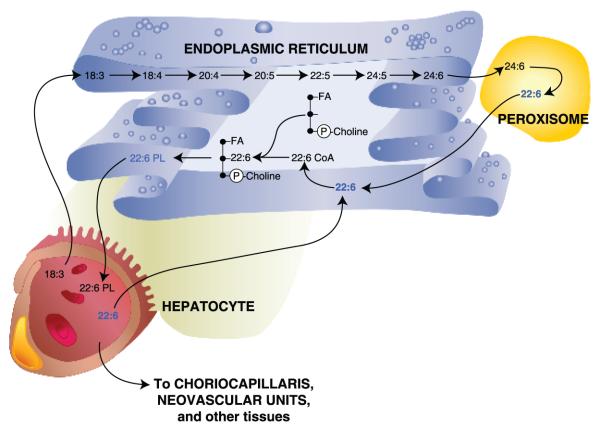
Figure 3.
Desaturation and elongation of α-linolenic acid (ALA) (18:3) within the hepatocyte. Systemic ALA is taken up by hepatocytes and transferred to the endoplasmic reticulum, where a series of desaturation and elongation steps occurs, leading to the formation of a 24-carbon, 6-double-bond FA [24:6, tetracosahexaenoic acid (THA)]. 24:6 is then conveyed to peroxisomes, converted to 22:6 by β-degradation, and delivered back to the endoplasmic reticulum. 22:6 (DHA) is then attached to the n-2 position of phosphatidyl choline to form a 22:6 phospholipid (22:6-PL), followed by release to the circulatory system for delivery to the choriocapillaris behind the retina and neovascular unit within the brain, as well as to other tissues.
THE LIVER SUPPLIES DOCOSAHEXAENOIC ACID TO THE CENTRAL NERVOUS SYSTEM
When dietary ALA reaches the liver, it is elongated and desaturated into DHA. Likewise, dietary DHA is taken up by the liver and then distributed to other tissues. The concept that the liver is a key “processor” and “distributor” of omega-3 FAs to the CNS (29, 183) was initially demonstrated using labeled ALA injected either intraperitoneally or into the stomachs of mouse pups. Strikingly, labeled FAs first appeared in the liver and subsequently decreased in a time-dependent manner, whereas labeled DHA evolved and appeared esterified to phospholipids and triglycerides. At 24 hours postinjection, liver DHA decreased by 50%, whereas brain and retinal DHA increased remarkably. At 72 hours postinjection, labeled DHA in the brain and retina accounted for 70% of the total labeled FA. Furthermore, rats intravenously infused with labeled ALA for two hours (69, 164, 165) confirmed the ability of the liver to supply DHA to the brain and showed the appearance of esterified DHA in a sigmoidal time-dependent manner. This was concurrent with the decrease of labeled ALA in plasma collected throughout the two-hour infusion. These studies allowed for mathematical modeling of liver synthesis and secretion rates of DHA from plasma ALA. By using positron emission tomography scans in unison with radio-tracer studies, relative incorporation rates have been estimated for humans and experimental animals. In healthy 15-week-old rats, liver conversion of ALA to DHA surpassed the brain consumption rate 30-fold (74, 164, 166). Similar studies in humans confirmed the liver’s ability to provide physiologically adequate amounts of DHA when ALA was present in the diet (166). The hepatic conversion of ALA to DHA in healthy individuals is sufficient to meet the demands of DHA in the brain and retina (165, 183).
DOCOSAHEXAENOIC ACID AVID RETENTION IN THE CENTRAL NERVOUS SYSTEM
The DHA Long Loop: DHA Is Supplied by the Liver for Synaptogenesis and Biogenesis of Photoreceptor and Other Membranes of the Nervous System
The FA transporter family of proteins has several isoforms with specific tissue expression profiles, ensuring appropriate and selective uptake of FA from VLDL in a variety of organs with specific FA metabolic requirements (89, 130, 194). Such transporters are present in the liver, kidney, adipose tissue, muscle, and the CNS (93, 135, 200). In light of the specific DHA enrichment in the CNS membranes, the existence of DHA-specific FA receptors in the CNS has been postulated and is a major topic of current interest.
Cellular and molecular events that selectively take up and retain DHA in the CNS are not completely understood. It seems that FAs esterified to VLDL or bound to albumin are cleaved by endothelial lipoprotein lipases of capillaries in the neurovascular unit. FAs are taken up by FATP1 expressed on the luminal side of the endothelial cells that line the neurovascular unit (108, 200); these FAs are then transferred to astrocytes that provide support to endothelial and other CNS cells. Astrocytes comprise a direct connection between the neurovascular unit, the synapse, and other parts of the neuronal network. Thus, DHA in astrocytes is incorporated into cellular membranes and transferred to neurons (Figure 4) (70, 117, 140, 141).
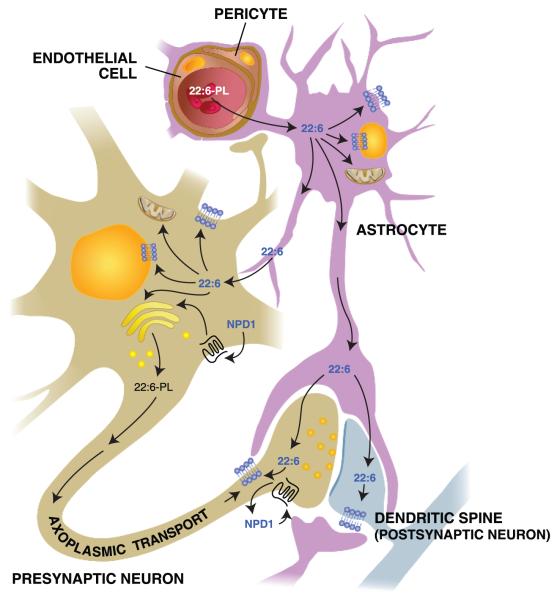
Figure 4.
Movement of docosahexaenoic acid (DHA) (22:6) through the neurovascular unit and its disposition within neurons and astrocytes. 22:6-phospholipids (22:6-PLs) are taken up by endothelial cells from the circulation and transferred to astrocytes within the central nervous system; from there, 22:6 is incorporated into astrocytes or transferred to neurons. 22:6 is also available for conversion to neuroprotectin D1 (NPD1) upon inductive signals. After packaging in the endoplasmic reticulum and the Golgi apparatus, 22:6 is also transported to the synaptic terminal to become incorporated into synaptic elements. Arrows represent possible routes. Molecular characterization of transporter(s) and receptors remains to be done.
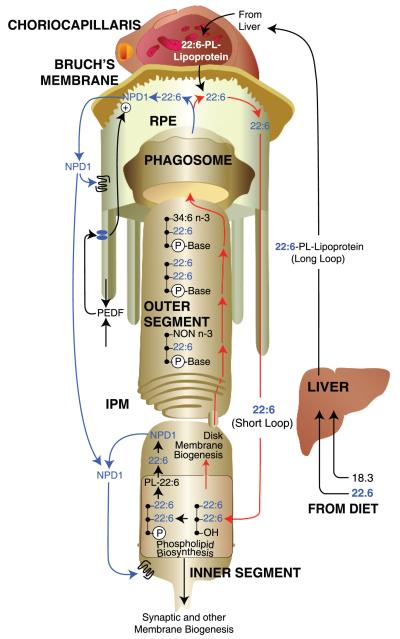
The mechanisms of DHA uptake and accretion in photoreceptors do not involve astrocytes (20, 31, 33, 34, 81, 82, 183). Instead, the liver provides DHA via the hepatic-photoreceptor long loop. DHA esterified to VLDL or bound to albumin arrives to the retinal pigment epithelial (RPE) cells via the choroidal plexus and is cleaved by lipoprotein lipases. DHA is then transported across the basolateral surface of the RPE cell by an unidentified DHA-specific transporter. RPE cells release DHA (most likely coupled to a binding protein) into the interphotoreceptor matrix (IPM), where the photoreceptor inner segment takes it up (Figure 5) (31, 32).
Figure 5.
Docosahexaenoic acid (DHA) (22:6) delivery to photoreceptors, photoreceptor membrane renewal, and synthesis of neuroprotectin D1 (NPD1). DHA (22:6) or immediate precursors are obtained by diet. Once within the liver, hepatocytes incorporate 22:6 into 22:6-phospholipid (22:6-PL)-lipoproteins, which are then transported to the choriocapillaris. 22:6 crosses Bruch’s membrane from the subretinal circulation and is taken up by the retinal pigment epithelium (RPE) cells lining the back of the retina to subsequently be sent to the underlying photoreceptors. This targeted delivery route from the liver to the retina is referred to as the 22:6 long loop. Then 22:6 passes through the interphotoreceptor matrix (IPM) and into the photoreceptor inner segment, where it is incorporated into phospholipids for cell membrane, organelles, and disk membrane biogenesis. As new 22:6-rich disks are synthesized at the base of the photoreceptor outer segment, older disks are pushed apically toward the RPE cells. Photoreceptor tips are phagocytized by the RPE cells each day, removing the oldest disks. The resulting phagosomes are degraded within the RPE cells, and 22:6 is recycled back to photoreceptor inner segments for new disk membrane biogenesis. This local recycling is referred to as the 22:6 short loop. Upon inductive signaling, such as pigment-epithelium derived factor (PEDF), 22:6 can be obtained from a phospholipid pool for the synthesis of neuroprotectin D1 (NPD1).
THE PHOTORECEPTOR CELL INNER SEGMENT UTILIZES DOCOSAHEXAENOIC ACID FOR PHOSPHOLIPID BIOSYNTHESIS TARGETED TO PHOTORECEPTOR DISC MEMBRANE BIOGENESIS AND THE SYNAPSE
Subsequent to the events outlined above, the photoreceptor cell inner segment uses DHA to synthesize phospholipids for photoreceptor disc membrane biogenesis or for other photoreceptor membranes (81, 82). This represents a critically important DHA supply site because the phototransduction apparatus is immersed into membranes richly endowed with docosahexaenoyl phospholipids. RPE cells actively interact with the photoreceptor cells through the phagocytosis of photoreceptor outer segment tips and by supporting the nourishment of these cells (31, 33, 81, 82). RPE cells are the most active phagocytes of the body and exhibit a phagocytosis distinct from the removal of debris associated with apoptosis. These cells engulf a mass equivalent of 5–10 erythrocytes in the photoreceptor outer-segment membrane daily, recycling vitamin A and DHA back into the photoreceptor as well as regenerating 11-cis-retinal from all-trans-retinal (Figure 5) (26, 31, 33, 82). This represents the DHA RPE-photoreceptor short loop (discussed below) (31, 33, 81, 82). This economic solution to the costly continuous nature of the visual cycle allows for the maintenance of a system with a very high metabolic demand and high exposure to oxidative stress (26, 31, 33).
THE DOCOSAHEXAENOIC ACID SHORT LOOP: INTERCELLULAR RECYCLING AND RETENTION
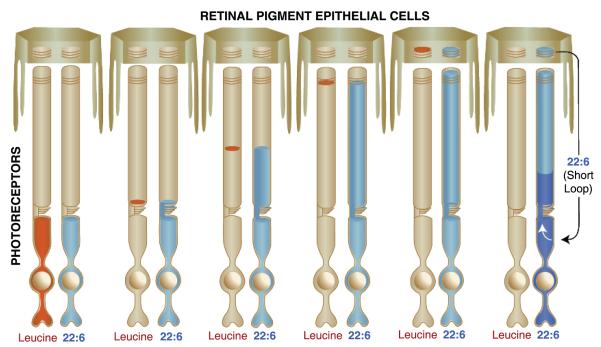
Amino acids (e.g., leucine) are taken up by the inner segments of photoreceptors for protein biosynthesis. Rhodopsin (by far the most abundant protein of the outer segment) and several other quantitatively smaller proteins are critical for phototransduction and synaptic transmission. Proteins are sorted and distributed to the synaptic apparatus and to outer segments, where they are incorporated into newly forming basal disk membranes. As additional disks are synthesized, older disks are pushed distally toward the RPE (Figure 6). Opsin synthesis and trafficking in photoreceptors can be followed by systemically injecting tritium-labeled amino acids. A short time after injection the membranes of newly formed disks form an autoradiographic band at the base of the outer segment. Then, over a period of about 10 days, this band migrates distally until it reaches the RPE. These overcapping RPE cells phagocytize the photoreceptor tips each day, taking in a small stack of photoreceptor disks (which match the number of newly forming basal disks) to maintain a constant outer segment length. Consequently, after about 10 days these phagosomes within the RPE cells exhibit an autoradiographic band of labeled amino acids; this band appears narrow because the pool of amino acids is quickly turned over so that the original label disappears (see red label, Figure 6).
Figure 6.
The incorporation of amino acids (e.g., leucine) and docosahexaenoic acid (DHA) during photoreceptor outer segment disk membrane biogenesis. Amino acids are utilized by photoreceptors in the synthesis of new outer segment disk membrane opsin and can be followed, when labeled with a tracking tag such as tritium, by autoradiography as they move into and through photoreceptor outer segments. The amino acid leucine (red), a component of the opsin protein, is avidly incorporated into disk membrane. However, because this amino acid is in great demand for many proteins, its photoreceptor pool is rapidly turned over. Initially, labeled leucine appears at the bottom of the outer segment, where the opsin protein is inserted into new membranes. A narrow band of label occurs as more membranes are synthesized, but labeling ceases as all labeled leucine is used up. The labeled band is slowly pushed apically as more membranes are synthesized, finally arriving at the photoreceptor tip in about 10 days in a warm-blooded animal. As the photoreceptor tip is phagocytized by the RPE cells, the labeled band appears within the RPE cell cytoplasm until degradation is complete. Leucine forms a tight band with no dispersion because the opsin molecule is locked into the structure of the disk membrane. Lipids do not follow this pattern. Labeled fatty acids (FAs) immediately disperse through the membranes of the outer segment, indicating that they exhibit free lateral and vertical movement (40). DHA (blue) is an exception. Once it enters the outer segment, it becomes integrated into the structure of the disk membrane and remains there until the disks are shed into the RPE cells. In addition, the pool of this FA is continually replenished (indicated by the white arrow) by recycling the 22:6 back to the inner segment from the retinal pigment epithelium, resulting in continual labeling of the new disk membranes. Importantly, the labeled front of the moving leucine band exactly matches the front of the 22:6 label. This occurs because the 22:6 becomes associated with the opsin molecule within the disk membrane and is thus, prevented from lateral and vertical free movement within the outer segment.
Non-omega 3 fatty acids behave differently when incorporated into photoreceptor outer segment disk phospholipids rapidly diffusing throughout the outer segment (40). DHA, however, is exceptional in its incorporation pattern. Phospholipids rich in DHA are taken up from the subretinal circulation within the choriocapillaris by the RPE (Figure 5). DHA is conveyed from the RPE cells to the IPM and ultimately enters the photoreceptor inner segment, where phospholipid biosynthesis occurs. If tritium-labeled DHA is made available to photoreceptors, it can also be followed autoradiographically. DHA is taken up by the photoreceptor inner segment, used for phospholipid biosynthesis, sorted, and then channeled to the synaptic terminal, where it becomes associated with synaptic membranes. The bulk of phospholipids are directed to the outer segments, where they become part of new disk membranes in close association with the visual pigment, rhodopsin. Because DHA is targeted to the retina, the pool of labeled DHA molecules is large and available continually for phospholipid synthesis for new disk membranes. Thus, over time autoradiographic images show DHA continuing to fill the outer segments from bottom to top. When labeled DHA is compared with labeled amino acids, the front of the DHA label corresponds to the position of the amino acid band (Figure 6). When the outer segment tip is phagocytized after about 10 days, the DHA-phospholipids in the phagosome are hydrolyzed, and DHA is recycled back to the photoreceptor inner segment for reuse.
Interestingly, autoradiographic analysis of DHA recycling demonstrates that the reuptake of this FA actually makes the label appear denser because the photoreceptor is continuously collecting DHA from the labeled pool within the retina/RPE complex. This is depicted in darker blue in Figure 6. This pattern of labeling indicates that, unlike quickly cycled amino acids, DHA is tenaciously retained and recycled back to photoreceptors for many cycles through the outer segments. This also underscores the importance of DHA in photoreceptor membrane synthesis.
An additional function of the RPE cell is to generate growth factors (204) to provide paracrine and autocrine signaling and hence promote communication between retina cells as well as photoreceptor survival (160). These growth factors, or neurotrophins, induce NPD1 synthesis and its apical polarized release in RPE cells (143-145). These observations were made in human RPE cells grown to confluence using a specialized culture (100) that allows the cells to develop a high degree of differentiation, preserving the apical-basolateral polarization. Among the neurotrophins tested, pigment epithelium-derived factor (PEDF) is by far the most potent stimulator of NPD1 synthesis. PEDF, a member of the serine protease inhibitor (serpin) family, was originally identified in human RPE cells. If PEDF or ciliary neurotrophic factor are added to the incubation media, bathing the basolateral side in increasing concentrations, they evoke a lesser degree of NPD1 release on the apical side. Conversely, if these neurotrophins are added to the apical side, they exert concentration-dependent increases in NPD1 release only on the apical side (143).
DOCOSAHEXAENOIC ACID AND GENE TRANSCRIPTION
PUFAs are transcriptional regulators acting through multiple interactions with nuclear receptors (56, 107, 209) [peroxisome proliferator active receptor (PPAR) family, retinoid X receptor α, liver X receptor α, and hepatocyte nuclear factor-4α (HNF-4α)] and transcription factors (105, 106, 153) [sterol regulatory element-binding protein 1 (SREBP-1), carbohydrate response element-binding protein] to modulate hepatic lipid metabolism. Specifically, PUFAs also activate PPAR alpha signaling, with EPA being its most potent PUFA agonist. In general, PUFAs exhibit antilipogenic effects via increased beta oxidation and decreased lipid synthesis secondary to down-regulation of elongase and desaturase enzymes. DHA has similar transcriptional effects to insulin in hepatocytes. However, unlike insulin, DHA decreases de novo lipogenesis through increased proteosomal degradation of SREBP-1 (105, 106, 153). The ligand-activated transcription factor PPARγ regulates lipoprotein metabolism, adipogenesis, and insulin sensitivity, and its activation underlies some of DHA’s anti-inflammatory actions (80, 102, 134, 211). Moreover, PPARγ has a fatty acid binding pocket for polyunsaturated fatty acids (134) and their derivatives, including DHA (36). Further studies on this nuclear receptor in relationship to DHA and NPD1 in AD models are discussed below. Also, PUFA peroxidation products are implicated in second messenger signaling at the level of transcription. Thus, DHA can act as a regulator of gene expression and as a precursor of docosanoids.
DECREASED PLASMA DOCOSAHEXAENOIC ACID IS ASSOCIATED WITH COGNITIVE DECLINE
DHA deficiency is associated with aging, AD, hyperactivity, schizophrenia, and peroxisomal disorders. Experimental models have shown that learning during aging is enhanced by dietary omega-3 FAs concomitant with higher DHA content in hippocampal and amygdala phospholipids. Thus the current concept is that dietary omega-3 PUFAs are associated with a delay in cognitive decline (159). It is remarkable that the CNS displays a high degree of fatty acid molecular structural recognition since omega-6 fails to substitute for DHA; this correlates with omega-3 deficiency that results in impaired performance in spatial learning (126, 127). The molecular characterization of the transporters/receptors engaged in these events remains to be ascertained. Higher dietary DHA intake is inversely correlated with risk of AD (67). DHA dietary supplementation is associated with improved immediate and delayed Verbal Recognition Memory scores but not working memory or executive function tests. For the CANTAB Paired Associate Learning (PAL), a visuospatial learning and episodic memory test, members of the DHA group scored higher on the test than controls. DHA was well tolerated, with no reported treatment-related adverse events. A current study during a 24-week supplementation with 900 mg/d DHA shows that improved learning and memory function occurred in patients with age-related cognitive decline, supporting the notion that DHA supplementation has benefits for supporting cognitive health during aging (213). The studies listed here and others described in this review provide a general overview on this subject. Overall, we aim to convey the clear need for further mechanistic studies to define at the molecular level precisely how DHA is avidly retained in the CNS and participates in successful aging, as well as the extent of its action/s.
ALZHEIMER’S DISEASE
Alzheimer’s disease is a multifactorial, genetically complex, progressive, late-onset neurodegeneration of the elderly that involves accumulation of 4 kDa peptide amyloid-beta (which is initially soluble but then becomes part of amlyoid plaques). In addition, somatodendritic hyperphosphorylated tau (neurofibrillary tangles) complement the hallmarks of the disease. Risk factors include apoE polymorphisms, age, hypercholesterolemia, and diet. A major clinical/behavioral feature is progressive cognitive impairment. At the cellular level, βAPP processing dysfunction leads to overabundance of the 42 amino acid Aβ42 peptide oligomer, which initially impairs synaptic function. These cellular impairments lead to apoptosis. Then the oligomer Aβ42 accumulates as an aggregate and becomes a major component of senile plaques (37, 75, 77, 85-87). Aβ42 peptides are generated from βAPP via sequential cleavage by beta- and gamma- (β- and γ-) secretases, or a second pathway may be activated through alpha-secretase distintegrin and metalloproteinase 10 (ADAM10) that cleaves βAPP to yield soluble amyloid precursor protein alpha (sAPPα) via the nonamyloidogenic or neurotrophic pathway.
Human neural (HN) progenitor cells in primary culture display an eightfold-enhanced synthesis and release of Aβ40 and Aβ42 peptides that resembles Aβ deposition during brain aging and in AD. In these cells, Aβ42 triggers damaging signals (accompanied by early onset of apoptosis) and changes in gene expression that emulate neurodegenerative events characteristic of AD. DHA partially counteracts cognitive decline in the elderly (66). Moreover, omega-3 essential FA-rich diets are associated with a trend in reduced risk for mild cognitive impairment (MCI) and with MCI conversion to AD, whereas DHA has been shown to be beneficial in transgenic AD models (2, 66, 83, 126, 176). The DHA-derived NPD1 displays neuroprotective bioactivity in brain and retinal cells against various insults, including oxidative injury, ischemia-reperfusion, and inflammation (7, 124, 127, 140, 176). Both AD brain (129) and the 3xTg-AD mouse exhibit reductions in DHA and NPD1. Moreover, the CNS response to injury and to the onset (and progression) of neurodegeneration involves the release of free DHA and AA along with the synthesis of stereospecific docosanoid derivatives. Therefore, the anti-inflammatory and antiapoptotic activity of NPD1 in cocultures of HN cells stressed with the Aβ42 oligomer was studied, including the NPD1-mediated modulation of α- and β-secretase activity that resulted in reduced shedding of Aβ42 (214).
Neuroinflammatory neurodegeneration associated with Aβ42 is an important contributory event to AD neuropathology (3, 208). Thus, using primary HN cells (129, 214) is a useful experimental approach for AD-related mechanisms. In HN cell models of Aβ42 toxicity, microarray analysis and Western blot analysis revealed down-regulation of proinflammatory genes (cyclooxygenase-2, tumor necrosis factor alpha, and B94), suggesting NPD1’s anti-inflammatory bioactivity targets this gene family, at least in part (129). These effects are persistent, as shown by time-course Western blot analysis in which protein expression was examined up to 12 hours after treatment by Aβ42 and NPD1 (214).
Although counteracting Aβ42-induced neurotoxicity is a promising strategy for AD treatment, curbing excessive Aβ42 release during neurodegeneration is also desirable. DHA could lower the Aβ42 load in the CNS by stimulating nonamyloidogenic βAPP processing, reducing PS1 expression, or by increasing the expression of the sortilin receptor, SorLA/LR11 (125, 131, 175, 215).
In contrast to a previous report (83), which suggested that steady state levels of PS1 result in Aβ peptide reductions in whole-brain homogenates of 3xTg AD after dietary supplementation with DHA, NPD1 had no effect on PS1 levels in primary human glial (HG) cells but rather a significant increase in ADAM10 occurred in conjunction with a decrease in betasite amyloid precursor protein-cleaving enzyme 1 (β-secretase-1) (BACE1). These later observations were further confirmed by both activity assays and siRNA knockdown. NPD1 reduced Aβ42 levels released from HN cells overexpressing APPsw in a dose-dependent manner. Moreover, other βAPP fragments revealed that after NPD1 addition, a reduction in the β-secretase products sAPPβsw and CTFβ occurred, along with an increase in α-secretase products sAPPα and C-terminal fragment (CTF)α, whereas levels of βAPP expression remained unchanged in response to NPD1. Hence these abundance- and activity-based assays indicate a shift by NPD1 in βAPP processing from the amyloidogenic to nonamyloidogenic pathway. sAPPα promotes NPD1 biosynthesis (129), and NPD1 stimulates sAPPα secretion (214), creating positive feedback and neurotrophic reinforcement. sAPPα enhances learning, memory, and displays neurotrophic properties (86). NPD1 further down-regulated BACE1 and activated ADAM10, a putative α-secretase. ADAM10 siRNA knock-down and BACE1 overexpression-activity experiments confirmed that both are required in NPD1’s regulation of βAPP. Therefore, NPD1 appears to function in both of these competing βAPP-processing events.
PPARγ activation leads to anti-inflammatory, antiamyloidogenic actions and antiapoptotic bioactivity, as does NPD1. Some FAs are ligands for PPARγ, which has a predilection for binding polyunsaturated fatty acids (45, 52, 92). NPD1 is a PPARγ activator, as shown by using both human adipogenesis and cell-based-transactivation assay (214). NPD1 may activate PPARγ via direct binding or other interactive mechanisms (20, 211). Analysis of βAPP-derived fragments revealed that PPARγ does play a role in the NPD1-mediated suppression of Aβ production. Overexpressing PPARγ or incubation with a PPARγ agonist leads to reductions in Aβ, sAPPβ, and CTFβ similar to that with NPD1 treatment, whereas a PPARγ antagonist abrogates these reductions. Activation of PPARγ signaling is further confirmed by the observation that PPARγ activity decreases BACE1 levels, and a PPARγ antagonist overturns this decrease. Thus, the antiamyloidogenic bioactivity of NPD1 is associated with activation of the PPARγ and the subsequent BACE1 down-regulation (Figure 7). The difference between the bioactivity of NPD1 concentrations for antiapoptotic and antiamyloidogenic activities ([50 nM] versus [500 nM]) may be due to the different cell models used (i.e., Aβ-peptide-stressed versus βAPPsw-overexpressing human neuronal glial (HNG cells) and/or related mechanisms. Although Aβ-lowering effects of PPARγ have been reported, the molecular mechanism of this action remains unclear. Induction of βAPP ubiquitination, which leads to enhanced βAPP degradation and reduced Aβ peptide secretion, has been suggested (52). Alternatively, Aβ clearance might be involved, or regulation by PPARγ may be due to enhancement of insulin sensitivity and increases in brain insulin degrading enzyme (45). The decreases in BACE1 may be the cause for Aβ reduction (180, 216). A reason for these conflicting reports may be that cell models and culture conditions used vary. HN cells that transiently overexpress βAPPsw and cell lines with stable βAPP expression are both utilized. Similar to the model of Sastre et al. (180), our cells underwent increases in Aβ overproduction. Excessive Aβ causes inflammatory responses in both neuronal and glial cells (216). Since inflammatory signaling plays a role in AD pathogenesis, HN cell cultures are a valuable model for Aβ42-mediated cellular actions. The fact that comparable results were obtained at a much lower concentration [0.5 μM] of rosiglitazone versus [10–30 μM] in previous reports (214) underscores the highly sensitive nature of HN cells after βAPP transfection. It is still possible that PPARγ may repress BACE1 by antagonizing activities of other transcription factors that promote BACE1 expression, such as signal transducer and activator of transcription-1 (STAT1), nuclear factor kappa-B (NF-κB) and activation protein-1 (AP1) (174). It is noteworthy that BACE1 expression in HN cells was increased after βAPP overexpression. The fact that PPARγ did not affect the levels of sAPPα and CTFα, aside from the PPARγ antagonist being unable to reverse NPD1-elicited increase in these fragments, clearly shows that PPARγ is not essential for NPD1’s regulation on the nonamyloidogenic pathway. Further analysis of ADAM10 showed no change occurring in ADAM10 following PPARγ activation, nor did PPARγ antagonists affect NPD1-enhanced expression of mature ADAM10. Therefore, modulation by NPD1 of α-secretase and βAPP processing is independent of PPARγ. ADAM10 is synthesized as an inactive zymogene and is processed to its mature form by cleavage of the prodomain by proprotein convertases (PPCs), such as furin and PC7 (4). Other evidence also demonstrates that protein kinase C and mitogen-activated protein (MAP) kinase, particularly extracellular signal-regulated kinases 1/2, are involved in regulation of α-secretase activity (20, 158, 212). No cross-talk between the PPCs and protein kinase C or MAP kinases has been reported. Since only the mature ADAM10 was increased, it is likely that the PPCs are implicated in NPD1 actions (214).
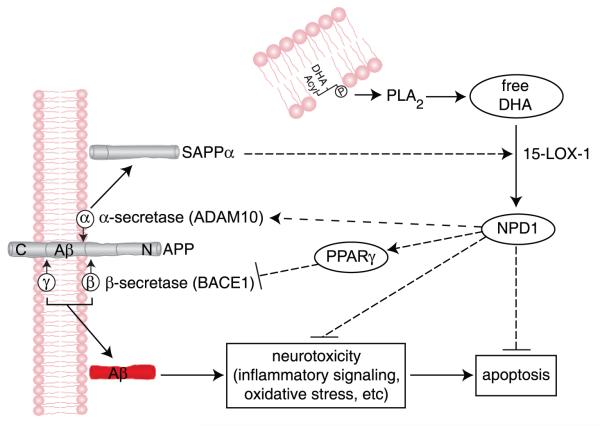
Figure 7.
Mechanism for neuroprotectin D1 (NPD1) induction of nonamyloidogenic and neurotrophic bioactivity. Docosahexaenoic acid (DHA) (22:6) is excised by phospholipase A2 (PLA2) to yield free DHA; in turn, free DHA is 15-lipoxygenated to generate NPD1, which then activates neuroprotective signaling. These events are mediated, in part, by shifting beta-amyloid precursor protein (βAPP) processing from an amyloidogenic into a neurotrophic, nonamyloidogenic pathway and by inhibiting apoptosis, blocking inflammatory signaling, and promoting cell survival. Beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) (β-secretase-1) activity is suppressed, and α-secretase distintegrin and metalloproteinase 10 (ADAM10) activity is stimulated, thus down-regulating amyloid-beta 42 (Aβ42) peptide release. NPD1 signaling to BACE1 and ADAM10 may be mediated via other neuromolecular factors. The ADAM10 cleavage product soluble amyloid precursor protein alpha (sAPPα) further induces the conversion of free DHA into NPD1, thus constituting a positive, neurotrophic feedback loop.
PPARγ antagonist GW9662 also failed to reverse the antiapoptotic effect of NPD1, indicating that PPARγ is not implicated in NPD1 antiapoptotic bioactivity. NPD1 attained this neuroprotection at a concentration of 50 nM, at which its PPARγ activity is far from physiologically relevant in the in vitro system. There is compelling evidence that NPD1 is endowed with strong anti-inflammatory, antiamyloidogenic, and antiapoptotic bioactivities in HN cells upon exposure to Aβ42 oligomers, or in HN cells overexpressing βAPPsw. These results suggest that the antiamyloidogenic effects of NPD1 are mediated in part through activation of the PPARγ receptor, whereas NPD1 stimulation of nonamyloidogenic pathways is PPARγ independent. NPD1 stimulation of ADAM10, coupled to suppression of BACE1-mediated Aβ42 secretion, clearly warrants further study since these dual secretase-mediated pathways may provide effective combinatorial or multitarget approaches in the clinical management of the AD process.
The hippocampal cornu ammonis 1 (CA1) region, the area of the cortex most heavily damaged by AD, displays one-twentieth of the NPD1 of age-matched controls, even though the difference in free DHA was only twofold lower; these changes were not present in other brain regions (129). Potent protective bioactivity of NPD1 was shown in various models of neuro-inflammatory pathology, including age-related macular degeneration (AMD) (25, 26, 145), stroke (24, 52, 127), epilepsy (26), AD (27, 55, 129), and oxidative stress (129, 132, 145). These observations implicate NPD1 as an integral homeostatic modulator of long-term function and highlight the needs of DHA accretion in the CNS.
NEUROINFLAMMATION
The proinflammatory cytokine interleukin (IL)-1ß stimulates Aβ40 and Aβ42 secretion as a function of HN cell aging. Conversely, DHA suppresses both Aβ40 and Aβ42 peptide release with concomitant NPD1 synthesis. Also, NPD1 inhibits Aβ42-induced apoptosis in HN cells. Pro- and antiapoptotic proteins are modulators proximal to mitochondrial and cellular damage. Proapoptotic Bik and Bax were enhanced by Aß42 but not affected by NPD1, whereas Bcl-2, Bcl-xL, and Bfl-1(A1) were increased in the presence of NPD1. Bfl-1(A1) increased almost sixfold (129). NPD1 also induces the antiapoptotic Bcl-2 family proteins Bcl-2 and Bcl-xL in oxidatively challenged human RPE cells and promotes cytoprotection (145). Because photoreceptor survival is dependent on RPE cell integrity, the protection of RPE cells has important implications in vision and AMD (26). A further suggestion for the significance of NPD1 in AD is the finding that hippocampal CA1 regions from AD patients show a dramatic reduction in NPD1. Thus the interplay of DHA-derived neuroprotective signaling aims to counteract proinflammatory, cell-damaging events triggered by multiple, converging cytokine and amyloid peptide factors in AD. Amyloid peptide mediates oxidative stress and induces microglial-derived cytokines, such as IL-1β and tumor necrosis factor alpha (TNF-α), supporting progressive inflammatory episodes in AD. These noxious stimuli further orchestrate pathogenic gene-expression programs in stressed brain cells, thereby linking a cascade of caspase-mediated cell death pathways with apoptosis and neuronal demise. Glial cells provide neuroprotective “shielding” as well, and both neuronal and glial cells release cytokines when exposed to Abeta42 that, in turn, activate more microglia and astrocytes that reinforce pathogenic signaling. NPD1 opposes and modulates consequences of these actions by its anti-inflammatory actions and by enhancing inflammatory resolution (132, 145, 182, 185). Therefore, neural mechanisms leading to NPD1 generation from DHA appear to redirect cellular fate toward successful brain cell aging. The Bcl-2 pro- and antiapoptotic gene families, neurotrophins, sAPPα, and NPD1 lie along a cell fate–regulatory pathway whose component members are highly interactive and have potential to function cooperatively in brain cell survival. Agonists of NPD1 biosynthesis, NPD1 analogs, or dietary regimens may be of importance for exploring new preventive/therapeutic strategies for stroke, AMD, AD, and other neurode-generative diseases. Other mechanisms have been proposed to explain the antiapoptotic and anti-inflammatory effects of DHA, including maintenance of plasma membrane integrity, activation of Akt signaling (62), and conversion into other derivatives (2, 147). These findings also provide clues for potential targets of NPD1. NPD1 inhibits NF-κB activation and cyclooxygenase-2 (COX-2) expression in brain ischemia-reperfusion (132), whereas Aβ peptide-induced apoptosis is associated with extracellular signal-regulated kinases and p38 MAPK-NF-κB-mediated COX-2 up-regulation (103). Neuroprotection mediated by NPD1 may further involve components of signaling pathways upstream of NF-κB activation and DNA binding (129).
The importance of DHA in maintaining cellular integrity and homeostasis has been underscored in many studies linking decreased levels of brain DHA to cognitive decline, specifically AD (Figure 7) (8, 129). When rats are fed a diet depriving them of both DHA and ALA precursors, specific physio-neurological changes take place. Rats show decreased performance in water-maze challenges, learning disabilities, decreased visual acuity, and increased tendencies toward aggression and depression (42, 55). Multiple studies have shown decreased levels of DHA in both the esterified phospholipid and free form in brains from Alzheimer’s patients compared with controls. Recently it has been shown that in AD, the levels of omega-3 precursors are similar in brain samples and increased in liver, whereas the amount of DHA (free and esterified) was decreased in both tissues. These changes are due to the fact that the liver from the Alzheimer’s patients displayed reduced expression of peroxisomal bidirectional protein (an enzyme involved in the final step of DHA synthesis from DPA, an ALA elongation product) and increased metabolic precursors of DHA (8). Various forms of retinitis pigmentosa and Usher’s syndrome type 1, two retinal pathologies, have been linked to decreased plasma DHA levels (26, 95). These data imply a link between impaired conversion of omega-3 precursors in the liver to decreased liver DHA bioavailability to the brain and retina.
PARKINSON’S DISEASE
Parkinson’s disease (PD) is also a complex age-related neurodegenerative disorder that results in movement, balance, and fine motor control alterations as a consequence of cell death of nigrostriatal dopamine-containing neurons of the substantia nigra pars compacta (SNpc). These neurons project their nerve terminals to the striatum. The initiating events of dopaminergic cell death involve oxygen reactive free radicals overproduction, mitochondrial dysfunction, and inflammation signaling enhancement. Reactive oxygen species up-regulates the redox-sensitive transcriptional DNA binding protein NF-κB that modulates expression of the proinflammatory genes inducible COX-2 and nitric oxide synthase. Nuclear translocation of NF-κBp65 in dopaminergic neurons of PD mice (72) has been reported to target microglia in the SNpc associated with COX-2 expression and prostaglandin E2 production (207). COX-2, an inducible early gene, is both physiological and part of the inflammatory response in the CNS (206). The MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) model affects mitochondria by inhibiting mitochondrial complex I or complex III (149), which induces neuronal cell death in the SNpc. DHA inhibits COX-2 expression and activity (121) and the activity of NF-κB p65 protein. DHA at lower concentrations induces NF-κB, whereas at higher concentrations, it exerts inhibitory actions on this transcription factor (121). DHA supplementation protects dopaminergic neurons in experimental PD models by targeting these inflammatory signaling pathways (46). In addition, DHA protects dopaminergic neurons by enhancing the expression of glial-derived neurotrophic factor (GDNF) and neurturin in MPTP-induced Parkinsonism (205). DHA in a nonhuman primate model (MPTP) reduces levodopa-induced dyskinesias, suggesting an innovative and safe approach to improve the quality of life of PD patients (178).
MACULAR DEGENERATION
Retinal degenerations are also a complex group of diseases with a neuroinflammatory/immune involvement that leads to photoreceptor apoptotic cell death (41, 47, 155, 161, 167). Retinitis pigmentosa is a group of inherited retinal degenerations caused by mutations in rhodopsin or other proteins. In these diseases, rod photoreceptor death initially occurs in the periphery, whereas in AMD, photoreceptor loss is initiated in the macula and then spreads throughout the retina (41, 167). AMD, the leading cause of blindness over the age of 65, is multifactorial (41, 167) and has two forms: the dry form (geographic atrophy, photoreceptors degenerate slowly and progressively) and the wet or exudative form (invasive choroidal neovascularization). The Age-Related Eye Disease Study (National Eye Institute, National Institutes of Health) indicates that the intake of antioxidants and zinc decreases the risk of advanced AMD, implying a role of oxidative stress in the pathogenesis. Moreover, oxidative damage markers are increased in retinas of patients with dry AMD (187). Thus, enhanced oxidative stress and mitochondrial dysfunction (54, 58, 63, 138, 187, 199) lead to apoptotic cell death both in photoreceptors and in the RPE (58). Initiation and progression of AMD underlies a failed resolution of the inflammatory response in conjunction with immune alterations. In this connection, single-nucleotide polymorphisms in the gene-encoding factor H (CFH/HF1) (59, 88, 89, 118) are a major risk factor for AMD. Factor H is an inhibitor of the alternative pathway of complement activation that in turn attenuates cell injury and inflammation. Factor B (BF) and complement component 2 (C2) (76, 198) display protection and reduce the risk of AMD to some extent. For example, the E318D variant of C2 (H10) as well as a variant in intron 10 of C2 and the R32Q variant of BF (H7) confer a reduced risk of AMD.
DHA is retained and protected from peroxidation in the photoreceptors and RPE cells. For example, trans-4-hydroxy-2-hexenal (HHE), a product of oxidative damage to DHA, is toxic to neurons, depletes GSH and increases reactive oxygen species (128), whereas the thiol scavengers N-acetyl cysteine and GSH provide protection (148). Moreover, in models of retinal degeneration (152), when lipid peroxidation takes place, perturbations of photoreceptor function, damage, and cell death occur. In several forms of retinitis pigmentosa (78, 95, 96, 136, 139, 188) and in Usher’s syndrome (35, 136), a decrease of DHA content in blood has been reported, implying that decreased DHA supply may impair photoreceptor function. However, the relationship between decreased DHA in the blood supply and disease initiation and progression remains unclear. Rats overexpressing rhodopsin mutations homologous to human RP display decreased amounts of DHA in photoreceptors (5). This could represent a retinal response to metabolic stress, whereby decreasing the amount of the major target of lipid peroxidation (DHA) contributes to the protection of photoreceptors (5). In addition, in constant-light-mediated retinal degeneration, there is loss of DHA from photoreceptors. Rats reared in bright cyclic light are protected from such loss and degeneration, suggesting that there is adaptation and/or a plasticity response (124).
Is the shortage of DHA in the blood of patients with Usher’s syndrome (35, 136) and retinitis pigmentosa (94, 139, 181) reflected in the relationship of very-long-chain DHA-derived acyl groups with rhodopsin? Or, as shown in experimental retinal degeneration, is the peroxidation of DHA closely associated with rhodopsin, perhaps impairing the function of this protein? Are these events critical in photoreceptors of the macula? Do the phospholipids containing di-DHA and/or very-long-chain omega-3 FA (34:6,n-3) play a role in early stages of AMD or of other retinal degenerative diseases? These questions remain to be answered.
NEUROPROTECTIN D1 INHIBITS EXPERIMENTAL CHOROIDAL NEOVASCULARIZATION
Neovascularization at or near the retina/vitreous interface takes place in retinopathy of prematurity and diabetic retinopathy, whereas new vessel growth from the choroid into the retina occurs in AMD. Choroidal neovascularization, as in wet or exudative AMD, involves inflammation as an early event (109). The proinflammatory cytokines TNF-α and IL-1β increase the permeability (i.e., vascular leakage) of the pericyte/endothelial cell unit through a PLA2-dependent mechanism (111). NF-κB, a downstream component of both the TNF-α and IL-1β pathways, is activated. In retinal choroidal cells, IL-1β-induced NF-κB activity results in up-regulated vascular endothelial growth factor (VEGF) expression (130). VEGF controls both physiological and pathological angiogenesis (71), and enhanced expression of VEGF is associated with AMD pathology (84). VEGF-neutralizing antibody-based approaches have been successful in treatment of AMD (6). Thus, one approach for managing AMD is to block the signaling that triggers vascularization (43), although this is a secondary event resulting from primary insults. A second possibility involves the upstream regulation of VEGF-triggering events, including up-regulation of protective signaling that can suppress/attenuate the initial triggers of proangiogenesis.
PUFAs, especially DHA and AA, are abundant in the retina, where they become concentrated in photoreceptor and RPE cells (65). Connor et al. (50) have shown that NPD1, resolvin D1, and resolvin E1 (all derivatives of omega-3 PUFAs) suppress angiogenesis, and Szymczak et al. (203) demonstrated mediation by up-regulation of cyclooxygenases. Because omega-3 PUFAs inhibit major proangiogenic processes in endothelial cells (203) but do not affect VEGF (50), omega-3 PUFA protection may involve a VEGF-independent mechanism.
NPD1 significantly inhibits choroidal neovascularization. At least two possible mechanisms could explain the neuroprotective action of NPD1. Ultimately, NF-κB could be inhibited with a reduction in COX-2 to reduce VEGF expression, and/or activation of the resolution phase of the inflammatory response/survival pathways could be upregulated. Moreover, NPD1 continued to be effective after treatment was concluded, suggesting sustained protection and highlighting the potential applicability of this lipid mediator in preventing or ameliorating endothelial cell growth in pathogeneses such as AMD.
PIGMENT EPITHELIUM-DERIVED FACTOR TRIGGERS NEUROPROTECTIN D1 SYNTHESIS FROM DOCOSAHEXAENOIC ACID AND IN TURN INDUCES REGENERATION OF CORNEAL NERVES
Trigeminal ganglion nerves innervate and modulate corneal cell integrity (146). More than 12 million patients have undergone laser-assisted in situ keratomileusis (LASIK) surgery since it was first approved, 8 million of whom are Americans (see http://www.laser-eye-surgery-statistics.com/). However, corneal nerve damage after surgery results in loss of sensitivity, visual halos, severe dry eye, neurotrophic keratitis, decreased blink reflex, and enhanced susceptibility to injury and damage. Dry eye due to corneal sensory denervation by refractive surgery can last up to one year. Corneal subbasal nerve density does not recover for up to five years (60), and the number of stromal nerves decreases by nearly 90% after LASIK surgery (120). Photorefractive kerotectomy (PRK) and the newer corneal transplant technique, deep anterior lamellar keratoplasty, require transection of the host’s corneal nerves. Regeneration of nerves in the graft is slow and incomplete, resulting in long-lasting abnormal corneal sensation (156) that contributes to complications (64). In addition, corneal nerves are also damaged under conditions such as neurotrophic keratitis, herpetic infection, injury to trigeminal nerves associated with cranial, orbital, or retinal surgery, chemical burns, multiple sclerosis, Sjogren syndrome, diabetes mellitus (173), and use of contact lenses (157). Patients with dry eye have been treated using artificial tears, ointments, punctal plugs, etc., that provide only symptomatic relief. Treatment with steroids, serum tears, and more recently, low-concentration cyclosporin A (177), aims at decreasing the inflammatory response triggered by dry eye conditions. In spite of the fact that some of these treatments alleviate symptoms, they must be continuously administered, and their effects cease with discontinuation of treatment. Corneal innervation disturbances recently have been shown to be beneficially affected by topical DHA administration. Treatment with nerve growth factor and DHA after experimental PRK produces faster nerve recovery (61). Moreover, DHA in combination with PEDF in a rabbit model of lamellar keratectomy promotes regeneration of corneal nerves. Neurotrophinmediated NPD1 synthesis was suggested to precede nerve regeneration since its formation is increased upon addition of DHA and PEDF. Therefore, this signaling mechanism up-regulates corneal nerve regeneration and may be targeted in neurotrophic keratitis, dry eye after refractive surgery, and other corneal diseases.
EPIDEMIOLOGICAL STUDIES
Emerging public health nutrition challenges are mainly due to the consequences of changes in the Western diet in recent decades with less fish consumption, more carbohydrates and saturated fatty acids, and overall less dietary omega-3 essential fatty acids, particularly during the perinatal period and aging. Epidemiological and longitudinal studies suggest that DHA is critical for healthy brain development and is closely associated with cognitive performance and memory. DHA supplementation has been linked with reduced attention deficit disorders (101); decreased levels have been associated with AMD (25), impaired postnatal development (197), cognitive decline, and AD (129). The current interest in the significance of DHA is reflected in the increasing number of publications and of clinical trials assessing DHA as well as fish oil interventions (see http://www.clinicaltrials.gov). Clinical trials indicate that DHA slows cognitive decline in the elderly without dementia (66), but they do not provide evidence for DHA prevention of dementia, including AD (210). Moreover, Mediterranean diets are associated with a trend for reduced risk for MCI and with reduced risk for MCI conversion to AD (129). Deficiencies in DHA biosynthesis by the liver correlate with cognitive impairment in AD patients (8). This implies that the liver DHA supply to the CNS may be a factor in AD, as discussed above. In AD transgenic mice, on the other hand, dietary DHA restores cerebral blood volume, reduces Aβ deposition, and ameliorates Aβ pathology. Moreover, some current studies such as the large randomized trial using fish oil supplementation during pregnancy (The DOMInO Study: DHA to Optimize Mother Infant Outcome) (163) have yielded either minor or no effects.
PERSPECTIVES
Homeostasis is sustained by omega-3 FAs in the CNS as well as in other organs and is disrupted by dietary deficits of these FAs to various degrees at different stages of life. The DHA physiological demand for membrane biogenesis, maintenance, and cell functions during pre- and postnatal development outpaces the rate at which DHA can be synthesized from ALA. Therefore, in these crucial stages of perinatal development, DHA is provided from maternal sources to cope with the requirements for photoreceptor development, synaptogenesis, and the building of other cellular membranes (1, 39, 42, 95). Thereafter, the liver supplies and replaces DHA lost from membrane phospholipids as long as omega-3 precursors or DHA is present in the diet. Lipid peroxidation targeting DHA-containing membrane phospholipids is the major cause of DHA loss in the body, and this process is mainly triggered by pathological conditions. The DHA pool size needed to sustain NPD1 synthesis is very small, since this potent lipid mediator is synthesized upon demand.
The ability of the liver to supply DHA is compromised by liver damage or mutations that impair DHA synthesis (8). Also, genetic predisposition to autoimmune diseases (e.g., multiple sclerosis, systemic lupus erythromatosus) or chronic nonresolving inflammation (e.g., HIV encephalopathy, AD) has been linked with increased PLA2 activity and elevated DHA turnover secondary to lipid peroxidation and oxidative stress. However, the body’s high need for DHA may surpass liver synthetic capacity, particularly during aging, enhancing vulnerability to DHA insufficiency. Defects in DHA accretion in the CNS are not well understood. For example, we have an incomplete understanding of how the availability of PUFA is regulated through binding proteins or transporters, as well as the age-related deficits of intestinal absorption that also may bottleneck the supply of omega-3 precursors.
Metabolic syndrome, a public health concern in the United States and the rest of the Western world, involves impaired lipid metabolism, insulin resistance, and hypertension that in turn leads to atherosclerosis and diabetes linked to cognitive decline. Defective desaturase and elongase activity in hypertensive patients links chronic disease to metabolic derangement (53, 195, 196). Since PUFAs affect hepatic lipogenesis, impairments in the response to dietary FA levels may also contribute to DHA shortage. Therefore, it is evident that DHA incorporation to specific CNS cellular membranes is an important process that is sensitive to noxious, disrupting conditions.
Although up-regulation of hepatic PUFA-synthesis enzymes occurs in response to a DHA-deficient diet in rats (69, 164-166), humans may exhibit genetic differences and gene polymorphisms reflected in enzyme activity and regulation, making some individuals more dependent on dietary DHA than others. Looking at the rodent DHA requirements during a relatively short period of time does not help us understand whether humans lose their synthetic capabilities to make DHA as they age, thus increasing the importance of dietary supplementation, especially in the elderly. Increased levels of omega-6 PUFAs have been linked with increased inflammatory signaling, decreased DHA levels, cardiovascular disease, and certain cancers (51, 53, 57, 73, 150, 189-194). Omega-6 PUFAs (such as AA) compete with omega-3 PUFAs (such as EPA and DHA) for esterification in membrane phospholipids. Increased dietary AA reduces DHA esterification in membranes, even when adequate amounts are present in the diet (150). In addition, because both omega-6 and omega-3 PUFA precursors use the same set of enzymes for conversion to AA and DHA, increased amounts of linoleic acid in the diet might impair conversion of ALA to DHA, further decreasing its abundance. PUFAs are substrates for a variety of enzymes involved in inflammatory signaling cascades. PLA2 cleaves the PUFAs esterified to the sn-2 position of phospholipids, releasing free PUFAs. Free AA is converted by COX-1/2 into prostaglandins, thromboxanes, and prostacyclins. EPA-derived series 3 prostaglandins counteract inflammatory signaling (53, 119, 137, 154). DHA is also a substrate of COX-2 in the presence of aspirin, forming resolvins that participate in the resolution phase of inflammation (186). In experimental stroke, two pathways for endogenous DHA oxygenation were discovered: NPD1 biosynthesis and 17-R aspirin-triggered NPD1 biosynthesis (132). It is of interest to point out that the experimental design of this study did not provide any exogenous DHA. Changes in the relative abundance of PUFAs have been linked to inflammatory and cardiovascular diseases. The genetic and molecular mechanisms that underlie DHA deficiency and various neurodegenerative disorders remain, for the most part, unknown. Therefore, due to the relative shortage of dietary omega-3 FA precursors, DHA supplementation is often referred to as an alternative.
DHA signalolipidomics advances are made possible by powerful new methodological tools that include electrospray ionization/mass spectrometry–based mediator lipidomic analysis and imaging MALDI mass spectrometry. Thus, determining the precise organization, metabolism, signaling pathways, and synthesis of stereospecific mediators (e.g., NPD1) in a given cell or part of a cell (e.g., dendrites) is approachable. The composition of lipid classes and molecular species can now be determined more accurately. As such, the identification of changes in the DHA lipidome during the development, function, aging, and dysfunctions of the nervous system can be tackled more readily. These studies will be integrated with nutritional approaches and clinical neurosciences.
An evolving area is the study of the fundamental inner workings of dendrites, which contain complex membranes rich in DHA phospholipids. Dendrites undergo profound changes during neuronal function, including the membrane vesicular transport of neurotransmitter receptors, ion channels, and other proteins destined to the dendritic spine, where critical postsynaptic elements of neurotransmission are located. Definition of the dendritic DHA lipidome will also define the participation of DHA signaling in dendritic development and the establishment of synaptic contacts as well as overall dendritic plasticity.
NMDA receptor activation promotes the release of free DHA and AA. Clearly, DHA-containing molecular species of phospholipids confer a unique environment for ion channels, receptors, transporters and protein-protein interactions critical in synaptic signaling. How these events are regulated, including the synthesis and remodeling of these highly unsaturated phospholipids, is not understood. The enzyme-mediated synthesis of the docosanoid, NPD1, raises the possibility of exploring the specific generation of other novel DHA-oxygenation derivatives. How these docosanoids are synthesized, through which receptors they elicit their actions, how they are affected by pharmacologic agents, and how the docosanoids themselves may become a template for drug design are questions for the near future.
Structural neurobiology will also come into play because the DHA lipidome will provide new insights into the precise stereochemical structure of lipids of excitable and structural membranes. There is also growing evidence for exquisite signaling interplay among neurons, astrocytes, oligodendrocytes, and microglia. For example, lipid messengers have been explored as modulators of astrocyte/neuronal interactions.
Dietary supplementation may positively influence dyslipidemia, atherosclerosis, hypertension, diabetes mellitus, obesity, metabolic syndrome, and other inflammatory diseases. Omega-3 FA ingestion during pregnancy reduces the risk of premature birth and improves cognitive maturation of the fetus (49). Fish, fish oils, and some vegetable oils are rich sources of omega-3 FAs. On the other hand, a recent prospective analysis does not support the concept that long-term dietary intake of omega-3 FAs decreases the risk of type 2 diabetes. In contrast, higher fish and omega-3 FA consumption appears to be associated with higher incidence of type 2 diabetes (110). Given the beneficial effects of fish and omega-3 FAs on multiple risk factors associated with diabetes, including triglycerides, high-density lipoprotein cholesterol, blood pressure, and inflammation, and on coronary heart disease, the major sequelae of diabetes—and the clinical relevance of this relation and its possible mechanisms—require further investigation (110).
Ultimately, clinical trials using various DHA formulations will need to demonstrate their safety and efficacy. Because omega-3 FAs (mainly DHA) are liable to peroxidation, DHA signalolipidomics research on the molecular details of DHA uptake, disposition, and action will likely identify other factors/molecules/systems that protect DHA integrity, particularly during the initiation and early progression of diseases. These new approaches may lead to novel multinutrient-based trials. Cofactor/s (e.g., antioxidants and others molecules) that may be depleted or near depletion in a clinical trial need to be defined. Thus, no matter how much DHA is provided, it just may not be effective because of a shortage of needed cofactors.
The macula—a highly pigmented conedominant region in the human central retina specialized for high-acuity vision—provides lessons regarding dietary supplementation with omega-3 FAs. The macula selectively accumulates the xanthophylls lutein and zeaxanthin from the diet to protect vision cells from macular degeneration (38, 104). It is interesting to note that the short loop of DHA conservation actively engages RPE cells (Figure 6). Significant effects were observed in these cells from monkeys treated with a combination of the xanthophylls and omega-3 FA (122). The complexity of the system is highlighted by the fact that neither xanthophyll supplementation nor low dietary omega-3 fatty acids yielded consistent actions on S-cone or rod density profiles. However, monkeys low in omega-3 FAs show enhanced variability of S-cone density in the fovea and low density of foveal rod outer segments. The high variability indicates that photoreceptors of some animals were resistant to the nutritional manipulations while others may have been affected. Thus, photoreceptor cells are less sensitive than the RPE to these nutritional manipulations (122, 123).
In conclusion, a new understanding, as well as a definition of required dietary components (including multinutrient approaches), is needed to determine effective DHA supplementation for sustaining CNS homeostasis.
SUMMARY POINTS.
Omega-3 FAs are critical nutritional lipids that must be present in the diet to sustain homeostasis. They are key components of biomembranes, required during development and to sustain functional cell integrity. The essential omega-3 FA family member, DHA, is avidly retained and uniquely concentrated in the nervous system, particularly in photoreceptors and synaptic membranes. DHA is necessary for vision, neuroprotection, corneal nerve regeneration, successful aging, memory, and other functions.
The synthesis of the docosanoid NPD1, a DHA-derived stereoselective lipid mediator, is induced by neurotrophins and oxidative stress. NPD1 elicits potent anti-inflammatory actions, targets BCL-2 proteins at the onset of apoptosis signaling, modulates AKT signaling, is antiangiogenic, is endowed with overall prohomeostatic bioactivity, and induces cell survival.
DHA counteracts cognitive decline in the elderly, and diets rich in omega-3 essential fatty acids are associated with reduced risk for mild cognitive impairment. DHA is beneficial in transgenic AD models; however, its clinical effectiveness in AD has been as yet inconclusive.
The brains of AD patients show a decrease in NPD1. DHA attenuates Aβ42 and Aβ40 secretion from human neuronal/astrocyte cultures with concomitant NPD1 synthesis. Also, NPD1 attenuates proinflammatory gene expression and up-regulates procell-survival Bcl-2 proteins in these cells. NPD1 also shifts the APP processing pathway to the nonamyloidogenic route, activates PPARγ, and induces APPα in brain cells.
DHA down-regulates events leading to the death of dopaminergic neurons.
Photoreceptor cell (rods and cones) renewal is mediated by intermittent shedding of the tips of the outer segment with subsequent phagocytosis in RPE cells. This renewal is essential for vision. Photoreceptor outer segment phagocytosis in an in vitro model remarkably attenuates oxidative stress-induced apoptosis in RPE cells. This phenomenon is not a generalized outcome of phagocytosis because nonbiological (polystyrene microsphere) phagocytosis did not elicit protection. The free DHA pool size and NPD1 increased during phagocytosis but not during microsphere phagocytosis. Thus, NPD1 may be a mediator that promotes homeostatic modulation of cell integrity during photoreceptor renewal. Moreover, NPD1 inhibits A2E-mediated RPE cell death but not A2E conversion to A2E epoxides, suggesting that its bioactivity is distinct from that of an antioxidant. These studies are relevant to retinal degenerations because RPE survival fails early in disease development. In addition, NPD1 is antiangiogenic and inhibits experimental choroidal neovascularization.
DHA displays anti-inflammatory inflammation-resolving properties, in contrast to proinflammatory actions of several members of the omega-6 PUFA family.
DHA signalolipidomics includes the cellular/tissue organization of DHA uptake, its distribution among different cellular compartments, the organization and function of membrane domains rich in DHA-containing phospholipids, and the cellular and molecular events revealed by the uncovering of signaling pathways regulated by DHA and NPD1.
FUTURE ISSUES.
What are the optimal conditions, including duration, schedule of administration (e.g., time of day), and composition, for DHA supplementation studies? Do we have to consider a multinutrient approach?
Will a better understanding of the dietary antioxidants such as the xanthophylls (lutein and zeaxanthin) that accumulate in the macula, in relationship to DHA signalolipidomics, contribute to defining issues relevant to macular degeneration as well as to multinutrient dietary supplementation?
Which targets emerging from DHA signalolipidomics are amenable to clinical translation and pharmaceutical intervention?
What are the molecular characteristics of the NPD1 receptor, receptors for other docosanoids, and of DHA transporters/specific binding proteins?
How do the DHA peroxidation products 4-hydroxyhexenal, neuroprostanes, and NPD1 function in the CNS? Do they counteract each other, or do they elicit synergies? Is HHE, like 4-HNE, a second messenger?
How do neurotrophins regulate the DHA signalolipidome?
How does the DHA signalolipidome regulate membrane ion channels, receptors, synaptic plasticity, and neurogenesis?
What factors regulate interorgan flux of DHA, particularly with attention to communication between the CNS and the liver for DHA supply? Are there specific signals from the CNS that evoke liver release of DHA as needed?
ACKNOWLEDGMENTS
N.G.B. is grateful for the invaluable contributions made by current and past laboratory members. Work from the authors’ laboratories was supported by funding from the National Institutes of Health, National Center for Complementary and Alternative Medicine (AT005909), the National Center for Research Resources (RR016816), the National Institute of Neurological Disorders and Stroke (NS046741), the National Eye Institute (EY005121), the Foundation Fighting Blindness (TA-NP-0808-0463-LSUNO), the American Health Assistance Foundation, the Eye, Ear, Nose & Throat Foundation, and the Ernest C. and Yvette C. Villere Endowed Chair of Retinal Degenerations. The task of writing this review was an enormous one because of the very rapid pace of the growth of this field, reflected in the increasing number of original publications and of clinical trials assessing DHA as well as fish oil interventions. We apologize to our colleagues if we have failed to cite papers; this was in large part due to space limitations.
Glossary
- Docosahexaenoic acid (DHA) signalolipidomics
includes the cellular organization of DHA uptake, its distribution among cellular compartments, the organization and function of membrane domains rich in DHA-containing phospholipids, and the signaling pathways regulated by DHA and by neuroprotectin D1 (NPD1). DHA signalolipidomics in the CNS offers emerging targets for clinical translation and pharmaceutical intervention
- Neuroprotectin D1 (NPD1;10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid)
a DHA-derived stereoselective mediator that acts as a sentinel to defend the maintenance of homeostasis at the onset of CNS injury, expression of neurodegenerative genes, oxidative stress, etc.
Footnotes
DISCLOSURE STATEMENT
The authors are unaware of any affiliation, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Agostoni C. Role of long-chain polyunsaturated fatty acids in the first year of life. J. Pediatr. Gastroenterol. Nutr. 2008;47:S41–44. doi: 10.1097/01.mpg.0000338811.52062.b2. [DOI] [PubMed] [Google Scholar]
- 2.Akbar M, Calderon F, Wen Z, Kim H. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. USA. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–39. doi: 10.1096/fj.01-0007fje. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM. Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol. Vis. 2002;8:351–58. [PubMed] [Google Scholar]
- 6.Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr. Opin. Ophthalmol. 2007;18:502–8. doi: 10.1097/ICU.0b013e3282f0ca54. [DOI] [PubMed] [Google Scholar]
- 7.Antony R, Lukiw WJ, Bazan NG. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J. Biol. Chem. 2010;285:18301–8. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astarita G, Jung K, Berchtold NC, Nguyen VQ, Gillen DL, et al. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS ONE. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aveldaño MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–39. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 10.Aveldaño MI, Bazan NG. High content of docosahexaenoate and of total diacylglycerol in retina. Biochem. Biophys. Res. Commun. 1972;48:689–93. doi: 10.1016/0006-291x(72)90403-2. [DOI] [PubMed] [Google Scholar]
- 11.Aveldaño MI, Bazan NG. Fatty acid composition and level of diacylglycerols and phosphoglycerides in brain and retina. Biochim. Biophys. Acta. 1973;296:1–9. doi: 10.1016/0005-2760(73)90038-6. [DOI] [PubMed] [Google Scholar]
- 12.Aveldaño MI, Bazan NG. Displacement into incubation medium by albumin of highly unsaturated retina free fatty acids arising from membrane lipids. FEBS Lett. 1974;40:53–56. doi: 10.1016/0014-5793(74)80892-6. [DOI] [PubMed] [Google Scholar]
- 13.Aveldaño MI, Bazan NG. Differential lipid deacylation during brain ischemia in a homeotherm and a poikilotherm. Content and composition of free fatty acids and triacylglycerols. Brain Res. 1975;100:99–110. doi: 10.1016/0006-8993(75)90244-9. [DOI] [PubMed] [Google Scholar]
- 14.Aveldaño MI, Bazan NG. Acyl groups, molecular species and labeling by 14C-glycerol and 3H-arachidonic acid of vertebrate retina glycerolipids. Adv. Exp. Med. Biol. 1977;38:397–404. doi: 10.1007/978-1-4684-3276-3_37. [DOI] [PubMed] [Google Scholar]
- 15.Aveldaño MI, Bazan NG. Composition and biosynthesis of molecular species of retina phosphoglycerides. Neurochem. Int. 1980;1:381–92. [PubMed] [Google Scholar]
- 16.Aveldaño MI, Giusto NM, Bazan NG. Polyunsaturated fatty acids of the retina. Prog. Lipid Res. 1981;20:49–57. doi: 10.1016/0163-7827(81)90013-8. [DOI] [PubMed] [Google Scholar]
- 17.Aveldaño MI, Bazan NG. Molecular species of phosphatidylcholine, -ethanolamine, -serine, and -inositol in microsomal and photoreceptor membranes of bovine retina. J. Lipid Res. 1983;24:620–27. [PubMed] [Google Scholar]
- 18.AveldaAveldaño MI, Pasquare de Garcia SJ, Bazan NG. Biosynthesis of molecular species of inositol, choline, serine, and ethanolamine glycerophospholipids in the bovine retina. J. Lipid Res. 1983;24:628–38. [PubMed] [Google Scholar]
- 19.AveldaAveldaño MI. Phospholipid species containing long and very long polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. Biochemistry. 1988;27:1229–39. doi: 10.1021/bi00404a024. [DOI] [PubMed] [Google Scholar]
- 20.Avramovich Y, Amit T, Youdim MB. Non-steroidal anti-inflammatory drugs stimulate secretion of non-amyloidogenic precursor protein. J. Biol. Chem. 2002;277:31466–73. doi: 10.1074/jbc.M201308200. [DOI] [PubMed] [Google Scholar]
- 21.Bazan NG, Silvia di Fazio de Escalante M, Careaga MM, Bazan HE, Giusto NM. High content of 22:6 (docosahexaenoate) and active [2-3H]glycerol metabolism of phosphatidic acid from photoreceptor membranes. Biochim. Biophys. Acta. 1982;712:702–6. doi: 10.1016/0005-2760(82)90301-0. [DOI] [PubMed] [Google Scholar]
- 22.Bazan HE, Sprecher H, Bazan NG. De novo biosynthesis of docosahexaenoyl-phosphatidic acid in bovine retinal microsomes. Biochim. Biophys. Acta. 1984;796:11–19. doi: 10.1016/0005-2760(84)90232-7. [DOI] [PubMed] [Google Scholar]
- 23.Bazan NG. Supply of n-3 polyunsaturated fatty acids and their significance in the central nervous system. In: Wurtman RJ, Wurtman JJ, editors. Nutrition and the Brain. Vol. 8. Raven; New York: 1990. pp. 1–24. [Google Scholar]
- 24.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 2003;44:2221–33. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 2007;48:4866–81. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- 27.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res. 2009;50:S400–5. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazan NG, Birkle DL, Reddy TS. Docosahexaenoic acid (22:6, n-3) is metabolized to lipoxygenase reaction products in the retina. Biochem. Biophys. Res. Commun. 1984;125:741–47. doi: 10.1016/0006-291x(84)90601-6. [DOI] [PubMed] [Google Scholar]
- 29.Bazan NG, Birkle DL, Reddy TS. Biochemical and nutritional aspects of the metabolism of polyunsaturated fatty acids and phospholipids in experimental models of retinal degeneration. In: LaVail MM, Hollyfield JG, Anderson RE, editors. Retinal Degeneration: Experimental and Clinical Studies. Liss; New York: 1985. pp. 159–87. [Google Scholar]
- 30.Bazan NG, Giusto NM. Docosahexaenoyl chains are introduced in phosphatic acid during de novo synthesis in retinal microsomes. In: Kates M, Kukis A, editors. Control of Membrane Fluidity. Humana; Totowa, NJ: 1980. pp. 223–36. [Google Scholar]
- 31.Bazan NG, Gordon WC, Rodriguez de Turco EB. Docosahexaenoic acid uptake and metabolism in photoreceptors: retinal conservation by an efficient retinal pigment epithelial cell-mediated recycling process. Adv. Exp. Med. Biol. 1992;318:295–306. doi: 10.1007/978-1-4615-3426-6_26. [DOI] [PubMed] [Google Scholar]
- 32.Bazan NG, Reddy TS, Redmond TM, Wiggert B, Chader GJ. Endogenous fatty acids are covalently and noncovalently bound to interphotoreceptor retinoid-binding protein in the monkey retina. J. Biol. Chem. 1985;260:13677–80. [PubMed] [Google Scholar]
- 33.Bazan NG, Rodriguez de Turco EB, Gordon WC. Pathways for the uptake and conservation of docosahexaenoic acid in photoreceptors and synapses: biochemical and autoradiographic studies. Can. J. Physiol. Pharmacol. 1993;71:690–98. doi: 10.1139/y93-103. [DOI] [PubMed] [Google Scholar]
- 34.Bazan NG, Scott BL. Dietary omega-3 fatty acids and accumulation of docosahexaenoic acid in rod photoreceptor cells of the retina and at synapses. Ups. J. Med. Sci. Suppl. 1990;48:97–107. [PubMed] [Google Scholar]
- 35.Bazan NG, Scott BL, Reddy TS, Pelias MZ. Decreased content of docosahexaenoate and arachidonate in plasma phospholipids in Usher’s syndrome. Biochem. Biophys. Res. Commun. 1986;141:600–4. doi: 10.1016/s0006-291x(86)80215-7. [DOI] [PubMed] [Google Scholar]
- 36.Bernardo A, Minghetti L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr. Pharm. Des. 2006;12:93–109. doi: 10.2174/138161206780574579. [DOI] [PubMed] [Google Scholar]
- 37.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Bettler J, Zimmer JP, Neuringer M, DeRusso PA. Serum lutein concentrations in healthy term infants fed human milk or infant formula with lutein. Eur. J. Nutr. 2010;49:45–51. doi: 10.1007/s00394-009-0047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bézard J, Blond JP, Bernard A, Clouet P. The metabolism and availability of essential fatty acids in animal and human tissues. Reprod. Nutr. Dev. 1994;34:539–68. doi: 10.1051/rnd:19940603. [DOI] [PubMed] [Google Scholar]
- 40.Bibb C, Young RW. Renewal of fatty acids in the membranes of visual cell outer segments. J. Cell Biol. 1974;61:327–43. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird AC. The Bowman Lecture. Towards an understanding of age-related macular disease. Eye. 2003;17:457–66. doi: 10.1038/sj.eye.6700562. [DOI] [PubMed] [Google Scholar]
- 42.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, et al. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J. Nutr. 1989;119:1880–92. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 43.Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009;116:S15–23. doi: 10.1016/j.ophtha.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 44.Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009;284:17877–82. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J. Neurosci. 2004;24:10908–17. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cansev M, Ulus IH, Wang L, Maher TJ, Wurtman RJ. Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson’s disease. Neurosci. Res. 2008;62:206–9. doi: 10.1016/j.neures.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang GQ, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 48.Chapus C, Rovery M, Sarda L, Verger R. Minireview on pancreatic lipase and colipase. Biochimie. 1988;70:1223–34. doi: 10.1016/0300-9084(88)90188-5. [DOI] [PubMed] [Google Scholar]
- 49.Coletta JM, Bell SJ, Roman AS. Omega-3 fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010;3:163–71. [PMC free article] [PubMed] [Google Scholar]
- 50.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crawford MA. The early development and evolution of the human brain. Ups. J. Med. Sci. Suppl. 1990;48:43–78. [PubMed] [Google Scholar]
- 52.d’Abramo C, Massone S, Zingg JM, Pizzuti A, Marambaud P, et al. Role of peroxisome proliferator-activated receptor gamma in amyloid precursor protein processing and amyloid beta-mediated cell death. Biochem. J. 2005;391:693–98. doi: 10.1042/BJ20050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Caterina R. n-3 Fatty acids and the inflammatory response—biological background. Eur. Heart J. Suppl. 2001;3:D42–49. [Google Scholar]
- 54.de Jong PT. Age-related macular degeneration. N. Engl. J. Med. 2006;355:1474–85. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 55.DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 2006;47:172–80. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–44. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 57.Drozdowski L, Thomson AB. Aging and the intestine. World J. Gastroenterol. 2006;12:7578–84. doi: 10.3748/wjg.v12.i47.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunaief JL, Dentchew T, Ying GS, Milam AH. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002;120:1435–42. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- 59.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–24. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 60.Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol. 2005;140:1059–64. doi: 10.1016/j.ajo.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 61.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 2005;46:3121–27. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 62.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp. Eye Res. 2010;90:718–25. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Balacco Gabrieli C. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol. Aging. 2006;27:983–93. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 64.Feiz V, Mannis MJ, Kandavel G, McCarthy M, Izquierdo L, et al. Surface keratopathy after penetrating keratoplasty. Trans. Am. Ophthalmol. Soc. 2001;99:159–68. [PMC free article] [PubMed] [Google Scholar]
- 65.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 66.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat. Clin. Pract. Neurol. 2009;5:140–52. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 67.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 2006;63:1402–8. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 68.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark. Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao F, Kiesewetter D, Chang L, Ma K, Bell JM, et al. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified alpha-linolenic acid in unanesthetized rats. J. Lipid Res. 2009;50:749–58. doi: 10.1194/jlr.D800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Garcia MC, Kim H. Mobilization of arachidonate and docosahexaenoate by stimulation of the 5-HT2A receptor in rat C6 glioma cells. Brain Res. 1997;768:43–48. doi: 10.1016/s0006-8993(97)00583-0. [DOI] [PubMed] [Google Scholar]
- 71.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–46. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007;104:18754–59. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gil A. Polyunsaturated fatty acids and inflammatory diseases. Biomed. Pharmacother. 2002;56:388–96. doi: 10.1016/s0753-3322(02)00256-1. [DOI] [PubMed] [Google Scholar]
- 74.Giovacchini G, Chang MC, Channing MA, Toczek M, Mason A, et al. Brain incorporation of [11C]arachidonic acid in young healthy humans measured with positron emission tomography. J. Cereb. Blood Flow Metab. 2002;22:1453–62. doi: 10.1097/01.WCB.0000033209.60867.7A. [DOI] [PubMed] [Google Scholar]
- 75.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–25. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, et al. Variation in factor B (BF) and complement 2 (C2) genes is associated with age-related macular degeneration. Nat. Genet. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Curr. Alzheimer Res. 2006;3:421–30. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 78.Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1992;33:2596–602. [PubMed] [Google Scholar]
- 79.González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–57. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–39. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 81.Gordon WC, Bazan NG. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. J. Neurosci. 1990;10:2190–202. doi: 10.1523/JNEUROSCI.10-07-02190.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordon WC, Rodriguez de Turco EB, Bazan NG. Retinal pigment epithelial cells play a central role in the conservation of docosahexaenoic acid by photoreceptor cells after shedding and phagocytosis. Curr. Eye Res. 1992;11:73–83. doi: 10.3109/02713689209069169. [DOI] [PubMed] [Google Scholar]
- 83.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, et al. Dietary docosa-hexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 2007;27:4385–95. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grisanti S, Tatar O. The role of vascular endothelial growth factor and other endogenous inter-players in age-related macular degeneration. Prog. Retin. Eye Res. 2008;27:372–90. doi: 10.1016/j.preteyeres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Haass C. Initiation and propagation of neurodegeneration. Nat. Med. 2010;16:1201–4. doi: 10.1038/nm.2223. [DOI] [PubMed] [Google Scholar]
- 86.Haass C, Mandelkow E. Fyn-tau-amyloid: a toxic triad. Cell. 2010;142:356–58. doi: 10.1016/j.cell.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 87.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 88.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisphotoreceptor outer segment predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 90.Hamilton J, Greiner R, Salem N, Kim H. N-3 Fatty acid deficiency decreases phosphatidylserine accumulation selectively in neuronal tissues. Lipids. 2000;35:863–69. doi: 10.1007/s11745-000-0595-x. [DOI] [PubMed] [Google Scholar]
- 91.Hassan IR, Gronert K. Acute changes in dietary omega-3 and omega-6 polyunsaturated fatty acids have a pronounced impact on survival following ischemic renal injury and formation of renoprotective docosahexaenoic acid-derived protectin D1. J. Immunol. 2009;182:3223–32. doi: 10.4049/jimmunol.0802064. [DOI] [PubMed] [Google Scholar]
- 92.Henke BR. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands and their therapeutic utility. Prog. Med. Chem. 2004;42:1–53. doi: 10.1016/S0079-6468(04)42001-3. [DOI] [PubMed] [Google Scholar]
- 93.Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc. Natl. Acad. Sci. USA. 1998;95:8625–29. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoffman DR, Birch DG. Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1995;36:1009–18. [PubMed] [Google Scholar]
- 95.Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:151–58. doi: 10.1016/j.plefa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 96.Hoffman DR, DeMar JC, Heird WC, Birch DG, Anderson RE. Impaired synthesis of DHA in patients with X-linked retinitis pigmentosa. J. Lipid Res. 2001;42:1395–401. [PubMed] [Google Scholar]
- 97.Hoffman DR, Uauy R, Birch DG. Red blood cell fatty acid levels in patients with autosomal dominant retinitis pigmentosa. Exp. Eye Res. 1993;57:359–68. doi: 10.1006/exer.1993.1135. [DOI] [PubMed] [Google Scholar]
- 98.Holman RT. Control of polyunsaturated acids in tissue lipids. J. Am. Coll. Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- 99.Horrocks LA, Farooqui AA. NMDA receptor-stimulated release of arachidonic acid: mechanisms for the Bazan effect. In: Municio AM, Miras-Portugal MT, editors. Cell Signal Transduction, Second Messengers, and Protein Phosphorylation in Health and Disease. Plenum; New York: 1994. pp. 113–28. [Google Scholar]
- 100.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- 101.Huss M, Volp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems—an observational cohort study. Lipids Health Dis. 2010;9:105. doi: 10.1186/1476-511X-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008;15:924–31. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang JH, Surh YJ. Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 upregulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic. Biol. Med. 2005;38:1604–13. doi: 10.1016/j.freeradbiomed.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 104.Jeffrey BG, Neuringer M. Age-related decline in rod phototransduction sensitivity in rhesus monkeys fed an n-3 fatty acid-deficient diet. Invest. Ophthalmol. Vis. Sci. 2009;50:4360–67. doi: 10.1167/iovs.09-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J. Nutr. 2005;135:2503–6. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 106.Jump DB, Botolin D, Wang Y, Xu J, Demeure O, Christian B. Docosahexaenoic acid (DHA) and hepatic gene transcription. Chem. Phys. Lipids. 2008;153:3–13. doi: 10.1016/j.chemphyslip.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jump DB, Thelen A, Ren B, Mater M. Multiple mechanisms for polyunsaturated fatty acid regulation of hepatic gene transcription. Prostaglandins Leukot. Essent. Fatty Acids. 1999;60:345–49. doi: 10.1016/s0952-3278(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 108.Kalant D, Cianflone K. Regulation of fatty acid transport. Curr. Opin. Lipidol. 2004;15:309–14. doi: 10.1097/00041433-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 109.Kanda A, Abecasis G, Swaroop A. Inflammation in the pathogenesis of age-related macular degeneration. Br. J. Ophthalmol. 2008;92:448–50. doi: 10.1136/bjo.2007.131581. [DOI] [PubMed] [Google Scholar]
- 110.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am. J. Clin. Nutr. 2009;90:613–20. doi: 10.3945/ajcn.2008.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kerkar S, Williams M, Blocksom JM, Wilson RF, Tyburski JG, Steffes CP. TNF-alpha and IL-1beta increase pericyte/endothelial cell co-culture permeability. J. Surg. Res. 2006;132:40–45. doi: 10.1016/j.jss.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 112.Kim H. Novel metabolism of docosahexaenoic acid in neural cells. J. Biol. Chem. 2007;282:18661–65. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 113.Kim HY, Akbar M, Kim KY. Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J. Mol. Neurosci. 2001;16:223–27. doi: 10.1385/JMN:16:2-3:223. [DOI] [PubMed] [Google Scholar]
- 114.Kim H, Akbar M, Kim Y. Phosphatidylserine-dependent neuroprotective signaling promoted by docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:165–72. doi: 10.1016/j.plefa.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim H, Akbar M, Lau A. Effects of docosapentaenoic acid on neuronal apoptosis. Lipids. 2003;38:453–57. doi: 10.1007/s11745-003-1083-z. [DOI] [PubMed] [Google Scholar]
- 116.Kim HY, Akbar M, Lau A, Edsall L. Inhibition of neuronal apoptosis by docosahexaenoic acid (22:6n-3). Role of phosphatidylserine in antiapoptotic effect. J. Biol. Chem. 2000;275:35215–23. doi: 10.1074/jbc.M004446200. [DOI] [PubMed] [Google Scholar]
- 117.Kim HY, Edsall L, Garcia M, Zhang H. The release of polyunsaturated fatty acids and their lipoxygenation in the brain. Adv. Exp. Med. Biol. 1999;447:75–85. doi: 10.1007/978-1-4615-4861-4_7. [DOI] [PubMed] [Google Scholar]
- 118.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–89. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Layé S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 120.Lee BH, McLaren JW, Erie JC, Hodge DO, Bourne WM. Reinnervation in the cornea after LASIK. Invest. Ophthalmol. Vis. Sci. 2002;43:3660–64. [PubMed] [Google Scholar]
- 121.Lee SA, Kim HJ, Chang KC, Baek JC, Park JK, et al. DHA and EPA down-regulate COX-2 expression through suppression of NF-kappaB activity in LPS treated human umbilical vein endothelial cells. Korean J. Physiol. Pharmacol. 2009;13:301–7. doi: 10.4196/kjpp.2009.13.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2004;45:3244–56. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- 123.Leung IY, Sandstrom MM, Zucker CL, Neuringer M, Snodderly DM. Nutritional manipulation of primate retinas. IV. Effects of n-3 fatty acids, lutein, and zeaxanthin on S-cones and rods in the foveal region. Exp. Eye Res. 2005;81:513–29. doi: 10.1016/j.exer.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 124.Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp. Eye Res. 2001;73:569–77. doi: 10.1006/exer.2001.1068. [DOI] [PubMed] [Google Scholar]
- 125.Lim GP, Calon F, Morihara T, Yang F, Teter B, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005;25:3032–40. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr. N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res. 2005;58:741–48. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 127.Lim SY, Hoshiba J, Salem N., Jr. An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: n-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance. J. Neurochem. 2005;95:848–57. doi: 10.1111/j.1471-4159.2005.03427.x. [DOI] [PubMed] [Google Scholar]
- 128.Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, et al. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008;105:714–24. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- 129.Lukiw WJ, Cui J, Marcheselli VL, Bodker M, Botkjaer A, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, et al. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest. Ophthalmol. Vis. Sci. 2003;44:4163–70. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 131.Ma QL, Teter B, Ubeda OJ, Morihara T, Dhoot D, et al. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer’s disease (AD): relevance to AD prevention. J. Neurosci. 2007;27:14299–307. doi: 10.1523/JNEUROSCI.3593-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 133.Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marion-Letellier R, Butler M, Dechelotte P, Playford RJ, Ghosh S. Comparison of cytokine modulation by natural peroxisome proliferator-activated receptor gamma ligands with synthetic ligands in intestinal-like Caco-2 cells and human dendritic cells. Am. J. Clin. Nutr. 2008;87:939–48. doi: 10.1093/ajcn/87.4.939. [DOI] [PubMed] [Google Scholar]
- 135.Masson CJ, Plat J, Mensink RP, Namiot A, Kisielewski W, et al. Fatty acid and cholesterol transporter protein expression along the human intestinal tract. PLoS ONE. 2010;5:e10380. doi: 10.1371/journal.pone.0010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maude MB, Anderson EO, Anderson RE. Polyunsaturated fatty acids are lower in blood lipids of Usher’s type I but not Usher’s type II. Invest. Ophthalmol. Vis. Sci. 1998;39:2164–66. [PubMed] [Google Scholar]
- 137.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukot. Essent. Fatty Acids. 2010;83:185–91. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mendes HF, van der Spuy J, Chapple JP, Cheetham ME. Mechanisms of cell death in rhodopsin retinitis pigmentosa: implications for therapy. Trends Mol. Med. 2005;11:177–85. doi: 10.1016/j.molmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 139.McColl AJ, Converse CA. Lipid studies in retinitis pigmentosa. Prog. Lipid Res. 1995;349:1–16. doi: 10.1016/0163-7827(94)00004-6. [DOI] [PubMed] [Google Scholar]
- 140.Moore SA. Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro. J. Mol. Neurosci. 2001;16:195–200. doi: 10.1385/JMN:16:2-3:195. [DOI] [PubMed] [Google Scholar]
- 141.Moore SA, Yoder E, Murphy S, Dutton GR, Spector AA. Astrocytes, not neurons, produce docosahexaenoic acid (22:6 omega-3) and arachidonic acid (20:4 omega-6) J. Neurochem. 1991;56:518–24. doi: 10.1111/j.1471-4159.1991.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 142.Moriguchi T, Lim S, Greiner R, Lefkowitz W, Loewke J, et al. Effects of an n-3-deficient diet on brain, retina, and liver fatty acyl composition in artificially reared rats. J. Lipid Res. 2004;45:1437–45. doi: 10.1194/jlr.M400087-JLR200. [DOI] [PubMed] [Google Scholar]
- 143.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl. Acad. Sci. USA. 2007b;104:13152–57. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc. Natl. Acad. Sci. USA. 2007a;104:13158–63. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. USA. 2004;101:8491–96. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp. Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 147.Musiek ES, Brooks JD, Joo M, Brunoldi E, Porta A, et al. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid. J. Biol. Chem. 2008;283:19927–35. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Neely MD, Zimmerman L, Picklo MJ, Ou JJ, Morales CR, et al. Congeners of N(alpha)-acetyl-L-cysteine but not aminoguanidine act as neuroprotectants from the lipid peroxidation product 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2000;29:1028–36. doi: 10.1016/s0891-5849(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 149.Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mito-chondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–8. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 150.Novak EM, Dyer RA, Innis SM. High dietary [omega]-6 fatty acids contribute to reduced docosa-hexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008;237:136–45. doi: 10.1016/j.brainres.2008.07.107. [DOI] [PubMed] [Google Scholar]
- 151.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, et al. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis. 2006;23:563–72. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 152.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest. Ophthalmol. Vis. Sci. 1996;37:2243–57. [PubMed] [Google Scholar]
- 153.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, et al. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA. 2001;98:6027–32. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Palacios-Pelaez R, Lukiw WJ, Bazan NG. Omega-3 essential fatty acids modulate initiation and progression of neurodegenerative disease. Mol. Neurobiol. 2010;41:367–74. doi: 10.1007/s12035-010-8139-z. [DOI] [PubMed] [Google Scholar]
- 155.Papermaster DS. The birth and death of photoreceptors: the Friedenwald Lecture. Invest. Ophthalmol. Vis. Sci. 2002;43:1300–9. [PubMed] [Google Scholar]
- 156.Patel SV, Erie JC, McLaren JW, Bourne WM. Keratocyte density and recovery of subbasal nerves after penetrating keratoplasty and late endothelial failure. Arch. Ophthalmol. 2007;125:1693–98. doi: 10.1001/archopht.125.12.1693. [DOI] [PubMed] [Google Scholar]
- 157.Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest. Ophthalmol. Vis. Sci. 2002;43:995–1003. [PubMed] [Google Scholar]
- 158.Peng Y, Lee DY, Jiang L, Ma Z, Schachter SC, Lemere CA. Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695. Neuroscience. 2007;150:386–95. doi: 10.1016/j.neuroscience.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 159.Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV. Effect of dietary n-3 polyun-saturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1153–60. doi: 10.1093/gerona/63.11.1153. [DOI] [PubMed] [Google Scholar]
- 160.Politi LE, Rotstein NP, Carri NG. Effect of GDNF on neuroblast proliferation and photoreceptor survival: additive protection with docosahexaenoic acid. Invest. Ophthalmol. Vis. Sci. 2001;42:3008–15. [PubMed] [Google Scholar]
- 161.Portera-Cailliau C, Sung CH, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA. 1994;91:974–78. doi: 10.1073/pnas.91.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin Q, Patil KA, Gronert K, Sharma SC. Neuroprotectin D1 inhibits retinal ganglion cell death following axotomy. Prostaglandins Leukot. Essent. Fatty Acids. 2008;79:201–7. doi: 10.1016/j.plefa.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 163.Quinn JF, Raman R, Thomas RG, Yurko-Mauro K, Nelson EB, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304:1903–11. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot. Essent. Fatty Acids. 2009;81:119–23. doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rapoport SI, Igarashi M, Gao F. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82:273–76. doi: 10.1016/j.plefa.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot. Essent. Fatty Acids. 2007;77:251–61. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006;7:860–72. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 168.Reddy TS, Bazan NG. Activation of polyunsaturated fatty acids by rat tissues in vitro. Lipids. 1984a;19:987–89. doi: 10.1007/BF02534740. [DOI] [PubMed] [Google Scholar]
- 169.Reddy TS, Bazan NG. Synthesis of arachidonoyl coenzyme A and docosahexaenoyl coenzyme A in retina. Curr. Eye Res. 1984b;3:1225–32. doi: 10.3109/02713688409000826. [DOI] [PubMed] [Google Scholar]
- 170.Reddy TS, Bazan NG. Synthesis of arachidonoyl coenzyme A and docosahexaenoyl coenzyme A in synaptic plasma membranes of cerebrum and microsomes of cerebrum, cerebellum, and brain stem of rat brain. J. Neurosci. Res. 1985a;13:381–90. doi: 10.1002/jnr.490130305. [DOI] [PubMed] [Google Scholar]
- 171.Reddy TS, Bazan NG. Synthesis of docosahexaenoyl-, arachidonoyl- and palmitoyl-coenzyme A in ocular tissues. Exp. Eye Res. 1985b;41:87–95. doi: 10.1016/0014-4835(85)90097-1. [DOI] [PubMed] [Google Scholar]
- 172.Roberts LJ, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Brain Pathol. 2005;15:143–48. doi: 10.1111/j.1750-3639.2005.tb00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rosenberg ME, Tervo TM, Immonen IJ, Muller LJ, Gronhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest. Ophthalmol. Vis. Sci. 2000;41:2915–21. [PubMed] [Google Scholar]
- 174.Rossner S, Sastre M, Bourne K, Lichtenthaler SF. Transcriptional and translational regulation of BACE1 expression-implications for Alzheimer’s disease. Prog. Neurobiol. 2006;79:95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 175.Sahlin C, Pettersson FE, Nilsson LN, Lannfelt L, Johansson AS. Docosahexaenoic acid stimulates non-amyloidogenic APP processing resulting in reduced Abeta levels in cellular models of Alzheimer’s disease. Eur. J. Neurosci. 2007;26:882–89. doi: 10.1111/j.1460-9568.2007.05719.x. [DOI] [PubMed] [Google Scholar]
- 176.Salem N, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 177.Sall K, Stevenson OD, Mundorf TK, Reis BL, CsA Phase 3 Study Group Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. Ophthalmology. 2000;107:631–39. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 178.Samadi P, Grégoire L, Rouillard C, Bédard PJ, Di Paolo T, Lévesque D. Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Biochemistry. 2006;45:15610–16. doi: 10.1002/ana.20738. [DOI] [PubMed] [Google Scholar]
- 179.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 180.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 2006;103:443–48. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schaefer EJ, Robins SJ, Patton GM, Sandberg MA, Weigel-DiFranco CA, et al. Red blood cell membrane phosphatidylethanolamine fatty acid content in various forms of retinitis pigmentosa. J. Lipid Res. 1995;36:1427–33. [PubMed] [Google Scholar]
- 182.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc. Natl. Acad. Sci. USA. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 185.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J. Immunol. 2006;176:1848–59. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 186.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histol. Histopathol. 2007;22:1301–8. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 188.Simonelli F, Manna C, Romano N, Nunziata G, Voto O, Rinaldi E. Evaluation of fatty acids in membrane phospholipids of erythrocytes in retinitis pigmentosa patients. Ophthalmic Res. 1996;28:93–98. doi: 10.1159/000267880. [DOI] [PubMed] [Google Scholar]
- 189.Simopoulos AP. Summary of the NATO advanced research workshop on dietary omega 3 and omega 6 fatty acids: biological effects and nutritional essentiality. J. Nutr. 1989;119:521–28. doi: 10.1093/jn/119.4.521. [DOI] [PubMed] [Google Scholar]
- 190.Simopoulos AP. n-3 fatty acids and human health: defining strategies for public policy. Lipids. 2001;36:S83–89. doi: 10.1007/s11745-001-0687-7. [DOI] [PubMed] [Google Scholar]
- 191.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002a;56:365–79. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 192.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002b;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 193.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 2006;60:502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 194.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 195.Singer P, Berger I, Lück K, Taube C, Naumann E, Gödicke W. Long-term effect of mackerel diet on blood pressure, serum lipids and thromboxane formation in patients with mild essential hypertension. Atherosclerosis. 1986;62:259–65. doi: 10.1016/0021-9150(86)90100-0. [DOI] [PubMed] [Google Scholar]
- 196.Singer P, Jaeger W, Voigt S, Thiel H. Defective desaturation and elongation of N-6 and N-3 fatty acids in hypertensive patients. Prostaglandins Leukot. Med. 1984;15:159–65. doi: 10.1016/0262-1746(84)90173-2. [DOI] [PubMed] [Google Scholar]
- 197.Singh M. Essential fatty acids, DHA and human brain. Indian J. Pediatr. 2005;72:239–42. [PubMed] [Google Scholar]
- 198.Spencer KL, Hauser MA, Olson LM, Schmidt S, Scott WK, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Hum. Mol. Genet. 2007;16:1986–92. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 199.Sreekumar PG, Kannan R, Yaung J, Spee CK, Ryan SJ, Hinton DR. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem. Biophys. Res. Commun. 2005;334:245–53. doi: 10.1016/j.bbrc.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 200.Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol. Metab. 2001;12:266–73. doi: 10.1016/s1043-2760(01)00427-1. [DOI] [PubMed] [Google Scholar]
- 201.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, et al. Identification of the major intestinal fatty acid transport protein. Mol. Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 202.Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 203.Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by ω-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111:3514–21. doi: 10.1182/blood-2007-08-109934. [DOI] [PubMed] [Google Scholar]
- 204.Tanihara H, Inatani M, Honda Y. Growth factors and their receptors in the retina and pigment epithelium. Prog. Retin. Eye Res. 1997;16:271–301. [Google Scholar]
- 205.Tanriover G, Seval-Celik Y, Ozsoy O, Akkoyunlu G, Savcioglu F, et al. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia. Histochem. Cytobiol. 2010;48:434–41. doi: 10.2478/v10042-010-0047-6. [DOI] [PubMed] [Google Scholar]
- 206.Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp. Neurol. 2003;179:167–75. doi: 10.1016/s0014-4886(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 207.Vijitruth R, Liu M, Choi DY, Nguyen XV, Hunter RL, Bing G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J. Neuroinflammation. 2006;3:6. doi: 10.1186/1742-2094-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Walsh DM, Selkoe DJ. A beta oligomers—a decade of discovery. J. Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 209.Wisely GB, Miller AB, Davis RG, Thornquest AD, Jr, Johnson R, et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 2002;10:1225–34. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 210.Yaffe K. Treatment of Alzheimer disease and prognosis of dementia: time to translate research to results. JAMA. 2010;304:1952–53. doi: 10.1001/jama.2010.1625. [DOI] [PubMed] [Google Scholar]
- 211.Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, et al. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg. Med. Chem. Lett. 2005;15:517–22. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 212.Yang HQ, Pan J, Ba MW, Sun ZK, Ma GZ, et al. New protein kinase C activator regulates amyloid precursor protein processing in vitro by increasing alpha-secretase activity. Eur. J. Neurosci. 2007;26:381–91. doi: 10.1111/j.1460-9568.2007.05648.x. [DOI] [PubMed] [Google Scholar]
- 213.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010;6:456–64. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 214.Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, et al. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE. 2010;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhao Y, Cui JG, Lukiw WJ. Reduction of sortilin-1 in Alzheimer hippocampus and in cytokine-stressed human brain cells. Neuroreport. 2007;18:1187–91. doi: 10.1097/WNR.0b013e32821c56c4. [DOI] [PubMed] [Google Scholar]
- 216.Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxi-some proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–75. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]