Abstract
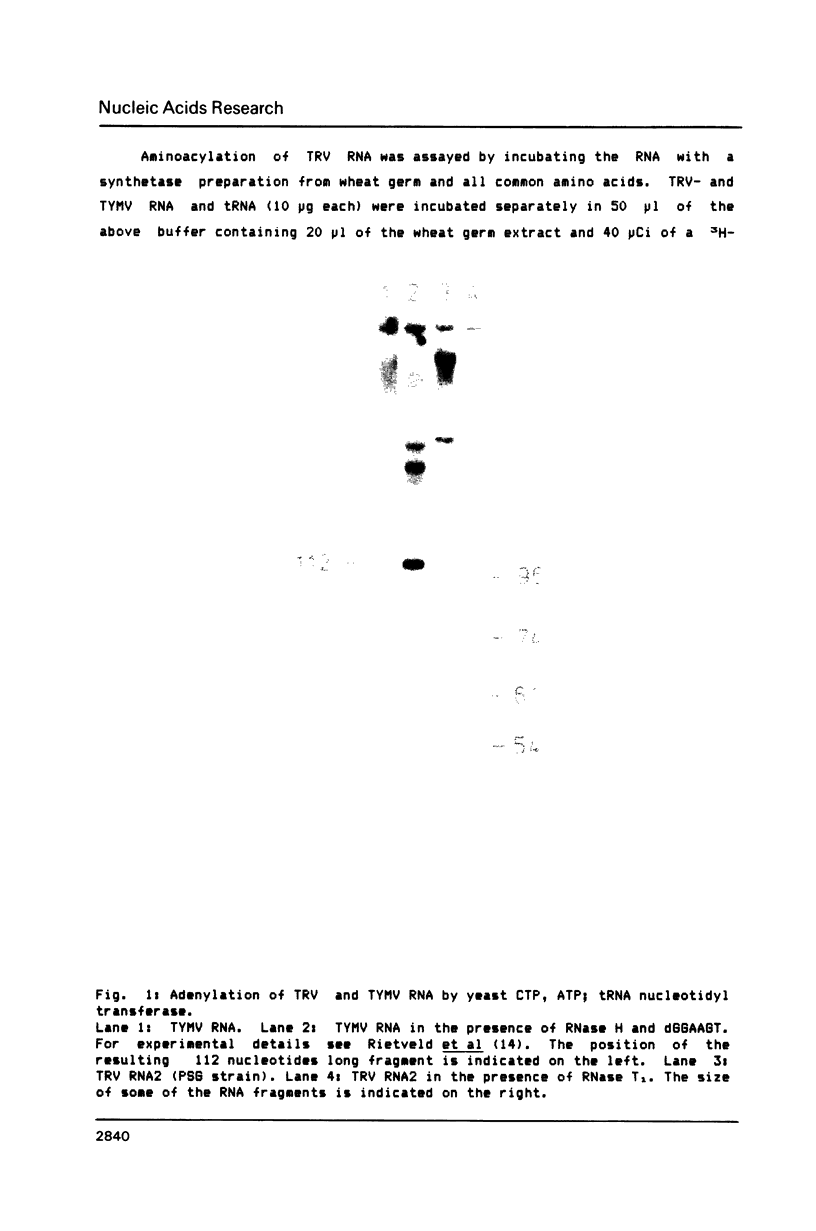
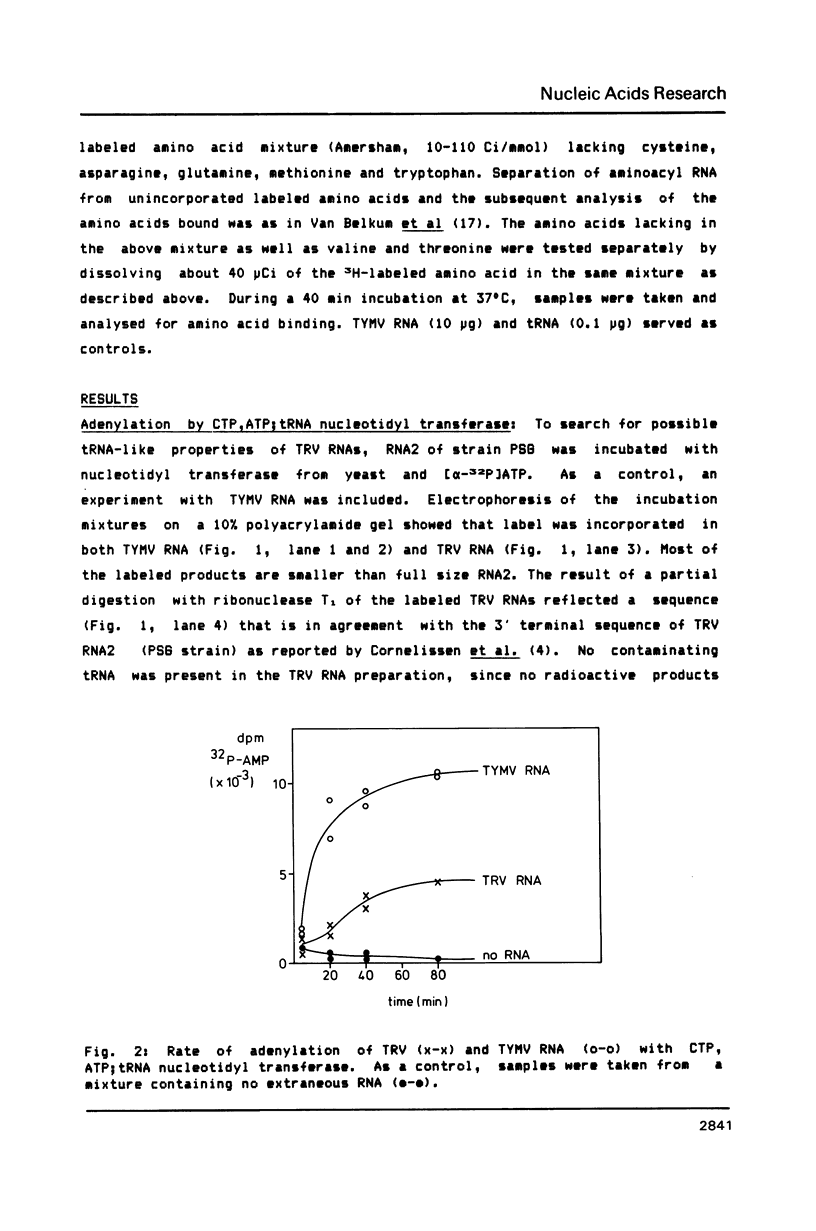
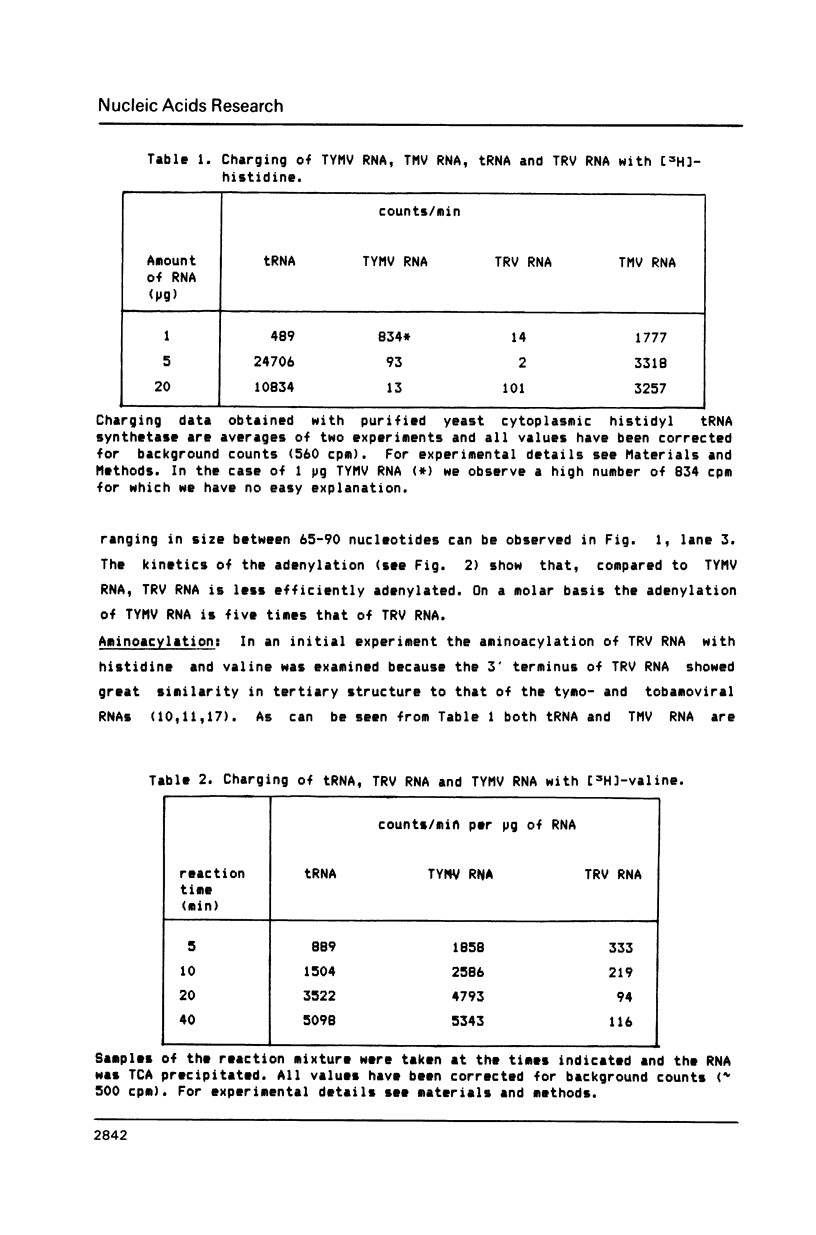
The 3' terminal forty nucleotides of tobraviral RNAs readily fold into a tertiary structure, resembling that of tymo- and tobamoviral RNAs. The latter RNAs possess a tRNA-like structure at their 3' end that is recognized by a number of tRNA-specific enzymes (Rietveld et al. (1984), EMBO J. 3, 2613-2619). Characteristic for their aminoacyl acceptor arm is the presence of a so-called pseudoknot which we now also find in a corresponding position at the 3' terminus of TRV RNA2 (PSG strain). The nucleotide sequences of all tobraviral RNAs analysed so far indicate that they all possess a similar 3' terminal structure. A domain resembling the anticodon arm of canonical tRNA is not readily recognizable. TRV RNA2 can be adenylated with CTP, ATP; tRNA nucleotidyl transferase and ATP. It is unable, however, to accept any of the twenty common amino acids when incubated with ATP and aminoacyl-tRNA synthetases from wheat germ or yeast. We conclude that TRV RNA contains a tRNA-like structure, which, in contrast to the tymo- and tobamoviral tRNA-like structures, cannot be aminoacylated. It is unlikely therefore, that aminoacylation of plant viral RNAs with a tRNA-like structure is a prerequisite for viral RNA replication.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Angenent G. C., Linthorst H. J., van Belkum A. F., Cornelissen B. J., Bol J. F. RNA 2 of tobacco rattle virus strain TCM encodes an unexpected gene. Nucleic Acids Res. 1986 Jun 11;14(11):4673–4682. doi: 10.1093/nar/14.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh S. T., Koziel M. G., Huang S. C., Thomas R. A., Gilley D. P., Siegel A. The nucleotide sequence of tobacco rattle virus RNA-2 (CAM strain). Nucleic Acids Res. 1985 Dec 9;13(23):8507–8518. doi: 10.1093/nar/13.23.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara M., Hamilton W. D., Baulcombe D. C. The organisation and interviral homologies of genes at the 3' end of tobacco rattle virus RNA1. EMBO J. 1986 Feb;5(2):223–229. doi: 10.1002/j.1460-2075.1986.tb04202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch L., Bonnet-Smits E. M., van Duin J. In situ breakage of turnip yellow mosaic virus RNA and in situ aggregation of the fragments. Virology. 1967 Mar;31(3):453–460. doi: 10.1016/0042-6822(67)90226-7. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Linthorst H. J., Brederode F. T., Bol J. F. Analysis of the genome structure of tobacco rattle virus strain PSG. Nucleic Acids Res. 1986 Mar 11;14(5):2157–2169. doi: 10.1093/nar/14.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. N., Williams K. L. Monoclonal antibody characterisation of slime sheath: the extracellular matrix of Dictyostelium discoideum. EMBO J. 1983;2(6):935–940. doi: 10.1002/j.1460-2075.1983.tb01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C. Transfer RNA-like structures in viral genomes. Int Rev Cytol. 1979;60:1–26. doi: 10.1016/s0074-7696(08)61257-7. [DOI] [PubMed] [Google Scholar]
- Harrison B. D., Robinson D. J. The tobraviruses. Adv Virus Res. 1978;23:25–77. doi: 10.1016/s0065-3527(08)60097-4. [DOI] [PubMed] [Google Scholar]
- Joshi R. L., Joshi S., Chapeville F., Haenni A. L. tRNA-like structures of plant viral RNAs: conformational requirements for adenylation and aminoacylation. EMBO J. 1983;2(7):1123–1127. doi: 10.1002/j.1460-2075.1983.tb01556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A., Gilbert W. Chemical probes for higher-order structure in RNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4679–4682. doi: 10.1073/pnas.77.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleij C. W., Rietveld K., Bosch L. A new principle of RNA folding based on pseudoknotting. Nucleic Acids Res. 1985 Mar 11;13(5):1717–1731. doi: 10.1093/nar/13.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rether B., Bonnet J., Ebel J. P. Studies on tRNA nucleotidyltransferase from baker's yeast. 1. Purification of the enzyme. Protection against thermal inactivation and inhibition by several substrates. Eur J Biochem. 1974 Dec 16;50(1):281–288. doi: 10.1111/j.1432-1033.1974.tb03896.x. [DOI] [PubMed] [Google Scholar]
- Rietveld K., Linschooten K., Pleij C. W., Bosch L. The three-dimensional folding of the tRNA-like structure of tobacco mosaic virus RNA. A new building principle applied twice. EMBO J. 1984 Nov;3(11):2613–2619. doi: 10.1002/j.1460-2075.1984.tb02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld K., Pleij C. W., Bosch L. Three-dimensional models of the tRNA-like 3' termini of some plant viral RNAs. EMBO J. 1983;2(7):1079–1085. doi: 10.1002/j.1460-2075.1983.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld K., Van Poelgeest R., Pleij C. W., Van Boom J. H., Bosch L. The tRNA-like structure at the 3' terminus of turnip yellow mosaic virus RNA. Differences and similarities with canonical tRNA. Nucleic Acids Res. 1982 Mar 25;10(6):1929–1946. doi: 10.1093/nar/10.6.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum A., Abrahams J. P., Pleij C. W., Bosch L. Five pseudoknots are present at the 204 nucleotides long 3' noncoding region of tobacco mosaic virus RNA. Nucleic Acids Res. 1985 Nov 11;13(21):7673–7686. doi: 10.1093/nar/13.21.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]