Abstract
Environmental neurotoxic exposure to agrochemicals has been implicated in the etiopathogenesis of Parkinson’s disease (PD). The widely used herbicide paraquat is among the few environmental chemicals potentially linked with PD. Since epigenetic changes are beginning to emerge as key mechanisms in neurodegenerative diseases, herein we examined the effects of paraquat on histone acetylation, a major epigenetic change in chromatin that can regulate gene expression, chromatin remodeling, cell survival and cell death. Exposure of N27 dopaminergic cells to paraquat induced histone H3 acetylation in a time-dependent manner. However, paraquat did not alter acetylation of another core histone H4. Paraquat-induced histone acetylation was associated with decreased total histone deacetylase (HDAC) activity and HDAC4 and 7 protein expression levels. To determine if histone acetylation plays a role in paraquat-induced apoptosis, the novel HAT inhibitor anacardic acid was used. Anacardic acid treatment significantly attenuated paraquat-induced caspase-3 enzyme activity, suppressed proteolytic activation and kinase activity of protein kinase C delta (PKCδ) and also blocked paraquat-induced cytotoxicity. Together, these results demonstrate that the neurotoxic agent paraquat induced acetylation of core histones in cell culture models of PD and that inhibition of HAT activity by anacardic acid protects against apoptotic cell death, indicating that histone acetylation may represent key epigenetic changes in dopaminergic neuronal cells during neurotoxic insults.
Keywords: Histone Modification, HDAC, apoptosis, pesticides, epigenetics, neurotoxicity
1. Introduction
Parkinson’s disease (PD) is a major neurodegenerative disorder among the elderly and is characterized mainly by progressive degeneration of dopaminergic cells in the substantia nigra pars compacta of the brain, resulting in irreversible motor dysfunction. Despite a very complex etiology of the disease, several gene mutations and environmental risk factors have been implicated (Brown et al., 2006; Le Couteur et al., 2002). In terms of environmental risk factors, epidemiological evidence supporting the possible involvement of agrochemicals exposure in the development of PD is overwhelming (Corrigan et al., 2000; Costello et al., 2009; Firestone et al., 2005; Fleming et al., 1994; Gorell et al., 1998; Hertzman et al., 1994; Priyadarshi et al., 2000; Semchuk et al., 1992). The herbicide 1,1′-dimethyl- 4,4′-bipyridium is widely used in agriculture and is marketed under the trade name paraquat; it is structurally similar to the parkinsonism-inducing neurotoxic agent 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) (Hertzman et al., 1990; Li et al., 2005; Liou et al., 1997; Peng et al., 2005). Like other dopaminergic neurotoxins, exposure to paraquat alone or together with other pesticides has been shown to cause nigral dopaminergic neuronal loss in cell culture and animal models (Dinis-Oliveira et al., 2006; Drechsel and Patel, 2008; Herrera et al., 2005; Peng et al., 2005; Thiruchelvam et al., 2000). Paraquat has further been shown to increase reactive oxygen species generation, caspase-3 activation and neurotoxicity in cell models (Castello et al., 2007; Drechsel and Patel, 2008; Miller et al., 2007; Yang and Sun, 1998a; Yang and Sun, 1998b). However, cellular and molecular mechanisms underlying the degenerative process induced by paraquat still remain unclear. Thus, clarification of the mechanisms and subcellular signal cascades that mediate paraquat-induced degeneration of dopaminergic cells may provide valuable insights into the molecular mechanisms underlying dopaminergic neurodegeneration following environmental chemical exposure in PD.
In recent years, epigenetic changes have been recognized as a key mechanism underlying the pathogenesis of chronic neurodegenerative diseases, including PD and Alzheimer’s disease (AD) (Abel and Zukin, 2008; Edwards and Myers, 2007; Mattson, 2003; Migliore and Coppede, 2009). However, little is known about epigenetic mechanisms underlying neurotoxic pesticide exposure in nigral dopaminergic systems and the relevance of these epigenetic changes to the pathogenesis of PD. A growing body of evidence from cell culture and animal models indicates that histone acetylation is linked with gene transcription, chromatin remodeling, embryonic development and oncogenesis (Gupta et al., 2008; Korzus et al., 2004; Yin et al., 2007). Acetylation of lysine residues on the N-terminal tail of core histones helps uncoil the chromatin to facilitate transcription. More strikingly, recent studies demonstrate that the balance in histone acetylation/deacetylation can be a critical factor in determining cell survival and cell death (Chen and Cepko, 2009; Marchion and Munster, 2007; Renthal et al., 2007; Soriano et al., 2009). Importantly, studies increasingly suggest that significant alterations in the critical balance of histone acetylation/deacetylation may contribute to chronic neurodegenerative processes (Saha and Pahan, 2006; Selvi and Kundu, 2009; Taylor et al., 2003). Histone acetyltransferase (HAT) and histone deacetylase (HDAC) are the opposing enzymes that dynamically regulate acetylation status of histones in cells. Maintaining the precise balance of HATs and HDACs is important for cell survival. Any aberrant changes in the homeostasis of HATs and HDACs might induce neuronal cell death (Boutillier et al., 2003; Rouaux et al., 2003; Saha and Pahan, 2006; Salminen et al., 1998).
The effect of environmental neurotoxic chemical exposure on histone acetylation and the functional consequences of histone acetylation in dopaminergic neuronal cell death have not been studied in detail. We recently reported that exposure to the organochlorine pesticide dieldrin hyperacetylates histone H3 and H4 via upregulation of CREB-binding protein (CBP), a transcriptional co-activator with intrinsic HAT activity, which causes apoptotic cell death in dopaminergic neuronal cells (Song et al., 2010). In the present study, we examined whether paraquat, a commonly used herbicide, alters histone acetylation in dopaminergic neuronal cells. Our results show paraquat induces histone hyperacetylation via a distinctive mechanism in which suppression of HDAC contributes to the hyperacetylation and neurotoxicity.
2. Materials and Methods
2.1. Chemicals
Paraquat was purchased from Sigma Chemical Co. (St. Louis, MO). Anacardic acid was purchased from Alexis Co. (Lausen, Switzerland). The caspase-3 substrate Ac-DEVD-AFC was obtained from Bachem Biosciences (King of Prussia, PA). RPMI 1640 medium, fetal bovine serum, L-glutamine, penicillin/streptomycin and Sytox green dye were obtained from Invitrogen (Carlsbad, CA). The Bradford protein assay kit was purchased from Bio-Rad (Hercules, CA). The primary antibodies used in this study were protein kinase C delta (PKCδ), caspase-3 (rabbit polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA), HDAC Antibody Sampler Kit (rabbit polyclonal, Cell Signaling Technology, Danvers, MA), β-actin (mouse monoclonal, Sigma, St. Louis, MO), acetyl-lysine (rabbit polyclonal) and Histone H3 (mouse monoclonal, Millipore, Charlottesville, VA). [3H]-dopamine ([3H]-DA) was purchased from PerkinElmer (Boston, MA). The Bradford protein assay kit was purchased from Bio-Rad (Hercules, CA). IRDye 800-conjugated anti-rabbit (Rockland labs, Gilbertsville, PA) and Alexa Fluor 680 conjugate anti-mouse (Licor, Lincoln, NE) were used.
2.2. Cell culture and treatment paradigm
Rat mesencephalic N27 dopaminergic cells were grown in RPMI-1640 medium containing 10% fetal bovine serum, 2 mM L-glutamine, 50 units penicillin and 50 μg/ml streptomycin and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells (2-3 days old) were used for experiments. N27 dopaminergic cells were treated with 400 μM paraquat for 12, 24 or 36 h. In the anacardic acid studies, cells with 60% confluency were pretreated with anacardic acid for 1 h and then exposed to 400 μM paraquat in media containing serum. Primary mesencephalic neuronal cultures were prepared from timed-pregnant C57BL/6 mice (gestation E14), as described previously (Zhang et al., 2007). Briefly, mesencephalic tissues were dissected from mouse embryos and maintained in ice-cold Ca2+-free HBSS; then HBSS solution containing trypsin-EDTA (0.25%) was used to dissociate the fetal brain tissues for 30 min at 37°C. The dissociated cells were then seeded at equal density (1 × 106 cells) in 30-mm-diameter tissue culture wells that were pre-coated with poly-D-lysine (1 mg/ml) and 10 μg/ml laminin. Cultures were maintained in a chemically defined medium consisting of neurobasal medium fortified with B-27 supplements, L-glutamine (500 μM), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (Invitrogen). The cells were then maintained in a humidified CO2 incubator (5% CO2, 37°C) for 6-7 d. Half of the culture medium was replaced every 2 days.
2.3. Histone extraction
After treatment, cells were collected by scraping and were washed thrice with ice-cold PBS. Whole histones were extracted with the PIERCE “NE-PER” kit and eventually dissolved into 0.2N HCl. Briefly, cell pellets were incubated with CERI buffer (supplied by NE-PER kit) plus 0.5% Triton X-100 for 10 min. Nuclei were collected by centrifugation at 2000 × g for 5 min. Then the pellet was resuspended in 0.2 N HCl and incubated on a rotator for 3 h at 4°C. After centrifuging for 10 min at maximum speed in a microfuge, supernatant was collected for further analysis.
2.4. Proteolytic activation of caspase-3 and PKCδ
After paraquat exposure, cells were washed with PBS (pH 7.4) and resuspended in caspase lysis buffer at 37°C for 20 min. Lysates were centrifuged at 14,000 rpm and the cell-free supernatants were incubated with 50 μM Ac-DEVD-AFC at 37°C for 1 h. Formation of 7-amino-4-methylcoumarin (AFC), resulting from caspase-3 activity, was measured at excitation 400 nm and emission 505 nm using a fluorescence plate reader. The caspase-3 cleavage and PKCδ cleavage were checked by Western blot (Kitazawa et al., 2003). Briefly, cell lysates containing equal amounts of protein were loaded in each lane and separated on a 10–12% SDS-PAGE gel. After separation, proteins were transferred to nitrocellulose membrane, and nonspecific binding sites were blocked by treating with Licor blocking buffer. The membranes then were incubated with primary antibodies directed against PKCδ (rabbit polyclonal, 1:2000 dilution) or caspase-3 (rabbit polyclonal, 1:1000). The primary antibody treatments were followed by treatment with secondary IR dye-800 conjugated anti-rabbit dye or Alexa Fluor 680 conjugated anti-mouse IgG for 1 h at room temperature (RT). To confirm equal protein loading, blots were reprobed with β-actin antibody (1:5000 dilution). Western blot images were captured with the Odyssey Infrared Imaging System (LI-COR) and data were analyzed using Odyssey 2.0 software.
2.5. Sytox cell death assay and morphometric studies
Cell death was determined by the cell-impermeable dye Sytox green (Invitrogen, Carlsbad, CA) after exposing the cells to paraquat with or without anacardic acid or sodium butyrate treatment. Sytox green enters only dead cells, and binds with DNA to produce green fluorescence (Roth et al., 1997; Sherer et al., 2002). Briefly, cells grown in 24-well plates were exposed to 400 μM paraquat with or without 8.5 μM anacardic acid or 1 mM sodium butyrate treatment together with 1 μM Sytox green in media containing serum. In the Sytox assay, dead cells can be viewed directly under the fluorescence microscope as well as quantitatively measured using the fluorescence microplate with excitation at 485 nm and emission at 538 nm using a fluorescent reader (SpectraMax Gemini XS Model, Molecular Devices, Sunnyvale, CA).
2.6. Nuclear extraction
After treatment, cells were collected by scraping and were washed thrice with ice-cold PBS. Nuclear and cytosolic fractions were separated with the Pierce NE-PER extraction kit. Briefly, cell pellets were dissolved in CERI solution containing protease inhibitor and HDAC inhibitor. CERII was added into each sample after 10 min incubation on ice for another 1 min. Cell suspension was then centrifuged at 16,000 × g for 5 min. Supernatant was discarded and cell pellets were dissolved in NERI solution and vortexed on the highest setting for 15 seconds every 15 min for a total of 4 times. The suspension was subjected to centrifugation at 16,000 × g for 10 min and the supernatant was collected as the nuclear fraction.
2.7. HAT assays
Each nuclear extract was harvested as described above and subjected to the assays. Nonradioactive HAT assays were carried out using a HAT assay kit (Millipore, Billerica, MA), according to the manufacturer’s instructions. Briefly, biotinylated histones were allowed to bind to the streptavidin-coated 96-well assay plates. After blocking and washing, each nuclear extract, together with acetyl-CoA and 1X HAT assay buffer, was added and incubated at RT for 30 min. After washing several times with PBS, acetylated histones were detected using an anti-acetyl-lysine rabbit polyclonal antibody, followed by the horseradish peroxidase-based secondary antibody. A colorimetric assay was carried out using TMB substrate mixture (10 min), and the reaction was stopped by adding 1 M sulfuric acid. The color development was measured at 450 and 570 nm. The 570-nm values were subtracted from the 450-nm values to avoid any variations.
2.8. HDAC assay
HDAC activity was examined in vitro using a commercial HDAC fluorescent activity assay/drug discovery kit (Enzo, Plymouth Meeting, PA), in which fluorescent product is generated from a deacetylation process of a synthetic, acetylated substrate. After 3, 6, 12, or 24 h exposure to paraquat, N27 dopaminergic cells were harvested and lysed in nuclear extraction buffer supplemented with protease inhibitor. The assay was conducted at RT according to the manufacturer’s protocol. The samples were prepared in triplicate and the fluorescence was measured on a fluorescence counter within 30 min, with excitation at 360 nm and emission at 460 nm after stopping the reaction.
2.9. Data analysis
Data analysis was performed using Prism 4.0 software (GraphPad Software, San Diego, CA). Data were first analyzed using one-way ANOVA and then Bonferroni’s post-test was performed to compare all treatment groups. Differences with p < 0.05 were considered significant.
3. Results
3.1. Paraquat-induced cytotoxicity in N27 dopaminergic cells
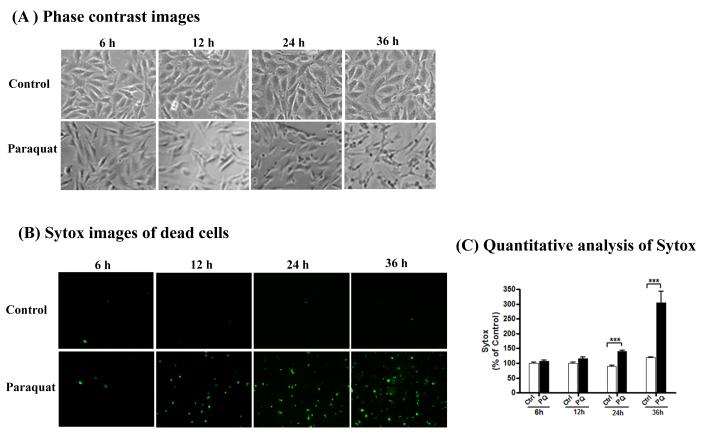
N27 dopaminergic cells were exposed to 400 μM paraquat for 6, 12, 24 and 36 h. The dose was selected from published literature and preliminary studies (Peng et al., 2004; Peng et al., 2005). Morphological changes were measured by phase-contrast microscopy and cytotoxicity was monitored both qualitatively and quantitatively with the Sytox fluorescence assay. Microscopic analysis clearly displayed cell death induced by paraquat, evidenced by Sytox positive green cells in paraquat-treated cells (Fig. 1A-B). Quantitative analysis of Sytox fluorescence using a fluorescence plate reader revealed a time-dependent increase in cytotoxicity in N27 dopaminergic cells following paraquat treatment (Fig. 1C). Treatment with 400 μM paraquat for 24 and 36 h increased cytotoxicity (sytox fluorescence) in cells by 140% and 300% compared with controls, respectively.
Fig. 1. Paraquat induced cytotoxicity in dopaminergic neuronal N27 dopaminergic cells.
N27 dopaminergic neuronal cells were exposed to 400 μM paraquat for indicated time. (A) Phase contrast images of N27 dopaminergic cells treated with paraquat, (B) Sytox fluorescence staining in N27 dopaminergic cells treated with paraquat, (C) Sytox green fluorescence in cells treated with paraquat was also quantified using a microplate reader. The data represent n =6. Asterisk ***p < 0.001 represents significant difference between the control and paraquat-treated group.
3.2. Paraquat induced proteolytic cleavage of PKCδ
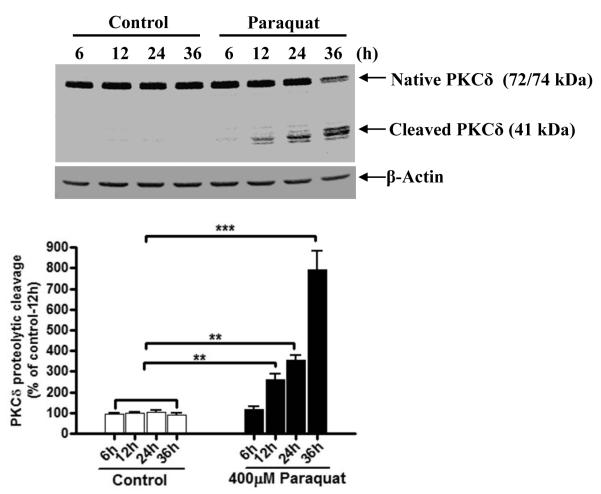
Previously we demonstrated that several dopaminergic toxicants induce apoptotic cell death by activating the mitochondrial-dependent pathway involving caspase-3 dependent proteolytic activation of PKCδ (Kaul et al., 2005; Kitazawa et al., 2003; Latchoumycandane et al., 2005). We routinely use PKCδ proteolytic activation as a key marker of apoptotic cell death. Exposure to paraquat over a 36 h time period resulted in the proteolytic cleavage of native PKCδ (74 kDa) into the 41 kDa persistently active catalytic fragment and 38 kDa regulatory fragment (Fig. 2). The proteolytic cleavage of PKCδ increased in a time-dependent manner. For example, 400 μM paraquat exposure for 12, 24 or 36 h resulted in 1.5-, 2.5-, and 7-fold increases in PKCδ proteolytic cleavage, respectively. Cleaved PKCδ was detected after 12 h following treatment with 400 μM paraquat and reached the maximum at 36 h in N27 dopaminergic cells. No proteolytic cleavage of PKCδ was measured in control N27 dopaminergic cells during the entire exposure period.
Fig. 2. Paraquat induced proteolytic cleavage of PKCδ.
N27 dopaminergic cells were treated with 400 μM paraquat for 6, 12, 24 or 36 h respectively. (A) PKCδ cleavage was examined in the cell lysate by immunoblotting against anti-PKCδ antibody. Equal loading of protein was demonstrated by using β-actin. (B) Densitometric quantification of cleaved PKCδ band statistical significance between control and the paraquat exposed groups was determined by ANOVA. Asterisks (*p < 0.05; ***p < 0.001) indicate significant difference compared with control and paraquat-treated cells.
3.3. Paraquat induces acetylation of core histones H3 and H4 in a time-dependent manner in N27 dopaminergic cells
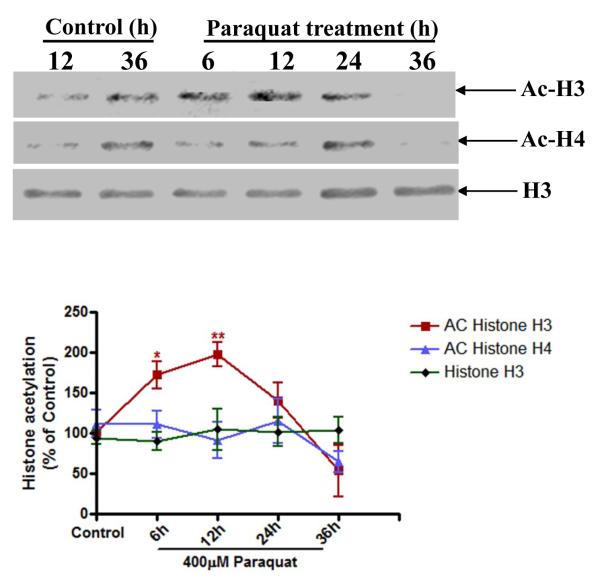
Emerging evidence indicates that acetylation and deacetylation of histones can profoundly influence various functions, including cell death, in response to stress (Chandramohan et al., 2007; Jakobsson et al., 2008; Mattson, 2003; Rouaux et al., 2003; Soriano et al., 2009). Our recent study showed that the organochlorine pesticide dieldrin induced hyperacetylation of core histones in N27 dopaminergic cells to promote apoptosis (Song et al., 2010). Therefore, we examined whether exposure to the neurotoxic herbicide paraquat also induces acetylation of histones. N27 dopaminergic cells were treated with 400 μM paraquat for 6, 12, 24 and 36 h and then the nuclear fraction was isolated. Histone acetylation was examined in the nuclear extract by Western blot analysis using anti-acetyl-lysine antibody. As shown in Fig. 3, a time-dependent acetylation of core histone H3 was observed in paraquat-treated cells as compared to control cells. The acetylation of histone H3 occurred as early as 6 h and resulted in a 70% increase after paraquat exposure. The acetylation of H3 reached the maximum at 12 h, which was 2-fold greater than control cells. However, 36 h after paraquat exposure, acetylation was dramatically reduced, possibly due to loss of integrity resulting from cell death during the longer exposure. Also, paraquat treatment did not alter acetylation of H4. Further, we observed a marginal increase in H3 and H4 acetylation levels in control samples between 12 and 36 h. The increase in the basal level of histone acetylation could be attributed to the on-going aging process in the cells. However, it is important to point out that at 12 and 24 h acetylated H3 and H4 are significantly increased in paraquat-treated cells compared to time-matched untreated controls. The expression levels of non-acetylated H3 bands were unaffected during paraquat exposure suggesting equal protein. Together, these results demonstrate that paraquat exposure induces histone acetylation in dopaminergic neuronal cells.
Fig. 3. Paraquat increases acetylation of core histones H3 in N27 dopaminergic cells.
(A) N27 dopaminergic cells were exposed to 400 μM paraquat and then acetylation of histones H3 and H4 was monitored at various time points. Native H3 was used as an internal control. (B) Densitometric quantification of acetylated H3 bands and H4 bands in Fig. 3A was analyzed. Statistical significance between the control group and each treatment group was determined by ANOVA, *p < 0.05; **p < 0.01.
3.4. Paraquat treatment represses HDAC activity and expression in N27 dopaminergic cells
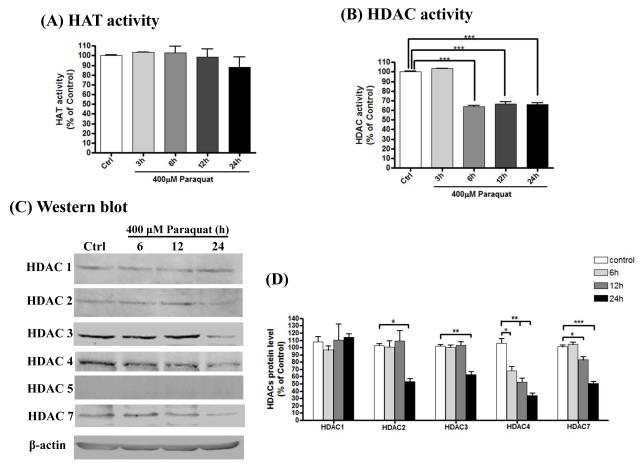
Histone hyperacetylation can be achieved by HAT activation or HDAC inactivation. HATs have been reported to play important roles in acetylation reactions in the neuronal system. We previously demonstrated that the HAT domain of CBP is critical to the hyperacetylation of histone H3 and H4 induced by dieldrin treatment. Therefore, first we examined whether the histone acetylation observed during paraquat exposure was due to an increase in the HAT activity. Surprisingly, total HAT activity after paraquat treatment was not increased (Fig. 4A). Next we examined whether the inactivation of HDAC plays any role in paraquat-induced histone acetylation. Measurement of HDAC activity in the nuclear extracts of paraquat-treated cells showed a significant reduction in HDAC activity following 400 μM paraquat exposures for 3, 6, 12 or 24 h (Fig. 4B). HDAC activity after 6 h of paraquat exposure was reduced to 60% compared with control cells, and remained reduced during the entire treatment period. In preliminary studies, we also observed that lower doses of paraquat (100-300 μM) for a longer period of time (24-72 hr) induced hyperacetylation and HDAC inhibition (data not shown).
Fig. 4. Paraquat treatment represses HDAC activity and expression in N27 dopaminergic cells.
N27 dopaminergic cells were exposed to 400 μM paraquat for 3, 6, 12 or 24 h respectively. (A) Total HAT activity was examined in the nuclear extractions from control or each treated samples by HAT assay, as described in the Methods section. (B) Nuclear HDAC activity was also detected in those nuclear extractions from each treatment. ***p < 0.001 indicates significant difference compared with control cells and paraquat-treated cells. (C) Various specific HDACs protein level was assessed in whole-cell lysates of each sample by immunoblotting. Equal loading of protein was demonstrated by using β-actin. (D) Densitometric quantification of each HDAC protein band was measured from triplicates and analyzed. Statistical significance between control and paraquat-exposed groups for individual HDAC was determined by ANOVA. Asterisks (*p < 0.05; ***p < 0.001) indicate significant difference compared with control and paraquat-treated cells.
We next examined whether reduced HDAC activity was a result of reduced expression of HDAC isoform protein levels. The expression of HDAC1, 2, 3, 4, 5, and 7 levels in control and paraquat-treated cells was measured and used to determine HDAC protein levels by Western blot using an HDAC Antibody Sampler Kit (Cell Signaling) and normalized to the β-actin protein. Paraquat exposure induced a time-dependent decrease in both HDAC4 and HDAC7 protein levels. For example, 6, 12 and 24 h exposure to paraquat resulted in reduction of the HDAC4 protein level to 60%, 50% and 30% of the control level. HDAC7 protein level decreased to 80% and 50% of the control level at 12 h and 24 h treatment, respectively. However, the expression level of HDAC1 did not change during the entire treatment period (Fig. 4C-D). HDAC2 and 3 only started to decrease until 24 h after paraquat exposure (Fig. 4C-D). HDAC5 was not detected in N27 dopaminergic cells. Comparison of the HDAC isoform expression levels and HDAC activity revealed a significant reduction in the protein expression levels of HDAC4 and HDAC7 as early as 6 h and 12 h, respectively. It is possible that HDAC4 and HDAC7 are predominantly affected isoforms during early stages of paraquat-induced toxicity. The HDAC activity kit we used in our study measures the total combined activity of all HDAC isoforms. At this point, published data describing the relative activity and expression levels of HDAC isoforms in neuronal cells during toxic insults are not available. As we have shown in Fig 4C, the different HDAC isoforms are differently expressed in untreated N27 dopaminergic cells (HDAC3=HDAC4 > HDAC7>HDAC1=HDAC2).
3.5. Hyperacetylation of histones promotes paraquat-induced caspase-3 activation and PKCδ proteolytic activation in dopaminergic cells
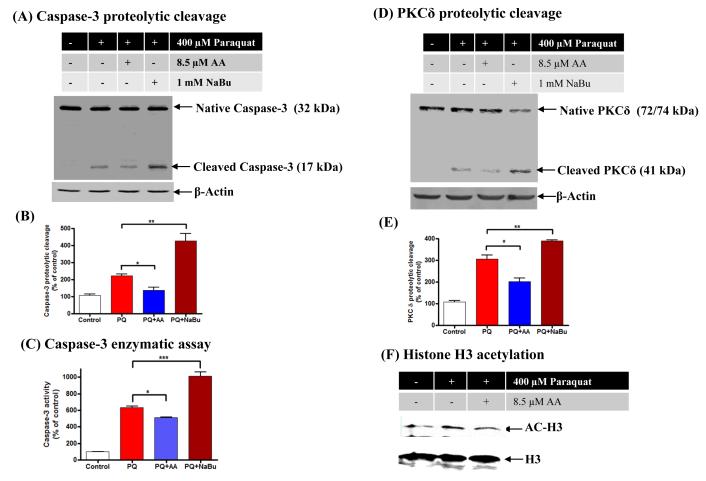
To further demonstrate that histone acetylation plays a role in paraquat-induced apoptosis, we used the HAT inhibitor anacardic acid, which reduces acetylation, and the HDAC inhibitor sodium butyrate (NaBu), which increases acetylation. The cleaved caspase-3 product was measured as a marker of apoptosis and the data revealed that 8.5 μM anacardic acid significantly attenuated acetylation, while NaBu significantly exacerbated paraquat-induced caspase-3 proteolytic cleavage (Fig. 5A-B). Furthermore, measurement of caspase-3 enzyme activity, using the fluorogenic caspase-3 substrate Ac-DEVD-AFC, also revealed that anacardic acid effectively attenuated paraquat-induced caspase-3 activity while NaBu exacerbated caspase-3 activation (Fig. 5C). These findings indicate that inhibition of histone acetylation protects dopaminergic cells against neurotoxic pesticide paraquat-induced apoptosis, whereas increased histone acetylation exacerbates the neurotoxicity.
Fig. 5. HAT inhibitor anacardic acid attenuates paraquat-induced PKCδ proteolytic cleavage and caspase-3 activation, and HDAC inhibitor NaBu enhances PKCδ proteolytic cleavage and caspase-3 activation.
N27 dopaminergic cells were pretreated with 8.5 μM anacardic acid or 1mM NaBu for 1 h and then exposed to 400 μM paraquat. (A) Caspase-3 proteolytic activation was examined by cleaved caspase-3 Western blot and (B) Densitometric quantification of cleaved caspase-3 band intensity then was analyzed. Statistical significance between the paraquat exposure groups with or without anacardic acid/NaBu co-treatment was determined by ANOVA, *p < 0.05, **p <0.01. (C) Measurement of caspase-3 enzyme activity by fluorogenic caspase-3 substrate Ac-DEVD-AFC. Asterisks (*p < 0.05, ***p < 0.001) indicate significant differences between anacardic acid or NaBu pretreated and paraquat-alone treated cells. (D) PKCδ cleavage was measured in the cell lysate by immunoblotting. Equal loading of protein was demonstrated by using β-actin. (E) Densitometric quantification of cleaved PKCδ band statistical significance between the paraquat exposed groups, in the presence and absence of anacardic acid or NaBu treatment, was determined by ANOVA, *p < 0.5, **p < 0.01. (F) H3 acetylation with or without anacardic acid treatment was measured from the nuclear histone extract by Western blot. Native H3 was used as an internal control.
Next, we examined if anacardic acid also can reduce paraquat-induced proteolytic activation of the proapoptotic kinase PKCδ. Anacardic acid effectively blocked paraquat-induced PKCδ proteolytic cleavage, but NaBu significantly increased the cleavage and activation of PKCδ (Fig. 5D-E). Anacardic acid suppressed paraquat-induced PKCδ proteolytic cleavage by 33% and NaBu increased the cleavage of PKCδ by 30%. Anacardic acid at 8.5 μM co-treatment also reduced hyperacetylation of histone H3 (Fig 5F). Taken together with caspase-3 cleavage, these results suggest that hyperacetylation may play a proapoptotic role in dopaminergic neuronal cells following treatment with the neurotoxic pesticide paraquat.
3.6. Inhibition of hyperacetylation protects dopaminergic cells from paraquat-induced apoptosis and neurotoxicity
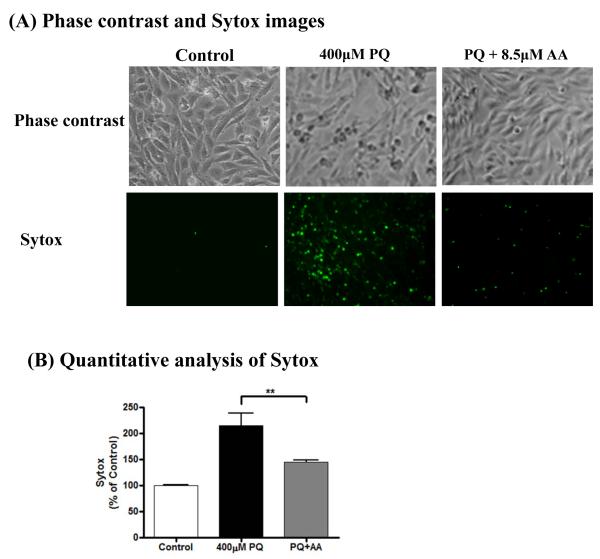
To further determine the role of hyperacetylation in the neurotoxicity induced by paraquat in dopaminergic cells, we used anacardic acid, which has been shown to effectively inhibit HAT activity (Balasubramanyam et al., 2003; Song et al., 2010; Sun et al., 2005). Anacardic acid at 8.5 μM effectively inhibits histone acetylation (Song et al., 2010). We examined whether anacardic acid can alter paraquat-induced neurotoxicity. The Sytox green cytotoxicity assay was carried out to determine the neurotoxic response. N27 dopaminergic cells were exposed to 400 μM paraquat in the presence or absence of 8.5 μM anacardic acid for 36 h. As shown in Fig. 6A, treatment with 8.5 μM anacardic acid for 36 h attenuated paraquat-induced morphological changes measured by phase-contrast microscopy and Sytox green fluorescence microscopy. Quantification of Sytox fluorescence (Fig. 6B) revealed significant protection from paraquat-induced neurotoxicity by anacardic acid, indicating that anacardic acid, as an inhibitor of histone acetylation, ameliorates cell death induced by paraquat in dopaminergic neuronal cells.
Fig. 6. Paraquat-induced dopaminergic neuronal cell death is attenuated by anacardic acid treatment.
N27 dopaminergic cells were pretreated with 8.5 μM anacardic acid for 1 h and then exposed to 400 μM paraquat for 36 h. (A) Phase contrast images and Sytox fluorescence staining of N27 dopaminergic cells treated with paraquat in the presence and absence of anacardic acid. (B) Sytox green fluorescence in cells treated with paraquat with or without anacardic acid was also quantified using a microplate reader. The data represent n =6. **p < 0.01 represents significant difference between the paraquat-treated group and the cells treated with paraquat plus anacardic acid.
3. Discussion
Our results demonstrate that the neurotoxic herbicide paraquat induces acetylation of histones in dopaminergic neuronal cells and that the hyperacetylation can contribute to paraquat-induced apoptosis. Histone acetylation status depends on the dynamic balance between the activity of HAT and HDAC. Maintaining the precise balance of HATs and HDACs is critical for normal functioning of cells. Any aberrant changes in the homeostasis of HATs and HDACs might result in cell death (Boutillier et al., 2003; Rouaux et al., 2003; Saha and Pahan, 2006; Salminen et al., 1998). We found that total HDAC activity was decreased, despite little change in HAT activity after paraquat exposure. Moreover, protein expression levels of HDAC4 and 7 were affected at the early time point after paraquat treatment. For the first time we report that HDACs are differentially expressed under basal conditions as well as during early and late stages of paraquat-induced cell death in N27 dopaminergic cells. Thus, the loss of equilibrium between HDAC activity and HAT activity leads to histone hyperacetylation in paraquat-treated cells.
The effect of environmental neurotoxic chemicals on histone acetylation has not been studied in detail. Recently, we reported that the environmental neurotoxic pesticide dieldrin induces acetylation of H3 and H4 in dopaminergic cells due to accumulation of the key histone acetyltransferase CBP (Song et al., 2010). In order to determine whether additional neurotoxic agents can induce histone acetylation, we examined the effect of the herbicide paraquat on histone acetylation in the present study. Although we found paraquat induces histone acetylation similar to dieldrin, some key differences we noted. First, paraquat-induced acetylation was limited to H3 histone as compared to acetylation of both H3 and H4 acetylation following dieldrin exposure. Second, paraquat reduced the HDAC level but did not alter HAT activity. Therefore, the mechanisms of histone acetylation used by various neurotoxicants appear to be distinct. Further characterization of the effect of other neurotoxicants on histone acetylation will provide greater insight into the role of histone acetylation and epigenetic changes underlying neurotoxic exposure. Comparison of the time course of paraquat-induced cell death and histone acetylation revealed that histone acetylation preceded the cell death, suggesting that histone modification is an early event in the neurotoxicity. Importantly, we found paraquat-induced PKCδ-dependent cleavage is significantly attenuated in the presence of the HAT inhibitor anacardic acid and exacerbated in the presence of the HDAC inhibitor NaBu. We further demonstrate that paraquat-induced cytotoxicity is blocked by the HAT inhibitor anacardic acid but is exacerbated by the HDAC inhibitor NaBu, indicating that histone hyperacetylation promotes the neuronal cell death processes during neurotoxic insult. The results of the present study suggest that core histone H3 hyperacetylation is related to reduced HDAC activity, which in turn contributes to apoptotic cell death.
Among various mammalian HDACs that regulate histone deacetylation, our data suggest that members of the class II family, HDAC4 and 7, play a key role in paraquat-induced hyperacetylation of histone H3. Our data also show paraquat-induced increases in core histone H3 acetylation, but not in other core histones: H2A, H2B and H4. In addition, we also have demonstrated that protein expression of two members from the mammalian class II HDAC family, HDAC4 and 7, is significantly reduced in paraquat-treated samples. HDAC4 is abundantly expressed in the brain, suggesting its critical role in neurons (Grozinger et al., 1999). A previous study reported that HDAC4 appears to mediate neuronal cell death, because inactivation of HDAC4 by small interfering RNA or HDAC inhibitors suppresses neuronal death induced by low-potassium or excitotoxic glutamate conditions (Bolger and Yao, 2005). On the contrary, later, more careful examination of HDAC4-null mice revealed a delayed formation of the folia, indicating that HDAC4 acts in a pro-survival manner rather than a pro-apoptotic one (Majdzadeh et al., 2008; Vega et al., 2004). A recent study confirmed the HDAC4 pro-survival role, in which overexpression of HDAC4 attenuates low-potassium-induced neuronal cell death through inactivation of cyclin-dependent kinase-1. Moreover, the study also demonstrated that HDAC4 protects neuroblastoma cells against cell death induced by oxidative stress (Majdzadeh et al., 2008). Similarly, studies using a model of mouse retinal degeneration showed that HDAC4 promotes survival of photoreceptor cells (Chen and Cepko, 2009). There are very few studies investigating the role of HDAC7 in neuronal systems (Ajamian et al., 2004; Hoshino et al., 2003; Sharma et al., 2008). HDAC7 associates with transcription factors such as myocyte enhancer factor 2 (MEF2) and regulates cell differentiation, cell migration, gene expression, and animal development (Gao et al., 2010; Gregoire et al., 2007). We also noted a significant reduction in HDAC2 and HDAC3 at 24 h, but the reduction was much lower than reductions of HDAC4 and HDAC7. Collectively, our results indicate that HDAC isoforms are differentially affected by neurotoxic insult induced by paraquat in dopaminergic neuronal cells.
Many different types of HDAC inhibitors (HDACi) have been discovered and described (Candido et al., 1978; Jeon et al., 2010; Kim et al., 1999; Remiszewski, 2003; Richon et al., 1998; Schlake et al., 1994). The effect of HDAC inhibitors on neuronal cell survival and death has been controversial. Some studies indicate that HDAC inhibitors are protective against neurodegeneration in a variety of cell culture and in vivo models (Butler and Bates, 2006; Langley et al., 2005; Steffan et al., 2001). However, other reports demonstrate that HDAC inhibitors induce neuronal cell death, and overexpression of HDAC can protect cultured neurons (Chen and Cepko, 2009; Majdzadeh et al., 2008; Wang et al., 2009). Our results indicate that HDAC inhibitors induce neuronal apoptosis. Notably, we observed that the classic HDAC inhibitor NaBu significantly augmented the paraquat-induced caspase-3 inhibitor, suggesting a proapoptotic function of HDAC in dopaminergic neurotoxicity. Inhibition of HDACs can result in the accumulation of hyperacetylated histones, followed by the transcriptional activation of certain genes by weakening histone-DNA interactions, relaxing the DNA conformation, and regulating the cell cycle and apoptosis (Butler and Bates, 2006; Langley et al., 2005; Steffan et al., 2001). To examine if the histone acetylation homeostasis is critical to dopaminergic neuronal cell survival following neurotoxic insults, we reduced the acetylation level by using the general HAT inhibitor anacardic acid and then determined the paraquat neurotoxicity. Anacardic acid effectively attenuated increases in the activation of caspase-3 and proapoptotic kinase PKCδ in dopaminergic cells treated with the neurotoxicant paraquat. Further studies are needed to elucidate how upstream signaling cascades and other regulatory mechanisms reduce activity of these HDACs and their influence on neuronal apoptosis after paraquat exposure.
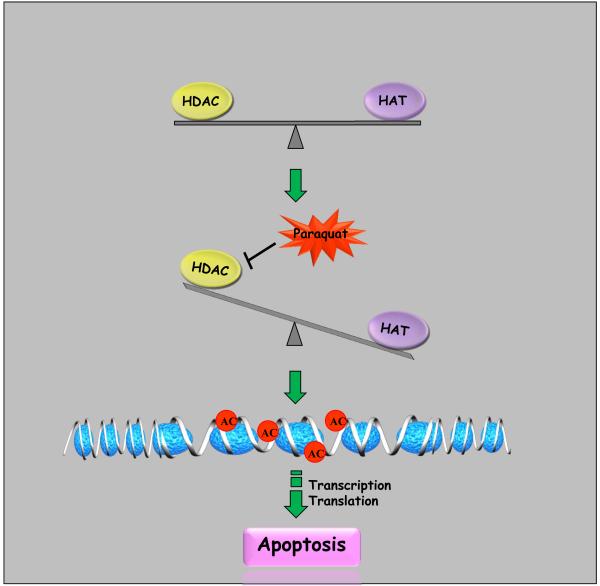
In summary, we have shown the neurotoxic herbicide paraquat induced hyperacetylation of core histone H3 in dopaminergic neuronal cells by reducing HDAC activity. Suppression of paraquat-induced H3 acetylation by the HAT inhibitor anacardic acid attenuated the apoptotic cell death. Alternatively, hyperacetylation caused by treatment with the HDAC inhibitor NaBu exacerbates apoptotic cell death. These findings suggest that core histone acetylation status plays a key role in determining cell survival or cell death. As summarized in Fig. 7, we propose that environmental neurotoxic agents can alter the homeostasis of histone acetylation by causing imbalance in HAT/HDAC levels, which might be important in dopaminergic neurotoxicity.
Fig. 7. Schematic representation of mechanisms underlying paraquat-induced histone H3 hyperacetylation.
Exposure to neurotoxic insult paraquat inhibits total HDAC activity, particular HDAC4 and 7, resulting in imbalance between HDAC and HAT. Decreased HDAC activity results in greater acetylation of nuclear histone H3 in the chromatin, which ultimately results in alterations of gene expression associated with the neurodegenerative process including oxidative damage and apoptosis in dopaminergic cells.
Acknowledgements
The authors also acknowledge Ms. Mary Ann deVries for her assistance in the preparation of this manuscript. This work was supported by National Institutes of Health (NIH) [Grants ES10586, NS65167 and ES 19267]. The W. Eugene and Linda Lloyd Endowed Chair to A.G.K. is also acknowledged.
Abbreviations
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- CBP
CREB-binding protein
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- PKCδ
protein kinase C delta
- paraquat
1,1′-dimethyl- 4,4′-bipyridium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- DA
Dopamine
- H3 and H4
Histone H3 and H4
- HDACi
HDAC inhibitor
- NaBu
sodium butyrate
- AFC
7-amino-4-methylcoumarin
- [3H]-DA
[3H]-dopamine
Footnotes
Conflict of interest There are no conflicts of interest to declare.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajamian F, Salminen A, Reeben M. Selective regulation of class I and class II histone deacetylases expression by inhibitors of histone deacetylases in cultured mouse neural cells. Neurosci Lett. 2004;365:64–8. doi: 10.1016/j.neulet.2004.04.087. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–40. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]
- Bolger TA, Yao TP. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci. 2005;25:9544–53. doi: 10.1523/JNEUROSCI.1826-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem. 2003;84:814–28. doi: 10.1046/j.1471-4159.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease--is there a link? Environ Health Perspect. 2006;114:156–64. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R, Bates GP. Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nat Rev Neurosci. 2006;7:784–96. doi: 10.1038/nrn1989. [DOI] [PubMed] [Google Scholar]
- Candido EP, Reeves R, Davie JR. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978;14:105–13. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–93. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Reul JM. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-D-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J Neurochem. 2007;101:815–28. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323:256–9. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59:229–34. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–26. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, et al. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27:1110–22. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44:1873–86. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ Health Perspect. 2007;115:1264–70. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT, Jr., Checkoway H. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol. 2005;62:91–5. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–3. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Gao C, Liu Y, Lam M, Kao HY. Histone deacetylase 7 (HDAC7) regulates myocyte migration and differentiation. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamcr.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–50. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Gregoire S, Xiao L, Nie J, Zhang X, Xu M, Li J, et al. Histone deacetylase 3 interacts with and deacetylates myocyte enhancer factor 2. Mol Cell Biol. 2007;27:1280–95. doi: 10.1128/MCB.00882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci U S A. 1999;96:4868–73. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AJ, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Inflammatory process as a determinant factor for the degeneration of substantia nigra dopaminergic neurons. J Neural Transm. 2005;112:111–9. doi: 10.1007/s00702-004-0121-3. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Bowering D, Snow B, Calne D. Parkinson’s disease: a case-control study of occupational and environmental risk factors. Am J Ind Med. 1990;17:349–55. doi: 10.1002/ajim.4700170307. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Snow B, Kelly S, Calne D. A case-control study of Parkinson’s disease in a horticultural region of British Columbia. Mov Disord. 1994;9:69–75. doi: 10.1002/mds.870090111. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Tagawa K, Okuda T, Murata M, Oyanagi K, Arai N, et al. Histone deacetylase activity is retained in primary neurons expressing mutant huntingtin protein. J Neurochem. 2003;87:257–67. doi: 10.1046/j.1471-4159.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- Jakobsson J, Cordero MI, Bisaz R, Groner AC, Busskamp V, Bensadoun JC, et al. KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron. 2008;60:818–31. doi: 10.1016/j.neuron.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Jeon HS, Ahn MY, Park JH, Kim TH, Chun P, Kim WH, et al. Anticancer effects of the MHY218 novel hydroxamic acid-derived histone deacetylase inhibitor in human ovarian cancer cells. Int J Oncol. 2010;37:419–28. doi: 10.3892/ijo_00000690. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Kanthasamy A, Kanthasamy AG. Wild-type alpha-synuclein interacts with pro-apoptotic proteins PKCdelta and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res. 2005;139:137–52. doi: 10.1016/j.molbrainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kim YB, Lee KH, Sugita K, Yoshida M, Horinouchi S. Oxamflatin is a novel antitumor compound that inhibits mammalian histone deacetylase. Oncogene. 1999;18:2461–70. doi: 10.1038/sj.onc.1202564. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–64. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–72. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley B, Gensert JM, Beal MF, Ratan RR. Remodeling chromatin and stress resistance in the central nervous system: histone deacetylase inhibitors as novel and broadly effective neuroprotective agents. Curr Drug Targets CNS Neurol Disord. 2005;4:41–50. doi: 10.2174/1568007053005091. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Muller M, Yang MC, Mellick GD, McLean AJ. Age-environment and gene-environment interactions in the pathogenesis of Parkinson’s disease. Rev Environ Health. 2002;17:51–64. doi: 10.1515/reveh.2002.17.1.51. [DOI] [PubMed] [Google Scholar]
- Li X, Yin J, Cheng CM, Sun JL, Li Z, Wu YL. Paraquat induces selective dopaminergic nigrostriatal degeneration in aging C57BL/6 mice. Chin Med J (Engl) 2005;118:1357–61. [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48:1583–8. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Majdzadeh N, Wang L, Morrison BE, Bassel-Duby R, Olson EN, D’Mello SR. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol. 2008;68:1076–92. doi: 10.1002/dneu.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchion D, Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007;7:583–98. doi: 10.1586/14737140.7.4.583. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Methylation and acetylation in nervous system development and neurodegenerative disorders. Ageing Res Rev. 2003;2:329–42. doi: 10.1016/s1568-1637(03)00013-8. [DOI] [PubMed] [Google Scholar]
- Mercille S, Massie B. Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotechnol Bioeng. 1994;44:1140–54. doi: 10.1002/bit.260440916. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res. 2009;674:73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–39. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Lopez C, Jammu A. Oxidative stress and activation of proteasome protease during serum deprivation-induced apoptosis in rat hepatoma cells; inhibition of cell death by melatonin. Apoptosis. 2003;8:497–508. doi: 10.1023/a:1025542424986. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280:29194–8. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology. 2000;21:435–40. [PubMed] [Google Scholar]
- Remiszewski SW. The discovery of NVP-LAQ824: from concept to clinic. Curr Med Chem. 2003;10:2393–402. doi: 10.2174/0929867033456675. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HEr, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–29. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci U S A. 1998;95:3003–7. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Poot M, Yue ST, Millard PJ. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–31. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Jokic N, Mbebi C, Boutillier S, Loeffler JP, Boutillier AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. Embo J. 2003;22:6537–49. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13:539–50. doi: 10.1038/sj.cdd.4401769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Tapiola T, Korhonen P, Suuronen T. Neuronal apoptosis induced by histone deacetylase inhibitors. Brain Res Mol Brain Res. 1998;61:203–6. doi: 10.1016/s0169-328x(98)00210-1. [DOI] [PubMed] [Google Scholar]
- Schlake T, Klehr-Wirth D, Yoshida M, Beppu T, Bode J. Gene expression within a chromatin domain: the role of core histone hyperacetylation. Biochemistry. 1994;33:4197–206. doi: 10.1021/bi00180a012. [DOI] [PubMed] [Google Scholar]
- Selvi RB, Kundu TK. Reversible acetylation of chromatin: implication in regulation of gene expression, disease and therapeutics. Biotechnol J. 2009;4:375–90. doi: 10.1002/biot.200900032. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–35. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Sharma R, Ottenhof T, Rzeczkowska PA, Niles LP. Epigenetic targets for melatonin: induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J Pineal Res. 2008;45:277–84. doi: 10.1111/j.1600-079X.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, et al. An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–15. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somech R, Izraeli S, A JS. Histone deacetylase inhibitors--a new tool to treat cancer. Cancer Treat Rev. 2004;30:461–72. doi: 10.1016/j.ctrv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol. 2010;77:621–32. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, Papadia S, Bell KF, Hardingham GE. Role of histone acetylation in the activity-dependent regulation of sulfiredoxin and sestrin 2. Epigenetics. 2009;4:152–8. doi: 10.4161/epi.4.3.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–43. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- Taylor JP, Taye AA, Campbell C, Kazemi-Esfarjani P, Fischbeck KH, Min KT. Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes Dev. 2003;17:1463–8. doi: 10.1101/gad.1087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000;20:9207–14. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–66. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang X, Liu L. HDAC inhibitor trichostatin A-inhibited survival of dopaminergic neuronal cells. Neurosci Lett. 2009;467:212–6. doi: 10.1016/j.neulet.2009.10.037. [DOI] [PubMed] [Google Scholar]
- Yang W, Sun AY. Paraquat-induced free radical reaction in mouse brain microsomes. Neurochem Res. 1998a;23:47–53. doi: 10.1023/a:1022497319548. [DOI] [PubMed] [Google Scholar]
- Yang WL, Sun AY. Paraquat-induced cell death in PC12 cells. Neurochem Res. 1998b;23:1387–94. doi: 10.1023/a:1020750706762. [DOI] [PubMed] [Google Scholar]
- Yin W, Barkess G, Fang X, Xiang P, Cao H, Stamatoyannopoulos G, et al. Histone acetylation at the human beta-globin locus changes with developmental age. Blood. 2007;110:4101–7. doi: 10.1182/blood-2007-05-091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V, Kanthasamy A. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J Neurosci. 2007;27:5349–62. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]