Abstract
Classical benzodiazepines, such as diazepam, interact with αxβ2γ2 GABAA receptors, x = 1, 2, 3, 5 and modulate their function. Modulation of different receptor isoforms probably results in selective behavioural effects as sedation and anxiolysis. Knowledge of differences in the structure of the binding pocket in different receptor isoforms is of interest for the generation of isoform-specific ligands. We studied here the interaction of the covalently reacting diazepam analogue 3-NCS with α1S204Cβ2γ2, α1S205Cβ2γ2 and α1T206Cβ2γ2 and with receptors containing the homologous mutations in α2β2γ2, α3β2γ2, α5β1/2γ2 and α6β2γ2. The interaction was studied using radioactive ligand binding and at the functional level using electrophysiological techniques. Both strategies gave overlapping results. Our data allow conclusions about the relative apposition of α1S204Cβ2γ2, α1S205Cβ2γ2 and α1T206Cβ2γ2 and homologous positions in α2, α3, α5 and α6 with C-atom adjacent to the keto-group in diazepam. Together with similar data on the C-atom carrying Cl in diazepam, they indicate that the architecture of the binding site for benzodiazepines differs in each GABAA receptor isoform α1β2γ2, α2β2γ2, α3β2γ2, α5β1/2γ2 and α6β2γ2.
Introduction
Benzodiazepines are widely used drugs. They exert sedative/hypnotic, anxiolytic, muscle relaxant, and anticonvulsant effects. Benzodiazepines are safe and effective in short term treatments even if some side effects as anterograde amnesia have been reported.
Benzodiazepines act at the major inhibitory neurotransmitter receptor, the γ-aminobutyric acid type A (GABAA) receptor. The GABAA receptors are composed of five subunits surrounding a central chloride ion selective channel [1]–[4]. A variety of subunit isoforms of the GABAA receptor has been cloned, leading to a multiplicity of receptor subtypes [1], [2], [5], [6]. The major receptor isoform in mammalian brain consists of α1, β2, and γ2 subunits [6], [7]. Different approaches have indicated a 2α:2β:1γ subunit stoichiometry for this receptor [8]–[13] with a subunit arrangement γβαβα anti-clockwise as seen from the synaptic cleft [11]–[13]. The classical benzodiazepine diazepam binds with high affinity and positively modulates recombinant α1βxγ2 (x = 1, 2, 3), α2βxγ2, α3βxγ2 and α5βxγ2 GABAA receptors. The high affinity binding site has been located between the α and γ subunit and is homologous to the agonist binding site located between the β and α subunit [14], [15]. Even if it is very common in the field to discuss e.g. “α1 receptors”, there is good evidence for the fact that many GABAA receptors contain two different α subunit isoforms (e.g. [16]). Exclusively the α subunit adjacent to the γ subunit defines the nature of the benzodiazepine site [17].
It has been demonstrated that the residue H101 within the α1 subunit [18] and the homologous residues α2H101, α3H126 and α5H105 are crucial for diazepam potentiation [19]. In α4 and α6 containing receptors the homologous residue is an arginine rendering these receptors insensitive to classical benzodiazepines [18], [20], [21]. Replacement of arginine by histidine in α4 and α6 confers benzodiazepine sensitivity [18] and replacement of histidine by arginine in α1, α2, α3 and α5 abolishes modulation by diazepam [18], [19].
Pharmacological and behavioral studies of knock-in mice in which the relevant histidine residue has been mutated to an arginine, have led to correlations between the α subunit isoform adjacent to the γ subunit and several of the behavioral effects mediated by benzodiazepines. These studies have revealed that GABAA receptors containing an α1 subunit in this position mediate the sedative, the anterograde amnesic and partly the anticonvulsive effects of diazepam [22], [23]. GABAA receptors containing an α2 subunit in this position mediate the anxiolytic effect and the myorelaxant effect [24], [25]. GABAA receptors containing either an α3 or an α5 subunit adjacent to the γ subunit contribute to the myorelaxant actions of benzodiazepines [24], [26], [27].
Diazepam is arranged in the binding pocket of α1β2γ2 receptors such as to allow interaction of a reactive group replacing the –Cl atom with a reactive residue in place of α1H101 [28]–[31] and interaction of a reactive group attached to the 3′-atom with a reactive residues in place of α1S205 or α1S206 [32]. It has also been shown that a reactive residue in place of the –Cl atom interacts with a reactive residue in place the histidine homologous to α1H101 in α2, α3 and the corresponding arginine residue in α6 but not to the histidine residue in α5 [33].
In addition to the high affinity binding site for benzodiazepines, two low affinity binding sites were identified; one has been described to be located in the lipid bilayer [34] and the second at the α/β subunit interface [33], [35].
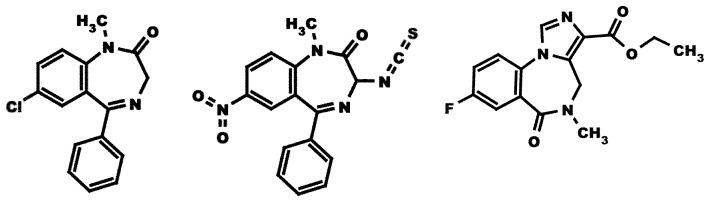
We used the proximity-accelerated chemical reaction approach to further characterize the architecture of the benzodiazepine binding site in different receptor isoforms. GABAA receptor residues thought to reside in the site were individually mutated to cysteine and combined with a modified benzodiazepine molecule carrying a substituent reactive with cysteine at the 3′ atom (3-NCS, Fig. 1). Direct apposition of target carbon atom of the NCS group and the reactive –S– group of cysteine is expected to lead to a covalent reaction.
Figure 1. Chemical structures of diazepam, 3-NCS and Ro15–1788.
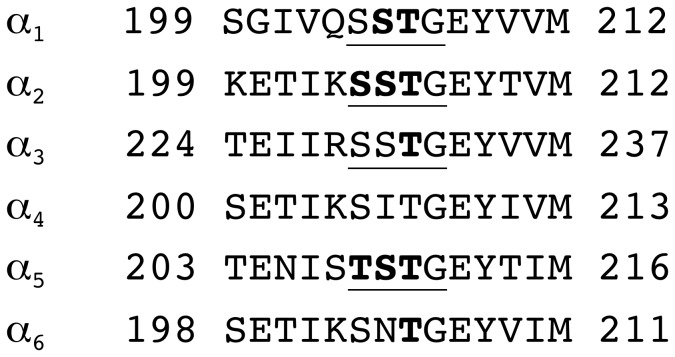
We studied interaction of 3-NCS compound with amino acid residues 204, 205 and 206 in α1β2γ2 receptors and the homologous residues in α2β2γ2, α3β2γ2, α5β1/2γ2 and α6β2γ2 (Fig. 2), using radioactive ligand binding studies at receptors expressed in HEK-cells and electrophysiological studies, using the two electrode voltage clamp technique at receptors expressed in Xenopus laevis oocytes. In each receptor isoform αxβ2γ2 (x = 1, 2, 3, 5, 6), we found a different interaction pattern of the corresponding α subunit with the cysteine reactive compound. This indicates a difference in shape of the benzodiazepine binding site in the region of the 3′ atom of diazepam in different receptor isoforms. We further observed disruption of benzodiazepine binding site in α1G207Cβ2γ2 and α2G207Cβ2γ2 receptors.
Figure 2. Alignment of Loop C of the α subunits.
The underlined residues were individually mutated to cysteine. The bold residues react covalently with 3-NCS. α4 was not investigated and is only shown for comparison.
Methods
Construction of the Mutated Receptor Subunits
The mutant subunits α1S204C, α1S205C, α1T206C, α1G207C, α2S204C, α2S205C, α2T206C, α2G207C, α3S229C, α3S230C, α3T231C, α5T208C, α5S209C, α5T210C, α6S203C, α6N204C and α6T205C were prepared using the QuikChange™ mutagenesis kit (Stratagene). For cell transfection, the cDNAs were subcloned into the polylinker of pBC/CMV [36]. This expression vector allows high-level expression of a foreign gene under control of the cytomegalovirus promoter.
The Cysteine-reactive Compound
The synthesis of the cysteine-reactive compound 3-NCS (Fig. 1) has been described before [32].
Transfection of GABAA Receptors in HEK293 Cells and Membrane Preparation
cDNAs coding for the αx (x = 1, 2, 3, 6), β2, and γ2S subunits DNA (20 µg : 20 µg : 20 µg) per 9 cm diameter dish were transfected in human embryonic kidney (HEK) 293 cells (American Type of Culture Collection, MD, USA, CRL 1573) using the calcium phosphate precipitation technique [37]. For α5 containing receptors β2 was replaced by β1. This resulted in higher expression levels. Culturing of cells and membrane preparation were done as described before [30].
Radioactive Ligand Binding Assay
The properties of the recombinant mutant receptors were only estimated. For this affinity estimate, membranes were re-suspended in phosphate buffer using a Teflon homogenizer. They were incubated in a total volume of 360 µl for 1 h on ice in the presence of [3H]Ro15–1788 (78.6 Ci/mmol; PerkinElmer Life Sciences). The final protein concentration was 0.1–1 mg of protein/ml. Total binding was measured at 0.5 and 5 nM [3H]Ro15–1788. Nonspecific binding was determined under the same condition but in the presence of 100 µM unlabeled Ro15–1788 and amounted to less than 10% of total binding, except for α2S206Cβ2γ2 and α5T208Cβ2γ2 where it amounted to 10–17%. Expression levels of α6β2γ2 receptors were estimated with [3H]Ro15–4513. Membranes were collected by rapid vacuum filtration on GF/C filters. After three washing steps (3 sec each) with 5 ml of phosphate buffer, the filter-retained radioactivity was determined by liquid scintillation counting.
Detection of a Covalent Reaction
As detailed in previous work [28], [30], [33] this procedure included three steps: incubation of membranes expressing recombinant wild type or mutant receptors with the reactive agent followed by extensive washing of the membranes in order to remove non-reacted compound and a radioactive ligand binding assay to determine residual binding. No covalent reaction would result in 100% residual binding, and 100% covalent reaction would result in 0% residual binding.
Briefly, the membranes were re-suspended in phosphate buffer (100 mM KCl, 10 mM KH2PO4, 0.1 mM EDTA, pH 7.4) using a Glass/Teflon homogenizer. 0.1–1.0 mg/mL of protein were incubated in a total volume of 360 µL with either 10 µM (determination of degree of covalent reaction) or several concentrations of 3-NCS for 30 min on ice. Membranes were collected with rapid filtration on a round 7 mm diameter glass fiber filter (GF/C; Whatman) that was placed on a round 24 mm diameter glass fiber filter (GF/C; Whatman), both pre-washed with phosphate buffer. The reaction of 3-NCS with the receptor was stopped by washing of the filters six times with 5 mL phosphate buffer, each. The small filters with the deposited membranes were incubated in 0.12 mL phosphate buffer containing 5 nM [3H]Ro15–1788. After 30 min the 7 mm filter was placed on a 24 mm filter and washed six times with 5 mL phosphate buffer each. Radioactivity was determined by liquid scintillation counting. Non-specific binding was determined in the presence of 100 µM Ro 15–1788. In control experiments, washing efficiency was estimated by placing radioactivity on the small filter. More than 99.95% of the radioactivity was removed (not shown).
Concentration response curves for 3-NCS were fitted with the equation C(c) = Cmax/(1+(EC50/c)), where c is the concentration of 3-NCS, EC50 the concentration of 3-NCS where half maximal covalent reaction was observed, Cmax is the maximal extent of the covalent reaction and C the measured extent of the covalent reaction.
Expression of GABAA Receptors in Xenopus Oocytes
Capped cRNAs were synthesized (Ambion, Austin, TX, USA) from the linearized plasmids with a cytomegalovirus promotor (pCMVvectors) containing the different subunits, respectively. A poly-A tail of about 400 residues was added to each transcript using yeast poly- A polymerase (United States Biologicals, Cleveland, OH, USA). The concentration of the cRNA was quantified on a formaldehyde gel using Radiant Red stain (Bio-Rad) for visualization of the RNA. Known concentrations of RNA ladder (Invitrogen) were loaded as standard on the same gel. cRNAs were precipitated in ethanol/isoamylalcohol 19∶ 1, the dried pellet dissolved in water and stored at −80°C. cRNA mixtures were prepared from these stock solutions and stored at −80°C. Xenopus laevis oocytes were prepared, injected and defolliculated as described previously [38], [39]. They were injected with 50 nL of the cRNA solution containing wild type or mutated α1 or α2, α3, α5, α6 and wild type β2 and γ2 subunits at a concentration of 10 nM : 10 nM : 50 nM [40] and then incubated in modified Barth’s solution at +18°C for at least 24 h before the measurements.
Functional Characterization of the GABAA Receptors
Currents were measured using a modified two-electrode voltage clamp amplifier Oocyte clamp OC-725 (Warner Instruments) in combination with a XY-recorder (90% response time 0.1s) or digitized at 100 Hz using a PowerLab 2/20 (AD Instruments) using the computer programs Chart (ADInstruments GmbH, Spechbach, Germany). Tests with a model oocyte were performed to ensure linearity in the larger current range. The response was linear up to 15 µA.
Electrophysiological experiments were performed by using the two-electrode voltage clamp method at a holding potential of −80 mV. The perfusion medium contained 90 mM NaCl, 1 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 5 mM Na-HEPES (pH 7.4) and was applied by gravity flow 6 ml/min. The perfusion medium was applied through a glass capillary with an inner diameter of 1.35 mm, the mouth of which was placed about 0.4 mm from the surface of the oocyte. Allosteric modulation via the benzodiazepine site was measured at a GABA concentration eliciting 2–5% of the maximal GABA current amplitude. GABA was applied for 20 s alone or in combination with allosteric compound. 6 ml of the covalent reacting compound was applied to the oocyte and incubated for 3 min while stopping the flow of the perfusion medium. Modulation of GABA currents was expressed as (I(modulator + GABA)/IGABA –1) * 100%. The perfusion system was cleaned between drug applications by washing with DMSO to avoid contamination.
Results
We wanted to derive information on part of the benzodiazepine binding pockets in different GABAA receptor isoforms. For this purpose, we characterized the covalent interaction of a benzodiazepine-like compound with αxβ1/2γ2 (x = 1, 2, 3, 5, 6) receptors containing a cysteine mutation in residues α1S204C, α1S205C and α1T206C and homologous positions in α2, α3, α5 and α6. The β2 subunit was used throughout except for expression of α5 containing receptors in HEK-cells where β1 was used instead to achieve higher expression levels. α1G207C and the homologous mutation α2G207C were also prepared. These mutated subunits did not confer to the expressed receptors high affinity [3H]Ro 15–1788 or [3H]flunitrazepam binding nor functional modulation by diazepam (not shown). This indicates that mutation in this position disrupts the binding site for benzodiazepines.
The Cysteine Reactive Compound
We used a modified nitrazepam molecule carrying a cysteine reactive isothiocyanate substituent at the C-3 carbon (3-NCS; Fig. 1). 3-NCS was able to displace [3H]Ro15–1788 or [3H]flunitrazepam from wild-type α1β2γ2 receptors. The Ki values were 340±16 nM and 240±55 nM [32], respectively. The Ki values for displacement of [3H]Ro 15–1788 by 3-NCS in α2β2γ2 were 1550±250 nM (n = 3) and in α5β1γ2 receptors 9840±1480 nM (n = 3). The determination of Ki values was based on the Kd values of 2.1±0.5 nM and 1.5±1.2 nM, respectively. The covalent reaction of 3-NCS with α1mβ2γ2 (m = S205C, T206C) receptor at the binding and functional level has previously been described [32].
Binding Properties of the GABAA Receptor Carrying a Cysteine Point Mutation
The homologous residues to α1S204, α1S205 and α1T206 in α2, α3, α5 and α6 were mutated individually to cysteine. α1 and α2 were expressed in combination with β2 and γ2 subunits, α5 for reasons mentioned above together with β1 and γ2 subunits. All these mutated αmβ2γ2 receptors bound [3H]Ro 15–1788 with an estimated affinity between 0.08 and 7.5 nM (data not shown). No specific binding was detected in α 3 containing receptors (data not shown). α6T205Cβ2γ2 bound [3H]Ro15–4513 with an estimated affinity 4 nM.
Covalent Reaction of 3-NCS at the Binding Level
First we tested reactivity of 3-NCS using a radioactive ligand binding assay. 3-NCS is expected to first occupy its binding site reversibly. Upon proper apposition of the –SH group of the cysteine from the mutated receptors with the –C atom of the NCS group from the 3-NCS compound, this is followed by covalent reaction. This reaction was determined at 10 µM concentration of 3-NCS. Preliminary experiments showed that at this concentration and at 1 µM 3-NCS, covalent reaction was reaching a maximum within 15 sec (not shown). Covalent reaction did not reach completion. This observation was made before and has been interpreted to indicate covalent reaction at a low affinity benzodiazepine binding site at the α/β subunit interface, which prevents covalent reaction on the classical benzodiazepine binding site [33]. Alternatively or in addition, the reactive compound may be consumed in non-specific reactions. Non-covalently reacted compound was removed by filtration (see methods). A covalent reaction of 3-NCS with a mutated cysteine residue is expected to prevent reversible binding of the [3H]Ro15–1788. If no covalent reaction occurs, the binding site should still be available for reversible binding. α5 containing receptors were expressed together with β1 instead of β2. α3 containing receptors did not express in HEK293-cells. As α6 subunit containing receptors do not bind [3H]Ro15–1788 with high affinity, we used [3H]Ro15–4513 instead.
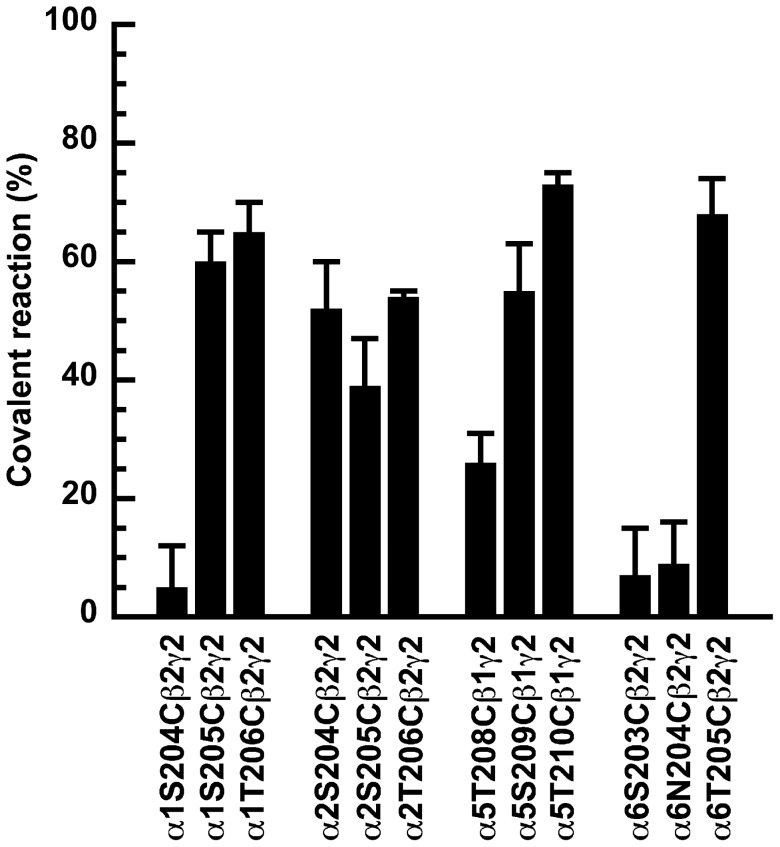
Figure 3 compares the percentage of covalent reaction, in mutated α1, α2, α5 and α6 containing receptors. For the α1β2γ2 receptor isoform cysteine mutation in residue S205 and T206 resulted in covalent reaction, while S204C did not covalently react with 3-NCS [32]. For α2β2γ2 receptor isoform the cysteine mutated residues homologous to α1S204, α1S205 and α1T206 showed covalent reaction amounting to 52%±8% (n = 3), 39%±8% (n = 3) and 54%±1% (n = 3). For α5β1γ2 receptors the cysteine mutated receptors homologous to α1S204, α1S205 and α1T206 reacted covalently amounting to 26%±5% (n = 3), 54%±8% (n = 3) and 73%±2% (n = 3). In the α6β2γ2 receptor isoform only the cysteine mutation homologous to α1T206 reacted covalently with 3-NCS amounting to 68% ±6% (n = 3).
Figure 3. Covalent reaction at the binding level.
Extent of covalent reaction of 3-NCS at α1S204Cβ2γ2, α1S205Cβ2γ2, α1T206Cβ2γ2 mutant GABAA receptors and homologous receptors formed with α2, α3, and α6. α5 was expressed together with β1 and γ2 subunits for reasons mentioned in the results section. Receptors were expressed in HEK-293 cells, membranes harvested and exposed to 10 µM 3-NCS. After incubation, the residual 3-NCS was removed by filtration. [3H]Ro15–1788 was used as radioactive ligand to determine the residual binding. Covalent binding is expressed as 100% minus % residual binding. Data are shown as mean ±SD for three experiments each (triplicates of each point in each experiment).
Concentration Dependence of the Covalent Reaction
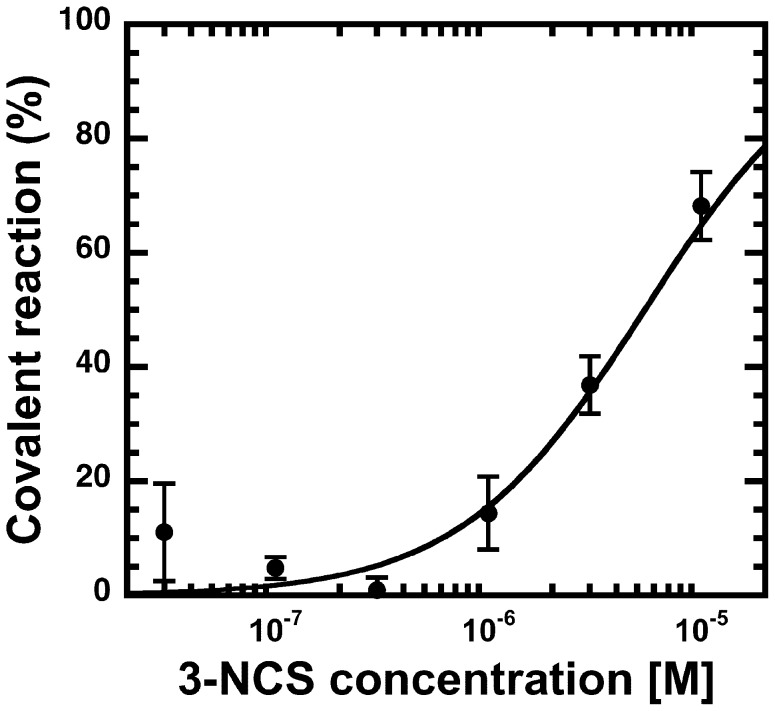
We next investigated the concentration dependence of the covalent reaction. Mutated receptors were exposed for 30 min to different concentrations of 3-NCS. Figure 4 documents such a concentration dependence in α6T205Cβ2γ2 receptors. The concentration dependence was also determined in mutated α1S204Cβ2γ2, α1S205Cβ2γ2, α1T206Cβ2γ2 and homologous mutations in α2, α5 and α6 containing receptors. Table 1 documents the calculated EC50 values of the covalent reaction, which are between 0.08 µM and 6.6 µM. For α1S204Cβ2γ2, α6S203Cβ2γ2, α6N204Cβ2γ2 the EC50 values could not be determined because we could not detect covalent reaction with 3-NCS.
Figure 4. Concentration dependence at the binding level.
Concentration dependence of the covalent reaction of the 3-NCS at α6T205Cβ2γ2 mutant GABAA receptors. Extent of covalent reaction was determined at different concentrations of 3-NCS as indicated below Fig. 3. Data are shown as mean ±SD for three experiments each (triplicates of each point in each experiment).
Table 1. Concentration dependence of the covalent reaction at mutant receptors at the binding level.
| mutated receptor | mutation homologous to | EC50 (µM) |
| α1S204Cβ2γ2 | n.r. | |
| α1S205Cβ2γ2 | 0.63±0.18 | |
| α1T206Cβ2γ2 | 0.082±0.028 | |
| α2S204Cβ2γ2 | α1S204C | 3.2±2.3 |
| α2S205Cβ2γ2 | α1S205C | 6.6±5.4 |
| α2T206Cβ2γ2 | α1S206C | 0.19±0.056 |
| α5T208Cβ1γ2 | α1S204C | 0.39±0.21 |
| α5S209Cβ1γ2 | α1S205C | 4.1±2.2 |
| α5T210Cβ1γ2 | α1S206C | 0.45±0.15 |
| α6S203Cβ2γ2 | α1S204C | n.r. a |
| α6N204Cβ2γ2 | α1S205C | n.r. a |
| α6T205Cβ2γ2 | α1S206C | 5.4±0.80a |
Left column: mutated receptors. Middle column: homology of the mutations to α1. Right column EC50 is given as mean ±SD for three to four experiments where each point in the dose-response curve was determined in triplicates. The receptors were exposed to increasing concentrations of 3-NCS compound for 30 min on ice and extensively washed. Residual binding was determined using [3H]Ro15–1788 as radioactive ligand and converted to percentage of binding sites covalently reacted. All wild type receptors showed no covalent reaction. n.r.: no covalent reaction.
[3H]15–4513 was used as a radioactive ligand.
Residue 206 is unique in that all the investigated subunit isoforms do show covalent reaction. In each case the absolute level of the reaction is relatively high (Fig. 3). It should be noted that residue 206 in the α1 subunit and homologous residues in α2 and α5 show a very high apparent affinity in the reaction with 3-NCS while the corresponding residue in α6 shows a lower apparent affinity (F-test; p<0.01). In α1, α2 and α5, the residue identical to or homologous to residue 206 in the α1 subunit has a lower EC50 than residue 205 in the corresponding α subunit (F-test; p<0.01). Residue 205 in the α1 subunit shows a higher apparent affinity in the reaction with 3-NCS than the homologous residue in α2 (F-test; p<0.02).
Irreversible Reaction of 3-NCS at the Functional Level
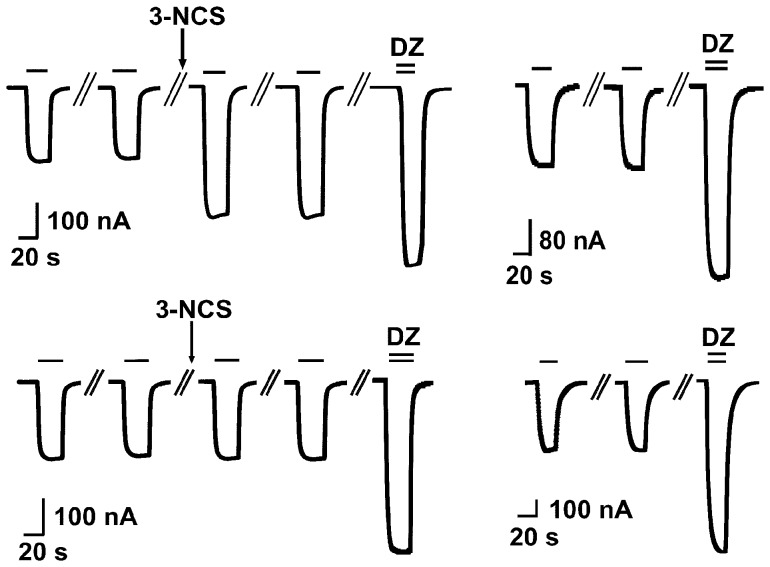
We also studied the covalent reaction at the functional level. All wild type receptors failed to show any changes in the current amplitude elicited by GABA, while some mutant receptors did. An example of this experiment is shown in Figure 5a for the α1S205Cβ2γ2 mutant receptor. 3-NCS led to an irreversible increase in the current amplitude and the relative stimulation by diazepam was smaller than observed in naïve oocytes (Fig. 5b). We interpret this as evidence for a covalent reaction. Fig. 5c shows the same experiment at the mutant receptor α1T204Cβ2γ2. 3-NCS did not increase the current amplitude and diazepam stimulated to the same extent as in naïve oocytes (Fig. 5d). Clearly, this mutant receptor did not show any covalent reaction. As α6 containing receptors did not respond to diazepam, we used 1 µM abercarnil instead.
Figure 5. Covalent reaction at the functional level.
Exposure of mutated α1S205Cβ2γ2 receptors to 3-NCS results in a covalent reaction. a) Receptors were exposed twice to 0.5 µM GABA followed by a washing period and a 3 min incubation in 10 µM 3-NCS. After this treatment GABA was applied twice with a 3 min interval. In some experiments GABA was applied up to six times. Thus, the current elicited by GABA was stimulated irreversibly. Application of 0.5 µM GABA in combination with 1 µM diazepam results in a further stimulation of the partially irreversibly reacted receptors that was smaller than that seen upon b) direct exposure to diazepam in independent experiments. Exposure of α1T204Cβ2γ2 receptors 3-NCS does not result in a covalent reaction. c) Receptors were exposed twice to 0.5 µM GABA followed by a 3 min incubation in 10 µM 3-NCS. Application of 0.5 µM GABA in combination with 1 µM diazepam results in stimulation similar to the stimulation seen in an oocyte that had not been previously exposed to 3-NCS (Fig. 4d).
Exposure of mutant receptors α1S205Cβ2γ2, α1T206Cβ2γ2, α2T205Cβ2γ2 (mutation homologous to α1S205), α2T206Cβ2γ2 (α1T206), α3T231Cβ2γ2 (α1T206), α5T208Cβ2γ2 (α1S204), α5T209Cβ2γ2 (α1S205), and possibly α3S230Cβ2γ2 (α1S205) and α6T205Cβ2γ2 (α1T206), to 3-NCS resulted in an irreversible positive allosteric modulation (Table 2) indicating a covalent reaction, the adduct acting as a positive allosteric modulator. If only a fraction of the receptors react with 3-NCS, diazepam is expected to stimulate the remaining receptors. If 3-NCS has a similar allosteric effect as diazepam the combined stimulations of 3-NCS and diazepam should be the same as stimulation by diazepam alone. This is the case for most mutated receptors studied. Exceptions are α1T206Cβ2γ2, α3T231Cβ2γ2 (homologous to α1T206), α5T208Cβ2γ2 (α1S204) and α5T210Cβ2γ2 (α1T206). In the last two cases, results may be explained if 3-NCS acts at these mutated receptors as a partial modulator and an antagonist, respectively, at the benzodiazepine site. In the first two cases combined stimulation by 3-NCS and diazepam is larger than expected from diazepam alone. The latter seems in both cases reduced by the mutation for unknown reasons. We can only hypothesize that covalent reaction with 3-NCS at the α/β subunit interface may allosterically stimulate the reaction to diazepam. It is interesting to note that at the functional level the identical residues were identified to react covalently as at the binding level. This in spite of the use of Ro15–1788 to detect covalent reaction at the binding level and the use of diazepam at the functional level. The only exception is α2S204C. We have no explanation for this discrepancy. Differences in the experimental conditions used in binding and functional experiments (total lipid content, lipid composition and temperature) may be responsible.
Table 2. Covalent reaction at the functional level.
| Mutated receptor | Mutation homolo-gous to | DZ | n | 3-NCS | p | 3-NCS +DZ | n |
| α1S204Cβ2γ2 + | 126±9 | 3 | 4±4 | n.s. | 115±34 | 3 | |
| α1S205Cβ2γ2 | 113±7 | 3 | 60±43 | <0.03 | 131±32 | 3 | |
| α1T206Cβ2γ2 | 82±18 | 6 | 119±51 | <0.01 | 204±62 | 4 | |
| α2S204Cβ2γ2 + | α1S204C | 215±42 | 3 | 1±10 | n.s. | 218±30 | 3 |
| α2S205Cβ2γ2 | α1S205C | 191±53 | 3 | 35±10 | <0.01 | 177±25 | 3 |
| α2T206Cβ2γ2 | α1S206C | 148±8 | 3 | 53±29 | <0.01 | 168±54 | 3 |
| α3S229Cβ2γ2 | α1S204C | l.e. | l.e. | l.e. | |||
| α3S230Cβ2γ2 | α1S205C | 149±44 | 3 | 15±11 | n.s. | 158±44 | 3 |
| α3T231Cβ2γ2 | α1S206C | 88±45 | 3 | 28±16 | <0.03 | 165±8 | 3 |
| α5T208Cβ2γ2 | α1S204C | 129±38 | 7 | 23±18 | <0.05 | 83±25 | 5 |
| α5S209Cβ2γ2 | α1S205C | 103±3 | 3 | 46±17 | <0.01 | 140±20 | 3 |
| α5T210Cβ2γ2 | α1S206C | 82±14 | 3 | 10±5 | n.s. | 47±8 | 3 |
| α6S203Cβ2γ2 + | α1S204C | 50±20* | 3 | 0±2 | n.s. | 57±21* | 3 |
| α6N204Cβ2γ2 + | α1S205C | 70±40* | 3 | −4±6 | n.s. | 71±40* | 3 |
| α6T205Cβ2γ2 | α1S206C | 48±6* | 3 | 14±8 | <0.05 | 58±21* | 3 |
Mutated GABAA receptors were expressed in Xenopus oocytes. Allosteric stimulation by 1 µM diazepam was determined (column labelled DZ (diazepam)) at EC2–5 for GABA. Data are given as % allosteric modulation. In independent experiments oocytes were exposed to GABA followed by 10 µM 3-NCS and after removing non-covalently reacted 3-NCS, allosteric stimulation was determined (column labelled 3-NCS). Subsequently the same oocyte was exposed to 1 µM diazepam (column labelled 3-NCS + diazepam) and allosteric stimulation was determined as compared to the initial application of GABA. *1µM Abecarnil was used. l.e. low expression, expressed currents were too small for measurement of covalent effects. p was determined with the one-way ANOVA followed by a post-hoc Dunnett’s test where the non-responsive receptors indicated with (+) served as one of the samples (mean ± mean SD, 0.25±5.5, n = 4). Data are given as mean ±SD.
Discussion
The most common GABAA receptors contain two identical or different α subunits [6]. Receptors containing different α subunit isoforms adjacent to the γ subunit mediate different effects of classical benzodiazepines. α1, α2, α3 or α5 are required in this position for the action of classical benzodiazepines. In order to be able to rationally design receptor subtype specific drugs more knowledge on the difference of the interaction of different receptor subtypes with classical benzodiazepines is required.
We aimed at finding differences or similarities in the shape of the benzodiazepine binding site in different receptor isoforms. We used the proximity-accelerated chemical coupling reaction at the binding and functional level. A Cysteine-mutated receptor is combined with a chemical modified cysteine reactive binding site ligand. Indentification of a covalent reaction implies apposition of the –S atom of cysteine and the reactive –C atom of the ligand [41]. After previous work in the α1H101 region [33], where we found that a NCS group introduced into diazepam at the –Cl position interacts subtly different with α1, α2, α3 and α6, but only very weakly with α5, we concentrated here on the C atom adjacent to the keto group in diazepam. We have previously shown that α1S205Cβ2γ2 and α1T206Cβ2γ2 receptors but not α1S204Cβ2γ2 receptors interact covalently with 3-NCS [32]. Now homologous mutations were introduced into other α subunit isoforms.
We studied the covalent reaction at the level of radioactive ligand binding at receptors expressed in transfected HEK 293 cells and at the functional level characterizing chloride current mediated by receptors expressed in Xenopus oocytes. In general, we found very good agreement of the results obtained by the two strategies. In case covalent reaction occurs not in the binding pocket, but on the access pathway of 3-NCS, we would not expect an allosteric modulation of the corresponding receptor. Thus the agreement between the strategies can be taken as evidence for a reaction in the binding pocket.
In the following we discuss in sequence our observations for the position homologous to α1S204, to α1S205, to α1T206 and to α1G207 in αxβ2γ2 (x = 1, 2, 3, 5, 6) receptors.
Position homologous to α1S204: At the binding level, mutated α2β2γ2 and α5β1γ2 showed covalent reaction, while α1β2γ2 and α6β2γ2 did not. At the functional level, exclusively mutated α5β2γ2 showed covalent reaction. We have no explanation for the discrepancy concerning α2β2γ2. Possibly, 3-NCS acts as an antagonist here and covalent reaction at the α/β subunit interface promotes stimulation by diazepam. α3β2γ2 could not be expressed at sufficient extent to determine reaction levels.
Position homologous to α1S205: At the binding level, mutated α1β2γ2, α2β2γ2 and α5β1γ2 showed covalent reaction, while mutated α6β2γ2 did not. At the functional level, the same result was obtained. In addition, α3β2γ2 might have reacted covalently at the functional level, but reaction was at the threshold for significance.
Position homologous to α1T206: At the binding level, all mutated receptors investigated α1β2γ2, α2β2γ2, α5β1γ2 and α6β2γ2 showed covalent reaction. At the functional level, the same result was obtained except for possibly α6β2γ2, which is at the threshold for significance. In addition, mutated α3β2γ2 reacted covalently.
Position homologous to α1G207: At the binding and at the functional level mutated α1β2γ2 and α2β2γ2 receptors were missing the benzodiazepine binding site. In functional experiments currents induced by GABA were not affected by the mutations. All other receptor isoforms were not tested.
In summary there is evidence for covalent reaction of the α1 subunit in residues 205 and 206; of the α2 subunit in residues 204, 205 and 206; of the α3 subunit at least in residue 206 and possibly 205; of the α5 subunit in residues 204, 205 and 206; and of the α6 subunit exclusively in residue 206. The residues highlighted in bold face react with a high apparent affinity. It is interesting to note that the residue homologous to 206 reacts covalently in all receptors. Unexpectedly, the α2 subunit showed a similar reactivity pattern as the α5 subunit.
These observations should be combined with data on another region of the benzodiazepine binding pocket. The region of the –Cl atom in diazepam has previously been investigated in different GABAA receptor isoforms [33]. A molecule made reactive in this position interacted best with α6 containing receptors and very little with α5 containing receptors while the variants with α1, α2, and α3 were intermediate.
The combined set of data is visualized in Fig. 6. The size of the lettering of the residues correlates qualitatively with the affinity of the covalent reaction. Our observations will help in modelling of the benzodiazepine binding pocket in different α subunit containing isoforms of the GABAA receptor.
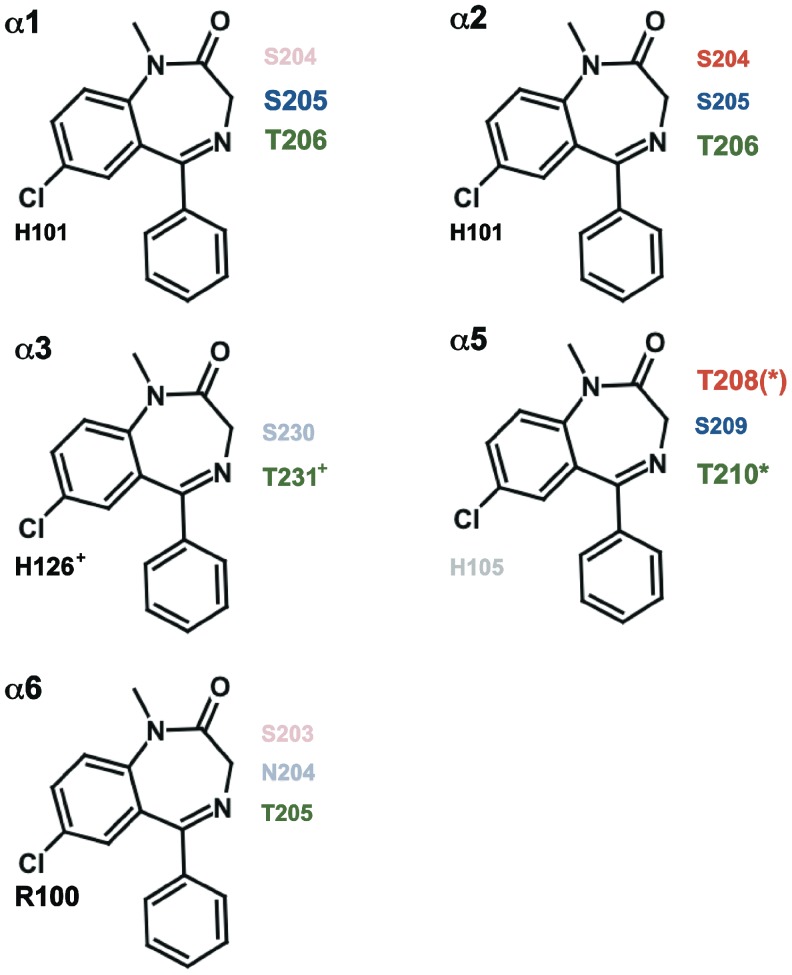
Figure 6. Summary.
The results obtained with NCS [33] and the results obtained with 3-NCS described here are summarized. For each receptor isoform αxβ1/2γ2 (x = 1, 2, 3, 5, 6) amino acid residues homologous to 101 in α1 are shown in black, those homologous to 204 are shown in red, those homologous to 205 are shown in blue and those homologous to 206 are shown in green. Residue 101 is located near to the C atom carrying the Cl atom in diazepam, and 204, 205 and 206 are near to C atom adjacent to the keto group. The residues showing covalent reaction are shown in full color and those showing no reaction coloured in reduced saturation. The residues in larger font size react with a high apparent affinity. The residues marked with * and (*) indicate an antagonistic effect and a partial modulatory effect by 3-NCS on the corresponding α5mβ2γ2 receptors, respectively. The residues marked with + indicate that the corresponding α3mβ2γ2 receptors were only investigated at the functional level.
Acknowledgments
We thank Dr. V. Niggli for carefully reading the manuscript.
Funding Statement
This work was supported by the Swiss National Foundation (http://www.snf.ch) grant 31003A_132806/1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Macdonald RL, Olsen RW (1994) GABAA receptor channels. Annu Rev Neurosci 17: 569–602. [DOI] [PubMed] [Google Scholar]
- 2. Rabow LE, Russek SJ, Farb DH (1995) From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse 21: 189–274. [DOI] [PubMed] [Google Scholar]
- 3. Sieghart W (1995) Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev 47: 181–233. [PubMed] [Google Scholar]
- 4. Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2: 795–816. [DOI] [PubMed] [Google Scholar]
- 5. Barnard EA, Skolnick P, Olsen RW, Möhler H, Sieghart W, et al. (1998) Intern ational Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50: 291–313. [PubMed] [Google Scholar]
- 6. Olsen RW, Sieghart W (2008) International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update Pharmacol Rev 60: 243–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKernan RM, Whiting PJ (1996) Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci 19: 139–143. [DOI] [PubMed] [Google Scholar]
- 8. Chang Y, Wang R, Barot S, Weiss DS (1996) Stoichiometry of a recombinant GABAA receptor. J Neurosci 16: 5415–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tretter V, Ehya N, Fuchs K, Sieghart W (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci 17: 2728–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM (1999) Stoichiometry of a Ligand gated ion channel determined by fluorescence energy transfer. J Biol Chem 274: 10100–10104. [DOI] [PubMed] [Google Scholar]
- 11. Baumann SW, Baur R, Sigel E (2001) Subunit arrangement of gamma-aminobutyric acid type A receptors. J Biol Chem 276: 36275–36280. [DOI] [PubMed] [Google Scholar]
- 12. Baumann SW, Baur R, Sigel E (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem 277: 46020–46025. [DOI] [PubMed] [Google Scholar]
- 13. Baur R, Minier F, Sigel E (2006) A GABA(A) receptor of defined subunit composition and positioning: concatenation of five subunits. FEBS Lett 580: 1616–1620. [DOI] [PubMed] [Google Scholar]
- 14. Sigel E, Buhr A (1997) The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 18: 425–429. [DOI] [PubMed] [Google Scholar]
- 15. Sigel E, Luscher BP (2011) A closer look at the high affinity binding site for benzodiazepines on GABAA receptors. Cur Top Med Chem 11: 241–246. [DOI] [PubMed] [Google Scholar]
- 16. Pöltl A, Hauer B, Fuchs K, Tretter V, Sieghart W (2003) Subunit composition and quantitative importance of GABAA receptor subtypes in the cerebellum of mouse and rat. J Neurochem 87: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 17. Minier F, Sigel E (2004) Positioning of the α-subunit isoforms confers a functional signature to γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 101: 7769–7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wieland HA, Lüddens H, Seeburg PH (1992) A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem 267: 1426–1429. [PubMed] [Google Scholar]
- 19. Benson JA, Low K, Keist R, Mohler H, Rudolph U (1998) Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS Lett 431: 400–404. [DOI] [PubMed] [Google Scholar]
- 20. Davies PA, Hanna MC, Hales TG, Kirkness EF (1997) Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature 385: 820–823. [DOI] [PubMed] [Google Scholar]
- 21. Dunn SMJ, Davies M, Muntoni AL, Lambert JJ (1999) Mutagenesis of the rat α1 subunit of the γ-amino butyric acid type A receptor reveals the importance of residue 101 in determining the allosteric effects of benzodiazepine site ligands. Mol Pharmacol 56: 768–774. [PubMed] [Google Scholar]
- 22. Rudolf U, Crestani F, Benke J, Brünig I, Benson JA, et al. (1999) Benzodiazepine actions mediated by specific μ-aminobutyric acidA receptor suptypes. Nature 401: 796–800. [DOI] [PubMed] [Google Scholar]
- 23. McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, et al. (2000) Sedative but not anxiolytic proprieties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nature Neurosci 3: 587–592. [DOI] [PubMed] [Google Scholar]
- 24. Löw K, Crestani F, Keist R, Benke D, Brünig I, et al. (2000) Molecular and Neuronal Substrate for the Selective Attenuation of Anexiety. Science 290: 131–134. [DOI] [PubMed] [Google Scholar]
- 25. Crestani F, Löw K, Keist R, Mandelli M-J, Möhler H, et al. (2001) Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol 59: 442–445. [DOI] [PubMed] [Google Scholar]
- 26. Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, et al. (2002) Traces fear conditioning involves hippocampal alpha 5 GABAA receptors. Proc Natl Acad Sci U S A 99: 8980–8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dias R, Sheppard WF, Fradley RL, Garett EM, Stanley JL, et al. (2005) Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci 25: 10682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Berezhnoy D, Nyfeler Y, Gonthier A, Schwob H, Goeldner M, et al. (2004) Towards a relative orientation of benzodiazepines in their binding pocket on GABAA receptors. J Biol Chem 279: 3160–3168. [DOI] [PubMed] [Google Scholar]
- 29. Berezhnoy D, Baur R, Gonthier A, Foucaud B, Goeldner M, et al. (2005) Conformational changes at the benzodiazepine binding site of GABAA receptors detected with a novel technique. J Neurochem 92: 859–866. [DOI] [PubMed] [Google Scholar]
- 30. Tan KR, Gonthier A, Baur R, Ernst M, Goeldner M, et al. (2007a) Proximity-accelerated chemical coupling reaction in the benzodiazepine binding site of GABAA receptors: superposition of different allosteric modulators. J Biol Chem 282: 26316–26325. [DOI] [PubMed] [Google Scholar]
- 31. Tan KR, Baur R, Gonthier A, Goeldner M, Sigel E (2007b) Two neighboring residues of loop A of the α1 subunit point towards the benzodiazepine binding site of GABAA receptors, FEBS Lett. 581: 4718–4722. [DOI] [PubMed] [Google Scholar]
- 32. Tan KR, Baur R, Charon S, Goeldner M, Sigel E (2009) Relative positioning of diazepam in the benzodiazepine binding pocket of GABAA receptors. J Neurochem 111: 1264–1273. [DOI] [PubMed] [Google Scholar]
- 33. Baur R, Tan KR, Lüscher BP, Gonthier A, Goeldner M, et al. (2008) Covalent modification of GABAA receptor isoforms by a diazepam analogue provides evidence for a novel benzodiazepine binding site that prevents modulation by these drugs. J Neurochem 106: 2353–2356. [DOI] [PubMed] [Google Scholar]
- 34. Walters RJ, Hadley SH, Morris KDW Amin J (2000) Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nature Neurosci 3: 1274–1231. [DOI] [PubMed] [Google Scholar]
- 35. Ramerstorfer J, Furtmüller R, Sarto-Jackson I, Varagic Z, Sieghart W, et al. (2011) The GABAA receptor Alpha+beta- interface: a novel target for subtype selective drugs. J Neurosci 31: 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm H-W, et al. (1991) Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci U S A 88: 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sigel E (1987) Properties of single sodium channels translated by Xenopus oocytes after injection with messenger ribonucleic acid. J Physiol 386: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sigel E, Minier F (2005) The Xenopus oocyte: system for the study of functional expression and modulation of proteins. Mol Nutr Food Res 49: 228–234. [DOI] [PubMed] [Google Scholar]
- 40. Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C (2002) The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology 43: 695–700. [DOI] [PubMed] [Google Scholar]
- 41. Foucaud B, Perret P, Grutter T, Goeldner M (2001) Cysteine mutants as chemical sensors for ligand-receptor interactions Trends Pharmacol Sci. 22: 170–173. [DOI] [PubMed] [Google Scholar]