Abstract
Culturing of microbes for food production, called cultivation mutualism, has been well-documented from eusocial and subsocial insects such as ants, termites and ambrosia beetles, but poorly described from solitary, non-social insects. Here we report a fungal farming in a non-social lizard beetle Doubledaya bucculenta (Coleoptera: Erotylidae: Languriinae), which entails development of a special female structure for fungal storage/inoculation, so-called mycangium, and also obligate dependence of the insect on the fungal associate. Adult females of D. bucculenta bore a hole on a recently-dead bamboo culm with their specialized mandibles, lay an egg into the internode cavity, and plug the hole with bamboo fibres. We found that the inner wall of the bamboo internode harboring a larva is always covered with a white fungal layer. A specific Saccharomycetes yeast, Wickerhamomyces anomalus ( = Pichia anomala), was consistently isolated from the inner wall of the bamboo internodes and also from the body surface of the larvae. Histological examination of the ovipositor of adult females revealed an exoskeletal pocket on the eighth abdominal segment. The putative mycangium contained yeast cells, and W. anomalus was repeatedly detected from the symbiotic organ. When first instar larvae were placed on culture media inoculated with W. anomalus, they grew and developed normally to adulthood. By contrast, first instar larvae placed on either sterile culture media or autoclaved strips of bamboo inner wall exhibited arrested growth at the second instar, and addition of W. anomalus to the media resumed growth and development of the larvae. These results strongly suggest a mutualistic nature of the D. bucculenta-W. anomalus association with morphological specialization and physiological dependence. Based on these results, we compare the fungal farming of D. bucculenta with those of social and subsocial insects, and discuss ecological factors relevant to the evolution of fungal farming in a non-social insect.
Introduction
Cultivation mutualism is a form of organism-organism symbiotic associations wherein an organism (normally called host) cultures another organism (often called symbiont or crop) as food source. Eusocial insects like ants and termites and subsocial insects like ambrosia beetles exhibit sophisticated forms of cultivation mutualism, which may be comparable to human agriculture in many aspects [1], [2]. These insects inoculate their specific fungal associates onto appropriate substrates, engineer the environmental conditions for their optimal growth, defend them against pests/parasites/pathogens by monitoring, sequestration and/or antibiotic application, harvest and consume them as food, and are obligatorily dependent on them [1]–[6]. Such specialized cultivation mutualisms are not found in non-social organisms. Only primitive forms of cultivation mutualism have been reported from non-social organisms like a marine snail with a fungus [7] and a damselfish with an alga [8], and also from a slime mold with food bacteria [9]. Among non-social insects, several cases of putative cultivation mutualism have been described [10], [11]. A well-documented case is the gall midges of the tribes Lasiopterini and Asphondyliini (Diptera: Cecidomyiidae). These insects carry their specific fungal associate and inoculate the fungus to their host plant upon oviposition. The fungus is suspected to be involved in the gall formation, proliferates in the inner space of the gall, and is consumed by the insect larvae [11]. Another well-documented case is the leaf-rolling weevils of the genus Euops [12]–[14]. External structures specialized for harboring fungal associates, designated as fungal pockets or mycangia, have been identified not only in social ants and ambrosia beetles with cultivation mutualism [15], [16] but also in various non-social insects such as lymexylid beetles, wood wasps, gall midges, leaf-rolling weevils, stag beetles and others [10], [12], [17]–[20]. In these non-social insects, however, biological aspects of the mycangium-associated microbes, in particular their physiological roles for their hosts, have been poorly investigated.
The lizard beetles of the tribe Languriini (Coleoptera: Erotylidae: Languriinae) constitute a moderately diversified taxon, consisting of more than 1000 species worldwide, and have been reported to be phytophagous [21]–[23]. Adult females of the lizard beetle Doubledaya bucculenta, which is endemic to Japan, have a large asymmetric head with enlarged mandibles and elongated forelegs (Fig. 1A). In spring, they excavate a small hole on a recently-dead culm of Pleioblastus and Semiarundinaria bamboos, lay an egg into the cavity of the bamboo internode via the hole, and plug the hole with bamboo fibres (Fig. 1B and C) [24]–[27]. Within the bamboo internode, a single larva of D. bucculenta develops and pupates, overwinters either as larva or adult, and an adult insect emerges by biting out the bamboo wall in spring. To our knowledge, there has been no report on beetles of the subfamily Languriinae associated with symbiotic microorganisms [28]–[30].
Figure 1. The lizard beetle Doubledaya bucculenta, the host bamboo Pleioblastus simonii, and the associated yeast Wickerhamomyces anomalus.
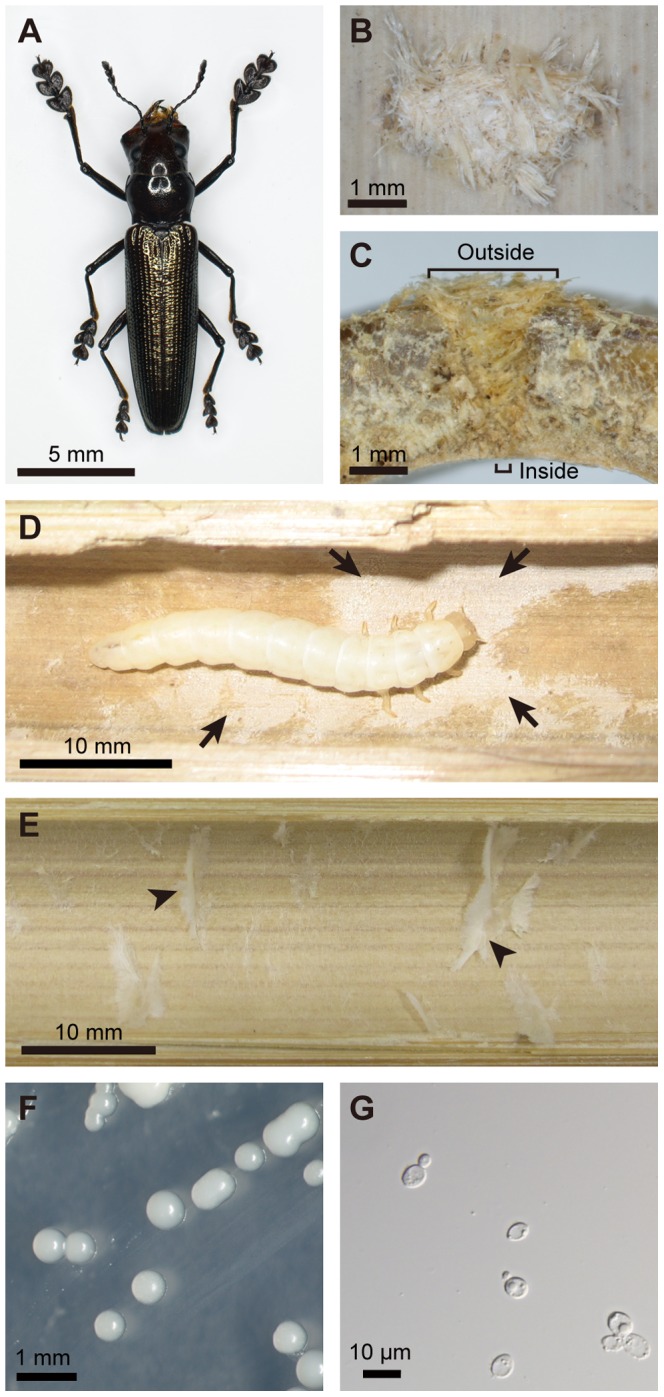
(A) An adult female of D. bucculenta. (B) An oviposition mark of D. bucculenta. An outside view of an oviposited dead bamboo culm is shown. (C) A cross section of an oviposited dead bamboo culm. The oviposition hole is wider outside and narrower inside. (D) A larva of D. bucculenta feeding on fungal layer on the inner surface of the bamboo internode. Arrows indicate the fungal layer. (E) The inner wall of the bamboo internode without oviposition mark. Arrowheads indicate pith tissues of the bamboo. (F) Yeast colonies isolated from D. bucculenta on a potato dextrose agar plate. (G) A light microscopic image of budding yeast cells obtained from D. bucculenta.
In this study, we demonstrate that (i) the inner surface of the bamboo internode harboring a larva of D. bucculenta is always covered with a fungal layer, (ii) a specific fungus is consistently isolated from the inner wall and the larva, (iii) adult females of D. bucculenta possess an ovipositor-associated mycangium that contains the fungal cells, and (iv) the fungus is essential as food source for growth and development of the larvae of D. bucculenta, which provides a novel case of cultivation mutualism in this non-social insect.
Materials and Methods
Materials
Almost all insect samples of D. bucculenta and internode samples of the bamboo Pleioblastus simonii were collected at Kawaminami, Miyazaki Prefecture, Japan [32°9′N, 131°29′E]. In addition, a larval sample was collected at Shirosato, Ibaraki Prefecture, Japan [36°38′N, 140°34′E] on 24th June 2007, and an adult female was sampled at Takanabe, Miyazaki Prefecture, Japan [32°6′N, 131°31′E] in September 2009. The relatively limited sample size of the beetles was due to difficulty in collecting a large number of D. bucculenta [24]. No specific permits were required for the described field studies. The locations are not privately-owned or protected in any way. The field studies did not involve endangered or protected species.
Microbial isolation
Seventeen bamboo internodes were collected on 4th December 2008 at Kawaminami, washed in running tap water, immersed in 70% ethanol and fire-sterilized. Then, each of the internodes was carefully cut open, and the inner wall was scraped with a tip of nichrome wire and transferred onto potato dextrose agar (PDA) (Difco, Detroit, MI, USA) plates containing no antibiotics. A larva collected from a dead P. simonii internode at Shirosato was placed on a 9 cm malt agar (Difco) plate containing no antibiotics, and allowed to walk for five minutes. Plates were incubated at 25°C until visible microbial colonies appeared. The microbial isolates were kept at 5°C during the experiments and stored in glycerol at −80°C for long-term maintenance.
Rearing experiments
The above-mentioned fungal isolate from a larva of D. bucculenta was used for rearing experiments. The fungal cells were suspended in sterilized water, and 9 cm PDA plates were wiped with tissue paper immersed with the cell suspension (about 1 ml each) and incubated at 25°C for two days. Eggs of D. bucculenta were collected from dead P. simonii internodes on 5th and 7th May 2008 at Kawaminami, surface-sterilized in 99.5% ethanol for one minute and then in 70% ethanol for one minute, and placed on wet filter paper in a sterilized Petri dish at 25°C. Newly hatched larvae were randomly allocated to either of three experimental groups. In the first group, each of five larvae was placed singly on a sterile 9 cm PDA plate. In the second group, each of five larvae was placed singly on a 9 cm PDA plate on which the fungal strain had grown. The larvae were transferred to fresh fungus-growing PDA plates at intervals of 13–15 days to avoid depletion of the fungus on the plates. In the third group, each of five larvae was placed singly on an autoclaved strip of P. simonii internode (about 15.5 cm×2.5 cm in size) in a sterilized test tube (3.0 cm in diameter and 20 cm tall) with moistened cotton placed at the bottom. These insects were reared at 25°C in the dark, observed daily to record molting, and weighed aseptically at intervals of 12–22 days during the initial 70 days. Since it was difficult to find larval shed skins in the test tubes, the duration of each instar could not be perfectly recorded. For the third group, after rearing for 70 days, the fungal suspension (about 1 ml each) was added to the bamboo strip in the test tube, and the rearing was continued.
Histology
Five adult males and five adult females were obtained from internodes of dead P. simonii culms at Kawaminami on 29th August 2009. These insects were dissected under a dissection microscope and carefully examined for any mycangial structures on the exoskeleton and internal organs. Dissected mycangia were fixed with 3% formalin in PBS, embedded in paraffin, processed into serial tissue sections, stained with periodic acid-Schiff reagent and haematoxylin, and observed under a light microscope as described [31].
DNA sequencing and phylogenetic analysis
Fungal DNA samples were prepared using lyticase (Sigma-Aldrich, St. Louis, MO, USA) and Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). The following primers were used for polymerase chain reaction (PCR): LS1 (5′ –AGT ACC CGC TGA ACT TAA G–3′) and NL4 (5′–GGT CCG TGT TTC AAG ACG G–3′) for D1/D2 domain of 26S rRNA gene; and 983 (5′–GCY CCY GGH CAY CGT GAY TTY AT–3′) and 2218 (5′–ATG ACA CCR ACR GCR ACR GTY TG–3′) for translation elongation factor-1α (EF-1α) gene [32]–[34]. PCR products were purified using Qiagen PCR Purification Kit (Qiagen, Hilden, Germany), and directly sequenced using ABI Prism BigDye Terminators (Applied Biosystems, Foster, CA, USA) and ABI PRISMR 3130xl Genetic Analyzer (Applied Biosystems). Nucleotide sequence data reported in this study have been deposited to the DNA Data Bank of Japan with accession numbers AB640725-AB640728, AB713902 and AB713903. Multiple alignments of the nucleotide sequences were generated using the program ClustalX 1.83 [35]. Molecular phylogenetic analyses were conducted by maximum parsimony method using the program PAUP 4.0b10 [36] with 1,000 bootstrap replicates, heuristic searches, and nearest neighbor interchange branch swapping.
Results
Observation of bamboo internodes harboring larvae of D. bucculenta
We collected more than 100 dead internodes of P. simonii that had an oviposition mark (Fig. 1B) at Kawaminami, Miyazaki, Japan. Many of them contained a larva of D. bucculenta, while the rest of them contained either an adult of D. bucculenta or no insect. In addition, we also sampled ten dead internodes of P. simonii with no oviposition mark. When they were cut open and inspected, a white layer always covered the inner surface of the larva-containing internodes with oviposition mark (Fig. 1D), whereas the white layer was not observed on the inner surface of the larva-absent internodes without oviposition mark (Fig. 1E). As for the internodes with oviposition mark that contained either an adult insect or no insect, the appearance of the inner surface was not uniform: some were clean while the others were dirtily contaminated, but none of them exhibited the typical white layer.
Microbial isolation from bamboo internodes harboring larvae of D. bucculenta
For microbial isolation, we collected the following samples at Kawaminami, Miyazaki, Japan: five living internodes without oviposition mark from three living culms of P. simonii, all of which contained no insect; five dead internodes without oviposition mark from three dead culms of P. simonii, all of which contained no insect; and seven dead internodes with oviposition mark from three dead culms of P. simonii, of which five contained a larva of D. bucculenta, one contained an adult male of D. bucculenta, and one contained no insect. When the inner surface of each of the bamboo internodes was scraped and inoculated onto PDA plates, no microbial colonies were obtained from all the five living and five dead internodes without oviposition mark. By contrast, all the five dead internodes containing a larva consistently yielded a number of uniform white colonies on the plates (Fig. 1F). Light microscopic observation revealed that the colonies consisted of yeast-like unicellular fungal cells (Fig. 1G). From the dead internodes with oviposition mark that contained either an adult or no insect, not only the yeast colonies but also filamentous fungi were obtained.
We also collected a larva of D. bucculenta from a dead internode of P. simonii at Shirosato, Ibaraki, Japan. When the larva was allowed to walk on a malt agar plate, a number of yeast colonies of the same type appeared on the plate.
Molecular characterization of the yeast isolates from D. bucculenta
A 0.5-kb fragment of 26S rRNA gene and a 0.8-kb fragment of EF-1α gene were amplified by PCR and sequenced from the yeast strains, one from a larva-containing bamboo internode collected at Kawaminami, Miyazaki, Japan, and the other from a larva collected at Shirosato, Ibaraki, Japan. The 530 bp sequences of 26S rRNA gene were identical between the yeast strains. The sequences were also identical to the sequence of Wickerhamomyces anomalus (formerly Pichia anomala [34]; accession number U74592 [37]) (Fig. 2A). Similarly, the 802 bp sequences of EF-1α gene were identical between the yeast strains except for one undetermined nucleotide site. The sequences were also identical to the sequence of W. anomalus (accession number EF552565 [34]) except for five nucleotide sites with ambiguous base reading (Fig. 2B).
Figure 2. Phylogenetic placement of the yeast strains associated with Doubledaya bucculenta.
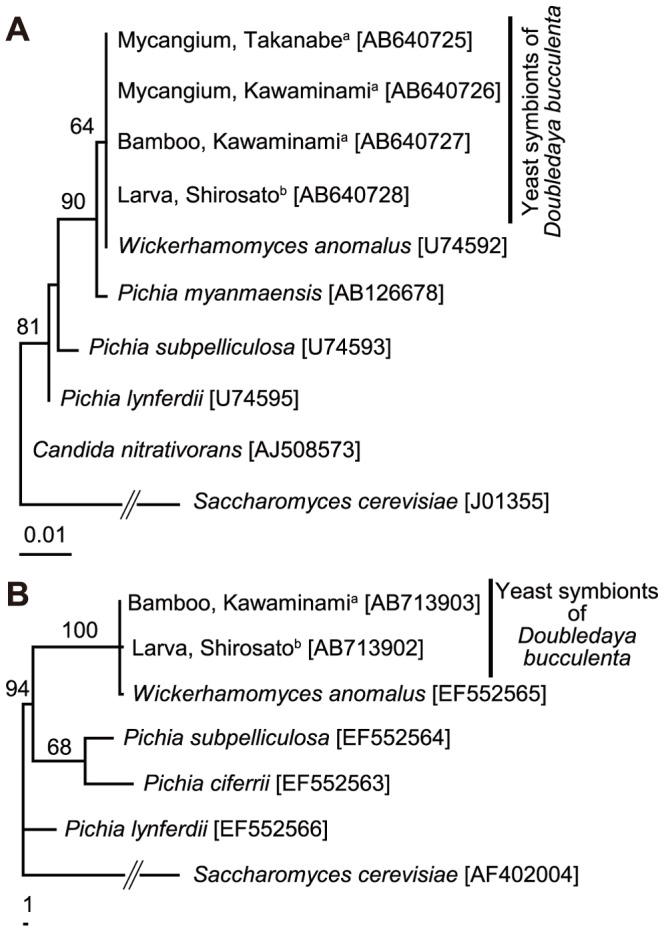
(A) A maximum parsimony phylogeny inferred from 26S rRNA gene sequences (530 bps). (B) A maximum parsimony phylogeny inferred from EF-1α gene sequences (802 bps). Bootstrap values of 50% or higher are shown at the nodes. For each yeast strain obtained from D. bucculenta, isolation source and collection locality are indicated. Sequence accession numbers are in brackets. a Miyazaki Prefecture, Japan; b Ibaraki Prefecture, Japan.
Discovery of a yeast-harboring mycangium associated with female ovipositor of D. bucculenta
In all five adult females of D. bucculenta collected at Kawaminami, Miyazaki, Japan, a yellowish exoskeletal pocket was present on the tergum of the eighth abdominal segment (Fig. 3A–C), while the structure was absent in adult males. Tissue sectioning identified yeast-like particles within the pocket (Fig. 3D). When the organ dissected from an adult female was subjected to DNA extraction, PCR and sequencing of the fungal 26S rRNA gene, the 530 bp nucleotide sequence obtained was identical to those of the already obtained yeast strains and also to that of W. anomalus (Fig. 2A). In addition, the same fungal sequence was obtained from the organ dissected from an adult female collected at a different locality, Takanabe, Miyazaki, Japan (Fig. 2A).
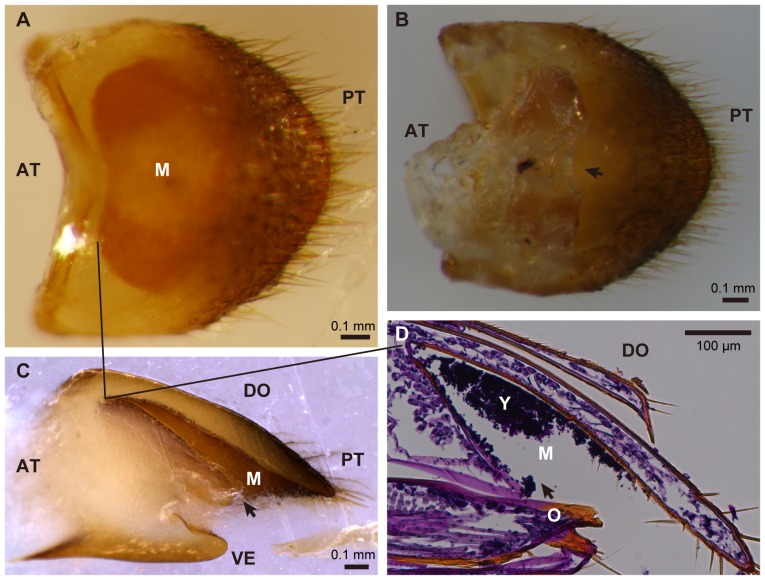
Figure 3. Mycangium associated with the ovipositor of adult females of Doubledaya bucculenta.
(A) A dorsal view of the tergum of the eighth abdominal segment. (B) A ventral view of the eighth abdominal segment. (C) A lateral view of the eighth abdominal segment that was embedded in paraffin and cut longitudinally. (D) A longitudinal tissue section of the eighth abdominal segment, stained with periodic acid-Schiff reagent and hematoxylin. Arrows in (B), (C) and (D) indicate the opening of mycangium. Solid lines in (A), (C) and (D) correspond to the front edge of mycangial cavity. Abbreviations: M, mycangium; O, ovipositor; Y, yeast cells; AT, anterior; DO, dorsal; PT, posterior; VE, ventral.
Rearing experiments of D. bucculenta larvae in the presence and absence of the yeast
When five first instar larvae of D. bucculenta were individually reared on sterile PDA plates, four larvae managed to become second instar (mean and standard deviation of first instar period, 8.0±2.0 days, n = 4) but neither grew nor developed further (Fig. 4A and B). Among them, only a larva survived for 70 days after hatching, but it was very frail and died soon. A larva died in first instar.
Figure 4. Effects of the yeast symbiont on larval growth of Doubledaya bucculenta.
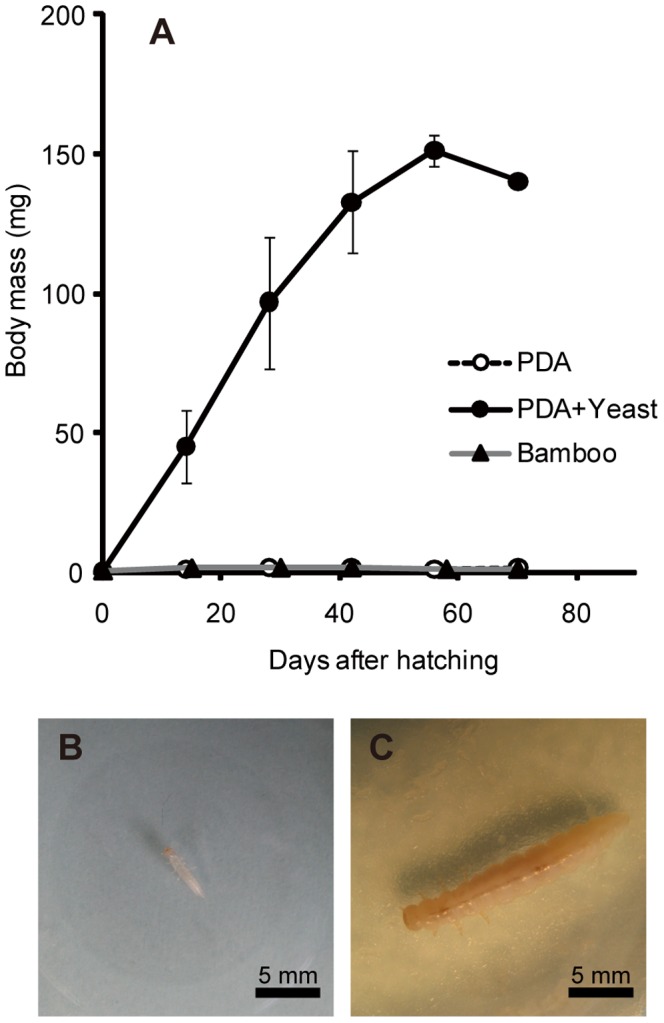
(A) Larval growth curves on sterile potato dextrose agar (PDA) plates (open circles), on PDA plates on which the yeast strain was fully grown (filled circles), and on sterilized bamboo strips (filled triangles). Means and standard deviations are shown. (B) A 14-day-old larva reared on a sterile PDA plate. (C) A 14-day-old larva reared on a yeast-inoculated PDA plate.
By contrast, when five first instar larvae were individually reared on PDA plates on which the larva-derived yeast strain had fully grown, all five larvae promptly became second instar (mean and standard deviation of first instar period, 5.6±1.3 days, n = 5) and, strikingly, normally grew, pupated, and reached adulthood (Fig. 4A and C). The final instar differed among the larvae: fifth instar for two insects (larval period 40 and 54 days for one female and one male, respectively) and sixth instar for three insects (larval period 60, 58 and 73 days for one male and two females, respectively). The elytral length ranged from 10.7 to 11.6 mm for the two males and 10.9 to 11.2 mm for the three females. These values were within the range of the elytral lengths of field-collected D. bucculenta [26].
When five first instar larvae were individually reared on autoclaved strips of P. simonii internodes, four larvae managed to develop to second instar, while a larva died in first instar. Three of the larvae remained at second instar for 70 days after hatching, while a larva died during the period (Fig. 4A). Then, we inoculated the cultured yeast strain to the bamboo strips, which resulted in, strikingly, restored growth and development of the second instar larvae. Finally, two of the three larvae became adult males, taking additional 74 and 201 days and attaining 9.9 and 7.0 mm of elytral length, respectively.
Discussion
In this study, we demonstrated that (i) the inner surface of the bamboo internode containing a larva of D. bucculenta is always covered with a white fungal layer (Fig. 1D), (ii) a specific yeast species, W. anomalus ( = P. anomala), is repeatedly isolated from the inner wall and also from the larva (Fig. 1F and G; Fig. 2), (iii) adult females of D. bucculenta possess an ovipositor-associated exoskeltal pocket on the tergum of the eighth abdominal segment (Fig. 3), (iv) the putative mycangium contains yeast-like particles (Fig. 3D), (v) PCR and sequencing of a fungal gene fragment identify the mycangium-associated yeast as W. anomalus (Fig. 2), (vi) larvae of D. bucculenta neither grow nor develop in the absence of W. anomalus (Fig. 4A and B), and (vii) larvae of D. bucculenta can normally grow, develop, pupate and become adult when cultured W. anomalus is provided as food (Fig. 4A and C). Based on these results, we conclude that D. bucculenta is in an obligate symbiotic association with W. anomalus. To our knowledge, this study is the first to report a symbiotic association with specific microorganisms in lizard beetles (Coleoptera: Erotylidae: Languriinae).
From distant geographic populations (Miyazaki and Ibaraki, Japan) and different isolation sources and developmental stages (bamboo inner surface, larval body surface and adult female mycangium), genetically indistinguishable strains of W. anomalus were consistently detected (Fig. 2), which indicate an intimate and stable (at least to some extent) association between D. bucculenta and W. anomalus. Here it should be noted that, since 26S rRNA and EF-1α genes are conservative and slow-evolving genes, some unrecognized genetic variations may exist among the fungal strains. It seems plausible that the female mycangium plays an important role for sustaining the host-symbiont association by mediating vertical transmission of the symbiont upon oviposition, although the exact transmission process and involvement of the mycangium require experimental verification. Judging from the anatomy of the female ovipositor (Fig. 3D), the yeast cells contained in the mycangium will be exposed to outside when the ovipositor is stretched for oviposition. It deserves future studies how and when the yeast cells are incorporated into the mycangium, whether W. anomalus is selectively incorporated and cultured in the symbiotic organ, and how the yeast cells are inoculated to the bamboo internode upon oviposition.
In a number of non-social beetles and other insects, although external exoskeletal pits, pockets and cavities have often been recognized and naively referred to as “mycangia”, their fungal storage/transport/inoculation roles have been rarely verified [20], with a few exceptions such as lymexylid beetles [10], wood wasps [17], gall midges [18], leaf-rolling weevils [14] and stag beetles [19]. The similarity in the mycangial configuration, namely fungus-carrying pouch(es) associated with female ovipositor, among the lymexylid beetles, stag beetles and D. bucculenta seems notable, plausibly reflecting their common habit of inoculating their microbial associates to woody substrate upon oviposition. The case of D. bucculenta is remarkable in that a specific mycangium-associated fungus is unequivocally identified and, furthermore, an essential biological role of the fungus for the host is experimentally demonstrated.
Mueller et al. [1] pointed out that the highest levels of cultivation mutualism as those found in ants, termites and ambrosia beetles entail the following features: (a) “habitual planting” or “inoculation” of specific fungal associates to appropriate substrates; (b) “conditioning” of the fungal associates by improving their growth and proliferation and/or “protection” of the fungal associates against pests/parasites/pathogens by monitoring, sequestration and/or antibiotic application; (c) “harvesting and consumption” of the fungal associates for food; and (d) “obligate nutritional dependence” on the fungal associates. Notably, the D. bucculenta-W. anomalus relationship evidently exhibits the features (a) inoculation, (c) harvesting/consumption and (d) obligate nutritional dependence, which indicate a high level of cultivation mutualism in this non-social beetle. Confirmation of the feature (b) conditioning/protection requires further studies, although the substantial monoculture of W. anomalus in larva-inhabiting bamboo internodes and the invasion of non-specific fungi into an adult-inhabiting bamboo internode suggest the possibility of larval conditioning of fungal growth and/or larval control over microbial contaminants in D. bucculenta. Alternatively, the fungus itself may be responsible for its own monoculture in the bamboo internodes, on the ground that W. anomalus ( = P. anomala) has often been referred to as “killer yeast” or “biocontrol yeast” on account of its inhibitory effects against molds and bacteria [38]–[42]. The inside of bamboo internode is a tightly packaged, firmly isolated, and substantially sterile ecological niche, and it is difficult to utilize for most animals. By evolving the exaggerated mandibles for biting the bamboo culm and the elongated forelegs for holding itself on the bamboo surface (Fig. 1A), D. bucculenta succeeded in exploitation of this untouched ecological niche that is suitable for monoculture of the fungal associate.
The relationship between D. bucculenta and W. anomalus seems certainly mutualistic, but the extents of their mutual interdependence are likely asymmetric. Since D. bucculenta cannot grow without the fungal symbiont (Fig. 4), the host is obligatorily dependent on the symbiont. On the other hand, although W. anomalus may benefit from the monoculturing and vectoring activities of D. bucculenta, the yeast can grow independently of the insect on standard microbiological media (Fig. 1F), suggesting that the symbiont is dependent on the host not obligatorily but only facultatively. It seems likely, although experimental confirmation is needed, that the fungal symbiont has both symbiotic and free-living life stages, like some fungal cultivars of leaf-cutting ants and ambrosia beetles [3], [25], potentially being subjected not only to vertical transmission but also to horizontal transmission across host insect generations. In many host-symbiont mutualisms among insects such as aphid-Buchnera and tsetse-Wigglesworthia associations, the symbionts are unable to survive outside their hosts, are subjected to strict vertical transmission through host generations, and often co-speciate with their hosts over evolutionary time [43], [44]. In host-symbiont mutualisms among many marine invertebrates and plants, and also in some insects, by contrast, the symbionts are freely present in the environment, the hosts acquire their symbionts from the environment every generation, and the host-symbiont relationship is consequently promiscuous both ecologically and phylogenetically [45], [46]. Probably the D. bucculenta-W. anomalus relationship represents an intermediate between these typical extremes of host-symbiont mutualism.
For a long time, lizard beetles were placed in the family Languriidae, but recent phylogenetic studies have revised the taxonomic treatment of the group as the subfamily Languriinae within the family Erotylidae, whose members, pleasing fungus beetles, are mostly fungivorous [47], [48]. Although ecological aspects of lizard beetles have been poorly documented, several literatures describe that members of the Languriinae are mostly phytophagous [21]–[23]. The discovery of the fungal farming on a plant substratum in D. bucculenta, together with its phylogenetic affinity to pleasing fungus beetles, suggests the possibility that some lizard beetles are actually not phytophagous but fungivorous.
Using light and scanning electron microscopy, van Zandt et al. [49] identified two pairs of deep pits on the ventral aspect of the gena between the eyes and the maxillae of the erotylid beetle Loberus impressus, and suggested their possible role as mycangia in addition to the role as glandular outlets. On the other hand, Grebennikov and Leschen [20] pointed out that the majority of reports on mycangial function of insect exoskeletal cavities are based solely on the observation of these structures and the fungus-associated ecology of the insects, usually without other biological evidence. Hence, whether the exoskeletal pits of L. impressus comprise mycangia is currently elusive. It is evident that the ovipositor-associated mycangium of D. bucculenta is structurally, developmentally and evolutionarily unrelated to the external pits on the head of the erotylid beetle.
In conclusion, we discovered a fungal cultivation mutualism in a non-social beetle D. bucculenta, which entails association with a specific yeast species, development of a mycangium adjacent to the female ovipositor, and obligate nutritional dependence on the fungal associate. The high level of cultivation mutualism in the non-social insect would provide a valuable insight into the evolutionary trajectories toward the highest levels of cultivation mutualisms, so-called agriculture, found in social insects, and ultimately, human beings.
Acknowledgments
The authors thank M. Komatsu for help with staining of insect tissues, N. Matsushita for help with taking pictures of the yeast, and K. Ichikawa, Y. Shintani and K. Toki for help with collecting insects.
Funding Statement
WT was supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (20-6541). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol 36: 563–595. [Google Scholar]
- 2.Schultz TR, Mueller UG, Currie CR, Rehner SA (2005) Reciprocal illumination: A comparison of agriculture in humans and ants. In: Insect-Fungal Associations: Ecology and Evolution. Vega F, Blackwell M, editors. New York: Oxford University Press. 149–190.
- 3. Mueller UG (2002) Ant versus fungus versus mutualism: Ant-cultivar conflict and the deconstruction of the attine ant-fungus symbiosis. Am Nat 160 Suppl.:S67–98. [DOI] [PubMed] [Google Scholar]
- 4. Leuthold RH, Badertscher S, Imboden H (1989) The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera, Macrotermitinae). Insect Soc 36: 328–38. [Google Scholar]
- 5.Norris DM (1979) The mutualistic fungi of Xyleborini beetles. In: Insect-Fungus Symbiosis. Batra LR, editor. Monclair: Allanheld, Osmun. pp. 53–65.
- 6. Biedermann PHW, Taborsky M (2011) Larval helpers and age polyethism in ambrosia beetles. Proc Natl Acad Sci USA 108: 17064–17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silliman BR, Newell SY (2003) Fungal farming in a snail. Proc Natl Acad Sci USA 100: 15643–15648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hata H, Kato M (2006) A novel obligate cultivation mutualism between damselfish and Polysiphonia algae. Biol Lett 2: 593–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brock DA, Douglas TE, Queller DC, Strassmann JE (2011) Primitive agriculture in a social amoeba. Nature 469: 393–396. [DOI] [PubMed] [Google Scholar]
- 10.Francke-Grosmann H (1967) Ectosymbiosis in wood-inhabiting insects. In: Symbiosis: Associations of invertebrates, birds, ruminants, and other biota. Henly SM, editor. New York: Academic Press. 141–205.
- 11. Rohfritsch O (2008) Plants, gall midges, and fungi: a three-component system. Entomol Exp Appl 128: 208–216. [Google Scholar]
- 12. Sawada Y, Morimoto K (1986) The mycetangia and the mode of the fungus transmission in the weevil genus Euops (Coleoptera: Attelabidae). Sci Bull Fac Agr Kyushu Univ 49: 197–205. [Google Scholar]
- 13. Kobayashi C, Fukasawa Y, Hirose D, Kato M (2008) Contribution of symbiotic mycangial fungi to larval nutrition of a leaf-rolling weevil. Evol Ecol 22: 711–722. [Google Scholar]
- 14. Li X, Guo W, Ding J (2012) Mycangial fungus benefits the development of a leaf-rolling weevil, Euops chinesis . J Insect Physiol 58: 867–873. [DOI] [PubMed] [Google Scholar]
- 15. Quinlan RJ, Cherrett JM (1978) Studies on the role of the infrabuccal pocket of the leaf-cutting ant Acromyrmex octospinosus (Reich) (Hym., Formicidae). Insect Soc 25: 237–245. [Google Scholar]
- 16. Batra LR (1963) Ecology of ambrosia fungi and their dissemination by beetles. Trans Kans Acad Sci 66: 213–236. [Google Scholar]
- 17. Morgan FD (1968) Bionomics of Siricidae. Annu Rev Entomol 13: 239–256. [Google Scholar]
- 18. Borkent A, Bissett J (1985) Gall midges (Diptera: Cecidomyiidae) are vectors for their fungal symbionts. Symbiosis 1: 185–194. [Google Scholar]
- 19. Tanahashi M, Kubota K, Matsushita N, Togashi K (2010) Discovery of mycangia and the associated xylose-fermenting yeasts in stag beetles (Coleoptera: Lucanidae). Naturwissenschaften 97: 311–317. [DOI] [PubMed] [Google Scholar]
- 20. Grebennikov VV, Leschen RAB (2010) External exoskeletal cavities in Coleoptera and their possible mycangial functions. Entomol Sci 13: 81–98. [Google Scholar]
- 21. Leschen RAB, Wegrzynowicz P (1998) Generic catalogue and taxonomic status of Languriidae (Coleoptera: Cucujoidea). Ann Zool 48: 221–243. [Google Scholar]
- 22.Leschen RAB (2003) Erotylidae (Insecta: Coleoptera: Cucujoidea): Phylogeny and review (Part 1). Fauna of New Zealand. Manaaki Whenua Press, Lincoln, New Zealand.
- 23. Leschen RAB, Buckley TR (2007) Multistate characters and diet shifts: evolution of Erotylidae (Coleoptera). Syst Biol 56: 97–112. [DOI] [PubMed] [Google Scholar]
- 24. Hayashi N (1974) Ecology of Doubledaya bucculenta . Nat Insect 9: 17. [Google Scholar]
- 25. Toki W (2009) New host plants and additional records of asymmetric lizard beetle Doubledaya bucculenta Lewis, 1884 (Coleoptera: Erotylidae: Languriinae) in distributional northernmost region. Biogeography 11: 109–111. [Google Scholar]
- 26. Toki W, Togashi K (2011) Exaggerated asymmetric head morphology of female Doubledaya bucculenta (Coleoptera: Erotylidae: Languriinae) and ovipositional preference for bamboo internodes. Zool Sci 28: 348–354. [DOI] [PubMed] [Google Scholar]
- 27. Toki W, Hosoya T (2011) New host plant and southernmost records of asymmetric lizard beetle Doubledaya bucculenta Lewis (Coleoptera: Erotylidae: Languriinae). Elytra New Ser 1: 253–254. [Google Scholar]
- 28.Batra LR (1979) Insect-fungus symbiosis: nutrition, mutualism and commensalism. John Wiley & Sons Inc. 288.
- 29.Buchner P (1965) Endosymbiosis of animals with plant microorganisms. New York: Wiley Interscience. 909.
- 30.Vega FE, Blackwell M (2005) Insect-fungal associations: ecology and evolution. New York: Oxford University Press. 333.
- 31. Sasaki T, Kawamura M, Ishikawa H (1996) Nitrogen recycling in the brown planthopper, Nilapawata lugens: involvement of yeast-like endosymbionts in uric acid metabolism. J Insect Physiol 42: 125–129. [Google Scholar]
- 32. Hausner G, Reid J, Klassen GR (1993) On the subdivision of Ceratocystis s. 1, based on partial ribosomal DNA sequences. Can J Bot 71: 52–63. [Google Scholar]
- 33. Suh S-O, Blackwell M (2004) Three new beetle-associated yeast species in the Pichia guilliermondii clade. FEMS Yeast Res 5: 87–95. [DOI] [PubMed] [Google Scholar]
- 34. Kurtzman CP, Robnett CJ, Basehoar-Powers E (2008) Phylogenetic relationships among species of Pichia, Issatchenkia and Williopsis determined from multigene sequence analysis, and the proposal of Barnettozyma gen. nov., Lindnera gen. nov. and Wickerhamomyces gen. nov. FEMS Yeast Res 8: 939–954. [DOI] [PubMed] [Google Scholar]
- 35. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The ClustalX windows interface: sexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swofford DL (2002) PAUP*, phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer, Sunderland, MA.
- 37. Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Van Leeuwenhoek 73: 331–371. [DOI] [PubMed] [Google Scholar]
- 38. Polonelli L, Morace G (1986) Reevaluation of the yeast killer phenomenon. J Clin Microbiol 24: 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersson S, Schnürer J (1995) Biocontrol of mold growth in high-moisture wheat stored under airtight conditions by Pichia anomala, Pichia guilliermondii, and Saccharomyces cerevisiae . Appl Environ Microbiol 61: 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Petersson S, Schnürer J (1998) Pichia anomala as a biocontrol agent of Penicillium roqueforti in high-moisture wheat, rye, barley and oats stored under airtight conditions. Can J Microbiol 44: 471–476. [Google Scholar]
- 41. Fredlund E, Druvefors U, Boysen ME, Lingsten KJ, Schnürer J (2002) Physiological characteristics of the biocontrol yeast Pichia anomala J121. FEMS Yeast Res 2: 395–402. [DOI] [PubMed] [Google Scholar]
- 42. Olstorpe M, Borling J, Schnürer J, Passoth V (2010) Pichia anomala yeast improves feed hygiene during storage of moist crimped barley grain under Swedish farm conditions. Anim Feed Sci Tech 156: 47–56. [Google Scholar]
- 43. Baumann P (2005) Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59: 155–189. [DOI] [PubMed] [Google Scholar]
- 44. Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- 45. Bright M, Bulgheresi S (2010) A complex journey: transmission of microbial symbionts. Nat Rev Microbiol 8: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73: 4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wegrzynowicz P (2002) Morphology, phylogeny and classification of the family Erotylidae based on adult characters (Coleoptera Cucujoidea). Genus 13: 435–504. [Google Scholar]
- 48. Robertson JA, Mchugh JV, Whiting MF (2004) A molecular phylogenetic analysis of the pleasing fungus beetles (Coleoptera: Erotylidae): evolution of colour patterns, gregariousness and mycophagy. Syst Entomol 29: 173–187. [Google Scholar]
- 49. van Zandt PA, Townsend JR, Carlton CE, Blackwell M, Mopper S (2003) Loberus impressus (LeConte) (Coleoptera: Erotylidae) fungal associations and presence in the seed capsules of Iris hexagona . Coleopts Bull 57: 281–288. [Google Scholar]