Abstract
The risk that the plant pathogen Phytophthora melonis develops resistance to carboxylic acid amide (CAA) fungicides was determined by measuring baseline sensitivities of field isolates, generating resistant mutants, and measuring the fitness of the resistant mutants. The baseline sensitivities of 80 isolates to flumorph, dimethomorph and iprovalicarb were described by unimodal curves, with mean EC50 values of 0.986 (±0.245), 0.284 (±0.060) and 0.327 (±0.068) µg/ml, respectively. Seven isolates with different genetic background (as indicated by RAPD markers) were selected to generate CAA-resistance. Fifty-five resistant mutants were obtained from three out of seven isolates by spontaneous selection and UV-mutagenesis with frequencies of 1×10−7 and 1×10−6, respectively. CAA-resistance was stable for all mutants. The resistance factors of these mutants ranged from 7 to 601. The compound fitness index (CFI = mycelial growth × zoospore production × pathogenicity) was often lower for the CAA-resistant isolates than for wild-type isolates, suggesting that the risk of P. melonis developing resistance to CAA fungicides is low to moderate. Among the CAA-resistant isolates, a negative correlation between EC50 values was found for iprovalicarb vs. flumorph and for iprovalicarb vs. dimethomorph. Comparison of the full-length cellulose synthase 3 (CesA3) between wild-type and CAA-resistant isolates revealed only one point mutation at codon position 1109: a valine residue (codon GTG in wild-type isolates) was converted to leucine (codon CTG in resistant mutants). This represents a novel point mutation with respect to mutations in CesA3 conferring resistance to CAA fungicides. Based on this mutation, an efficient allelic-specific PCR (AS-PCR) method was developed for rapid detection of CAA-resistance in P. melonis populations.
Introduction
The oomycete Phytophthora melonis Katsura, which is conspecific with P. sinesis, causes a severe disease of cucumber (Cucumis sativus) which has been reported in China, Japan, Egypt, Turkey, Korea, India and Iran [1]. In addition to cucumber, P. melonis infects other cucurbits including zucchini (Cucurbita pepo L.), hami melon (Cucumis melo L.), wax gourd (Benincasa hispida (Thunb.) Cogn.) [2]–[5], and pointed gourd (Trichosanthes dioica Roxb.) [6]. It also infects pistachio (Pistacia vera L) [7], causing blight, dieback, root rot, foot rot and crown rot. The use of resistant cultivars and chemical fungicides are two efficient control methods [2], [5], [8]. Phenylamides (e.g. metalaxyl) have been widely used for P. melonis disease control. However, metalaxyl-resistance of P. melonis has been reported in China [9]. Since the early 1980s, the efficacy of phenylamides has declined due to the emergence of resistant populations of oomycete pathogens in fields [10], [11].
The current study concerns resistance of P. melonis to the carboxylic acid amide (CAA) fungicides, which are divided into three different chemical groups based on differences in structure: the cinnamic acid amides (e.g., dimethomorph and flumorph), the valine amide carbamates (e.g., benthiavalicarb, benthiavalicarb-isopropyl and iprovalicarb) and the mandelic acid amides (e.g., mandipropamid) (FRAC Code List, www.frac.info). These fungicides are used to control the pathogens in the families Peronosporaceae (e.g., Plasmopara viticola and Bremia lactucea) and Pythiaceae (e.g., Phytophthora spp., but not Pythium spp.) [12]. All CAA fungicides strongly inhibit all asexual stages of susceptible pathogens but do not inhibit zoospore release and mobility [13]–[16]. Inhibition by CAA fungicides results from the interruption of cellulose biosynthesis and the disruption of cell wall structure [17].
istance to phenylamide fungicides, resistance to CAA fungicides is an important problem. Since dimethomorph’s introduction in the 1980s, CAA-resistant isolates of P. viticola have been detected in most areas of Europe (FRAC web). In China, flumorph-resistant isolates of Pseudoperonospora cubensis were obtained after successive applications of flumorph in a greenhouse [18]. P. viticola and Ps. cubensis are classified by FRAC as being at high risk to develop resistance to CAA fungicides, but P. infestans is considered to have a low risk of developing such resistance (FRAC pathogen risk list, www.frac.info). No resistant isolates of P. infestans have been detected in field since the introduction of CAA fungicides over 15 years ago (CAA Minutes 2010 final RG, www.frac.info). Using UV- and EMS- mutagenesis, researchers obtained stable CAA-resistant mutants of P. infestans only with difficulty [19]–[21] and an isolate of P. capsici failed to develop dimethomorph-resistance after repeated exposure to the fungicide [19]. However, P. capsici mutants with high CAA-resistance were obtained by mass selection from zoospores and oospores, and the resistance risk was considered low to moderate to CAAs in P. capsici [22], [23].
Cross-resistance among CAA fungicides has been reported in P. viticola [24] and P. capsici [22]. The resistance mechanism of some pathogens to CAA fungicides has been elucidated in recent reports. Amino acid substitutions at codon position 1105 (G1105V or G1105A) in cellulose synthase (CesA3) were responsible for resistance to the CAA fungicide mandipropamid in EMS-generated mutants of P. infestans [17]. Changes of G1105V or G1105W led to CAA-resistance in Ps. cubensis [25]. In P. viticola, changes of G1105S in PvCesA3 conferred CAA-resistance [26]. Based on the point mutation, a PCR-RFLP method has been developed for detecting CAA-resistant isolates in P. viticola populations [27].
The objectives of the present study were to (i) determine the baseline sensitivity of P. melonis to the CAA fungicides flumorph, dimethomorph and iprovalicarb; (ii) assess the risk of resistance to the three CAA fungicides; (iii) investigate the CAA-resistance mechanism in P. melonis; and (iv) develop a rapid and reliable method for detection of CAA-resistant isolates in populations of P. melonis.
Results
Baseline Sensitivity of P. melonis to Flumorph, Dimethomorph and Iprovalicarb
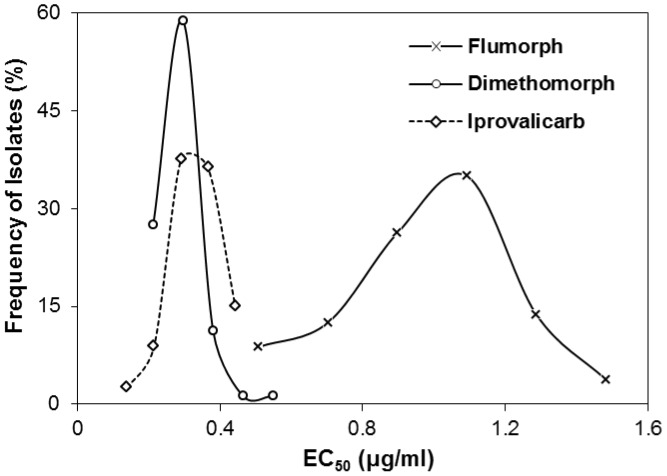
For the 80 P. melonis isolates investigated, the frequency distribution of EC50 values for each of the three CAA fungicides were described by unimodal curves (Figure 1), indicating the absence of CAA-resistant subpopulations among these isolates. The mean and range of EC50 values were 0.986±0.245 µg/ml and 0.410–1.577 µg/ml for flumorph, 0.284±0.060 µg/ml and 0.171–0.590 µg/ml for dimethomorph, and 0.327±0.068 µg/ml and 0.100–0.482 µg/ml for iprovalicarb. The highest EC50 value was 3.45-, 3.85-, and 4.82-times greater than the smallest value for flumorph, dimethomorph and iprovalicarb, respectively.
Figure 1. Frequency distributions of EC50 values (the effective concentration causing 50% inhibition of mycelial growth of Phytophthora melonis) for flumorph, dimethomorph and iprovalicarb.
In total, 80 isolates of P. melonis were collected from areas never exposed to carboxylic acid amide fungicides.
Development of CAA-resistant Isolates of P. melonis in vitro
Random amplified polymorphic DNA (RAPD)
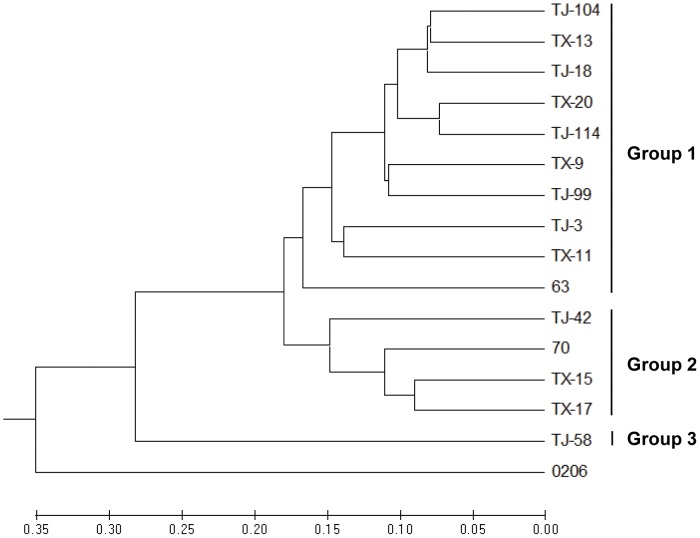
RAPD analysis was used to identify isolates with different genetic backgrounds; these isolates would be used as the parents for the generation of CAA-resistant mutants. Sixteen primers (Table 1) that produced easily recognizable and consistent banding patterns were used for RAPD analysis of 16 isolates from different geographic origins (Table 2). RAPD analysis using primer combinations clearly separated these isolates. A dendrogram based on UPGMA analysis indicated that the 15 isolates of P. melonis formed three major groups (Figure 2). One isolate of P. drechsleri was separated from P. melonis. RAPD groups were not related to the geographic origins or sensitivity to CAA fungicides of these isolates. Seven isolates (TJ-58, TX-11, TJ-99, TJ-104, TJ-114, 63 and 70) from different RAPD groups were selected for generation of CAA-resistant mutants.
Table 1. Nucleotide sequences and characteristics of primers used in this study.
| Primers | Sequence (5′–3′) | Description | Source or reference |
| ABA3 | AGTCAGCCAC | Primer for RAPD analysis | [42] |
| ABA7 | GAAACGGGTG | Same as for ABA3 | [42] |
| ABA9 | GGGTAACGCC | Same as for ABA3 | [42] |
| ABA10 | GTGATCGCAG | Same as for ABA3 | [42] |
| ABA13 | CAGCACCCAC | Same as for ABA3 | [42] |
| ABA17 | GACCGCTTGT | Same as for ABA3 | [42] |
| ABA18 | AGGTGACCGT | Same as for ABA3 | [42] |
| ABA20 | GTTGCGATCC | Same as for ABA3 | [42] |
| Y11 | AGACGATGGG | Same as for ABA3 | [43] |
| Y04 | GGCTGCAATG | Same as for ABA3 | [43] |
| OPG-16 | AGCGTCCTCC | Same as for ABA3 | [44] |
| OPS-14 | AAAGGGGTCC | Same as for ABA3 | [44] |
| OPX-12 | TCGCCAGCCA | Same as for ABA3 | [44] |
| OPG-11 | TGCCCGTCGT | Same as for ABA3 | [44] |
| OPG-14 | GGATGAGACC | Same as for ABA3 | [44] |
| OPG-15 | ACTGGGACTC | Same as for ABA3 | [44] |
| PmA3F1 | TCTCGTGTCGGACGGACCAA | Primer for amplification and partial sequencingof PmCesA3 gene | This study |
| PmA3S1 | ATCATCGCGTGCTACCTGC | Sequencing primer for PmCesA3 gene | This study |
| PmA3S2 | CCGTCTTTGTTGTTGGCGGACTG | Same as for PmA3S1 | This study |
| PmA3S3 | TCGACGTACTCGATCGCCA | Same as for PmA3S1 | This study |
| PmA3S4 | TCACTACATGGAACCGGTGACG | Same as for PmA3S1 | This study |
| PmA3S5 | TGGACGGTGGAGGTCGTCAG | Same as for PmA3S1 | This study |
| PmA3S6 | AAGCCGTCGCTTGCGTTCC | Same as for PmA3S1 | This study |
| PmA3R1 | TCTGCATGTCCAGCCTTCC | Same as for PmA3F1 | This study |
| PMF | ATCTACGCTCGCGGTACCAAG | Primer for rapid detection of resistance | This study |
| PMR1109A | CGAACACCACGATGTACACCAG | Same as for PMF | This study |
| PMR1109B | CGAACACCACGATGTACACCTG | Same as for PMF | This study |
| PMR1109C | CGAACACCACGATGTACACCCG | Same as for PMF | This study |
| PMR1109D | CGAACACCACGATGTACACCGG | Same as for PMF | This study |
Table 2. Isolates of Phytophthora melonis used for RAPD analysis and their sensitivities to flumorph, dimethomorph and iprovalicarb.
| Isolate | EC50 b (µg/ml) for the three fungicides | Origin | ||
| Flumorph | Dimethomorph | Iprovalicarb | ||
| TX-9 | 1.240 | 0.280 | 0.230 | Xiqing 1c, Tianjin |
| TX-11 | 1.080 | 0.260 | 0.310 | Xiqing 2, Tianjin |
| TX-13 | 1.193 | 0.280 | 0.330 | Xiqing 3, Tianjin |
| TX-15 | 0.983 | 0.250 | 0.330 | Xiqing 4, Tianjin |
| TX-17 | 1.161 | 0.319 | 0.340 | Xiqing 5, Tianjin |
| TX-20 | 0.857 | 0.240 | 0.320 | Xiqing 6, Tianjin |
| TJ-3 | 1.091 | 0.296 | 0.354 | Hexi 1, Tianjin |
| TJ-18 | 1.182 | 0.281 | 0.311 | Hexi 2, Tianjin |
| TJ-42 | 0.760 | 0.300 | 0.370 | Hexi 3, Tianjin |
| TJ-58 | 1.010 | 0.360 | 0.240 | Hexi 4, Tianjin |
| TJ-99 | 0.552 | 0.250 | 0.400 | Nankai 1, Tianjin |
| TJ-104 | 0.775 | 0.270 | 0.360 | Nankai 2, Tianjin |
| TJ-114 | 1.022 | 0.280 | 0.360 | Nankai 3, Tianjin |
| 63 | 0.440 | 0.360 | 0.290 | UC Riverside, USA |
| 70 | 1.410 | 0.590 | 0.370 | UC Riverside, USA |
| 0206a | 0.710 | 0.680 | 0.420 | Nanjing |
One isolate of Phytophthora drechsleri was used as an outgroup control.
EC50 values, the effective concentration for causing 50% inhibition of mycelial growth inhibition of P. melonis.
Number represents a different field in the same district.
Figure 2. Genetic relationships among 15 isolates of Phytophthora melonis.
The denrogram (UPGMA) shows the relationships among the isolates of P. melonis based on randomly amplified polymorphic DNA (RAPD) analysis with 16 decamer primers. Scale at the bottom depicts the genetic distance.
Generation of CAA-resistant isolates
When the seven isolates were exposed to the CAA fungicides, spontaneous mutation resulted in five isolates with flumorph-resistance, one with dimethomorph resistance and three with iprovalicarb resistance. These nine isolates with spontaneous mutations were obtained from TJ-58, 63 and 70, and the survival frequency was approximately 1×10−7 for mutants exposed to the three fungicides (Table 3). No resistant isolates were derived from the other parent isolates. Following the UV treatment, however, the survival frequency was increased to approximately 1×10−6, and 19 flumorph-resistant, 17 dimethomorph-resistant and 10 iprovalicarb-resistant mutants were obtained from the parent isolates TJ-58, 63 and 70 (Table 3).
Table 3. Results of the experiments conducted to induce resistance against flumorph, dimethomorph, and iprovalicarb in Phytophthora melonis.
| Parental isolates | Fungicides | Type of inductiona | No. of mutants | Survival frequencyb (×10−6) | EC50 c (µg/ml) | RFd |
| TJ-58 | Flumorph | SM | 2 | 0.40 | 114∼151 | 113∼150 |
| UV | 5 | 1.00 | 48∼155 | 133∼431 | ||
| Dimethomorph | SM | 0 | – | – | – | |
| UV | 5 | 1.00 | 40∼151 | 111∼419 | ||
| Iprovalicarb | SM | 1 | 0.25 | 101 | 421 | |
| UV | 2 | 0.40 | 46∼193 | 192∼804 | ||
| 63 | Flumorph | SM | 2 | 0.40 | 58∼159 | 132∼361 |
| UV | 10 | 2.00 | 16∼174 | 35∼395 | ||
| Dimethomorph | SM | 1 | 0.25 | 47 | 131 | |
| UV | 8 | 1.60 | 5∼194 | 14∼539 | ||
| Iprovalicarb | SM | 2 | 0.40 | 74∼104 | 206∼289 | |
| UV | 3 | 0.60 | 43∼174 | 119∼483 | ||
| 70 | Flumorph | SM | 1 | 0.25 | 63 | 45 |
| UV | 4 | 0.80 | 22∼57 | 16∼41 | ||
| Dimethomorph | SM | 0 | – | – | – | |
| UV | 4 | 0.80 | 9∼76 | 15∼129 | ||
| Iprovalicarb | SM | 0 | – | – | – | |
| UV | 5 | 1.00 | 8∼42 | 22∼114 |
SM, spontaneous mutation. UV, UV-mutagenesis.
Survival frequency, number of mutants/total number of zoospores used for mutant generation.
EC50, the effective concentration for causing 50% inhibition of mycelial growth inhibition of P. melonis.
Resistance factor = EC50 of resistant isolates at the 10th transfer/EC50 of its parent.
Characteristics of CAA-resistant Mutants
Resistance stability and resistance factor
After 10 transfers on non-amended medium, the mutant isolates grew as well on fungicide-amended medium as on non-amended medium, indicating that the resistance to CAA fungicides was stable. The level of resistance, as indicated by the resistance factor (RF = EC50 of mutant at the 10th transfer/EC50 of its parent), ranged from 7 to 601 (Table 3).
Mycelial growth, sporulation and virulence in vitro
Compared to the mycelial growth of the corresponding parents (TJ-58, 63 and 70), mycelial growth was faster for some resistant isolates and slower for others. For example, the mycelial growth rate relative to the parent TJ-58 was significantly decreased for F58-1, F58-3, F58-4 and I58-2 (p<0.05), but significantly increased for D58-2 and I58-1 (p<0.05) on the non-amended medium (Table 4). Virulence also increased or decreased, depending on the resistant isolate. Lesions were significantly larger for the resistant isolates D58-3, D58-2, I58-1, I58-2 and F58-1 than for their parent TJ-58 (p<0.05) (Table 4). The resistant isolates F58-3, D58-3, D58-5 and I58-1, however, produced fewer zoospores than the wild-type isolate (Table 4). A compound fitness index (CFI) was calculated: CFI = in vitro mycelia growth × zoospore production × lesion area on cucumber leaves. CFI values of resistant isolates were significantly lower for two of nine mutants derived from parent isolate TJ-58, for five of nine mutants derived from parent isolate 63, and all eight isolates derived from parent isolate 70 (Table 4). CFI values were never significantly greater for the mutants than for the parents and were frequently lower but without statistical significance.
Table 4. Fitness of CAA-resistant and -sensitive isolates of Phytophthora melonis in vitro.
| Isolates a | Mycelial growth (mm)b | Zoospore production (×105/cm2) | Lesion area on cucumber leaves (mm2) | CFIc (×105) |
| TJ-58 | 77 c | 1.53 a | 370 d | 43566 ab |
| F58-1 | 73 e | 1.30 abcd | 398 bc | 37728 bc |
| F58-3 | 72 e | 1.22 bcd | 380 cd | 33684 c |
| F58-4 | 74 de | 1.44 ab | 383 cd | 40980 abc |
| D58-2 | 81 a | 1.41 abc | 420 ab | 47483 a |
| D58-3 | 76 cd | 1.20 bcd | 425 a | 38712 abc |
| D58-5 | 77 c | 1.16 cd | 383 cd | 34205 c |
| I58-1 | 80 ab | 1.13 d | 420 ab | 37743 bc |
| I58-2 | 73 e | 1.28 abcd | 398 bc | 36977 bc |
| I58-3 | 78 bc | 1.35 abcd | 368 d | 38660 abc |
| 63 | 78 c | 0.76 a | 555 c | 32988 ab |
| F63-1 | 73 f | 0.42 c | 532 c | 16296 e |
| F63-3 | 76 de | 0.48 bc | 473 d | 17381 e |
| F63-5 | 75 e | 0.75 a | 476 d | 27014 abc |
| D63-1 | 76 de | 0.42 c | 462 d | 14893 e |
| D63-2 | 77 de | 0.53 bc | 457 d | 18652 de |
| D63-8 | 81 b | 0.51 bc | 476 d | 19648 cde |
| I63-2 | 83 a | 0.39 c | 583 b | 18784 de |
| I63-5 | 77 cd | 0.62 ab | 542 c | 25872 bcd |
| I63-9 | 72 f | 0.76 a | 621 a | 34020 a |
| 70 | 87 c | 0.87 a | 575 d | 43719 a |
| F70-1 | 82 e | 0.74 b | 497 e | 30125 cd |
| F70-5 | 91 a | 0.58 de | 619 bc | 32809 bc |
| F70-11 | 83 e | 0.70 bc | 561 d | 32795 bc |
| D70-1 | 87 cd | 0.53 e | 639 b | 29280 cd |
| D70-5 | 79 f | 0.68 bc | 681 a | 36690 b |
| I70-1 | 89 b | 0.52 e | 567 d | 26310 d |
| I70-5 | 85 d | 0.53 e | 564 d | 25505 d |
| I70-9 | 87 cd | 0.63 cd | 607 c | 33155 bc |
Isolates in bold font are parents of the resistant isolates listed under them in regular font. Isolates starting with the letter F, D, and I, are flumorph-resistant mutants, dimethomorph-resistant mutants, and iprovalicarb-resistant mutants, respectively.
For each parent and its resistant progeny, means followed by same letters are not significantly different according to Fisher’s least significance difference (α = 0.05).
CFI (compound fitness index) = mycelial growth × zoospore production × lesion area on cucumber leaves.
Cross-resistance
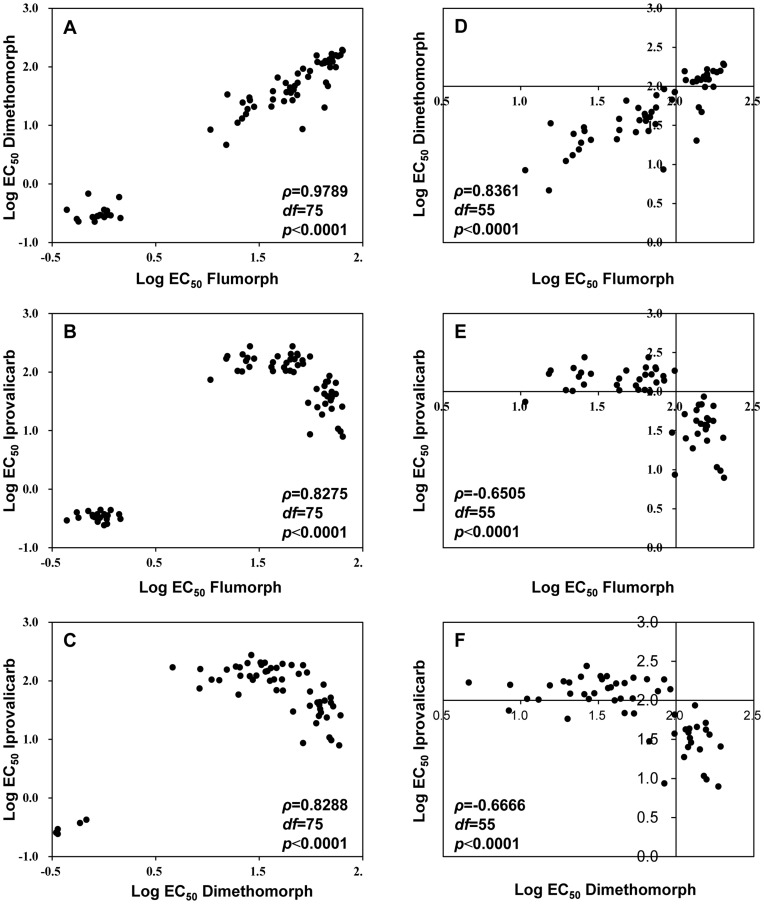
There was a high level of cross-resistance among all three CAA fungicides: the values of Spearman’s rho (ρ) were all >0.8000 (p<0.0001) (Figure 3 A to C). Examination of the EC50 values (those for CAA-resistant isolates and clustered on the right side of Figure 3 A, B and C) once again indicated a positive correlation between resistance to dimethomorph and flumorph (Figure 3 D), but a negative correlation between resistance to iprovalicarb and flumorph (Figure 3 E) and between resistance to iprovalicarb and dimethomorph (Figure 3 F). No cross-resistance was detected between CAA fungicides and non-CAA fungicides such as metalaxyl, cymoxanil, azoxystrobin and cyazofamid (p>0.05) (data not shown).
Figure 3. Cross-resistance among flumorph, dimethomorph and iprovalicarb.
Log-transformed EC50 values (the effective concentration for causing 50% inhibition of mycelial growth inhibition of Phytophthora melonis) for isolates of P. melonis were compared among the three carboxylic acid amide fungicides using Spearman’s rank correlation coefficients. (A), (B), and (C) indicate positive cross-resistance among flumorph, dimethomorph, and iprovalicarb; (D-F) include only the higher EC50 values from (A-C), i.e., EC50 values from CAA-resistant isolates. (D) reveals a positive correlation between the EC50 values for dimethomorph and flumorph among CAA-resistant isolates, while (E) and (F) reveals a negative correlation between iprovalicarb and flumorph and between iprovalicarb and dimethomorph among CAA-resistant isolates.
Analysis of the CesA3 Gene in P. melonis
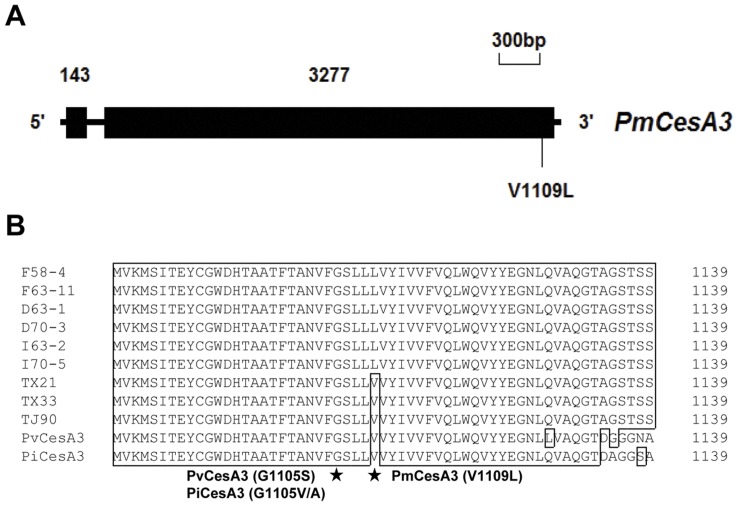
The full-length of PmCesA3 gene contained 3550 bp, with one intron of 130-bp located after nucleotide 143 (Figure 4 A). The PmCesA3 gene coded for a polypeptide chain of 1139 amino-acids and had a predicted molecular weight of 126.5 kDa. The analysis of identities between the PmCesA3 amino acid sequence and those of the closest organisms found in the NCBI GenBank database revealed that homologies were higher with the CesA3 in oomycetes than with CesA3 in Arabidopsis thaliana (Table 5). Compared to the CesA3 in sensitive isolates, only one amino-acid substitutions was detected in the CesA3 in the CAA-resistant isolates: this substitution (a GTG to CTG mutation) occurred at codon 1109 and resulted in the replacement of a valine residue with a leucine residue (Figure 4B).
Figure 4. Structure and site of mutation in the PmCesA3 gene associated with carboxylic acid amide (CAA) fungicide resistance.
(A) Intron/exon structure of the PmCesA3 gene. Numbers represent the size in base pairs. Point mutations in CAA-resistant mutants and the predicted amino acid substitution in the mutant gene products are indicated. (B) Alignment of partial amino acid sequences of CesA3 in P. melonis (PmCesA3), P. infestans (PiCesA3), and P. viticola (PvCesA3). TJ-90, TX-21, and TX-33 were wild-type isolates. D63-1 and D70-3 were dimethomorph-resistant mutants. F58-4 and F63-11 were flumorph-resistant mutants. I63-2 and I70-5 were iprovalicarb-resistant mutants. Mutations in CAA-resistant mutants of P. infestans, P. viticola and P. melonis are indicated by asterisks.
Table 5. Predicted amino acid sequence identities (%) among known CesA3s from four Phytophthora species, Plasmopara viticola, and Arabidopsis thaliana.
| PmCesA3 | PiCesA3 | PrCesA3 | PvCesA3 | AtCesA3 | |
| PmCesA3 | 100 | – | – | – | – |
| PiCesA3 | 82 | 100 | – | – | – |
| PrCesA3 | 95 | 95 | 100 | – | – |
| PvCesA3 | 81 | 95 | 94 | 100 | – |
| AtCesA3 | 14 | 16 | 15 | 16 | 100 |
Values indicate identity expressed as a percentage.
AS-PCR for Rapid Detection of CAA-resistant Isolates of P. melonis
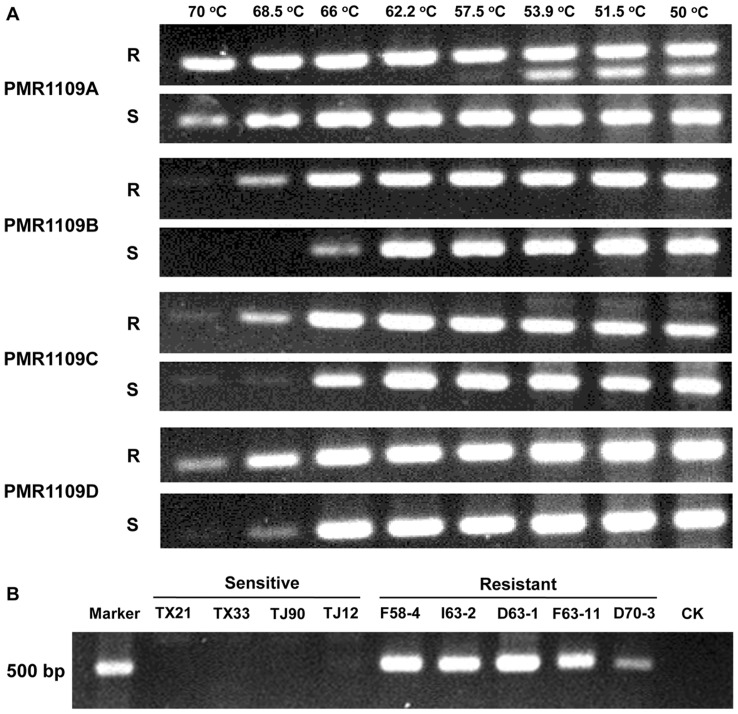
Four pairs of allele-specific primers, designed according to the single mutation in the PmCesA3 gene, were used for PCR with DNA template from CAA-resistant and -sensitive isolates. Using the primer pair PMR1109A + PMF, a 500-bp fragment was amplified at different annealing temperatures whether the template DNA was from resistant or sensitive isolates (Figure 5A), indicating that primers designed by the traditional method could not discriminate between sensitive and resistant alleles. The introduction of an artificial mismatch base at the second nucleotide at the 3′-end of the primers improved specificity at various annealing temperatures (Figure 5 A). As the annealing temperature increased, the reverse primer with artificial mismatch ‘T’ at the second nucleotide showed more specificity than the primers with mismatch ‘C’ or ‘G’. At the annealing temperature of 68.5°C, the primer PMR1109B was optimal for distinguishing the mutation at codon 1109. With the primer pairs PMF + PMR1109B, the 500-bp fragment was amplified from CAA-resistant isolates F58-4, I63-2, D63-1, F63-11 and D70-3 but not from CAA-sensitive isolates TX21, TX33, TJ90 and TJ12 (Figure 5 B).
Figure 5. Specificity of four allele-specific PCR primer pairs for the detection of carboxylic acid amide (CAA)-resistant isolates of Phytophthora melonis.
(A) Specificity of the four primer pairs for the CAA-sensitive isolate TJ-58 (S) and the CAA-resistant isolate F58-4 (R) at gradient annealing temperatures. (B) Specificity of primer pair (PMF + PMR1109B) for four CAA-sensitive and five CAA-resistant isolates at 68.5°C.
Discussion
The sensitivity of 80 P. melonis isolates (collected from 13 fields in China) to the CAA fungicides flumorph, dimethomorph and iprovalicarb was determined by measuring EC50 values. The frequency distributions of the EC50 values were described as unimodal curves with a narrow range for each fungicide, indicating the absence of CAA-resistant subpopulations among the 80 isolates. Therefore, these results can be used as baselines for tracking future sensitivity shifts of P. melonis populations to these three CAA fungicides. Mycelial growth was inhibited more strongly by dimethomorph than by iprovalicarb or flumorph. Similar results were reported for P. capsici [22], [23], [28], Bremia lactucae [29], P. infestans [21], [30] and Peronophythora litchi [15], indicating that dimethomorph is generally more effective than iprovalicarb or flumorph for control of oomycete plant pathogens.
Although RAPD analysis revealed a high degree of genetic diversity in P. melonis collected from different geographical regions, the groups defined by RAPD markers did not share CAA-sensitivity. A likely reason for this lack of correlation is that RAPD markers could not reflected the defined loci responding to sensitivity to fungicides [31]. The RAPD results, however, made it possible to select isolates with different genetic backgrounds for resistance generation.
Isolates with resistance to CAA fungicides were generated from three of the seven isolates used, suggesting that the risk of P. melonis resistance to CAA fungicides may be associated with an isolate’s genetic background. This would explain why dimethomorph-resistant mutants of P. capsici could not be obtained from only one isolate by taming [21], but why CAA-resistance could be obtained by mass selection from zoospores and sexual progeny [22], [23].
The risk of fungicide resistance also depends on the pathogen species and its biological characteristics. Based on disease cycles, dispersal ability, frequency of sexual recombination and the competitive ability, P. viticola and Ps. cubensis have been considered high risk pathogens, while P. infestans, P. capsici and P. melonis have been considered low risk pathogens (FRAC, www.frac.info). Assessments of the risk of fungicide resistance are also based on field observations. Thus, CAA-resistant isolates that are stable and competitive have been detected among field populations of P. viticola [24] and Ps. cubensis [18] but not among field populations of P. infestans [20] (FRAC, www.frac.info), indicating a high risk of resistance to CAAs in P. viticola and Ps. cubensis but a low risk in P. infestans. Until now, no CAA-resistant isolates of P. capsici have been reported in the field, but P. capsici mutants with high CAA-resistance were obtained by mass selection from zoospores and oospores, and the risk of resistance to CAAs was considered low to moderate in P. capsici [22], [23]. For P. melonis in the current study, CAA-resistant mutants were generated in vitro with a frequency of 1×10−7 by spontaneous selection and 1×10−6 by UV-mutagenesis of zoospores. That the frequency was higher with UV-mutagenesis than with spontaneous selection suggests that UV radiation can increase the probability of CAA fungicide resistance in P. melonis. The CFIs (compound fitness indices) were often lower for the CAA-resistant isolates than the wild-type isolates, indicating that CAA-resistance in this study was generally associated with reduced fitness. This supports our inference that the risk of resistance to CAA fungicides in P. melonis is low to moderate.
Mutants resistant to one of the CAA fungicides in the current study were resistant to other CAA fungicides but not non-CAA fungicides, indicating that there was cross-resistance among flumorph, dimethomorph and iprovalicarb but not between the CAA and non-CAA fungicides. Similar results have been reported for P. viticola [24], Ps. cubensis [18] and P. capsici [22], [23], [32]. Although the cross-resistance suggests that the CAA-resistant isolates have a similar resistance mechanism, the negative correlation between higher EC50 values for iprovalicarb and flumorph and between higher EC50 values for iprovalicarb and dimethomorph but not between those for flumorph and dimethomorph suggests that the resistance mechanism may differ somewhat between the cinnamic acid amides (dimethomorph and flumorph) and the valine amide carbamates (iprovalicarb).
We amplified and sequenced the CesA3 gene of P. melonis. Analysis of the CesA3 amino acid sequence revealed that the wild-type and CAA-resistant isolates of P. melonis differed only in the V1109L substitution (Figure 5B). Previous studies reported that resistance to CAA fungicides was conferred by G1105V or G1105A substitution in CesA3 of P. infestans [17], G1105S in CesA3 of P. viticola [26] and G1105V or G1105W in CesA3 of Ps. cubensis [25]. The substitution of V1109L in PmCesA3 would therefore represent a novel mutation causing resistance to CAA fungicides. The finding of only one mutation and the detailed cross-resistance results suggest that other genes might also be involved in CAA resistance. In addition, CAA resistance was considered to be controlled by a recessive gene in P. infestans [17] and P. viticola [26], but by two dominant genes in P. capsici [23]. In this study, we did not find any CAA-resistant isolates with a heterozygous mutation at codon position 1109 on PmCesA3, suggesting that CAA resistance in P. melonis may also be controlled by a recessive gene(s). Confirming this will require further genetic experiments, but genetic manipulation of P. melonis is difficult because it is homothallic.
Several methods such as AS-PCR and PCR-RFLP have been developed for detecting isolates with mutations associated with fungicides resistance [33]. A recent study described a PCR-RFLP method that rapidly detects CAA resistance in P. viticola populations [27]. In our study, AS-PCR primers were designed (based on the mutation of V1109L); these primers effectively identified CAA resistance in P. melonis. Compared with the traditional AS-PCR primers, the new reverse primer contained an additional mismatch at the second nucleotide of the 3′-end; the introduction of this mismatch was previously reported to increase specificity of the allele-specific primer [34]–[36]. In our trial, the mismatch nucleotide ‘T’ was more optimal than the mismatch nucleotides of ‘C’ and ‘G’. However, different mismatches can increase or decrease the specificity of the primer, indicating that the most suitable mismatch must be tested in different cases [37]. The AS-PCR primers described here will be useful for detecting CAA-resistant isolates of P. melonis from field populations.
Materials and Methods
Isolates and Culture Conditions
Roots and stems of cucumber (Cucumis sativus Linn.) with typical signs and symptoms of infection by P. melonis were collected from 13 fields in Xiqing, Hexi and Nankai districts in Tianjin of China in 2005, where CAA fungicides had never been used. Tissue plugs were cut from the margin of lesions on stems and roots. The plugs were disinfested for 3 min in 0.5% (vol/vol) NaClO. After being rinsed three times with sterile water, these plugs were placed on white kidney bean agar (WKB) (60 g of white kidney bean, 7 g of agar and distilled water up to 1 liter) plates amended with 50 µg/ml of ampicillin (98% a.i., Tuoyingfang Biotech Co., Ltd., Beijing), 50 µg/ml of rifampicin (98% a.i., Tuoyingfang Biotech Co., Ltd., Beijing) and 50 µg/ml of pentachloronitrobenzene (PCNB) (40% a.i., Sanli Chemical Industry Co., Ltd., Shanxi, China). After the cultures had been incubated at 25°C in darkness for 5 d, mycelial plugs were cut from margin of the culture and transferred to a new WKB agar plate. In total, 80 isolates of P. melonis were obtained. The isolates were identified using specific primers and morphology as described previously [1], [38].
For acquisition of single-zoospore isolates, a zoospore suspension was prepared by placing mycelial plugs into sterile soil extract (10 g of soil per liter of water). After incubation under light at 25°C for 72 h, sporangia formed. Following incubation at 4°C for 1 h and at 25°C for 40 min, zoospores were discharged from sporangia. A 0.2-ml volume of the zoospore suspension was placed on water-agar plate at 25°C. After 12 h, single germinated zoospores and associated agar were transferred to fresh WKB agar plate. Two single-zoospore isolates of P. melonis (68 and 70) were kindly provided by Dr. Michael D. Coffey (University of California, Riverside), and one single-zoospore isolate of P. drechsleri (0206) was kindly provided by Dr. Zheng Xiaobo (Nanjing Agricultural University). These three isolates were also single-zoospore cultures. All isolates were maintained on WKB agar medium. For long-term storage, each culture was transferred to WKB agar slants, covered with sterile mineral oil, and stored at room temperature.
Fungicides
The following technical-grade fungicides were individually dissolved in dimethyl sulfoxide (DMSO) to prepare stock solutions (1×104 µg/ml) and stored at 4°C in the dark: flumorph (96% a.i., Research Institute of Chemical Industry, Shenyang, China), dimethomorph (95% a.i., Frey Agrochemicals Ltd), iprovalicarb (98% a.i.; Sigma-Aldrich Shanghai Trading Co. Ltd), metalaxyl (97% a.i., Agrolex P. Ltd., Beijing), azoxystrobin (96% a.i., Syngenta Biotechnology Co. Ltd., Shanghai, China), cyazofamid (96% a.i., Sigma-Aldrich Shanghai Trading Co. Ltd) and cymoxanil (98% a.i., Xinyi Agrochemicals Company, Jiangsu, China). The final concentration of DMSO in the WKB agar medium was adjusted to 0.1% (vol/vol) throughout this study. WKB agar plates amended with fungicides were prepared by adding the same volume of serially diluted solutions to the molten agar medium at ≈50°C. WKB agar medium without fungicide but with the same volume of DMSO was used as a control.
Baseline Sensitivities of P. melonis to Flumorph, Dimethomorph and Iprovalicarb
The sensitivities of 80 P. melonis isolates to the CAA fungicides flumorph, dimethomorph and iprovalicarb were determined by measuring mycelium growth on fungicide-amended medium. Fresh mycelial plugs (5 mm in diameter) were cut from the edge of an actively growing colony and placed face up in the center of WKB agar medium plates, which were amended with flumorph (0, 0.50, 0.70, 0.90, 1.00, 1.25, 1.50 µg/ml), dimethomorph (0, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35 µg/ml) or iprovalicarb (0, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45 µg/ml). Each treatment was represented by four replicate plates. After incubation for 4 days at 25°C in darkness, colony diameter was measured at perpendicular angles, and the average of the two measurements (minus 5 mm for the mycelial plug) was used for data analysis. The percentage of inhibition was calculated for each concentration and the concentration of each fungicide causing 50% inhibition (EC50) was estimated from the regression of the probit of the percentage of growth inhibition against the logarithmic value of fungicide concentration. For each of the three CAA fungicides, the frequency distribution of 80 EC50 values was plotted as a representation of baseline sensitivity.
Development of CAA-resistant Mutants of P. melonis in vitro
RAPD. To select P. melonis isolates with different genetic background for generation of CAA-resistant mutants, 15 isolates collected from different fields were randomly chosen for genetic relationship analysis by using RAPD, and one isolate of P. drechsleri was used as the outgroup control (Table 2). Mycelia were frozen in liquid nitrogen and ground into fine powder with mortar and pestle, which has been previously sterilized at 160°C for 2 h. Genomic DNA was extracted according to the modified Ristaino’s CTAB protocol [39]. About 100 mg of mycelial powder was placed in a 1.5-ml centrifuge tube. A 150-µl volume of extraction buffer (0.35 M sorbitol, 0.1 M Tris, 0.005 M EDTA [pH 7.5], and 0.02 M sodium bisulfite) was added, and the tube was then mixed with a vortex mixer. A 150-µl volume of nuclear lysis buffer (0.2 M Tris, 0.05 M EDTA [pH 7.5], 2.0 M NaCl and 2% CTAB [pH 7.5]) and 60 µl of 20% SDS (20 g SDS per 100 ml water) was added, and the tube was mixed again. After incubation at 65°C for 30 min, an isopyknic mixture of chloroform-isoamyl alcohol (24∶1, v/v) was added, and the tube was centrifuged for 15 min at 13,000 g. The aqueous phase was transferred to a new tube, and the chloroform extraction was repeated. After adding 0.1 volume of 3 M sodium acetate (pH 8.0) and 0.6 volume of cold isopropyl alcohol, DNA was precipitated at −20°C for 2 h. The tube was centrifuged at 13,000 g for 15 min, and the precipitate was washed with 75% ethanol and then dried at room temperature. DNA was resuspended using 50 µl of TE buffer (10 mM Tris-HCl, 0.1 mM EDTA [pH 8.0]) for PCR.
RAPD-PCR was performed with each of 16 decamer primers (Table 1). The primers were synthesized by Beijing Sunbiotech Co. Ltd. (Beijing, China). PCR was performed in a 25-µl volume containing 50 ng of template DNA, 1 µl of primer (10 µM), 2 µl of dNTP mixture (2.5 mM of each dNTP and 20 mM Mg2+), 2.5 µl of Easy Taq DNA Polymerase Buffer (10×), and 2.5 U of EasyTaq DNA Polymerse (TransGen Biotech, Beijing, China). Amplification was performed in a MyCycler™ Thermal Cycler (Bio-Rad) with the following parameters: 94°C for 6 min; followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 36°C for 1 min, and extension at 72°C for 2 min and a final cycle of extension at 72°C for 10 min. Amplification products were separated on 1.5% agarose gels in Tris-acetate (TAE) buffer at 110 V for 2 h and were visualized under UV light after being stained with ethidium bromide. All PCRs were repeated at least twice.
Differences in fingerprinting patterns among isolates were assessed based on the clear and reproducible bands. Presumed homologous bands were scored zero (absent) or one (present) and then transformed into a binary matrix. Genetic distance coefficients were calculated for all pairwise comparisons by Nei’s method [40]. The phylogenic tree was generated based on the genetic distance coefficients by using UPGMA (unweighted pair-group method arithmetic averages) and MEGA (molecular evolutionary genetics analysis) software (version 5).
Generation of CAA-resistant isolates
Based on the genetic analysis, seven isolates of P. melonis were selected for generation of CAA-resistant mutants. In the case of flumorph, zoospores suspensions were prepared as described above, and 100 µl of a zoospore suspension (approximately 1.0×106 zoospores/ml) was inoculated onto WKB plates amended with 10 µg/ml of flumorph (WKBF). After incubation at 25°C in darkness for 5 days, the emergent colonies were transferred to a fresh WKBF plate. Single-zoospore isolates were obtained. The same procedure was used for generation of resistant mutants to dimethomorph (10 µg/ml of medium) and iprovalicarb (5 µg/ml of medium). This selection procedure was performed twice.
Ultraviolet (UV)-mutagenesis of zoospores
Zoospore suspensions were continuously agitated while they were exposed to UV irradiation (TUV Philips, 15 W, 254 nm) for 1 min at a distance of 30 cm. The suspensions were then spread on WKB medium plates amended with the corresponding CAA fungicide as described in the previous section. These plates were incubated in the dark for 30 min to minimize light repair of DNA damage. This selection procedure was performed twice and included control plates that were not exposed to UV.
Biological Characteristics of CAA-resistant Mutants
Stability and level of resistance
For determination of the stability of CAA resistance of the mutants, the mutants were subjected to 10 successive transfers on fungicide-free medium before mycelium growth was measured on WKB agar medium amended with each corresponding fungicide at the concentrations described previously. The experiment was done twice. EC50 values of mutants were estimated by measuring mycelium growth on fungicide-amended medium at 0, 5, 10, 20, 40, 80 and 100 µg/ml of each CAA fungicide. The level of resistance was described by the resistance factor: RF = EC50 of mutant at the 10th transfer/EC50 of its parent.
In addition, one spontaneous and one UV-induced mutant resistant to each of fungicide was randomly selected for determination of resistance stability of zoospore progeny. At least 20 single-zoospore isolates randomly sampled from each mutant were grown on WKBF medium.If these single-zoospore isolates grew on WKBF medium, their resistance was considered to be stable; otherwise their resistance was unstable. The same procedure was followed with dimethomorph at 10 µg/ml and with iprovalicarb at 5 µg/ml. This experiment was performed twice.
Mycelial growth and zoospores production
For determination of mycelial growth, the 26 CAA-resistant isolates and their parents were transferred to WKB medium in plates as described in the section concerning baseline sensitivities except that the medium did not contain fungicide. Each isolate was represented by three replicated plates. After incubation in the darkness at 25°C for 5 days, the colony diameter was measured at perpendicular angles, and the average of the two measurements was used to compare the mycelial growth of each resistant isolate and its parent. For comparison of zoospore production, 10 plugs (5-mm in diameter) from the colony margin and 10 plugs from the area near the initial inoculum plug were harvested, and zoospore production was induced as described above and quantified with a hemacytometer. The number of zoospores per cm2 of culture was calculated. These experiments were conducted at least twice.
Virulence
The second or third true leaf from a cucumber plant (cv. Changchunmici) at the fifth true leaf stage was used for virulence tests. The leaves were harvested and rinsed three times with sterile-distilled water. Zoospore suspensions were prepared as described earlier for each of the 26 CAA-resistant mutants and their parent isolates. Four 10-µl droplets of a zoospore suspension (1.0×105 zoospores/ml) were placed on the upper surface of leaves. One half of each leaf was inoculated with a resistant mutant and the other half was inoculated with the corresponding parent. Ten replicate leaves were used for each combination of mutant and parent. After 5 days in a moist chamber at 20°C with 12 h of light and 12 h of darkness, the lesion areas on each leaf were measured. This experiment was conducted at least twice for each combination of mutant and parent.
Cross-resistance
The 55 CAA-resistant mutants and 20 wild-type isolates were cultured on WKB agar medium amended with the non-CAA fungicides metalaxyl (0, 0.01, 0.02, 0.05, 0.10, and 0.20 µg/ml). azoxystrobin (0, 0.01, 0.05, 0.10, 0.50, and 1.00 µg/ml), cymoxanil (0, 10, 20, 40, 80, and 100 µg/ml), or cyazofamid (0.01, 0.02, 0.05, 1.00, and 2.00 µg/ml) or with the CAA fungicides at the concentrations described above. After incubation in darkness at 25°C for 4 days, the colony diameters were measured and the EC50 values were calculated as described above. Each treatment was represented by three replicate plates. The experiment was conducted at least twice for each isolate.
Amplification of the CesA3 gene of P. melonis
Based on the conserved sequence of the CesA3 genes in P. infestans (ABP96904), P. ramorum (ABP96912) and P. sojae (ABP96908) in the Genbank/EMBL data libraries, homologous primers were designed for amplification of the partial PmCesA3 gene fragment. The 5′and 3′end of the PmCesA3 gene were acquired using SiteFinding-PCR [41]. The full-length PmCesA3 gene was amplified and sequenced using primers listed in Table 1. All primers were synthesized by Beijing Sunbiotech Co. Ltd. (Beijing, China). Primers PmA3F1 and PmA3R1 were used to amplify the PmCesA3 gene. PCRs were performed in a 50-µl volume containing 50 ng of template DNA, 1 µl of each primer (10 µM), 4 µl of dNTP mixture (2.5 mM each dNTP), 1×Easy Taq DNA Polymerase Buffer, and 2.5 U of EasyTaq DNA Polymerase (TransGen Biotech, Beijing, China). The PCR was performed in a MyCycler™ Thermal Cycler (Bio-Rad) with the following parameters: an initial preheating for 5 min at 95°C; followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 4 min; and with a final extension at 72°C for 10 min. All PCR products were separated and purified by electrophoresis in a 1% agarose gel in Tris-acetate (TAE) buffer and were cloned into the pEASY-T3 Vector (TransGen Biotech, Beijing, China) and sequenced by Beijing Sunbiotech Co. Ltd. (Beijing, China). The programs in the DNAMAN software were used to predict the PmCesA3 amino acid sequences and to compare the amino acid sequences of the wild-type isolates with those of the CAA-resistant mutants.
Molecular Detection of Resistance Mutation in PmCesA3 by Allele-specific PCR
According to the single mutation in the PmCesA3 gene, allele-specific primers were designed with the match the nucleotide ‘C’ at the 3′-end of the reverse primers. The specificity of the primers was improved by introducing an artificial mismatch base at the second nucleotide at the 3′-end of the primers (Table 1). To test the specificity of the primers, all the primer pairs were used for gradient PCR using the DNA templates from wild-type isolate TJ-58 and CAA-resistant mutant F58-4. PCR amplification was performed in a MyCycler™ Thermal Cycler (Bio-Rad) with the following parameters: an initial preheating for 5 min at 95°C; followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 50 to 70°C for 30 s, and extension at 72°C for 30 s; and terminated with a final extension at 72°C for 10 min. A 5-µl volume of PCR product from each sample was analyzed by electrophoresis using a 1.5% agarose gel in TAE buffer.
Statistical Analysis
Data were analyzed by using the general linear model (GLM) procedure with Statistical Analysis System software (version 9; SAS Inc., Cary, NC, USA). Means were separated using Fisher’s protected least significant difference (LSD, α = 0.05). Cross-resistance between fungicides was analyzed using Spearman’s rank correlation coefficient for log-transformed EC50 values.
Acknowledgments
We thank M. D. Coffey and X. Zheng for kindly providing strains. We thank B. Jaffee and J. Hao for reviewing and providing professional opinions on this manuscript.
Funding Statement
This work was funded by the China National Science Foundation (NO. 30671390 and 30800731). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ho HH, Gallegly ME, Hong CX (2007) Redescription of Phytophthora melonis . Mycotaxon 102: 339–345. [Google Scholar]
- 2. Mohaghegh P, Khoshgoftarmanesh AH, Shirvani M, Sharifnabi B, Nili N (2011) Effect of silicon nutrition on oxidative stress induced by Phytophthora melonis infection in cucumber. Plant Dis 95: 455–460. [DOI] [PubMed] [Google Scholar]
- 3. Ho HH (1986) Phytophthora melonis and P. sinensis synonymous with P. drechsleri . Mycologia 78: 907–912. [Google Scholar]
- 4. Li G, Xue YL (1989) Research on induced immunization of hami melon against Phytophthora melonis . Chin Sci Bull 34: 253–256. [Google Scholar]
- 5. Wang R, Wang RP (2000) Induction of resistance to Phytophthora melonis in Hami melon (Cucumis melo L.). Zhi Wu Bao Hu 26: 9–11. [Google Scholar]
- 6. Guharoy S, Bhattacharyya S, Mukherjee SK, Mandal N, Khatua DC (2006) Phytophthora melonis associated with fruit and vine rot disease of pointed gourd in India as revealed by RFLP and sequencing of ITS region. J. Plant Pathol 154: 612–615. [Google Scholar]
- 7. Mirabolfathy M, Cooke DEL, Duncan JM, Williams NA, Ershad D, et al. (2001) Phytophthora pistaciae sp. nov. and P. melonis: The principal causes of pistachio gummosis in Iran. Mycol Res 105: 1166–1175. [Google Scholar]
- 8. Shaofeng L, Yongguan W, Siliang H, Lizhi L (2007) Reasearch Progress in Control of Wax Gourd Phytophthora Blight. Zhongguo Nong Xue Tong Bao 23: 301–304. [Google Scholar]
- 9. Wu Y, Lu S, Huang S, Fu G, Chen L, et al. (2011) Field resistance of Phytophthora melonis to metalaxyl in South China. Weishengwu Xuebao 51: 1078–1086. [PubMed] [Google Scholar]
- 10. Georgopoulos S, Grigoriu A (1981) Metalaxyl-Resistant Strains of Pseudoperonospora cubensis in Cucumber Greenhouses of Southern Greece. Plant Dis 65: 729–730. [Google Scholar]
- 11. Shattock RC (2002) Phytophthora infestans: populations, pathogenicity and phenylamides. Pest Manag Sci 58: 944–950. [DOI] [PubMed] [Google Scholar]
- 12. Gisi U, Lamberth C, Mehl A, Seitz T (2007) Carboxylic acid amide (CAA) fungicides. Modern crop protection compounds. 651–674. [Google Scholar]
- 13. Dutzmann S (1999) Iprovalicarb (SZX 0722) - a novel fungicide with specific activity against oomycetes. Pflanzenschutz-Nachrichten Bayer 52: 15–32. [Google Scholar]
- 14. Reuveni M (2003) Activity of the new fungicide benthiavalicarb against Plasmopara viticola and its efficacy in controlling downy mildew in grapevines. Eur. J. Plant Pathol. 109: 243–251. [Google Scholar]
- 15. Wang HC, Sun HY, Stammler G, Ma JX, Zhou MG (2009) Baseline and differential sensitivity of Peronophythora litchii (lychee downy blight) to three carboxylic acid amide fungicides. Plant Pathol 58: 571–576. [Google Scholar]
- 16. Zhu SS, Chen L, Lu XH, Li JQ, Liu XL (2010) Effect of three carboxylic acid amide fungicides on different life stages of Phytophthora melonis and determination of the sensitivities. Nongyaoxue Xuebao 12: 168–172. [Google Scholar]
- 17. Blum M, Boehler M, Randall E, Young V, Csukai M, et al. (2010) Mandipropamid targets the cellulose synthase-like PiCesA3 to inhibit cell wall biosynthesis in the oomycete plant pathogen, Phytophthora infestans . Mol Plant Pathol 11: 227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu SS, Liu XL, Wang Y, Wu XH, Liu PF, et al. (2007) Resistance of Pseudoperonospora cubensis to flumorph on cucumber in plastic houses. Plant Pathol 56: 967–975. [Google Scholar]
- 19. Young DH, Spiewak SL, Slawecki RA (2001) Laboratory studies to assess the risk of development of resistance to zoxamide. Pest Manag Sci 57: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 20. Cohen Y, Rubin E, Hadad T, Gotlieb D, Sierotzki H, et al. (2007) Sensitivity of Phytophthora infestans to mandipropamid and the effect of enforced selection pressure in the field. Plant Pathol 56: 836–842. [Google Scholar]
- 21. Yuan SK, Liu XL, Si NG, Dong J, Gu BG, et al. (2006) Sensitivity of Phytophthora infestans to flumorph: In vitro determination of baseline sensitivity and the risk of resistance. Plant Pathol 55: 258–263. [Google Scholar]
- 22. Lu XH, Zhu SS, Bi Y, Liu XL, Hao JJ (2010) Baseline sensitivity and resistance-risk assessment of Phytophthora capsici to iprovalicarb. Phytopathology 100: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 23. Meng QX, Cui XL, Bi Y, Wang Q, Hao JJ, et al. (2011) Biological and genetic characterization of Phytophthora capsici mutants resistant to flumorph. Plant Pathol 60: 957–966. [Google Scholar]
- 24. Gisi U, Waldner M, Kraus N, Dubuis PH, Sierotzki H (2007) Inheritance of resistance to carboxylic acid amide (CAA) fungicides in Plasmopara viticola . Plant Pathol 56: 199–208. [Google Scholar]
- 25. Blum M, Waldner M, Olaya G, Cohen Y, Gisi U, et al. (2011) Resistance mechanism to carboxylic acid amide fungicides in the cucurbit downy mildew pathogen Pseudoperonospora cubensis . Pest Manag Sci 67: 1211–1214. [DOI] [PubMed] [Google Scholar]
- 26. Blum M, Waldner M, Gisi U (2010) A single point mutation in the novel PvCesA3 gene confers resistance to the carboxylic acid amide fungicide mandipropamid in Plasmopara viticola . Fungal Genet Biol 47: 499–510. [DOI] [PubMed] [Google Scholar]
- 27. Aoki Y, Furuya S, Suzuki S (2011) Method for rapid detection of the PvCesA3 gene allele conferring resistance to mandipropamid, a carboxylic acid amide fungicide, in Plasmopara viticola populations. Pest Manag Sci 67: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 28. Sun H, Wang H, Stammler G, Ma J, Zhou M (2010) Baseline Sensitivity of Populations of Phytophthora capsici from China to Three Carboxylic Acid Amide (CAA) Fungicides and Sequence Analysis of Cholinephosphotranferases from a CAA-sensitive Isolate and CAA-resistant Laboratory Mutants. J Plant Pathol 158: 244–252. [Google Scholar]
- 29. Cohen Y, Rubin A, Gotlieb D (2008) Activity of carboxylic acid amide (CAA) fungicides against Bremia lactucae . Eur J Plant Pathol 122: 169–183. [Google Scholar]
- 30. Cohen Y, Gisi U (2007) Differential activity of carboxylic acid amide fungicides against various developmental stages of Phytophthora infestans . Phytopathology 97: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 31. Mahuku G, Peters RD, Platt HW, Daayf F (2000) Random amplified polymorphic DNA (RAPD) analysis of Phytophthora infestans isolates collected in Canada during 1994 to 1996. Plant Pathol 49: 252–260. [Google Scholar]
- 32. Bi Y, Cui X, Lu X, Cai M, Liu X, et al. (2011) Baseline sensitivity of natural population and resistance of mutants in Phytophthora capsici to zoxamide. Phytopathology 101: 1104–1111. [DOI] [PubMed] [Google Scholar]
- 33. Ma Z, Michailides TJ (2005) Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot 24: 853–863. [Google Scholar]
- 34. Drenkard E, Richter BG, Rozen S, Stutius LM, Angell NA, et al. (2000) A simple procedure for the analysis of single nucleotide polymorphism facilitates map-based cloning in Arabidopsis. Plant Physiol 124: 1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayashi K, Hashimoto N, Daigen M, Ashikawa I (2004) Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor Appl Genet 108: 1212–1220. [DOI] [PubMed] [Google Scholar]
- 36. Zhu LX, Zhang ZW, Liang D, Jiang D, Wang C, et al. (2007) Multiplex asymmetric PCR-based oligonucleotide microarray for detection of drug resistance genes containing single mutations in Enterobacteriaceae. Antimicrob Agents Chemother 51: 3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yin Y, Liu X, Shi Z, Ma Z (2010) A multiplex allele-specific PCR method for the detection of carbendazim-resistant Sclerotinia sclerotiorum . Pestic Biochem Physiol 97: 36–42. [Google Scholar]
- 38. Wang Y, Ren Z, Zheng X (2007) Detection of Phytophthora melonis in samples of soil, water, and plant tissue with polymerase chain reaction. Can J Plant Pathol 29: 172–181. [Google Scholar]
- 39. Ristaino JB, Madritch M, Trout CL, Parra G (1998) PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora . Appl Environ Microbiol 64: 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nei M (1972) Genetic distance between populations. Am Nat 106: 283–292. [Google Scholar]
- 41. Tan G, Gao Y, Shi M, Zhang X, He S, et al. (2005) SiteFinding-PCR: A simple and efficient PCR method for chromosome walking. Nucleic Acids Res 33: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silvar C, Merino F, Díaz J (2006) Diversity of Phytophthora capsici in Northwest Spain: Analysis of virulence, metalaxyl response, and molecular characterization. Plant Dis 90: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 43. Bagirova S, Li AZ, Dolgova A, Elansky S, Shaw D, et al. (2001) Mutants of Phytophthora infestans resistant to dimethomorph fungicide. J Russ Phytopathol Soc 2: 19–24. [Google Scholar]
- 44. Linde C, Soo SH, Drenth A (2001) Sexual recombination in Phytophthora cinnamomi in vitro and aggressiveness of single-oospore progeny to eucalyptus. Plant Pathol 50: 97–102. [Google Scholar]