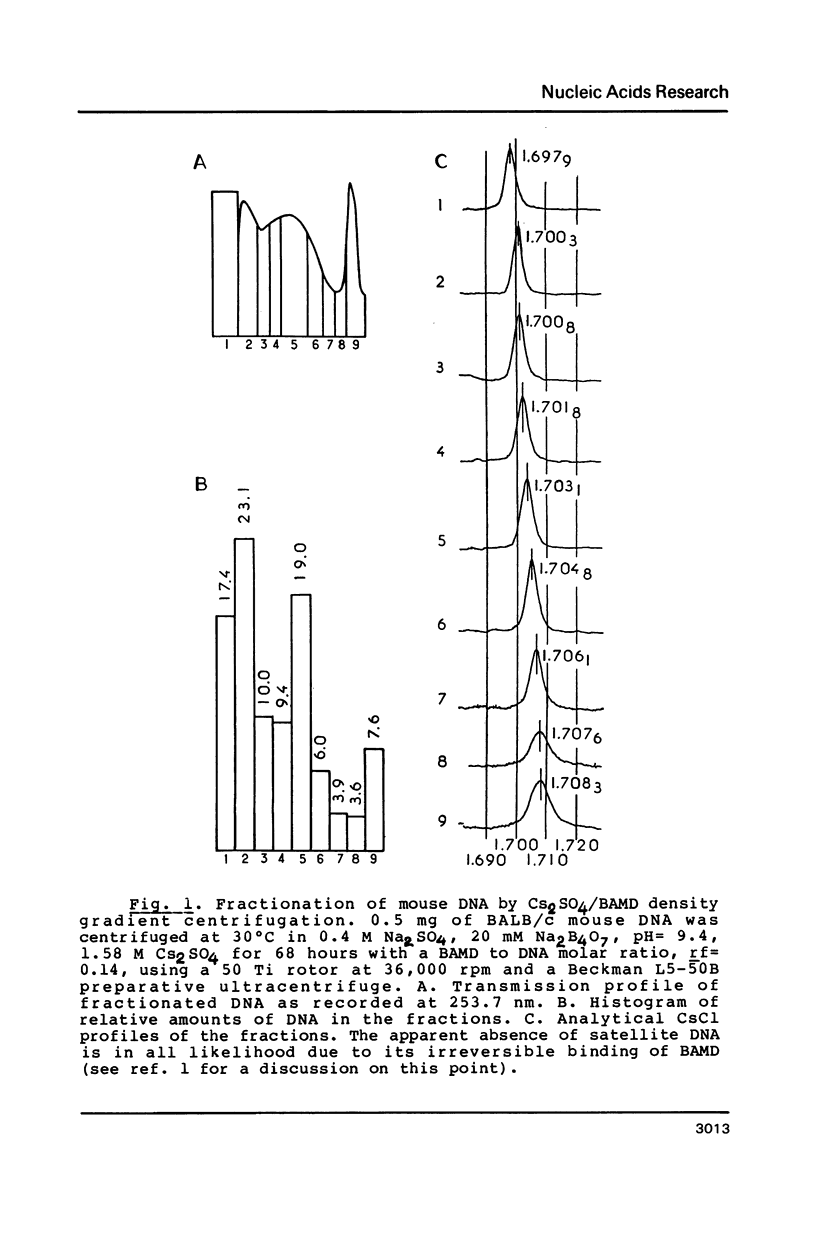
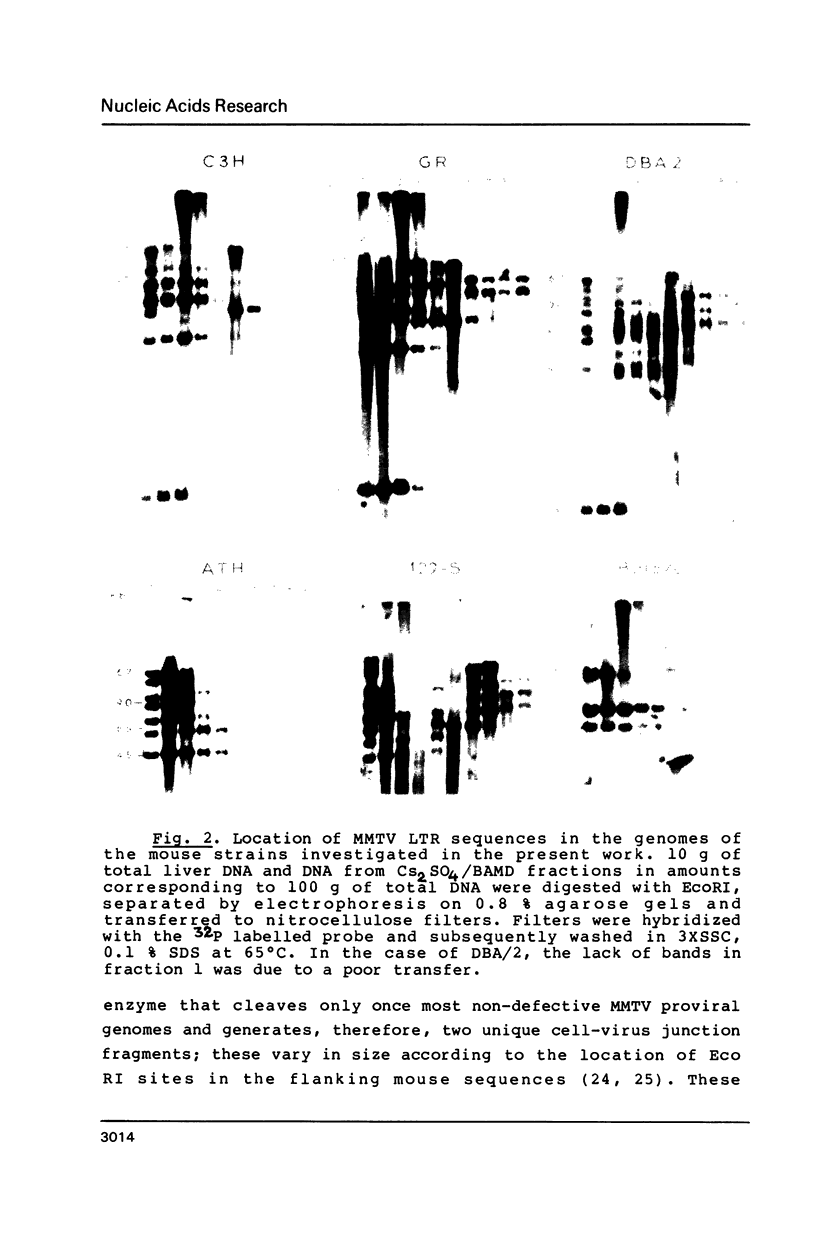
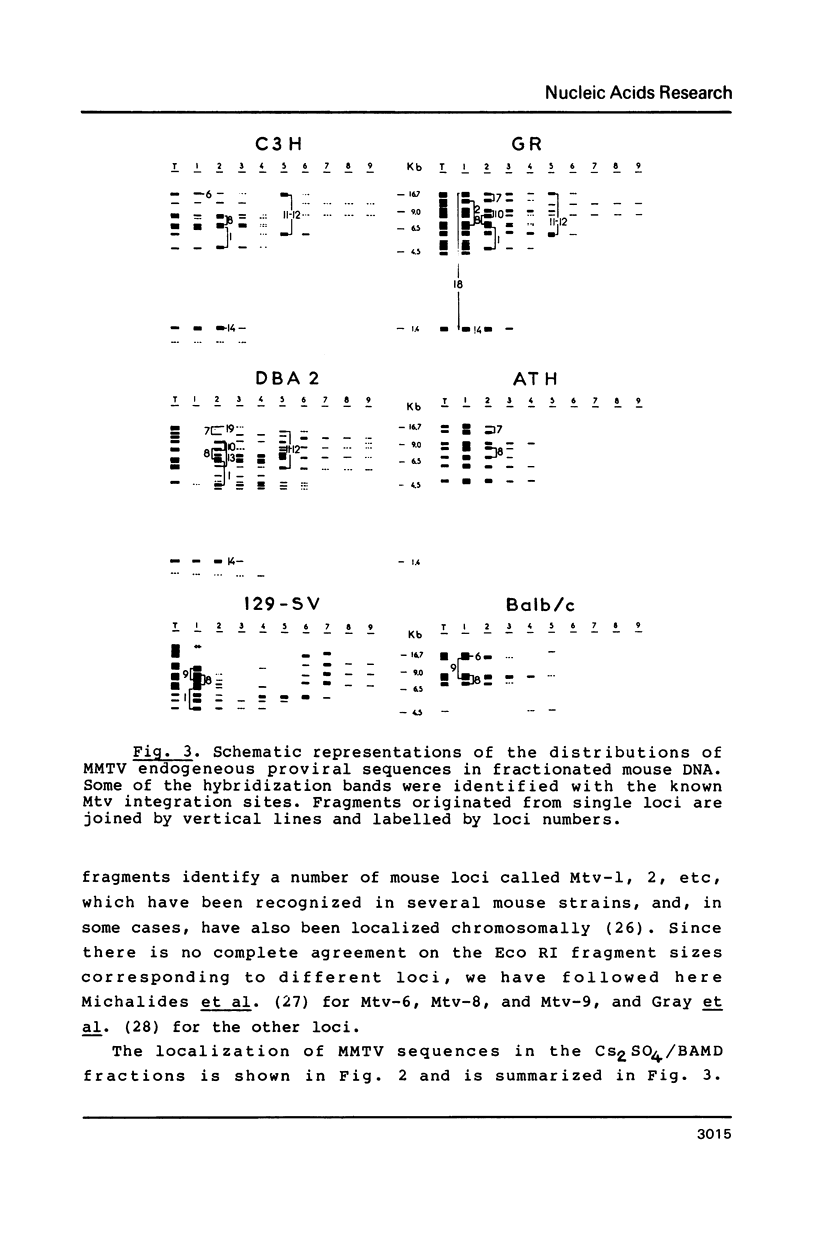
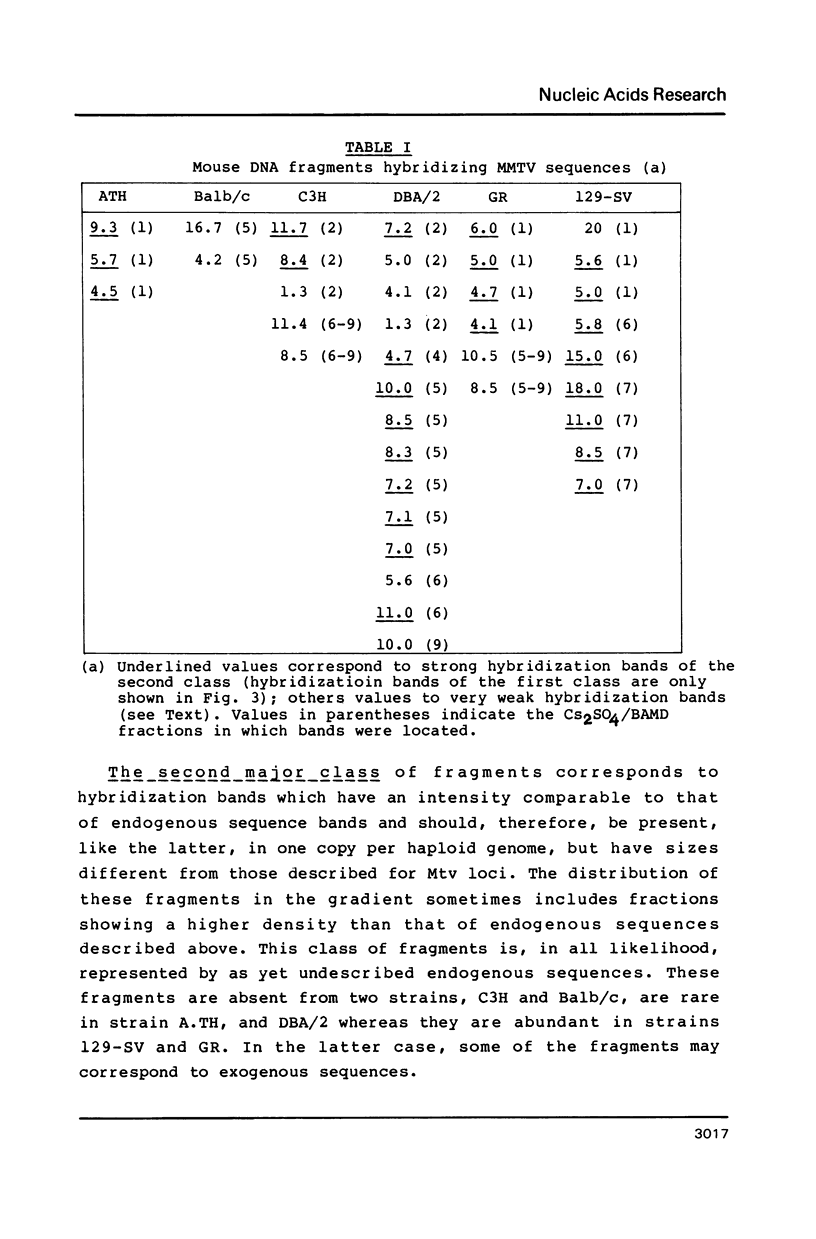
Abstract
Integrated sequences of mouse mammary tumor virus (MMTV) have been localized in the genomes of five inbred mouse strains (Balb/c, C3H, DBA/2, A.TH, 129-SV) and one mammary tumor cell line (GR). Two major classes of MMTV sequences have been detected in mouse DNA fractions as obtained by Cs2SO4/BAMD (3,6-bis-(acetatomercurimethyl)dioxane) density gradient centrifugation. The first one corresponds to previously described endogenous sequences (Mtv loci), whereas the second one corresponds to endogenous sequences not previously known, and/or recently acquired; in the case of GR cells exogenous sequences may also be present in this class. The genome distribution is somewhat different for the two classes of sequences, the first one being practically only present in the lightest DNA segments of the mouse genome (GC congruent to 38%); the second one being also represented in heavier segments (GC congruent to 43%). This integration pattern suggests that "ancient" endogenous sequences are practically only localized in genome segments of roughly matching composition, whereas exogenous and recently acquired endogenous MMTV sequences may also be present in heavier fractions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985 May 24;228(4702):953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cuny G., Soriano P., Macaya G., Bernardi G. The major components of the mouse and human genomes. 1. Preparation, basic properties and compositional heterogeneity. Eur J Biochem. 1981 Apr;115(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05227.x. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Sarkar N. H. Integration of new endogenous mouse mammary tumor virus proviral DNA at common sites in the DNA of mammary tumors of C3Hf mice and hypomethylation of the endogenous mouse mammary tumor virus proviral DNA in C3Hf mammary tumors and spleens. J Virol. 1983 Jan;45(1):114–123. doi: 10.1128/jvi.45.1.114-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Vassos A. B., Cardiff R. D. Methylation and amplification of mouse mammary tumor virus DNA in normal, premalignant, and malignant cells of GR/A mice. J Virol. 1982 Mar;41(3):1007–1013. doi: 10.1128/jvi.41.3.1007-1013.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Buetti E., Firzlaff J., Pearson K., Diggelmann H. Nucleotide sequence of the 5' noncoding region and part of the gag gene of mouse mammary tumor virus; identification of the 5' splicing site for subgenomic mRNAs. Nucleic Acids Res. 1983 Oct 25;11(20):6943–6955. doi: 10.1093/nar/11.20.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Wellinger R., Vessaz A., Diggelmann H. A new site of integration for mouse mammary tumor virus proviral DNA common to BALB/cf(C3H) mammary and kidney adenocarcinomas. EMBO J. 1986 Jan;5(1):127–134. doi: 10.1002/j.1460-2075.1986.tb04186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Gray D. A., Chan E. C., MacInnes J. I., Morris V. L. Restriction endonuclease map of endogenous mouse mammary tumor virus loci in GR, DBA, and NFS mice. Virology. 1986 Jan 15;148(1):237–242. doi: 10.1016/0042-6822(86)90421-6. [DOI] [PubMed] [Google Scholar]
- Gray D. A., McGrath C. M., Jones R. F., Morris V. L. A common mouse mammary tumor virus integration site in chemically induced precancerous mammary hyperplasias. Virology. 1986 Jan 30;148(2):360–368. doi: 10.1016/0042-6822(86)90332-6. [DOI] [PubMed] [Google Scholar]
- Günzburg W. H., Groner B. The chromosomal integration site determines the tissue-specific methylation of mouse mammary tumour virus proviral genes. EMBO J. 1984 May;3(5):1129–1135. doi: 10.1002/j.1460-2075.1984.tb01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Michalides R. Mouse mammary tumor virus: transcriptional control and involvement in tumorigenesis. Adv Cancer Res. 1984;41:155–184. doi: 10.1016/s0065-230x(08)60016-0. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Kettmann R., Meunier-Rotival M., Cortadas J., Cuny G., Ghysdael J., Mammerickx M., Burny A., Bernardi G. Integration of bovine leukemia virus DNA in the bovine genome. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4822–4826. doi: 10.1073/pnas.76.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Verstraeten R., Shen F. W., Hilgers J. Characterization and chromosomal distribution of endogenous mouse mammary tumor viruses of European mouse strains STS/A and GR/A. Virology. 1985 Apr 30;142(2):278–290. doi: 10.1016/0042-6822(85)90336-8. [DOI] [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S. M., Dickson C. Sequence and expression of the mouse mammary tumour virus env gene. EMBO J. 1983;2(1):125–131. doi: 10.1002/j.1460-2075.1983.tb01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. A straight LINE story. Nature. 1983 Nov 10;306(5939):113–114. doi: 10.1038/306113a0. [DOI] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas J., Zerial M., Filipski J., Bernardi G. Gene distribution and nucleotide sequence organization in the mouse genome. Eur J Biochem. 1986 Nov 3;160(3):469–478. doi: 10.1111/j.1432-1033.1986.tb10063.x. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Traina V. L., Taylor B. A., Cohen J. C. Genetic mapping of endogenous mouse mammary tumor viruses: locus characterization, segregation, and chromosomal distribution. J Virol. 1981 Dec;40(3):735–744. doi: 10.1128/jvi.40.3.735-744.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M., Salinas J., Filipski J., Bernardi G. Gene distribution and nucleotide sequence organization in the human genome. Eur J Biochem. 1986 Nov 3;160(3):479–485. doi: 10.1111/j.1432-1033.1986.tb10064.x. [DOI] [PubMed] [Google Scholar]
- Zerial M., Salinas J., Filipski J., Bernardi G. Genomic localization of hepatitis B virus in a human hepatoma cell line. Nucleic Acids Res. 1986 Nov 11;14(21):8373–8386. doi: 10.1093/nar/14.21.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., Kwee V., Nusse R. The nucleotide sequence of the human int-1 mammary oncogene; evolutionary conservation of coding and non-coding sequences. EMBO J. 1985 Nov;4(11):2905–2909. doi: 10.1002/j.1460-2075.1985.tb04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]