Abstract
Purpose
To implement and characterize a fluorine-19 (19F) magnetic resonance imaging (MRI) technique and to test the hypothesis that the 19F MRI signal in steady state after intravenous injection of a perfluoro-15-crown-5 ether (PCE) emulsion may be exploited for angiography in a pre-clinical in vivo animal study.
Materials and Methods
In vitro at 9.4T, the detection limit of the PCE emulsion at a scan time of 10 min/slice was determined, after which the T1 and T2 of PCE in venous blood were measured. Permission from the local animal use committee was obtained for all animal experiments. 12 µl/g of PCE emulsion was intravenously injected in 11 mice. Gradient echo 1H and 19F images were obtained at identical anatomical levels. Signal-to-noise (SNR) and contrast-to-noise (CNR) ratios were determined for 33 vessels in both the 19F and 1H images, which was followed by vessel tracking to determine the vessel conspicuity for both modalities.
Results
In vitro, the detection limit was ∼400 µM, while the 19F T1 and T2 were 1350±40 and 25±2 ms. The 19F MR angiograms selectively visualized the vasculature (and the liver parenchyma over time) while precisely coregistering with the 1H images. Due to the lower SNR of 19F compared to 1H (17±8 vs. 83±49, p<0.001), the 19F CNR was also lower at 15±8 vs. 52±35 (p<0.001). Vessel tracking demonstrated a significantly higher vessel sharpness in the 19F images (66±11 vs. 56±12, p = 0.002).
Conclusion
19F magnetic resonance angiography of intravenously administered perfluorocarbon emulsions is feasible for a selective and exclusive visualization of the vasculature in vivo.
Introduction
A considerable research effort is directed at improving the detection of vascular disease with different modalities such as X-ray, intravascular ultrasound (IVUS), computed tomography (CT) and magnetic resonance angiography (MRA) [1]. Because of its non-invasive nature and lack of harmful radiation, MRA is considered the most patient-friendly among these techniques, while soft-tissue characteristics and blood flow can easily be exploited for contrast generation.
The main challenge for MRA is associated with the fact that signal originates from water protons, which are abundant in the entire body. Inevitably, this leads to background signal in the image and the blood-pool cannot be seen exclusively. This means that some strategy is needed to attenuate the signal from static tissue in close proximity to the blood vessels. Contemporary MRA is therefore commonly performed using intravascular [2] or extracellular [3] T1-lowering gadolinium (Gd) contrast agents, time-of-flight (TOF) imaging, or phase contrast (PC) imaging.
Instead of lowering the T1 relaxation time or using flow characteristics, the frequency of the magnetic resonance imaging (MRI) signal itself can also be shifted and exploited for contrast generation. This should be feasible, since in addition to 1H, magnetic resonance imaging can also detect several other nuclei. Fluorine (19F) is such a nucleus and when imaged has 85% of the MRI sensitivity of 1H, as well as MRI-negligible natural concentrations in the body [4]. When incorporated in perfluorocarbons (PFC, carbohydrates in which all hydrogen has been replaced by fluorine) the results are biochemically inert, non-toxic molecules of which some can be used as blood volume expanders and oxygen carriers, and have been safely and successfully used in several phase 3 trials in human patients [5]. Since PFCs are both hydrophobic and lipophobic, they are usually incorporated in emulsions to allow for a longer circulation time in blood. Such PFC emulsions have already been used for angiography in perfused excised organs such as the heart and kidneys [6], [7], as well as in ‘first-pass’ regional angiography of carotid arteries in rabbits in combination with a gadolinium contrast agent for fast imaging [7].
These non-toxic emulsions can thus be directly injected intravenously and 19F MRI of the blood vessels might also be performed once they have spread through the entire vasculature. In a manner similar to off-resonance angiography with iron-oxide nanoparticles [8], the 19F signal will only originate from the injected PFC that will remain in the vascular system; no background signal is expected, and direct flow-independent imaging of the blood vessels may be feasible. Furthermore, if no T1-lowering gadolinium is added to the injection, the relaxation times T1 and T2 of the 19F agent remain constant, and no adaptations of the pulse sequence parameters as a function of time after injection will be necessary, and anatomy distant from the injection site (such as the heart chambers and great vessels) can also be visualized. Therefore, in this this pre-clinical study in rodents we wanted to develop, characterize and test a novel 19F MRI technique in vitro to test the hypothesis in vivo that the 19F MR signal after systemic perfluorocarbon injection may be exploited for angiography with conventional non-contrast 1H MRA as a reference.
Methods
All experiments were performed on a Varian (Palo Alto, CA, USA) 9.4T animal spectrometer. A dedicated custom-built quadrature surface coil with 18-mm diameter loops was used for 19F and 1H RF transmission and reception. Preparation of a 10% perfluoro-15-crown-5 ether (crown ether or PCE) emulsion was carried out as previously described [9].
To determine its T1 and T2 in an oxygenated and deoxygenated environment, 0.5 ml of the emulsion was added to 1.4 ml venous blood as well as to 1.4 ml of saline (to simulate oxygenated blood) for a final PCE concentration of 50 mM. An adiabatic inversion-recovery spectroscopy sequence with a repetition time (TR) of 10 s and an inversion time (TI) that increased from 0.001 to 20 s was used for T1 determination, while an adiabatic Carr-Purcell-Meiboom-Gill (CPMG) spectroscopy sequence was applied for T2 measurements with TR = 10 s and echo times (TE) ranging from 0.004 to 2 s.
Next, a dilution series helped ascertain the detection limit of the MRI pulse sequence within a 10 min measurement time limit. 50 mM PCE in the saline sample described above was diluted to a series with PCE concentrations of 10, 2.0, 0.80 and 0.40 mM. Subsequently, 19F gradient echo (GRE) images (30×30 mm2 field of view, 64×64 resolution, TR/TE = 5.4/2.5 ms, slice thickness = 3 mm, 1750 averages, 10 min/slice, 2 slices) were acquired and the signal-to-noise ratio (SNR) of the unprocessed images was determined. These in vitro images were then postprocessed and thresholded at 90% of the maximum signal intensity to improve detection of weaker signals; the detection limit with the postprocessing was experimentally determined as SNR = 2 in the original images [10].
For the in vivo part of these studies, which was approved by the state animal use committee (‘Service de la Consommation et des Affaires Vétérinaires du canton de Vaud’ authorization number 2374), male balb/c mice (11 animals, 28±2 g bodyweight) were anesthetized with isoflurane and a catheter was inserted into their tail vein. ECG leads were attached to their paws and the animal was placed in a dedicated holder on top of the surface coil, centered between the heart and kidneys. Next, 12 µl/g bodyweight of the PCE emulsion was infused through the catheter over ∼3 min.
Non-triggered 1H 128×128 TOF-GRE (TR/TE = 6.1/2.8 ms) and 64×64 TOF-GRE (TR/TE = 4.7/2.0 ms) images with a high radiofrequency excititation angle (not quantifiable since it varies with distance from the surface coil) were acquired in the 3 principal directions (field of view 30×30 cm2, slice thickness 2 or 3 mm, 32 averages, 25 s/slice and 10 s/slice, respectively) as well as 64×64 ECG-triggered axial TOF-GRE images through the heart at the same location (4 averages, ∼1 min/slice). The coil was then tuned to 19F, after which the PCE 19F resonance frequency was determined with a pulse-acquire [11] sequence (1 shot). 19F GRE imaging at the same exact anatomical level was then repeated (64×64 resolution, TR/TE = 4.3/1.8 ms, 1024 averages, 4.7 min/slice).
The SNR and contrast-to-noise ratio (CNR) of a total of 33 vessels (i.e. 3 per animal) were determined in both the 1H and 19F images. The CNR was defined as (SL−SS)/N, where SL is the intensity of an ROI in the lumen, SS the intensity of an ROI immediately adjacent to the vessel and N the standard deviation in the background. The “pure” contrast, defined as SL/SS, was also determined for all vessels. The 33 vessels were then tracked with Soap-Bubble to determine vessel sharpness [12] (conspicuity) on both 1H and 19F images.
Two-sided, unpaired, non-equal-variance student's t-tests were used for statistical comparison of the quantitative findings between 19F and 1H MRA.
In vivo 19F images were post-processed for visualization with a 3-pixel-diameter Gaussian filter, fourfold cubic interpolation and thresholding at 20% of the maximum signal, while a maximum intensity projection (MIP) was generated from all multi-slice datasets.
Results
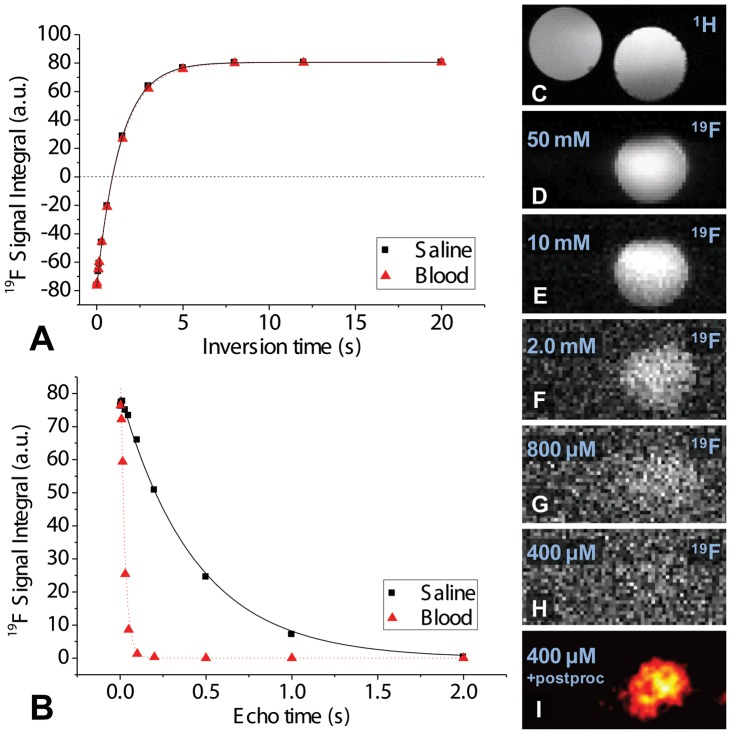
The in vitro 19F PCE T1 relaxation time was very similar in saline and blood (1400±30 and 1350±40 ms (Fig. 1a)), while the T2 was significantly reduced in venous blood from 440±25 to 25±2 ms (Fig. 1b). In the dilution series, the SNR ranged from ∼86 to ∼2 and the PCE detection limit within 10 min of scanning time was 400 µM PCE (Fig. 1c–i).
Figure 1. In vitro characterization of the PCE emulsion.
a) 19F inversion recovery curve fits to determine T1 in saline (▪,T1 = 1400±30 ms) and venous blood (▴,T1 = 1350±40 ms). b) 19F spin-echo decay curve fits to determine T2 in saline and venous blood, resulting in T2 = 440±25 ms in saline and T2 = 25±2 ms in venous blood. (c–i) Images of dilution series in two Eppendorf tubes, one with pure saline, the other with a decreasing concentration of PCE. c) A 1H reference image, d) Unprocessed 19F MR image with signal only from the tube with 50 mM PCE; the other tube contains no 19F and therefore generates no signal. e–h) 19F images of different dilutions of the PCE. i) Postprocessed 19F image at the detection limit of 400 µM.
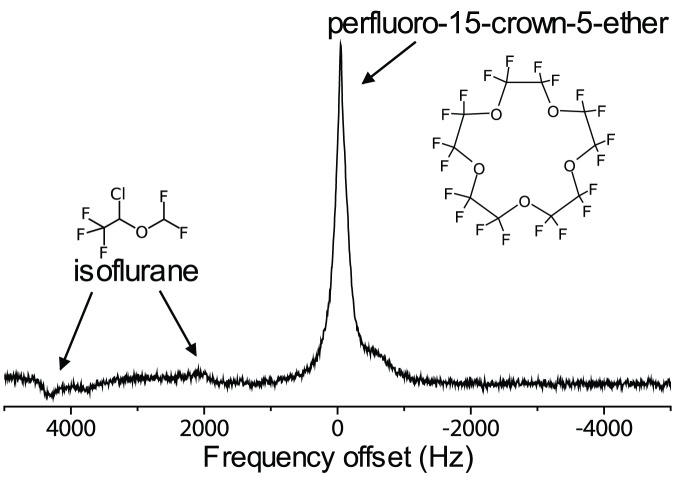
In the in vivo spectra, the PCE's single resonance was visible with high SNR (Fig. 2). The resonances of the isoflurane anesthetic, which is known to build up in the subcutaneous fat [13], were substantially smaller.
Figure 2. Representative unlocalized in vivo spectrum (single acquisition) acquired in the mouse.
The crown ether singlet in the center is several times higher than and well-separated from the isoflurane resonances to the left.
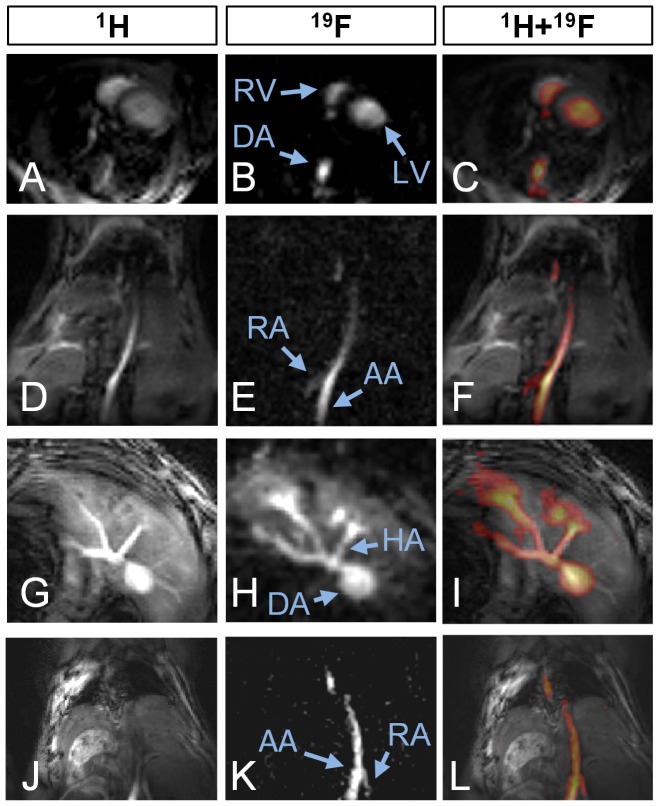
The in vivo 19F MRA protocol was successfully completed in all 11 animals and exclusively visualized the blood-pool and liver at different anatomical levels (Fig. 3, middle column) while consistently coregistering with the corresponding anatomy on the 1H TOF images (Fig. 3, left column). The PCE appeared to be well-distributed throughout the vasculature immediately after injection and 19F imaging was successful until 5 hours after injection. In the axial 19F images at the level of the heart, both ventricles were visible and coregistered with their ECG-triggered anatomical 1H counterpart (Fig. 3a–c). Similar results were found on coronal slices where the iliac and renal arteries were visible within the sensitive volume of the coil (Fig. 3d–f). Slices at the level of the liver showed the celiac trunk with its three branches, while a signal increase in the liver was observed over time (Fig. 3h).
Figure 3. Three series of 3 in vivo mouse images, with 1H reference images in the left column, 19F images in the middle and a color image fusion of the two in the right column.
a–c) axial slice through the heart, with a ECG-triggered to counter motion blurring, which is not needed in b because of the averaging (RV = right ventricle, LV = left ventricle, AA = abdominal aorta). d–f) Coronal slice through the lungs (dark at the top), DA and the kidneys (at the bottom), where the renal artery (RA) can be discerned. g–i) Axial slice at the level of the liver. In h, the hepatic artery (HA) and several others can be discerned while PCE is also temporarily stored in the surrounding liver tissue, thus decreasing the conspicuity of the vessels. j–l) Coronal slice through the abdominal aorta. Varying vessel contrast can be observed in j, which is not present in k.
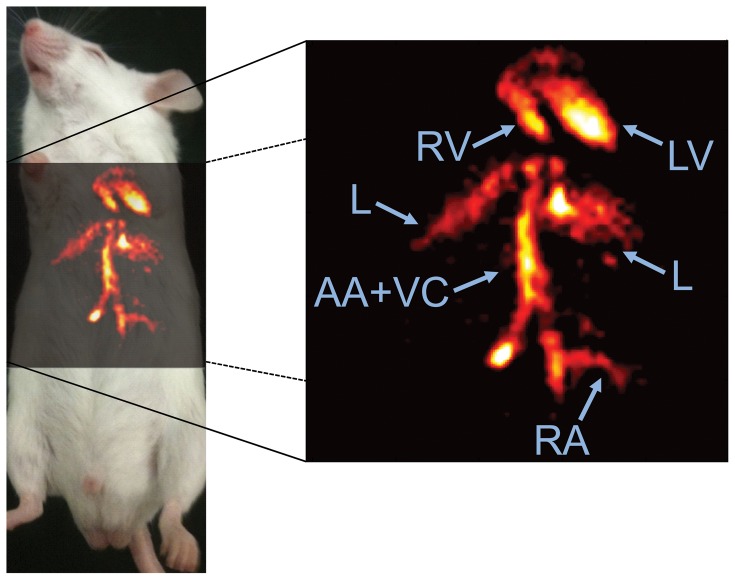
In the MIPs, long contiguous segments of thoracic and abdominal vasculature could be discerned with minimal background contamination (Fig. 4). Consistent with the spectra, no signal from isoflurane was observed in regions of subcutaneous fat.
Figure 4. Distribution of crown ether in the vasculature of the thorax in a mouse in vivo, 3h after injection.
Shown here is a 19F MRA coronal maximum intensity projection created from 6 adjacent slices with a thickness of 2mm each. The image shows the lumen of several cardiovascular structures including the right ventricle (RV), left ventricle (LV), abdominal aorta and vena cava (AA+VC), and a renal artery (RA), as well as the liver (L).
The average SNR of the vessels was 17±8 and 83±49 (p<0.001) for the 19F and 1H images respectively, while the CNR was 15±8 and 52±35 (p<0.001). The “pure” contrast was 17±8 and 3±1 for 19F and 1H, respectively (p<0.001). With tracking, an average vessel sharpness of 66±11 and 56±12(p = 0.002) was measured in the 19F and 1H images.
Discussion
A novel 19F MRA protocol for the detection of well-circulated intravascular PCE without the use of gadolinium was successfully designed, implemented and tested in vivo, while a quantitative analysis including a comparison with 1H images is provided. The GRE protocol had a similar in vitro detection limit as previously and theoretically predicted for a TSE sequence [9]. If an average human body weight of 70 kg and blood volume of 5 L are assumed, the detection limit of 400 µM used in this study is well below the dose of (1.35 g/kg)/(499 g/mol)*70 kg/5 L = 38 mM that has already been administered in humans [14], although such 19F MRA in humans would of course need a separate study on dose dependence. The main factors that allowed the GRE protocol to perform with similar sensitivity as TSE are most likely related to a higher number of signal averages, the use of a sensitive quadrature surface coil and the fact that TSE sequences with increased echo train length may suffer from a penalty in SNR. The measured in vitro 19F relaxation times were in agreement with the literature [15], [16] and are expected to be constant over the concentration range of PCE that can be found in vivo. The short T2 in venous blood may be explained by the presence of deoxyhemoglobin.
In vivo, the PCE resonance peak was more than one order of magnitude higher than those from isoflurane resonances in the spectra, indicating that the concentration of isoflurane was low when compared to that of PCE. This is consistent with the observation that there was no visible signal from fat or lungs in the 19F images. However, a PCE accumulation was observed in the liver, most likely due to uptake in the Kuppfer cells for clearance from the body [17].
The scanning time of ∼5 min per slice may be acceptable for in vivo human scanning and is consistent with the expected ∼7 h intravascular retention time of the PCE emulsion in the blood-pool [17]. However, our results were obtained on an experimental high-field animal system. In humans, the translation of this technology to lower field strengths (3T) may be necessary, but will inevitably lead to a penalty in SNR, although our injected dosage of 12 µl/g×1.78 g/ml = 0.021 g PFC/kg bodyweight is considerably lower than the 1.35 g PFC/kg bodyweight that has already been used in clinical trials [14]. In the current study we were unfortunately not able to use such doses both due to technical limitations in the production of stable emulsions with high PCE content and due to animal ethics committee limitations on administering higher volumes. It should also be noted that such large volume injections are contraindicated in patients who have coronary artery, renal, or pulmonary disease and in those who have severe hepatic disease [18]. However, with advanced and commercially available hardware (32-channel coil tunable to both 1H and 19F frequencies, parallel imaging, partial k-space sampling) on human high-field systems, imaging of one slice in ∼10 s may be feasible using contrast agent concentrations similar to our study. Furthermore, direct 3D GRE imaging will result in a similar SNR as 2D GRE imaging albeit with an increased volumetric coverage. Using a volume rather than a surface coil, the technique presented here may be extended for direct perfusion imaging as was recently partially demonstrated for the kidneys [19], since the signal can be quantified and the exact concentration determined with the addition of an external reference phantom.
The SNR of the 19F images was considerably lower than that of the 1H images (17 vs. 83), as expected and mainly caused by the lower concentration of PCE relative to water in the lumen blood-pool. The image quality is still limited, but longer scanning times, higher concentrations of contrast media or advances in hardware and software are likely to contribute to further improvement, although it has to be considered that prolonged scanning times may adversely affect image quality due to motion artifacts. The large difference in SNR between the 1H and 19F images is also the reason why the CNR is higher in the 1H images despite their lower “pure” contrast: the noise term appears to be more dominant than the difference in signal. However, the vessel tracking in the 19F and 1H images resulted in significantly higher vessel sharpness in the 19F images, most likely since the “pure” contrast between the vessel and the adjacent tissue is the dominant factor.
In conclusion, 19F magnetic resonance angiography of an intravenously administered perfluorocarbon emulsion supports an exclusive visualization of the vasculature in vivo due to the lack of background signal and provides a promising alternative, flow-independent angiographic MR technique. Since perfluoro-15-crown-5-ether is inert and non-toxic, a translation to the human setting seems feasible.
Funding Statement
Funding source: Department of Radiology, University (UNIL) and University Hospital (CHUV) of Lausanne, Switzerland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, et al. (2008) Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 118: 586–606. [DOI] [PubMed] [Google Scholar]
- 2. Lewis M, Yanny S, Malcolm PN (2012) Advantages of blood pool contrast agents in MR angiography: A pictorial review. J Med Imaging Radiat Oncol 56: 187–191. [DOI] [PubMed] [Google Scholar]
- 3. Gerretsen SC, le Maire TF, Miller S, Thurnher SA, Herborn CU, et al. (2010) Multicenter, double-blind, randomized, intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for MR angiography of peripheral arteries. Radiology 255: 988–1000. [DOI] [PubMed] [Google Scholar]
- 4. Holland GN, Bottomley PA, Hinshaw WS (1977) 19F magnetic resonance imaging. J Magn Reson 28: 133–136. [Google Scholar]
- 5. Riess JG (2006) Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol 34: 567–580. [DOI] [PubMed] [Google Scholar]
- 6. Joseph PM, Fishman JE, Mukherji B, Sloviter HA (1985) In vivo 19F NMR imaging of the cardiovascular system. J Comput Assist Tomogr 9: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 7. Neubauer AM, Caruthers SD, Hockett FD, Cyrus T, Robertson JD, et al. (2007) Fluorine cardiovascular magnetic resonance angiography in vivo at 1.5 T with perfluorocarbon nanoparticle contrast agents. J Cardiovasc Magn Reson 9: 565–573. [DOI] [PubMed] [Google Scholar]
- 8. Korosoglou G, Shah S, Vonken EJ, Gilson WD, Schar M, et al. (2008) Off-resonance angiography: a new method to depict vessels–phantom and rabbit studies. Radiology 249: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flogel U, Ding Z, Hardung H, Jander S, Reichmann G, et al. (2008) In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118: 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watts R, Wang Y (2002) k-space interpretation of the Rose Model: noise limitation on the detectable resolution in MRI. Magn Reson Med 48: 550–554. [DOI] [PubMed] [Google Scholar]
- 11. Spees WM, Gade TP, Yang G, Tong WP, Bornmann WG, et al. (2005) An 19F magnetic resonance-based in vivo assay of solid tumor methotrexate resistance: proof of principle. Clin Cancer Res 11: 1454–1461. [DOI] [PubMed] [Google Scholar]
- 12. Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, et al. (2002) “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 48: 658–666. [DOI] [PubMed] [Google Scholar]
- 13. Eger EI 2nd, Saidman LJ (2005) Illustrations of inhaled anesthetic uptake, including intertissue diffusion to and from fat. Anesth Analg 100: 1020–1033. [DOI] [PubMed] [Google Scholar]
- 14. Keipert PE (1995) Use of Oxygent, a perfluorochemical-based oxygen carrier, as an alternative to intraoperative blood transfusion. Artif Cells Blood Substit Immobil Biotechnol 23: 381–394. [DOI] [PubMed] [Google Scholar]
- 15. Morawski AM, Winter PM, Yu X, Fuhrhop RW, Scott MJ, et al. (2004) Quantitative “magnetic resonance immunohistochemistry” with ligand-targeted (19)F nanoparticles. Magn Reson Med 52: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 16. Giraudeau C, Flament J, Marty B, Boumezbeur F, Meriaux S, et al. (2010) A new paradigm for high-sensitivity 19F magnetic resonance imaging of perfluorooctylbromide. Magn Reson Med 63: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 17. Mattrey RF, Long DM, Multer F, Mitten R, Higgins CB (1982) Perfluoroctylbromide: a reticuloendothelial-specific and tumor-imaging agent for computed tomography. Radiology 145: 755–758. [DOI] [PubMed] [Google Scholar]
- 18. D'Ambra MN, Kaplan DK (1995) Alternatives to allogeneic blood use in surgery: acute normovolemic hemodilution and preoperative autologous donation. Am J Surg 170: 49S–52S. [DOI] [PubMed] [Google Scholar]
- 19. Hu L, Chen J, Yang X, Caruthers SD, Lanza GM, et al. Evaluating Endothelial Damage in Acute Kidney Injury with Perfluorocarbon (PFC) Nanoparticles (NP) and 19F MRI; 2012; Melbourne, Australia. [Google Scholar]