Abstract
The OsAPx2 gene from rice was cloned to produce PBI121::OsAPx2 dual-expression plants, of which expression level would be increasing under stressful conditions. The enzyme ascorbate peroxidase (APX) in the leaves and roots of the plants increased with increasing exposure time to different sodium chloride (NaCl) and hydrogen peroxide (H2O2)concentrations, as indicated by protein gel blot analysis. The increased enzyme yield improved the ability of the plants to resist the stress treatments. The OsAPx2 gene was localized in the cytoplasm of epidermal onion cells as indicated by the instantaneous expression of green fluorescence. An 80% regeneration rate was observed in Medicago sativa L. plants transformed with the OsAPx2 gene using Agrobacterium tumefaciens, as indicated by specific primer PCR. The OsAPx2 gene was expressed at the mRNA level and the individual M. sativa (T#1,T#2,T#5) were obtained through assaying the generation of positive T2 using RNA gel blot analysis. When the seeds of the wild type (WT) and the T2 (T#1,T#5) were incubated in culture containing MS with NaCl for 7 days, the results as shown of following: the root length of transgenic plant was longer than WT plants, the H2O2 content in roots of WT was more than of transgenic plants, the APX activity under stresses increased by 2.89 times compared with the WT, the malondialdehyde (MDA) content of the WT was higher than the transgenic plants, the leaves of the WT turned yellow, but those of the transgenic plants remained green and remained healthy. The chlorophyll content in the WT leaves was less than in the transgenic plants, after soaking in solutions of H2O2, sodium sulfite (Na2SO3), and sodium bicarbonate (NaHCO3). Therefore, the OsAPx2 gene overexpression in transgenic M. sativa improves the removal of H2O2 and the salt-resistance compared with WT plants. A novel strain of M. sativa carrying a salt-resistance gene was obtained.
Introduction
Under drought and salt stress conditions, plants become dehydrated, which lowers the water potential of cells and organs. Furthermore, different physiologic processes are changed in vivo [1] and the APX (EC1.11.1.11) of the active oxygen detoxification system is activated. The plant antioxidant is an important aspect in the plant’s self-adaptive adjustment [2] The APX is the main enzyme for removing intracellular H2O2. APX has a high specificity and affinity for H2O2 and it catalyzes the reduction of H2O2 into water using reduced ascorbate acid as the reaction substrate. The reaction generates monodehydroascorbate which can be reduced to ascorbic acid using different methods [3], [4], [5]. APX is mainly localized in the cytoplasm, peroxisome, chloroplast, and mitochondria [6], [7], [8], [9]. These enzymes have the same function, but they play a discriminating role in the anti-oxidation of plants because of their different intracellular locations. APX provides cross-protection to the cytoplasm, thylakoid, and stromal mitochondria under light stress [10]. APX has a high affinity for H2O2, capturing H2O2 within many subcellular regions [11]. APX belongs to a multi-gene family [9]. APx gene overexpression improves enzyme activity for cleaning up hydrogen peroxide and it alleviates membrane lipid peroxidation injury caused by MDA [12]. At present, recombinant APX isozymes had been obtained in vitro. Some researchers had compared their structure and enzymatic kinetics, which revealed that they have consistent enzymatic properties and that APX isozymes have higher specificity for reduced ascorbic acid. However, APX isozymes are inactivated easily in the absence of substrate [13]. Different GSTs, such as OsAPXa and OsAPXb proteins for purification and fusion, have been expressed in prokaryotic cells by Lu et al. [14]. The comparative analysis of the enzyme kinetics showed that these proteins exhibited differences. The cytosolic APX of spinach leaves have significantly increased transcriptional levels under the same light and ultraviolet ray treatments. However, the chloroplast and peroxisome expression of APX is not affected [15]. Ma et al. [16] cloned the full-length complementary DNA (cDNA) of Suaeda salsa APX. RNA gel blot analysis showed that APX expression increases under salt stress and the enzyme activity also increases significantly, which indicated that the gene is induced by salt. APX was speculated to play a role in protecting S. salsa from oxidative damage caused by salt stress. Moving Arabidopsis from weak light to strong light induces the expression of two nuclear-encoded APX genes within 15 minutes. The expression was accompanied by a decrease in the ratio of reduced and oxidized glutathione [17]. Hong and Kao et al. [18] analyzed eight rice OsAPx genes using semi-quantitative PCR. The differences in the mRNA exhibited the dissimilarity between the OsAPx1 and the OsAPx2 gene under 150 mM NaCl stress. A study [19] showed that the transcriptional expression of the OsAPx2 rice gene was more rapid than that of the OsAPx1 gene under diseases stress. Lu et al. [20]. discovered that the root length and chlorophyll content of transgenic Arabidopsis plants overexpressing the OsAPXb gene was superior to those of plants overexpressing the OsAPXa gene in salt tolerance research. Differences in the salt and alkali tolerance of transgenic tobacco plants with over-expressed OsAPx1 and OsAPx2 genes have not been reported. M. sativa is an excellent pasture plant from the Leguminosae family that promotes nitrogen-fixing microorganisms. It is one of the most important crops in agriculture, which have an effect on soil improvement through nitrogen fixation. Furthermore, the areas cultivated with legumes constitute more than 3.2×107 hectares worldwide [21].
Table 1. stress treatments of different oxidants.
| oxidants | Concentration (mM) | |||||
| H2O2 | 0 | 1632 | 3263 | 4895 | 6527 | 8158 |
| Na2SO3 | 0 | 5 | 10 | 20 | 30 | 40 |
| NaHCO3 | 0 | 75 | 100 | 150 | 200 | 250 |
Salinization is a serious problem in soil management, it can decrease agricultural yield and ecological environment. Therefore, breeding different varieties of M. sativa with resistance plants is of particular significance to improving the characteristics of M. sativa and enlarging the arable land area. Currently, the resistance of M. sativa is improved by breeding new varieties via plant genetic engineering. Since the first Agrobacterium-mediated M. sativa translation experiment has been successful [22], heterogeneous gene in M. sativa has been studied gradually. The experiment involved the transfer of the A. thaliana sAPX gene into M. sativa, which resulted greater mRNA expression. The enzyme activity was improved by 3.8-fold and the reactive oxygen species produced by stresses was efficiently cleaned up [23]. The yield and survival of M. sativa treated with drought stress for three years was clearly improved with Mn-SOD cDNA [24]. M. sativa treated with Alfinl enhanced the expression of MsPRP2 induced by saline, the salt tolerance, growth velocity [25]. Thus far, no experiment has reported on transgenic M. sativa about OsAPx2. In this paper the OsAPx2 gene was transferred into M. sativa cotyledon mediated by A. tumefaciens and the generation of T1 was assayed using specific primer PCR. The generation of T2 transcript levels was assayed using RNA blot analysis. Under salt stress, APX activity, physiologic and anti-oxidation indices in the recombinant M. sativa T2 generation were analyzed.
Materials and Methods
Chemicals
DIG-labelled and CDP-StarTM were purchased from Amersham Pharmacia. Trizol was purchased from Invitrogen. 3,3′-diaminobenzidine staining (DAB) was purchased from Sigma. The other chemicals are AR grade.
Plant Material
The M. sativa seeds were obtained from the Academy of Agricultural Sciences of Changchun (Jilin Province, China). The A. tumefaciens (EHA105 strain) containing the dual-expression plasmid PBI121-OsAPx2 was obtained. The probe with OsAPx2 was marked by Domain Information Groper (DIG) from the library.
Rice seedlings at the three-leaf stage (two weeks) were used in the subsequent stress tolerance assays. The plants were treated with different stresses, namely, 100 mM NaCl, 60 mM NaHCO3, and 10% (w/v) polyethylene glycol 6000(PEG 6000) for 6, 12, 24, 48, and 72 h. Plants treated with water were used as the controls. All treatments were performed in triplicate. The roots and leaves of rice seedlings were harvested, immediately frozen in liquid nitrogen, and stored in a freezer at −80°C to be used for testing.
Culture Medium
The callus induction medium used was Murashige and Skoog (MS) medium containing the following: 2 mg/L 2,4-dichlorophenoxyacetic acid, 1 mg/L benzylaminopurine (6-BA), 8 g/L agar, and 30 g/L sucrose. The screening culture medium for bud differentiation contained MS with 2 mg/L kinetin, 0.15 mg/L 6-BA, 0.3 mg/L α-naphthalene acetic acid, 50 mg/L kanamycin, 500 mg/L carbenicillin, 100 µM acetosyringone, 8 g/L agar, and 30 g/L sucrose. The culture medium for promoting root growth contained 1/2 MS and 0.5 g/L yeast extract.
Methods
Enzymatic and mRNA Transcription Expression Analysis of the OsAPx2 Gene Under Salt Stress
The specific primers for the gene probe were designed based on the GenBank login gene OsAPx2 (AB053297). The variance probe was signed with DIG. (OsAPx2. P1∶5′-aagcctttagagagcggga-3′, P2∶5′-gcttggtaactttgaaactcc-3′) 5 µg of RNA was used in the denature electrophoresis, membrane transfer, variance probe hybridization, membrane cleaning, and signal detection by CDP-Star [26].
The quality assay was performed on the plant protein via the Bradford method [27]. The materials were treated as follows: 500 mg of the finely cut leaves and roots were weighed, and then soaked in NaCl and H2O2, respectively. At the same time, an equal amount of sample was soaked in water as the control. The proteins were extracted using phosphate-buffered saline (PBS) contain 5 mM Ethylenediaminetetraacetic acid disodium salt (EDTANa2), 0.001% Phenylmethanesulfonyl fluoride (PMSF), pH 8.0. The materials were rubbed rapidly in Potter-Elvehjem Tissue Grinders under ice-cold conditions, and the supernatant liquid was collected after filtration at 4°C and 12000 rpm for 20 min in a 1.5 mL tube. A similar volume of crude protein was extracted for the sodium dodecyl sulfate polyacrylamide gel electrophoresis(SDS-PAGE). The protein was transferred onto a pyroxylin membrane using semi-electric transfer method instrument [28]. The expressed OsAPx2 under stresses was assayed using primary anti-OsAPx2 polyclonal antibodies. Secondary rabbit anti-goat antibodies AP-labeled were used for protein blot analysis with NBT as the substrate (Kit supplied by the Tiangen Corporation).
Subcellular Localization of OsAPx2 Genes
The subcellular location of OsAPx2 gene expression was predicted based on its amino acid sequence in NCBI (http://www.ncbi.nlm.nih.gov/). To further verify the subcellular location, a Kpn I site was designed before the 5′-end ATG of the OsAPx2 genes for synthesizing upstream primer: OsAPx2 (P3∶5′-ggatccatgggcagcaagtc-3′); An Spe I site was designed after the 3′-end for synthesizing downstream primer: (P4∶5′-actagtttcctcagcaaatcc-3′), and then the primers were cloned into a pMD18-T vector. If the sequence was correct, pMD18-T::OsAPx2 and PBT121-35SMCS::GFP were hydrolyzed by Kpn I and Spe I. Destination DNA was recycled for connecting, transforming, and constructing PBI121::OsAPx2::GFP expression plasmids. The building plasmids were introduced into onion epidermal cells via gene gun transformation. The green fluorescence, which demonstrated the expression location, was observed by a confocal laser scanning microscope (Olympus).
Heredity Transformation
The method was performed based on the study of Jin et al. [29].
Molecular Examination
PCR assay
Genomic DNA from the recombinant and wild-type (WT) plants of T1 generation was diluted 100-fold. Then, 1 µL was withdrawn for PCR template. Both upstream and downstream primers were designed based on the ORF sequences of OsAPx2 (AB053297) as follows: P5∶5′-gagcatgggcagcaagtcgt-3′: P6∶5′-cttattcctcagcaaatccc-3′, which were synthesized by Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. The agarose gel electrophoresis carried out for 45 minutes with 1× TAE buffer contains 0.8%(w/v) agarose. Pictures of the samples were taken using VILBER LOURMAT.
RNA gel blot assay
The total RNA was obtained using Trizol. Denaturing gel electrophoresis was carried out to examine the transformation of OsAPx2 gene, which was marked with an probe in the DIG with RNA gel blot analysis, as referred from Sambrook et al. [26].
Anti-salinity Analysis
The resistant OsAPx2 overexpression under salt stress was analyzed to study the positive generation of T2 under Promoter 35S from Cauliflower Mosaic (CaMV35S). The seeds of the WT and transgenic OsAPx2 (T#1, T#5) were sown in 1/2 MS culture medium containing 0, 125, 150, and 175 mM NaCl for 7 days. Then, the effects of OsAPx2 overexpression on the ability to resist salt stress were analyzed in detail.
The Hydrogen Peroxide Content was Assayed through DAB According to Hückelhoven [30].
The plants (WT, T#1, and T#5) were cultured at 24°C ±2°C with illumination for 10 h at a light intensity of 30 µmol·m−2·s−1 to 40 µmol·m−2·s−1 after soaking in 200 mM NaCl. The enzyme properties were assayed using the following: The total enzyme activity of APX [3]; the content of MDA [31] at 0, 12, 24, 48, and 72 h, respectively. The enzyme solution contained 0.5 mM l-ascorbic acid and 50 mM phosphate equilibrated with 0.2 mM H2O2 buffer (pH 7.0) with suitable crude enzyme extract.
Analysis of Anti-oxidation Injury in Transgenic OsAPx2 Plant Leaves
To verify if the plant overexpressing OsAPx2 is resistant to stress, their ability to scavenge the H2O2 produced during stress and the ability to resist anti-oxidation injury were compared. The leaves of the WT and transgenic plants were soaked in solutions with different concentrations, as shown Table1.
Data Analysis
Analysis of variance (AVONA) and multiple comparison by software data processing system(DPS) (version 7.05).
Results and Analysis
Expression and Analysis of OsAPx2 Genes in Rice Under Stress
Total RNA was extracted from the root and leaf samples. Then, the total RNA concentration was determined using a ultraviolet-visible spectrophotometer. Up to 10 µg of each sample was prepared for the formaldehyde denatured electrophoresis, membrane transfer, and DIG variance probe hybridization. RNA gel blot analysis showed that the induction of gene expression (Figure. 1).
Figure 1. RNA gel blot analysis of OsAPx2 gene expression under different stresses.
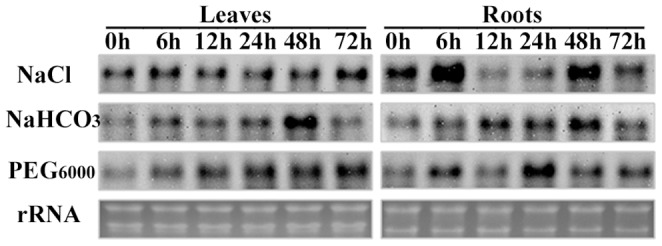
RNA gel blot analysis of OsAPx2 gene expression in rice roots and leaves treated with 100 mM NaCl, 20 mM NaHCO3, and 10% PEG 6000 for 0, 6, 12, 24, 48, and 72 h. 10 µg sample volume of total RNA.
With prolonged exposure to the NaCl, NaHCO3, and PEG 6000 stress treatments, OsAPx2 expression increased at the transcriptional level. The gene expression with the NaCl solution was significantly increased after 6 h, which is likely close related to salt and alkali stress.
Analysis of OsAPX2 Protein Expression Under Stress
The results of the protein gel blot analysis are shown in Figure 2. The signals of the leaf sample under both 100 mM NaCl and 3263 mM H2O2 were more distinct with time, by which the amount of APX2 increased gradually. The APX2 signal in the root sample also increased gradually under 100 mM NaCl and 3263 mM H2O2 with time. The salt stress likely stimulated the APX2 expression. More H2O2 was produced with prolonged exposure to the salt stress [32]. The H2O2 is converted into water by the APX isoenzyme under normal physiologic conditions [9]. Increasing the amount of APX2 reduces the injury caused by the H2O2 accumulation. Conversely, the increased H2O2 concentration increases the amount of APX2. Therefore, OsAPx2 overexpression may improve the ability of plants resisting salt stress.
Figure 2. Protein gel blot analysis of OsAPx2 expression in the leaves and roots of rice under NaCl and H2O2 stress.
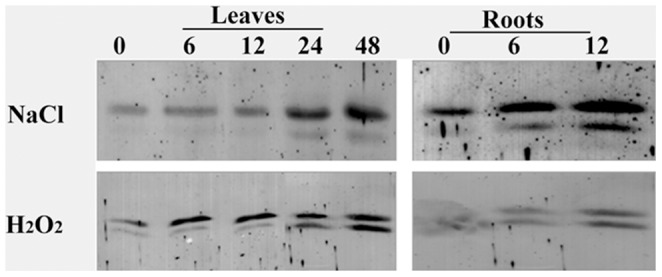
The leafs were treated for 0,6,12,24,48 h respectively and the roots were treated for 0,6,12 h respectively.
Subcellular Localization of OsAPx2::GFP in Onion Epidermal Cells
OsAPx2 genes were predicted to be localized in the cytoplasm with a reliability index of 0.45 (http://www.ncbi.nlm.nih.gov/).
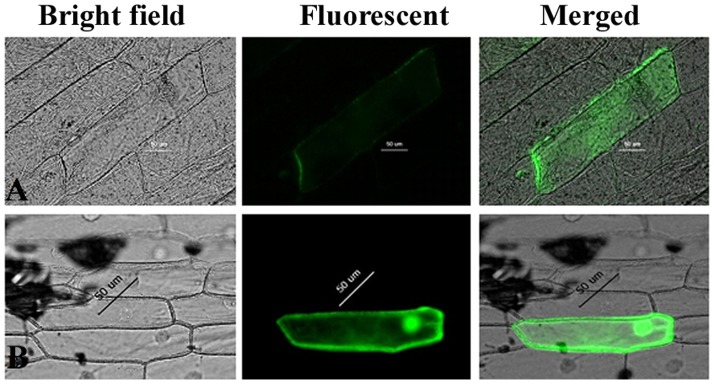
PBI121::OsAPx2::GFP plasmid DNA was constructed under the control of the CaMV35S promoter. The plasmid DNA was wrapped with aurum powder to replace arginine and CaCl2. The plasmid DNA was introduced into onion epidermal cells using a gene gun, and cultured in the dark for 18 h to 24 h. The onion epidermal cells were torn and squashed. GFP fluorescence was observed using a confocal laser scanning microscope. The results of subcellular localization analysis revealed that the OsAPx2::GFP fusion protein showed green fluorescence in the cytoplasm (Figure 3-A) and the control GFP protein in the cytoplasm and the whole cells were green (Figure 3-B), which indicated the cytoplasmic localization of the OsAPx2 gene. The results coincided with the results predicted from the network information software.
Figure 3. Subcellular localization of GFP fusion protein in the onion epidermis instant expression system.
A: Localization of pBI121-OsAPx2::GFP protein in the cytoplasm in the micrographs; B: pBI121-GFP proteins were located throughout the cytoplasm.
Rice OsAPx2 Gene Genetic Transformation
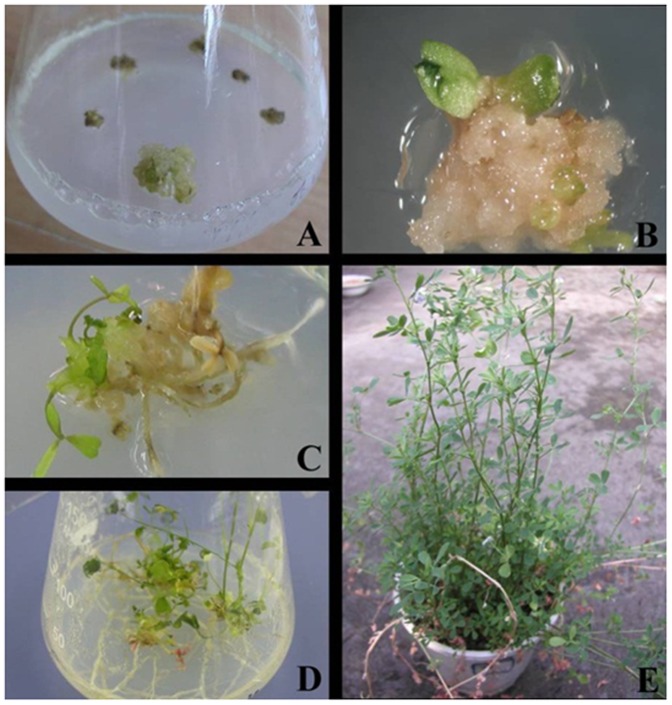
The white-green callus was transformed by A.tumefaciens EHA105 performed in the dual-expression plasmid PBI121-OsAPx2. Up to 60 samples carrying the OsAPx2 gene were obtained through the tissue culture technique (Figure 4.). The callus was incubated in three days. Then, the differentiation screening was performed on the regeneration bud in the medium for three weeks. The non-kanamycin-resistant callus turned brown and eventually died. Kanamycin, may have caused division in the cell mass (Figure 4-A as indicated by arrows). After five weeks, the embryo emerged with kanamycin-resistant cells (Figure 4-B as indicated by an arrow). Then the regenerating bud carrying the OsAPx2 gene was be obtained (Figure 4-C). After which, the transgenic regenerating bud was transferred into the medium for improving root growth for four weeks (Figure 4-D).
Figure 4. Procedure for obtaining M. sativa carrying the OsAPx2 gene.
A: The kanamycin-resistant callus, B: The kanamycin-resistant embryo, C: The kanamycin-resistant bud of resistant kana, D: the transgenic youth plant with OsAPx2, E: The transgenic plant with OsAPx2.
PCR Assay
The OsAPx2 gene was transformed into M. sativa callus by means of genetic transformation, 60 recombinants were obtained through Kanamycin resistance differentiation and screening, all of them and WT samples were PCR analysis. 48 samples contained OsAPx2 gene, which were identified with the same segment of plasmid pBI121-OsAPx2 (750 bp) however, WT (CK-) was not seen in the band (proportion results of PCR assay was shown as Figure 5.). Thus the OsAPx2 gene was transferred successfully into the 48 individual samples and the transformation ratio reached up to 80%. The T1 generation seeds were obtained.
Figure 5. PCR identification of transgenic alfalfa with rice OsAPx2.
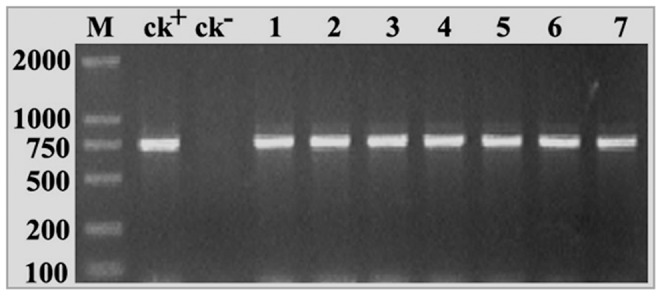
CK+: pBI 121-OsAPx2 plasmid, CK-: untransformed alfalfa, 1–7: transgenic alfalfa with rice OsAPx2. The volume was diluted up to100 then measured in a pipette with 1 µL for the PCR template. The result shows that 48 amplification products by PCR are identified with pBI121-OsAPx2’s (750 bp), and none of the products was identified with negative blank (ck-).
RNA Gel Blot Analysis
The total RNA of T2 positive plants and the blanks were extracted by Trizol Probes. The RNA gel blot analysis results were shown as Figure 6. The control samples had no signals but the transgenic plants all expressed the gene, which showed that the rice OsAPx2 gene has been transformed successfully. The heterogeneous genes were transcribed and expressed, and the new M. sativa lines carrying the rice OsAPx2 gene were obtained.
Figure 6. RNA gel blot analysis on T2 transgenic alfalfa expressing OsAPx2.
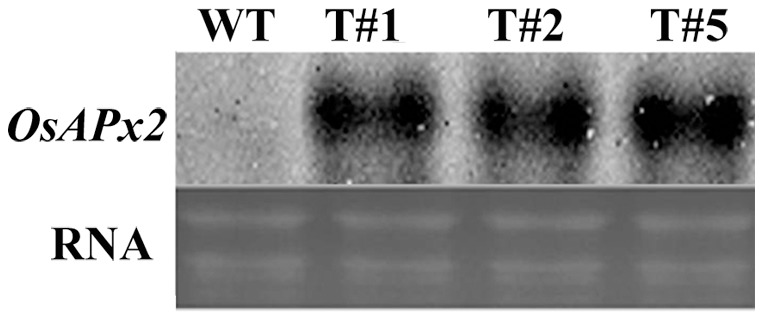
WT: untransferred alfalfa #1, 2, and 5:T2 transferred alfalfa with OsAPx2.
Salt Resistance Analysis
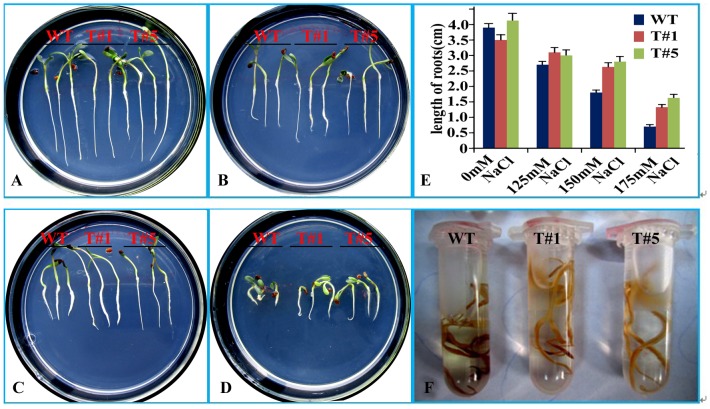
The root lengths under the 7-day stress with different NaCl concentrations were shown in Figure 7. The root lengths of the WT plants were affected significantly by the 150 mM and 175 mM NaCl treatments.This shows that the root lengths of the transgenic plants were longer than those of the WT plants ( Figure 7-A,B,C,D,E). However, the roots were unaffected by the 125 mM NaCl treatment. Analysis of theH2O2 content via DAB showed that WT had higher content than transgenic plants(Figure 7-F). Therefore, OsAPx2 gene overexpression removed the H2O2 produced during NaCl stress, and reduced plant injury.
Figure 7. Relative salt tolerance of wild type (WT) and transgenic plants with OsAPx2 overexpression in Medicago sativa (T#1 and T#5) under salt stress.
A: WT and transgenic seeds germinated in 1/2 ·MS solid medium agar plates for 1 week. B, C, D: WT and transgenic seeds germinated in 1/2 MS with 125, 150, and 175 mM NaCl. E: Effects of salt on the root lengths of the WT and transgenic plants overexpressing the OsAPx2 gene. The figure shows the root lengths of the WT and two transgenic lines (T#1 and T#5) after 1 week of salt treatment. This shows that the root lengths of the transgenic plants are longer than those of the WT plants. Data on the profile E note mean ±SD (n = 20). F: Analysis of the content of H2O2 via DAB shows that the WT has higher content than the transgenic type.
The salt resistance was tested by NaCl treatments with the same duration. The results were shown in Fig. 8, which demonstrated that the WT plants were chlorotic, whereas the T2 plants looked healthy.
Figure 8. The growth status of the WT and T2 plants under NaCl stress.

WT = wild type; T2 = the first generation carried OsAPx2, the root of the adult plants were soaked in 200 mMNaCl solution and the plants were maintained in natural light for 7 days.
APX Activity Assay
The APX activity of WT and transgenic plants were assayed and analyzed as shown in Table 2. The multiple comparison analysis as shown in table 3.With the CaMV35S promoter, the OsAPx2. gene was overexpressed, and the APX activity was clearly improved. The APX activity of T#1 and T#5 were more than WT plants. By means of multiple comparison of Duncan’s method, which showed there was a significant variation between T(T#1,T#5) and WT, but it was insignificant between T#1 and T#5 plants.
Table 2. APX activity AVONA.
| source of variation | SS | freedom | MS | F value | p-value |
| Interblock | 0.1371 | 2 | 0.0686 | 12.186 | 0.0077 |
| Within treatment | 0.0338 | 6 | 0.0056 | ||
| Total variation | 0.1709 | 8 |
Table 3. result of APX activity multiple comparison by Duncan’s method.
| treatments | mean | Significance level |
| T#1 | 0.4095 | a |
| T#5 | 0.3896 | a |
| WT | 0.1383 | b |
Note: a and b in this table indicate variation significance at α = 0.05 level(the same below).
Assay Content of MDA
MDA, which causes membrane lipid peroxidation, is a final product of the accumulation of reactive oxygen species under salt stress [33]. against the stress. The MDA content under the different NaCl treatments were shown in Table 4.
Table 4. MDA content multiple comparison by Duncan’s method.
| MDA(µmol/g) | Treatment time(h) | ||||
| 0 | 12 | 24 | 48 | 72 | |
| WT | 2.0838a | 2.3015a | 3.3893a | 6.8098a | 11.632a |
| T#1 | 2.0586a | 2.0867b | 2.5194b | 3.8703b | 4.3147b |
| T#5 | 1.9854a | 2.0551b | 2.4791b | 3.7678b | 3.9883b |
The MDA content in cells under stress can directly indicate the resistance of the plant. In all treatments, the MDA content increased with exposure time to the NaCl stress. After treating 24 h, the MDA content of the WT was higher than that of the transgenic plants. This indicated that the presence of the OsAPx2 gene can inhibit or eliminate the accumulation of reactive oxygen species in the cell. There was not significant variation of MDA content between the T#1 and T#5 treatments, but there was a significant variation between the WT and the other treatments after 12 h in stress.
Comparative Analysis of Leaf Anti-oxidation
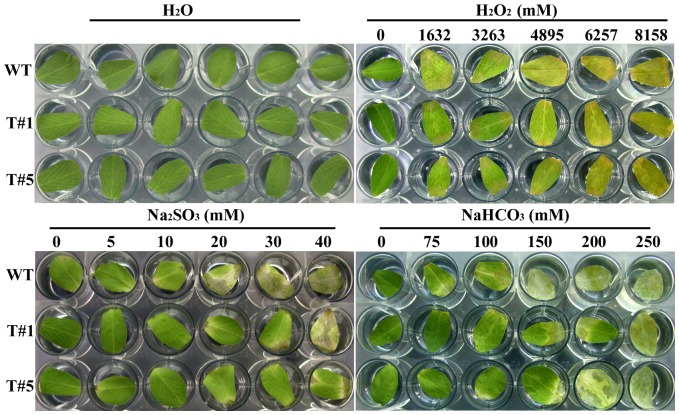
The leaves of the WT and the transgenic plants were soaked in solutions containing H2O2, Na2SO3, NaHCO3, and water (control). The results were shown in Figure 9. Based on the results, the OsAPx2 gene improved the capacity of alfalfa to eliminate reactive oxygen species, thereby increasing the salt resistance of the plants.
Figure 9. Antioxidative activity in the leaves of transgenic and WT alfalfa under different salinity levels.
In the 18-hour treatment, the differences in stress resistance were significant among the different treatments. The injury to the leaves increased with increasing salt concentrations and they gradually turned brown. The WT leaves turned yellow faster than the transgenic leaves under the 4895 mM H2O2. When the NaHCO3 and Na2SO3 concentrations reached 150 mM and 20 mM, respectively, the WT leaves experienced more injury than the transgenic leaves, and the chlorophyll content was evidently decreased.
Discussion
Reactive oxygen species accumulated at high levels in plant cells during salt stress. The proteins, membrane lipids, and other cell components are reduced, experiencing serious injury. However, the levels of reactive oxygen species in plant cells can reach equilibrium through enzyme and non-enzyme systems under normal conditions [34], [35]. The rice OsAPx gene belongs to a multi-gene family. RNA gel blot analysis showed that the transcription of the OsAPx2 gene increased significantly with time of exposure to NaCl, NaHCO3, and PEG 6000. This result coincides with the results of Székely et al. [36], Nounjan et al. [37], and Chen et al. [38]. The yield of APX2 enzyme increased gradually under the NaCl and H2O2 stress, as indicated by the protein gel blot analysis [32]. Longer stress time promoted the accumulation of hydrogen peroxide. The H2O2 is converted into water by APX isozyme under normal physiologic conditions [39]. Increasing the amount of APX2 reduces the injury caused by H2O2 accumulation. Conversely, increasing H2O2 concentrations increases the amount of APX2 such that OsAPx2 overexpression likely improves the ability to resistant salt.
Currently, plant genetic engineering is one of the most important ways for in improving the resistance of M. sativa. Agrobacterium-mediated transfection has greatly improved the study of transgenic crops [40]. In 1986, Deak et al. [41] obtained progenitive resistant samples.
After the first successful Agrobacterium-mediated transformation [42], more studies were performed on the subject. Furthermore, the seedling are not infected by the bacterium and harmed by the antibiotics [43].
In this paper, the T2 generation overexpressing the OsAPx2 gene was more resistance than the WT under the 200 mM NaCl stress. The regenerated plants were obtained using calli from organ transgenic OsAPx2 M. sativa. The transformation rate was up to 80% under specific primer PCR. OsAPx2 was overexpressed in the generation T2 as indicated by RNA gel blot analysis. Under NaCl stress, the potential for budding of the transgenic plants was higher than the WT. The H2O2 content of the transgenic plants was lower than that of the WT under DAB analysis. The transgenic plants were more resistant as indicated by the 200 mM NaCl using a pot test. The APX activity of the transgenic plants was 2.89-fold that in the WT [44]. This is also achieved when the CaMV35S was combined with the OsAPx2 gene, which improved enzyme production. Therefore, the plant carrying the OsAPx2 gene can improve the ability to detoxify reactive oxygen species. OsAPx2 gene overexpression promotes the removal of hydrogen peroxide [45]. Plant resistance to salt depends on the stability of the cell membrane, or the ability to maintain the integrity of the system, ion selective absorption, and other functions [46]. MDA, the finally product of membrane lipid peroxidation caused by accumulating reactive oxygen species, cross-links lipids, nucleic acid, saccharides, and proteins. MDA reacts strongly with many types of materials in the cell, which reduces the available unsaturated fatty acids, which changes the lipid membranous structures and functions. It can cause a series of changes in the physiology and metabolism [47], [48].
The MDA content in the WT and transgenic M. sativa was greater than that before the 48-hour and 72-hour treatments with the different NaCl concentrations. However, the content was lower in the transgenic than the WT plants. The reduction of accumulated reactive oxygen species in the cell during stress is caused by the OsAPx2 overexpression in M. sativa. The membrane lipid peroxidation in the cell is observed as leaf chlorosis. SOD overexpression in the chloroplast of transgenic M. sativa improves their tolerance to oxidative stress and freeze injury [49]. The leaves were soaked in different concentrations of H2O2, Na2SO3, and NaHCO3, indicated that the transgenic plants were significantly more tolerant of oxidative stress than the WT. OsAPx2 overexpression improves the detoxification of reactive oxygen species and reduces membrane lipid peroxidation. The gene incorporated into M. sativa is of great significance to the development of Chinese graziery.
Acknowledgments
We would like to express our great thanks to the anonymous reviewers for their helpful comments which improved this paper greatly.
Funding Statement
This work was supported by the Fundamental Research Funds for the Central Universities (Grant no. DL11CA05) and the State Forestry Administration 948 Program of China (Grant no. 2008429) to Professor Shenkui Liu.”The funders had no role in study design, data collection and analysis, decision to publish and preparation of the manuscript.
References
- 1. Ling JS, Zhang JH (1998) Production, Transport and Physiological Fuctions of Stress Signal Abscisic Acid in Roots.plant physiology communications. 34(5): 329–338. [Google Scholar]
- 2. Gasparsd S, Monzani E, Casella L, Gullotti M, Maritano S, et al. (1997) Inhibition of ascobate oxidase by phenolic compounds. Enzymatic and spectrospic studies.Biochemistry 36(16): 4852–4859. [DOI] [PubMed] [Google Scholar]
- 3. Asada K (1992) Ascorbate Peroxidase, A Hydrogen Peroxide Scavenging Enzyme in Plants. Physiologia Plantarum 85: 235–241. [Google Scholar]
- 4. Shigeoka S, Nakano Y, Kitaoka S (1980a) Metabolism of Hydrogen Peroxide in Euglena gracilis Z by L-ascorbic Acid. Peroxidase. Biochem J 186: 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shigeoka S, Nakano Y, Kitaoka S (1980b) Purification and some properties of L-ascorbic acid peroxidase in Euglena gracilis Z. Arch Biochem Biophys. 201: 121–127. [DOI] [PubMed] [Google Scholar]
- 6. Mittler R, Zilinkas BA (1992) Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem 267: 21802–21807. [PubMed] [Google Scholar]
- 7. Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radical in thylakoids. Plant Cell Physiol 33: 541–553. [Google Scholar]
- 8. Bunkelmann JR, Trelease RN (1996) Ascorbate peroxidase: a prominent membrane protein in oilseed glyoxysomes. Plant Physiol 110: 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teixeira FK, Menezes-Benavente L, Galvão VC, Margis R, Margis-Pinheiro M (2006) Rice ascorbate peroxidase gene family encodes functionally diverse isoforms localized in different subcellular comparetments. Planta 224: 333–344. [DOI] [PubMed] [Google Scholar]
- 10. Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, et al. (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of arabidopsis. The Plant Cell 17: 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mittler R (2002) Oxidative stress, antioxidants, and stress tolerance. Ternds Plant Sci 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 12. Ghazi HB, Naoyoshi K, Yasuo Y, Emi S, Ryozo, et al (2004) Over-expression of Ascorbate Oxidase in Tobacco Chloroplasts Enhances the Tolerance to Salt Stress and Water Deficit, Physiolgia Plantrum. 121: 231–238. [DOI] [PubMed] [Google Scholar]
- 13. Nishikawa F, Kata M, Wang R, Hyodo H, Ikoma Y, et al. (2003) Two APX from Brocoli: Idetification, Expression and Characterization of Their Recombinant Proteins. Postharv Biol Technol 27: 147–156. [Google Scholar]
- 14. Lu Z, Takano T, Liu S (2005) Purification and characterization of two ascorbate peroxidases of rice (Oryza sativa L.) expressed in Escherichia coli . Biotechnol Lett 27(1): 63–67. [DOI] [PubMed] [Google Scholar]
- 15. Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2000) Expression of Spinach Ascorbate Peroxidase Isoenzymes in Response to Oxidative Stresses. Plant Physiol 123: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma CL, Wang PP, Cao ZY, Zhao YX, Zhang H (2002) cDNA Cloning and Gene Expression of APX in Suaeda salsa in Response to Salt Stress. J Plant Physio and Molecu Biol 28: 261–266. [Google Scholar]
- 17. Brawn K, Fridovich I (1981) DNA strand scission by enzymically generated oxygen radicals. Arch. Biochem Biophys 206: 414. [DOI] [PubMed] [Google Scholar]
- 18. Chwan YH, Ching HK (2008) NaCl-induced expression of ascorbate peroxidase 8 in roots of rice (Oryza sativa L.) seedlings is not associated with osmotic component, Plant Signaling & Behavior 3: 3, 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agrawal GK, Jwa NS, Iwahashi H, Randeep R (2003) Importance of ascorbate peroxidases OsAPx1 and OsAPx2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling Science Gene. 322: 93–103. [DOI] [PubMed] [Google Scholar]
- 20. Lu Z, Liu D, Liu S (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26(10): 1909–1917. [DOI] [PubMed] [Google Scholar]
- 21. Xie CP (2003) King of Grass–Alfalfa. Xinjiang Agricultural Science and Technology5: 43. [Google Scholar]
- 22. Liang Z, Luo A L, Zhao Y, Tang L (1996) Accumulation of Betaine Aldehyde Dehydrogenase Induced by Drought and Salt Stress in Sugar Beet Leaves. Acta Phytophisiologica Sinica 22(2): 161–164. [Google Scholar]
- 23. Wang J, Zhang H, Allen RD (1999) Over-expression of an Arabidopsis Peroxisomal Ascorbate Peroxidase Gene in Tobacco Increases Protection against Oxidative Stress. Plant Cell Physiology 40(7): 725–732. [DOI] [PubMed] [Google Scholar]
- 24. Hightower R, Cathy B, Ranela D (1991) Expression of antifreeze proteins in transgenic plants. Plant Molecular Biology 17 (5): 1013–1021. [DOI] [PubMed] [Google Scholar]
- 25. Winicowi Bastoladr (1999) Transgenic Over-expression of the Transcription Factor Alfinl Enhances Expression of the Endogenous MsPRP-gene in Alfalfa and Improves Salinity Tolerance of the Plants. Plant Physiol 120: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd. New York: Cold Spring Harbor Laboratory Press.pp.1566–1573. [Google Scholar]
- 27. Bradford M (1976) A rapid and sensitive method for the quantification of g quantities of protein. Anal Chem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 28. Sambrook J, Ruessell DW (2001) Molecular Cloning: A Laboratory Manual, 3rd.Cold Spring Harbor Laboratory New York Press. 1474–1480. [Google Scholar]
- 29. Jin SJ, Guan QJ, Luo QX, Wang T, Takano T, et al. (2006) Study on High Frequency Regeneration and Transformation System of Alfalfa from Embryo genic Callus.Molecular Plant Breeding. 4(4): 571–578. [Google Scholar]
- 30. Ralph H, József F (1999) Christine Preis and Karl-Heinz Kogel. Hypersensitive cell death and papilla formation in barley attached by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiology 119: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao SJ, Dong XC (1998) Laboratory Manual of Plant Physiology, Beijing: China Agriculture Press. 305–306. [Google Scholar]
- 32. Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, et al. (2002) Regulation and Function of Ascorbate Peroxidase Isozymes. J Exp Bot 53: 1305–1319. [PubMed] [Google Scholar]
- 33. Hodges DM, De Long JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611. [DOI] [PubMed] [Google Scholar]
- 34. Wu GH, Zhan FJ, Huang ZL, Luo HL, Shao ZF (2001) Study on the Effects of Chilling Stress on the Metabolism of Acacia mangium . Forest Research 14(6): 633–640. [Google Scholar]
- 35. Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58: 79–110. [DOI] [PubMed] [Google Scholar]
- 36. Sze K, A braha M, Cse P, Rigo G, Zsigmond L, et al. (2008) Duplicated P5CS Genes of Arabidopsis Play Distinct Roles in Stress Regulation and Developmental Control of Proline Biosynthesis. Plant J 53: 11–28. [DOI] [PubMed] [Google Scholar]
- 37. Nounjan N, Nghia PT, Theerakulpisut P (2012) Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol 169(6): 596–604. [DOI] [PubMed] [Google Scholar]
- 38. Chen JB, Wang SM, Jing RL, Mao XG (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166: 12–19. [DOI] [PubMed] [Google Scholar]
- 39. Chou TS, Chao YY, Kao CH (2012) Involvement of hydrogen peroxide in heat shock-and cadmium induced expression of ascorbate peroxidase and glutathione reductase in leaves of rice seedlings. J Plant Physiol 169(5): 478–486. [DOI] [PubMed] [Google Scholar]
- 40. Trinh TH, Ratet P, Kondorosi E, Durand P, Kamaté K, et al. (1998) Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssa.falcate lines improved in somatic embryogenesis. Plant Cell Reports 17: 345–355. [DOI] [PubMed] [Google Scholar]
- 41. Deak M, Kiss G, Koncz C, Dudits D (1986) Transformation of Medicago by Agrobacterium mediated gene transfer. Plant Cell Reports 5(2): 97–100. [DOI] [PubMed] [Google Scholar]
- 42. Ganesh KA, Nam SJ, Hitoshi I, Randeep R (2003) Importance of ascorbate peroxidases OsAPx1 and OsAPx2 in the rice pathogen response pathways and growth and reproduction revealed by their transcriptional profiling. Science Gene 322: 93–103. [DOI] [PubMed] [Google Scholar]
- 43. Li Bl, Mei HS (1989) Relationship between Oat Leaf Senescence and Activated Oxygen Metabolism. Acta Photophysiologica Sinica 15(1): 62–72. [Google Scholar]
- 44. Zhao J, Wang C, Bedair M, Welti R, Sumner LW, et al. (2011) Suppression of Phospholipase Dys Confers Increased Aluminum Resistance in Arabidopsis thaliana. PLoS ONE 6(12): e28086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qian H, Lu T, Peng X, Han X, Fu Z, et al. (2011) Enantioselective Phytotoxicity of the Herbicide Imazethapyr on the Response of the Antioxidant System and Starch Metabolism in Arabidopsis thaliana. PLoS ONE 6(5): e19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang YX (2002) Main Characteristics, Progress and Prospect of International Wetlands Science Research.Progress in Geography. 21(2): 111–120. [Google Scholar]
- 47. Scandalios JG (1993) Oxygen stress and superoxide dismutase.Plant Physiol. 101: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu ZZ, Yu ZW, Dong QY, Qi XH, Yu SL (1997) Effects of Water on Cell Membrane and the Ultrastructure of Flag Leaf Cell Winter Wheat. Acta Agronomica Sinica 23(3): 370–375. [Google Scholar]
- 49. Du XM, Yin WX, Zhang H, Zhao YX (2003) The Researching Progress of superoxide Dismutase.Journal of Chinese Biotechnology. 23(1): 49–51. [Google Scholar]