Abstract
Background and Aim
The levels of interleukin (IL)-6 and IL-11 in the gastric mucosa are related to mucosal inflammation; however, the chronological changes in cytokine expression during different phases of Helicobacter pylori infection and the effects of H. pylori virulence factors, particularly those of outer membrane proteins, remain obscure. The aim of this study was to clarify the chronological changes in cytokine levels in relation to several H. pylori outer membrane proteins.
Methods
We studied Mongolian gerbils inoculated with wild-type H. pylori 7.13 for up to 48 weeks and then examined animals infected with oipA, babA, or alpAB isogenic mutants for 12 weeks. Mucosal IL-6 and IL-11 mRNA levels were measured using real-time reverse transcription-polymerase chain reactions.
Results
High levels of gastric mucosal IL-6 and IL-11 mRNA in gerbils infected with wild-type H. pylori were observed during the chronic phase of infection, reaching maximums at 12 and 6 months, respectively. Infection with oipA and babA mutants resulted in significantly reduced cytokine levels and inflammatory cell infiltrations compared to gerbils infected with wild-type strains, and this persisted throughout the observation period. The alpAB mutants did not infect gerbils. Mucosal IL-6 and IL-11 levels were significantly associated with the grade of inflammatory cell infiltration.
Conclusions
OipA and BabA result in more severe H. pylori infection and increased IL-6 and IL-11 levels, which in turn may increase the risk of developing H. pylori-induced gastrointestinal diseases.
Keywords: Helicobacter pylori, interleukin, Mongolian gerbil, outer membrane protein
Introduction
Helicobacter pylori infections are characterized by infiltration of inflammatory cells that release proinflammatory cytokines into the gastric mucosa.1–3 Upregulation of inflammatory cytokine mRNA and protein in the gastric mucosa was shown during H. pylori infections in humans, mice, and Mongolian gerbils,2–8 and this was thought to be involved in the development of H. pylori-associated gastroduodenal diseases.3, 6, 9 Animal models provide an opportunity to explore the relationship between chronological changes in cytokine profiles and gastroduodenal disease. As in humans, Mongolian gerbils infected with H. pylori initially develop antralpredominant gastritis that progresses to pangastritis, atrophic gastritis, metaplastic epithelia, gastric ulcers,6, 7, 10–13 and gastric cancer.14, 15 Thus, a gerbil model is often used to investigate the time course and pattern of H. pylori-associated acute and chronic gastric mucosal inflammations.3, 4 The expression patterns of different cytokines have been reported to vary over time in H. pylori-infected gerbils, with maximum levels of mucosal interleukin (IL)-1b and interferon (IFN)-γ mRNA observed early after inoculation. IL-4, IL-6, and IL-10 levels have been reported to peak from 8 to 26 weeks.3
Specific α-receptor subunits and gp130 homodimers of the signal transducing receptor are necessary for the signaling of the IL-6 family cytokines, IL-6 and IL-11.16 Recently, mice with a gp130 mutation (gp130 757F/F mice) were found to develop chronic gastric inflammation and gastric neoplasms, which were independent of the presence of H. pylori. This effect was reportedly due to an imbalance in signal transducer and activator of transcription 3 (STAT3) and Src homology-2 domain containing tyrosine phosphatase (SHP2) signaling,17 illustrating the potentially important role of IL-6 family pathways in gastric mucosal damage. We previously reported that increased IL-6 levels in H. pylori-infected mice are primarily regulated by OipA, an outer membrane protein (OMP), of H. pylori.18 OipA is a proinflammatory response-inducing protein associated with high H. pylori density and severe neutrophil infiltration in humans.19 Here, we used Mongolian gerbils to investigate the relationship among IL-6, IL-11, and OMPs and chronological changes in the levels of gastric mucosal IL-11, a member of the IL-6 family, in different phases of H. pylori infection.
Many OMPs are adhesins including OipA,20, 21 BabA,22, 23 and the adherence-associated proteins AlpA and AlpB, which are encoded by two adjacent homologous genes.24–26 All these proteins probably contribute to the pathogenesis of H. pylori-associated gastroduodenal disease. The functional status of oipA is regulated by slipped-strand repair according to the number of cytosine-thymine (CT) dinucleotide repeats in the 5′ region of the gene.19–21 BabA functions by facilitating adherence to the host Lewis-associated antigens (Leb and H-1).27 The expression of BabA is associated with severe inflammation and an increased risk of gastrointestinal disease, suggesting that BabA-Leb binding is involved in the pathogenesis of mucosal injury.22, 23 Although the presence of H. pylori OMPs is associated with enhanced mucosal inflammation, it is unknown whether these OMPs affect mucosal cytokine levels or functions in Mongolian gerbils. In the present study, we investigated the levels of expression of IL-6 and IL-11 during acute and chronic phases of H. pylori infection in Mongolian gerbils and the effect of OMP status on gastric mucosal inflammatory cytokine production.
Methods
Animals
Six-week-old male specific pathogen-free Mongolian gerbils (MGS/Sea) with an average weight of 50 g were purchased from Charles River Laboratories (Wilmington, MA, USA). They were housed in polypropylene cages on hardwood chip bedding in groups of five per cage under a 12-h light/12-h dark cycle. The animals used in this study were cared for in accordance with our institutional guidelines. Gerbils had free access to food and drinking water throughout the experiment. The experimental protocol was approved by the Animal Care Committee of the Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas.
Bacteria and bacterial inoculation
We used H. pylori strain 7.13, which was provided by Dr Richard M. Peek and which has been shown to cause reproducible mucosal damage and gastric cancer in 17%, 59%, and 59% of H. pylori-infected gerbils by 4, 8, and 16 weeks, respectively, after inoculation. 28, 29 In addition, we constructed isogenic oipA, babA, and alpAB mutants of this strain as previously described20 and used wild-type H. pylori strain 7.13 and its isogenic oipA, babA, and alpAB mutants.
Helicobacter pylori were grown in brain-heart infusion (BHI) broth (BD Diagnostics, Sparks, MD, USA) containing 15% fetal bovine serum (FBS) for 20 h at 37°C under microaerobic conditions (12% CO2) and saturated humidity (95%), shaking at 200 rpm. After fasting, each animal was inoculated three times daily with 1 mL of medium containing H. pylori (109 CFU/mL) using a metal stomach catheter. The control group was inoculated three times daily with BHI-broth medium.
Experimental design
We performed two experiments. In the first experiment, which was performed over a period of 48 weeks, Mongolian gerbils were randomly divided into an uninfected group (negative control) and a wild-type H. pylori 7.13-infected group. At each time point, five to seven gerbils were killed 4, 12, 24, and 48 weeks after inoculation. Three age- and sex-matched uninfected gerbils were killed at each time point. In the second experiment, Mongolian gerbils were inoculated with 7.13 oipA, babA, or alpAB mutants, and five to seven gerbils were killed after 4 and 12 weeks. In this study, we used part of the samples used previously6 (wild-type [the first experiment] and oipA mutants [part of the second experiment]) to clarify the natural history of the IL-6 inflammatory cytokine family.
At necropsy, the stomach was opened along the greater curvature, and the longitudinal half of the stomach was fixed in 10% buffered formalin for histological examination. The other half was divided into the pyloric gland mucosa (antrum) and the fundic mucosa (corpus) and stored at −80°C. A small amount of gastric mucosa was used for culturing H. pylori.30 DNA was extracted from the mucosa if H. pylori was not detected by culturing.
Histological examination
Tissues were sectioned (4 µm) for hematoxylin and eosin (HE) staining and Genta staining. The presence of inflammatory mononuclear cells (MNCs), polymorphonuclear cells (PMNs), and intestinal metaplasia were graded from 0 (absent/normal) to 5 (maximal intensity).31 Each strip from the antral and the corporal mucosa (both 3 points) was graded, and the results were averaged. These scores were used as the mean gastric mucosal PMNs/MNCs scores.
Sequence analysis of Mongolian gerbil IL-11
To identify the Mongolian gerbil IL-11 mRNA sequence, we selected conserved regions among mouse, rat, and human IL-11 mRNA. We designed a forward primer (IL-11-F, 5′-TGA ACT GTG TTT GTC GCC TG-3′) and a reverse primer (IL-11-R, 5′-TTG TCT CTC ATC TGT GCA GC-3′) for polymerase chain reaction (PCR) and amplified a segment of approximately 186 base pairs (bp) from the cDNA. PCR fragments were purified and directly sequenced at Macrogen, Inc. in Seoul, Korea.
Real time RT-PCR analysis
After the gastric tissue was divided into the antrum and the corpus and the mucosa was homogenized, total RNA was isolated using the RNeasy Mini Kit (Qiagen, Germantown, MD, USA). RNA (0.5 µg) from each sample was reverse transcribed (RT) using 50 units of SuperScript III RT (Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. cDNA (1 µL) was amplified by PCR using qPCR MasterMix Plus for SYBR Green I Kit (Eurogentec, San Diego, CA, USA), and specific primers for each cytokine (IL-6, forward: 5′-GAG GTG AAG GAT CCA GGT CA-3′, reverse: 5′-GAG GAA TGT CCT CAG CTT GG-3′; IL-11, forward: 5′-TGA ACT GTG TTT GTC GCC TG-3′, reverse, 5′-TTG TCT CTC ATC TGT GCA GC-3′) or β-actin (forward: 5′-TCC TCC CTG GAG AAG AGC TA-3′, reverse: 5′-CCA GAC AGC ACT GTG TTG GC-3′) using the Applied Biosystems 7300 real-time PCR system (Life Technologies Corporation, Carlsbad, CA, USA). Each PCR cycle consisted of a denaturation step (94°C, 1 min), an annealing step (55°C, 30 s), and an elongation step (72°C, 30 s). There were a total of 50 cycles, which were followed by an extension step (72°C, 7 min).
Data analysis
Data are presented as medians and ranges or as mean and standard errors of the mean (SEM) depending on whether the distribution was normal. Statistical differences were determined using a Mann–Whitney U-test or Student’s t-test depending on the dataset. Statistical associations between gastric mucosal cytokine mRNA levels and the pathological grading score of the gastric mucosal inflammation were assessed using Spearman’s correlation coefficients. P-values less than 0.05 were considered statistically significant. Calculations were conducted using the statistical software StatView 5.0 (SAS Institute Inc., Cary, NC, USA).
Results
Effect of H. pylori infection on macroscopic and pathological findings
In the H. pylori 7.13 wild-type and 7.13 oipA and babA mutant inoculation groups, H. pylori status was confirmed by culture and/or histology for all cases. However, no H. pylori were detected in gerbils inoculated with the alpAB mutants. Following inoculation with wild-type H. pylori, peptic ulcers were observed in the antrum in 4 of 9 gerbils (44%) at 3 months, 3 of 10 gerbils (30%) at 6 months, 3 of 5 gerbils (60%) at 12 months and in the corpus of 5 of 9 gerbils (56%) at 3 months and 1 of 10 gerbils (10%) at 6 months (Table 1). Although all gerbils infected with wild-type H. pylori had intestinal metaplasia, which is thought to increase the risk of developing gastric cancer, at the 6-month and 12-month time points, no wild-type-infected gerbils developed gastric cancer during the observation period in contrast to previous studies using strain 7.1328, 29 (Table 1). No ulceration or gastric cancer was observed in gerbils inoculated with the 7.13 oipA, babA, and alpAB mutant H. pylori (Table 1).
Table 1.
Incidence of gastric ulcer after inoculation with Helicobacter pylori
| Strain | Period (months) | Number | Successful infection by culture |
Gastric ulcer | Intestinal metaplasia | Dysplasia | Gastric cancer | |
|---|---|---|---|---|---|---|---|---|
| Corpus | Antrum | |||||||
| Wild-type | 1 month | 6 | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Wild-type | 3 months | 9 | 9 (100%) | 5 (56%) | 4 (44%) | 3 (33%) | 0 (0%) | 0 (0%) |
| Wild-type | 6 months | 10 | 10 (100%) | 1 (10%) | 3 (30%) | 10 (100%) | 0 (0%) | 0 (0%) |
| Wild-type | 12 months | 5 | 5 (100%) | 0 (0%) | 3 (60%) | 5 (100%) | 0 (0%) | 0 (0%) |
| oipA mutants | 1 month | 6 | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| oipA mutants | 3 months | 5 | 5 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| babA mutants | 1 month | 6 | 6 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| babA mutants | 3 months | 5 | 5 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| alpAB mutants | 1 month | 6 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| alpAB mutants | 3 months | 5 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
There was no infiltration of MNCs and PMNs in control animals. Infiltration of MNCs and PMNs reached maximum levels 3 to 12 months after inoculation of wild-type H. pylori (Fig. 1). The inflammation scores for MNCs and PMNs were higher in the antrum than in the corpus. Gastric antral-wall thickness was significantly greater at 6 to 12 months in the wild-type-infected group than in the controls (Fig. 1e). The thickness of the gastric corpus was significantly greater in the wild-type-infected group than in the controls during the experimental period (Fig. 1f).
Figure 1.
Infiltration of inflammation cells (a and b in the antrum, d and e in the corpus) and gastric wall thickness (c in the antrum and f in the corpus) in Mongolian gerbils infected with 7.13 wild-type and 7.13 oipA, babA, or alpAB mutants (KO). In Helicobacter pylori 7.13 wild-type infection, mononuclear cell (MNC) and polymorphonuclear (PMN) cell levels and gastric wall thickness significantly increased throughout the observation period. *P < 0.05 compared with week 0.
In our previous reports, the H. pylori-induced gastric mucosal inflammation reached a maximum 3 months after inoculation with H. pylori.3, 6, 13, 32 Thus, we monitored gastric inflammation for 3 months following inoculation with 7.13 oipA, babA, and alpAB mutants. The oipA and babA mutants induced mild inflammation compared with wild-type infection (Fig. 1). No gastric inflammatory cell infiltrations were observed in gerbils inoculated with alpAB mutants. Compared with the uninfected control group, infiltration scores and gastric wall thicknesses were significantly greater in the 7.13 oipA or babA mutant groups after 3 months (Fig. 1).
Sequence analysis of Mongolian gerbil IL-11 gene
Partial gerbil-specific IL-11 cDNA sequences were successfully cloned. The sequences of the Mongolian gerbil and the murine IL-11 were closely related (165 of 179 [92.2%] at the nucleotide level). A high degree of homology was also observed for IL-11 sequences between rats and Mongolian gerbils (163 of 179 [91.1%] at the nucleotide level) (Fig. 2).
Figure 2.
Sequences of 179-bp interleukin (IL)-11 mRNA from Mongolian gerbil, human, rat, and mouse. Oligonucleotides in rat and mouse are different from those of the Mongolian gerbil. A greater than 90% homology in the oligonucleotide identities was observed between Mongolian gerbil and mouse/rat.
Gastric mucosal cytokine mRNA levels in H. pylori-infected Mongolian gerbils
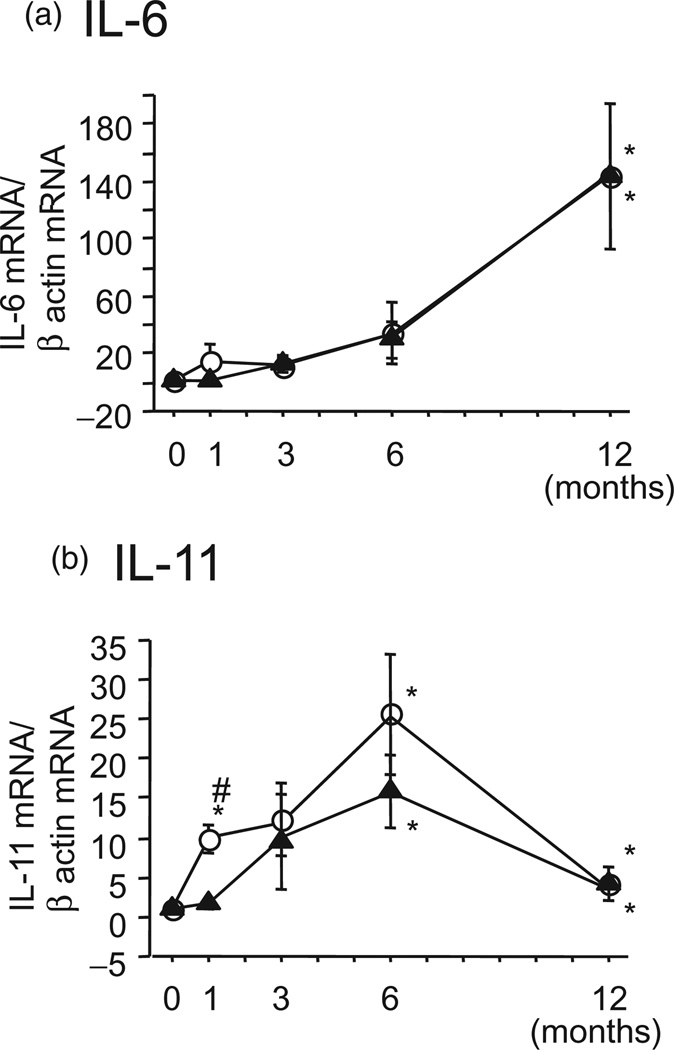
Interleukin-6 and IL-11 mRNA levels were not induced in the gastric mucosa of the uninfected group during the 12-month observation period. In infected animals, the IL-6 levels gradually increased while they remained similar in the antral and the corporal mucosa during the observation period (Fig. 3a). IL-11 mRNA levels in the antrum were strongly induced 6 months after wildtype inoculation, and they returned to near baseline levels at 12 months (Fig. 3b). IL-11 mRNA levels in the corpus were consistently lower than those in the antrum (Fig. 3b). After infection with the oipA or babA mutants, IL-6 and IL-11 mRNAlevels in the antrum and in the corpus were lower than after wild-type infection (Fig. 4). Inoculation of alpAB mutants did not result in IL-6 and IL-11 mRNA induction throughout the 12-week observation period.
Figure 3.
Gastric antral (white circles) and corporal (black triangles) mucosal cytokine mRNA levels in Mongolian gerbils infected with 7.13 wild-type Helicobacter pylori over 12 months. *P < 0.05 compared with week 0, and #P < 0.05 compared with the corpus.  , Antrum;
, Antrum;  , Corpus.
, Corpus.
Figure 4.
Cytokine mRNA levels in the Mongolian gerbil gastric antral (a and b) and corporal (c and d) mucosa when infected with 7.13 wild-type (white circle) and 7.13 oipA (black triangle), babA (white square), or alpAB (black circle) mutants (KO) over 3 months. Cytokine mRNA levels in 7.13 wild-type infections were higher throughout the experimental period. *P < 0.05 compared with week 0.  , 713 wt;
, 713 wt;  , 713.oipA-KO;
, 713.oipA-KO;  , 713.babA-KO;
, 713.babA-KO;  , 713.alpAB-KO.
, 713.alpAB-KO.
In infected gerbils, IL-6 mRNA levels correlated with IL-11 mRNA levels in the corporal mucosa (r = 0.47, P < 0.01), but not in the antrum (r = 0.13, P = 0.39).
Cytokine mRNAs and inflammatory cell infiltration in gastric mucosa of infected gerbils
Both the PMN and MNC scores were significantly correlated with IL-6 mRNA levels in the corpus (r = 0.635 and 0.581, respectively). These scores were also significantly correlated with IL-11 mRNA levels in the corpus and the antrum. Interestingly, mucosal IL-6 (80.69 ± 31.19) and IL-11 (73.33 ± 42.06) mRNA levels in the antrum were significantly higher in the ulcer group than in the nonulcer group (IL-6, 7.35 ± 2.05, P = 0.008; IL-11, 19.63 ± 11.74, P = 0.004; Fig. 5).
Figure 5.
Gastric antral mucosal cytokine levels in Mongolian gerbils in the ulcer group (grey area) and the nonulcer group (white area). Interleukin (IL)-6 and IL-11 mRNA levels in the antrum were higher in the ulcer group than in the non-ulcer group. *P < 0.05 versus nonulcer group.  , Non-peptic ulcer;
, Non-peptic ulcer;  , Peptic ulcer.
, Peptic ulcer.
Discussion
Helicobacter pylori infections induce inflammatory cytokines from activated neutrophils or MNCs, and they are thought to be involved in the pathogenesis of H. pylori-related gastroduodenal diseases.1–3, 7, 33 The expression of the IL-6 family cytokines was increased in various cancer cells and tissues and in some gastrointestinal cancer cell lines.34, 35 The IL-6 cytokine family includes IL-6, IL-11, ciliary neurotrophic factor, cardiotrophin-1, cardiotrophin-like cytokine, leukemia inhibitory factor, oncostatin M, and IL-27, all of which act as ligands for the signaling receptor subunit gp130.16, 36 IL-6 and IL-11 bind to the IL-6 and IL-11 receptor α subunits, respectively, and heterodimerize with the gp130 homodimeric receptor that is located on gastric epithelial cells.16 Recently, an imbalance in IL-6 signaling systems was shown to be related to the development of enhanced gastric mucosal inflammation and the development of gastric cancer in mice in the absence of H. pylori infection.16, 17 This mechanism may be related to the fact that a Y757F mutation in the gp130 receptor failed to enhance the phosphorylation of SHP2 and the activation of proapoptotic pathways, resulting in overactivation of STAT3. STAT3 hyperactivity induced by IL-6-family cytokines can suppress cytostatic effects of the stroma on cell proliferation.37 Moreover, STAT3 induced the epithelial cell expression of IL-11, creating a feed-forward mechanism that fueled persistent cellular proliferation.38 These signaling events promote an oncogenic program in which the expression of antiapoptotic, proangiogenic, and proproliferative genes results in inflammation-associated gastric tumorigenesis.38
Because gp130 757F/F mice naturally develop chronic gastric inflammation and gastric neoplasm without the need for H. pylori infection through an imbalance in STAT3 and SHP2 signaling,16, 17 the IL-6 signaling systems have become a focus in inflammation-associated gastric cancer research. We cloned partial sequences of the Mongolian gerbil IL-11 mRNA and measured IL-11 mRNA levels using real-time RT-PCR. Thus, the gerbil is a suitable model for investigating IL-6-family signaling in gastric cancer pathogenesis.
Mongolian gerbils infected with H. pylori developed antral-predominant gastritis, which progressed to corpus gastritis.7, 10–13 Maximal gastric mucosal inflammation in the antrum appeared early in chronic H. pylori infection and decreased dramatically after 48 weeks.3 In contrast, gastric mucosal inflammation in the corpus plateaued after reaching maximum levels.3 We previously reported that the pattern of chronic gastric inflammation generally mirrored the patterns of IFN-γ3 and IL-17 expression.6 In this study, we found that chronic gastric inflammation and atrophic changes also appeared to be related to IL-6 and IL-11. Chronic atrophic gastritis is associated with a higher risk for gastric ulcers and gastric cancer of increasing severities and extents. Long-period follow-up studies conducted in various populations have consistently confirmed that the extent of gastric mucosal atrophy paralleled the risk of gastric cancer development.39–43 Thus, it appears that the IL-6 family plays an important role in gastric ulcer and gastric cancer and is strongly related to gastric mucosal atrophy and intestinal metaplasia.
In previous reports, gastric dysplasia and cancer developed in about 70% and 50%, respectively, of gerbils at 12 to 16 weeks after inoculation of H. pylori wild-type 7.13 and 65% and 60%, respectively, of gerbils at 40 to 52 weeks.28, 29 In this study, the same H. pylori strain (7.13), the same animal model (Mongolian gerbil), and a similar infection period were examined. Although all gerbils infected with wild-type H. pylori had intestinal metaplasia, which is thought to increase the risk of developing gastric cancer, at the 6-month and 12-month time points, no infected gerbils developed gastric cancer during the observation period. The pathological diagnosis of gastric cancer in the Mongolian gerbil model remains unclear because of a lack of consensus in the diagnostic criteria. Therefore, this discrepancy may have been caused by different assessments of pathological gastric cancer. Moreover, we purchased specific pathogen-free Mongolian gerbils (MGS/Sea) from Charles River Laboratories (Wilmington). In contrast, Franco et al. used Mongolian gerbils from Harlan Laboratories (Indianapolis) 28, 29 or multiple cohorts of Mongolian gerbil.28, 29 Therefore, different strains of gerbil may have caused a discrepancy.
At least one function of the OipA, BabA, and AlpAB proteins is to act as adhesins.19, 30, 44 The inability of AlpAB mutants to infect gerbils prevented assessments of their role in this model. We were able to confirm that oipA and babA mutants did not induce gastric inflammation or peptic ulcers in Mongolian gerbils. IL-6 and IL-11 levels in tissues infected with the oipA and babA mutants were also significantly lower in oipA mutants- and babA mutants-infected strains during the acute phase than in those infected with wild-type strains. These data are consistent with the hypothesis that gastric cytokine mRNA expression is linked to inflammation induced by H. pylori infection and that the presence of a number of OMPs enhances gastric inflammation, resulting in the development of gastric ulcers in Mongolian gerbil models.19, 30, 44
These experiments showed that IL-6 and IL-11 are important in the chronic phase of H. pylori-related gastric inflammation in Mongolian gerbils. The specific outcome of an infection may be related to differences in the pattern and severity of the inflammation; both cytokines increased the risk of gastric ulcer and gastric cancer with severe gastric mucosal atrophy.
Acknowledgments
This material is based on work supported in part by the Office of Research and Development of the Medical Research Service Department of Veterans Affairs and by a Public Health Service grant, DK56338, which funds the Texas Medical Center Digestive Diseases Center. Dr Yamaoka is supported in part by a National Institutes of Health grant, DK 62813.
References
- 1.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–492. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 2.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaoka Y, Yamauchi K, Ota H, et al. Natural history of gastric mucosal cytokine expression in Helicobacter pylori gastritis in Mongolian gerbils. Infect. Immun. 2005;73:2205–2212. doi: 10.1128/IAI.73.4.2205-2212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree JE, Court M, Aboshkiwa MA, Jeremy AH, Dixon MF, Robinson PA. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J. Pathol. 2004;202:197–207. doi: 10.1002/path.1498. [DOI] [PubMed] [Google Scholar]
- 5.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–1803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 6.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and -18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer Sci. 2009;100:2152–2159. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–773. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Furuta T, Takashima M, et al. Relation between interleukin-1beta messenger RNA in gastric fundic mucosa and gastric juice pH in patients infected with Helicobacter pylori. J. Gastroenterol. 1999;34(Suppl. 11):10–17. [PubMed] [Google Scholar]
- 9.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 10.Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J. Gastroenterol. 1996;31:755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- 11.Ikeno T, Ota H, Sugiyama A, et al. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am. J. Pathol. 1999;154:951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai T, Yan J, Graham DY, et al. Serum immunoglobulin G immune response to Helicobacter pylori antigens in Mongolian gerbils. J. Clin. Microbiol. 2001;39:1283–1288. doi: 10.1128/JCM.39.4.1283-1288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai T, Fukui H, Franceschi F, et al. Cyclooxygenase expression during Helicobacter pylori infection in Mongolian gerbils. Dig. Dis. Sci. 2003;48:2139–2146. doi: 10.1023/b:ddas.0000004517.83166.26. [DOI] [PubMed] [Google Scholar]
- 14.Ogura K, Maeda S, Nakao M, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J. Exp. Med. 2000;192:1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howlett M, Judd LM, Jenkins B, et al. Differential regulation of gastric tumor growth by cytokines that signal exclusively through the coreceptor gp130. Gastroenterology. 2005;129:1005–1018. doi: 10.1053/j.gastro.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka Y, Kita M, Kodama T, et al. Helicobacter pylori infection in mice: role of outer membrane proteins in colonization and inflammation. Gastroenterology. 2002;123:1992–2004. doi: 10.1053/gast.2002.37074. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34 000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7533–7538. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kudo T, Nurgalieva ZZ, Conner ME, et al. Correlation between Helicobacter pylori OipA protein expression and oipA gene switch status. J. Clin. Microbiol. 2004;42:2279–2281. doi: 10.1128/JCM.42.5.2279-2281.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aspholm-Hurtig M, Dailide G, Lahmann M, et al. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto S, Olaniyi Ojo O, Arnqvist A, et al. Helicobacter pylori BabA expression, gastric mucosal injury, and clinical outcome. Clin. Gastroenterol. Hepatol. 2007;5:49–58. doi: 10.1016/j.cgh.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odenbreit S, Till M, Hofreuter D, Faller G, Haas R. Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol. Microbiol. 1999;31:1537–1548. doi: 10.1046/j.1365-2958.1999.01300.x. [DOI] [PubMed] [Google Scholar]
- 25.Odenbreit S, Faller G, Haas R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 2002;292:247–256. doi: 10.1078/1438-4221-00204. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Wu JY, Beswick EJ, et al. Functional and intracellular signaling differences associated with the Helicobacter pylori AlpAB adhesin from Western and East Asian strains. J. Biol. Chem. 2007;282:6242–6254. doi: 10.1074/jbc.M611178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 28.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030–1043. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 31.el-Zimaity HM, Graham DY, al-Assi MT, et al. Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Hum. Pathol. 1996;27:35–41. doi: 10.1016/s0046-8177(96)90135-5. [DOI] [PubMed] [Google Scholar]
- 32.Kudo T, Lu H, Wu JY, et al. Pattern of transcription factor activation in Helicobacter pylori-infected Mongolian gerbils. Gastroenterology. 2007;132:1024–1038. doi: 10.1053/j.gastro.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand. J. Gastroenterol. 1995;30:1153–1159. doi: 10.3109/00365529509101624. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo K, Oka M, Murase K, et al. Expression of interleukin 6 and its receptor in human gastric and colorectal cancers. J. Int. Med. Res. 2003;31:69–75. doi: 10.1177/147323000303100202. [DOI] [PubMed] [Google Scholar]
- 35.Huang SP, Wu MS, Wang HP, Yang CS, Kuo ML, Lin JT. Correlation between serum levels of interleukin-6 and vascular endothelial growth factor in gastric carcinoma. J. Gastroenterol. Hepatol. 2002;17:1165–1169. doi: 10.1046/j.1440-1746.2002.02873.x. [DOI] [PubMed] [Google Scholar]
- 36.Peters M, Muller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood. 1998;92:3495–3504. [PubMed] [Google Scholar]
- 37.Jenkins BJ, Grail D, Nheu T, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat. Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- 38.Tebbutt NC, Giraud AS, Inglese M, et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 39.Filipe MI, Munoz N, Matko I, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int. J. Cancer. 1994;57:324–329. doi: 10.1002/ijc.2910570306. [DOI] [PubMed] [Google Scholar]
- 40.Miehlke S, Hackelsberger A, Meining A, et al. Severe expression of corpus gastritis is characteristic in gastric cancer patients infected with Helicobacter pylori. Br. J. Cancer. 1998;78:263–266. doi: 10.1038/bjc.1998.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J. Clin. Pathol. 2002;55:770–773. doi: 10.1136/jcp.55.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sipponen P, Kekki M, Haapakoski J, Ihamaki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int. J. Cancer. 1985;35:173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 43.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 44.Dossumbekova A, Prinz C, Mages J, et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J. Infect. Dis. 2006;194:1346–1355. doi: 10.1086/508426. [DOI] [PubMed] [Google Scholar]