Abstract
Study Objectives:
To explore the utility of exercise training for improving daytime functioning in adults with obstructive sleep apnea (OSA).
Methods:
Forty-three sedentary and overweight/obese adults aged 18-55 years with at least moderate-severity untreated OSA (apnea-hypopnea index ≥ 15) were randomized to 12 weeks of moderate-intensity aerobic and resistance exercise training (n = 27) or low-intensity stretching control treatment (n = 16). As part of a trial investigating the efficacy of exercise training on OSA severity, daytime functioning was assessed before and following the intervention. Sleepiness, functional impairment due to sleepiness, depressive symptoms, mood, and quality of life (QOL) were evaluated with validated questionnaires, and cognitive function was assessed with a neurobehavioral performance battery. OSA severity was measured with one night of laboratory polysomnography before and following the intervention.
Results:
Compared with stretching control, exercise training resulted in significant improvements in depressive symptoms, fatigue and vigor, and aspects of QOL (p < 0.05). Sleepiness and functional impairment due to sleepiness also were improved following exercise versus control to a similar degree in terms of effect sizes (d > 0.5), though these changes were not statistically significant. No neurobehavioral performance improvements were found. Reduced fatigue following exercise training was mediated by a reduction in OSA severity, but changes in OSA severity did not significantly mediate improvement in any other measure of daytime functioning.
Conclusions:
These data provide preliminary evidence that exercise training may be helpful for improving aspects of daytime functioning of adults with OSA. Larger trials are needed to further verify the observed improvements.
Trial Registration:
Clinicaltrials.gov identification number NCT00956423.
Citation:
Kline CE; Ewing GB; Burch JB; Blair SN; Durstine JL; Davis JM; Youngstedt SD. Exercise training improves selected aspects of daytime functioning in adults with obstructive sleep apnea. J Clin Sleep Med 2012;8(4):357-365.
Keywords: exercise training, obstructive sleep apnea, mood, sleepiness, quality of life, cognitive performance
The most common complaint among individuals with obstructive sleep apnea is impaired daytime functioning,1 which includes excessive daytime sleepiness and fatigue,2 as well as decrements in cognitive function,3 mood,4 and quality of life (QOL).5 Unfortunately, continuous positive airway pressure (CPAP), the standard first-line treatment for OSA, does not always improve daytime functioning. Improvements are related to compliance to CPAP use,6 as improvements in daytime functioning can be reversed by even a single night of CPAP non-use.7 Moreover, residual symptoms often remain despite optimal compliance.6
Exercise training has been shown to reduce OSA severity, even in the absence of significant weight loss.8–10 As a result, improvements in daytime functioning might follow.8 However, it is plausible that exercise training might improve daytime functioning in individuals with untreated OSA even in the absence of a reduction in OSA severity, since exercise training has been shown to improve cognitive function,11 reduce daytime sleepiness,12 increase energy and reduce fatigue,13 improve mood,14 and enhance QOL15 in various adult populations.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Impaired daytime functioning is the most common complaint of obstructive sleep apnea (OSA). Exercise has been shown to improve numerous components of daytime functioning in other populations, but there is little supporting evidence that it improves daytime functioning in adults with OSA.
Study Impact: Exercise training significantly reduced depressive symptoms and fatigue while increasing vigor and selected aspects of quality of life, largely independent of the effects of exercise on OSA severity. These data provide preliminary evidence that exercise training may be a useful adjunct or alternative treatment to improve daytime functioning in adults with OSA.
The limited available research is supportive of this hypothesis. In epidemiologic studies of individuals with OSA, physical activity has been significantly associated with higher levels of vigor, vitality, and QOL, and lower levels of fatigue.16,17 Conversely, lack of exercise has been associated with higher daytime sleepiness in obese males with OSA.18 Moreover, in an uncontrolled study of adults with OSA, six months of exercise training elicited significant improvements in QOL, mood, and daytime sleepiness.8
We present here the daytime functioning outcomes from a randomized controlled trial in which the primary aim was to examine the efficacy of exercise training on OSA severity.9 The daytime functioning variables were regarded as secondary outcomes, and impairments in daytime functioning were not required for inclusion in the study. Nonetheless, we hypothesized that exercise training would result in moderate-sized improvements in these measures compared to the control treatment. An additional aim of the study was to determine whether daytime functioning improvements were mediated by exercise-induced reductions in OSA severity.
METHODS
A detailed description of the overall study methodology can be found elsewhere.9 The study utilized a randomized controlled experimental design involving assignment of sedentary adults with untreated OSA to an exercise training or stretching control treatment, with assessments of daytime functioning prior to and following a 12-week intervention. The study was approved by the Institutional Review Boards of the University of South Carolina and the WJB Dorn VA Medical Center, and participants provided informed consent before participation.
Participants
Participants in the current study are from the same cohort whose OSA severity and sleep quality data were previously published.9 Individuals aged 18-55 years who had at least moderate-severity OSA (apnea-hypopnea index [AHI] ≥ 15), were sedentary (i.e., self-reported exercise < 2 days/week), and were overweight/obese (i.e., body mass index [BMI] > 25) were eligible to participate in the study. Exclusion criteria included current treatment for OSA, uncontrolled hypertension (i.e., > 159/99 mm Hg), plans to lose weight, pregnancy, inability to exercise, and known or suspected significant cardiovascular, pulmonary, or metabolic disease. Medication use, including antidepressant or anxiolytic medications, was not an exclusion criterion as long as the medication dose was stable before (i.e., > 3 mo) and throughout the study.
Recruitment and Screening
Participants were recruited through local sleep clinics, media advertisements, and word of mouth. Initial eligibility was determined via a brief standardized phone screen. Individuals eligible following the phone screen were mailed a packet of questionnaires, including the Berlin Questionnaire.19 Individuals who were previously diagnosed with OSA or classified as “high risk” for OSA based on the Berlin Questionnaire were invited to the laboratory to review the protocol and provide informed consent. Individuals then were screened for OSA with one night of laboratory polysomnography (PSG), with an AHI ≥ 15 required for inclusion. As an additional inclusion criterion, individuals underwent a physical and physician-supervised graded exercise test for detection of underlying cardiovascular disease and/or adverse responses to exercise.
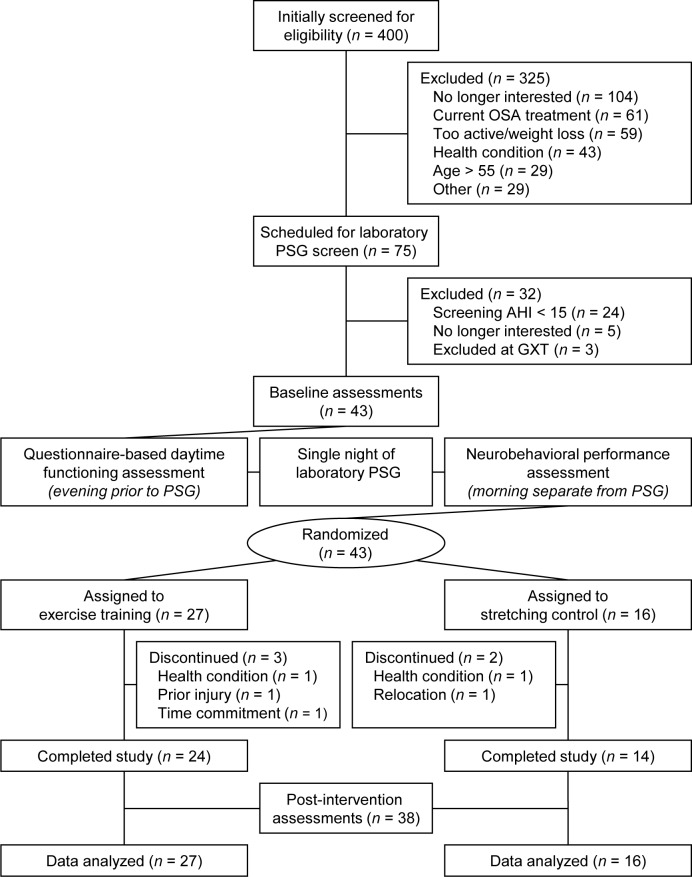
Enrolled participants visited the laboratory on 2 occasions before baseline assessment. During these visits, participants were familiarized with the experimental questionnaires and practiced the neurobehavioral performance battery used in this study. Following the introductory visits, participants completed baseline assessments of PSG, daytime functioning, and neurobehavioral performance, and were randomized to one of the 12-week treatments. Once the intervention was completed, the same assessments that were performed at baseline were repeated following a day of non-exercise. A summary of the study flow is provided in Figure 1.
Figure 1. Summary of recruitment and participant flow through study.
AHI, apnea-hypopnea index; GXT, graded exercise test; OSA, obstructive sleep apnea; PSG, polysomnography.
Interventions
Prior to randomization, treatment allocations were prepared by an individual otherwise unaffiliated with the study and placed in sealed opaque envelopes. Participants were randomly assigned by a 3:2 ratio to an exercise training treatment or a stretching control treatment, respectively.
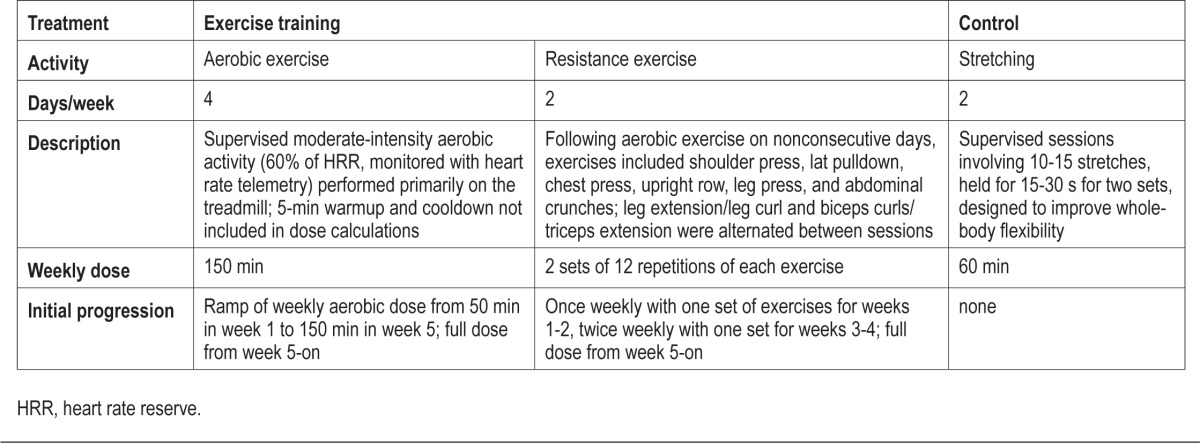
Descriptions of the exercise training and stretching control treatments are provided in Table 1. Although no change in OSA was expected from the stretching control treatment, it was chosen to control for the potential confounding influence of interpersonal interaction on outcomes related to the study. However, because blinding of treatment was not possible, both interventions were presented as active treatments. Expectancy regarding changes in daytime sleepiness, mood and health was assessed with 5-point Likert scales (1: much worse; 5: much better) at the time of randomization allocation.
Table 1.
Description of treatments
Laboratory Polysomnography
A single night of laboratory PSG (Alice 5, Philips Respironics, Murrysville, PA), fixed at 8 h time in bed, was performed at baseline and post-intervention. A standard recording montage was used,20 which was previously described.9 Assessments were evaluated using standard criteria for the scoring of sleep stages and respiratory events20 by a single registered PSG technician blinded to treatment assignment.
An apnea was defined as a ≥ 90% reduction in airflow for ≥ 10 s, and a hypopnea was defined as a ≥ 30% reduction in airflow accompanied by a ≥ 4% drop in oxygen saturation (SpO2). The AHI (i.e., number of apneas and hypopneas per hour of sleep), oxygen desaturation index (ODI; i.e., the number of SpO2 reductions ≥ 4% per hour of sleep), and percentage of total sleep time spent with SpO2 < 90% (TST90) were retained for analysis.
Questionnaire-Based Measures of Daytime Functioning
Mood, sleepiness and QOL questionnaires were administered in the evening prior to overnight laboratory PSG at baseline and post-intervention. Questionnaires were introduced using a standardized script and administered in sequential order.
Mood
The Center for Epidemiological Studies—Depression scale (CES-D)21 is a questionnaire that assesses the frequency of 20 depressive symptoms, with scores ranging from 0 to 60. The questionnaire has excellent reliability and validity.21 Scores ≥ 16 indicate significant depressive symptoms.21
The Profile of Mood States (POMS)22 assesses a wide spectrum of mood dimensions. The questionnaire assesses the intensity of 65 different emotions/feelings on a 5-point scale (0: not at all; 4: extremely). Subscale scores of fatigue and vigor, as well as a global Total Mood Disturbance (TMD) score, were retained for analysis.22
Daytime Sleepiness
The Epworth Sleepiness Scale (ESS) assesses the likelihood of falling asleep during 8 everyday sedentary activities. Response options for each activity range from 0 (would never dose) to 3 (high chance of dozing). Scores > 10 represent excessive daytime sleepiness. The ESS has a 5-month test-retest reliability of r = 0.82, differentiates between adults with and without OSA, and has been validated against the Multiple Sleep Latency Test.23
The 10-item Functional Outcomes of Sleep Questionnaire (FOSQ-10)24 is a shortened version of the 30-item FOSQ that estimates how daytime sleepiness affects one's ability to perform certain tasks in the dimensions of general productivity, social outcomes, activity level, vigilance, and intimacy. Individual questions are rated on a scale of 1 (yes, extreme difficulty) to 4 (no difficulty), averaged among the 5 subscales, and then multiplied by 5 to derive a total score. Lower scores indicate greater impairment from sleepiness. The questionnaire has been validated by its ability to distinguish between controls and OSA patients, is sensitive to change following OSA treatment, and has adequate internal consistency (α = 0.87).24
Quality of Life
The Medical Outcomes Study 36-item Short Form survey (SF-36) is a scale that evaluates 8 various health-related QOL concepts.25 Individual responses in each concept are calculated to form subscale scores that range from 0 to 100. Lower scores indicate a greater likelihood of disability due to illness. Subscales have good internal consistency (α = 0.73–0.96) and test-retest reliability (r = 0.60–0.81).25 The questionnaire has been validated by its ability to differentiate between clinical morbidities.25
Neurobehavioral Performance Battery
Neurobehavioral performance was assessed on a morning separate from laboratory PSG at baseline and post-intervention. All testing was conducted between 07:00-09:00, and participants arrived following an overnight fast. Participants went through abbreviated practice versions of each test before the full versions were administered.
Psychomotor Vigilance Task
The Psychomotor Vigilance Task (PVT; Ambulatory Monitoring, Inc., Ardsley, NY)26 is an electronic 10-min simple visual reaction time test that assesses sustained attention. Measures of median reaction time (RTmed), lapses (i.e, responses > 500 ms), and the reciprocal of the slowest 10% of responses (RRT10slow) were analyzed. Performance on this task has been shown to be impaired in adults with OSA.27
Stroop Color-Word Test
The Stroop Color-Word Test (SCWT)28 is a test of cognitive interference in which participants are required to read aloud words or colors while timed during 3 different tasks: randomly ordered words (task A); randomly ordered blocks of colors (task B); and randomly ordered words with conflicting colors (e.g., the word red in green ink). An interference score, calculated as the ratio of time on task C to the time on task B, was retained for analysis. Performance on the SCWT is impaired in individuals with OSA.29
Trail-Making Test
The Trail-Making Test (TMT)30 is a task that assesses frontal-lobe function. In Part A of the TMT, participants connect 25 consecutively numbered circles in ascending numerical order. In Part B, participants connect 25 circles, alternating between ascending sequences of numbers and letters (i.e., 1, A, 2, B). The difference between the time to complete Part B and Part A was retained for analysis. Performance on the TMT has been shown to be impaired in adults with OSA.31
Statistical Analysis
Participant sample size was estimated based upon the primary outcome for the parent study, change in AHI.9 Statistical power was 83% to detect the expected change in AHI with a total of 40 participants randomized by a 3:2 ratio. The unbalanced treatment allocation allowed for similar power as a 1:1 allocation, but permitted a more thorough investigation into the possible benefits of exercise training on OSA severity and its associated consequences.
Data from all randomized participants were utilized for all analyses with one exception: because one participant's baseline PVT scores were > 3 standard deviations different from the remaining sample, PVT data for this participant were excluded from analysis. Otherwise, all analyses were based on the intent-to-treat principle, and baseline data for participants who discontinued the study were carried forward for analysis. Participants who discontinued the study (n = 5) did not differ from those who completed the study on any demographic, OSA severity, or daytime functioning measures at baseline. Moreover, analyses restricted to those who completed the study (n = 38) did not differ from those presented here. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). Data are presented as mean ± standard error.
Baseline participant characteristics were compared between treatments with independent sample t-tests. Changes in outcome variables were evaluated with generalized linear models, with the post-intervention value for each variable as the dependent variable, treatment condition as the independent variable, and baseline value as a covariate. All statistical tests were 2-tailed. Results were considered to be statistically significant when p ≤ 0.05. Due to the small sample size and the fact that the study was not powered to detect these secondary outcomes, no statistical adjustment was made for multiple outcome measures.
There are inherent limitations of a study with multiple outcome measures and small sample size, leading to increased probability of type I and type II errors, respectively. Therefore, effect sizes, which characterize the magnitude of a treatment effect and are independent of sample size, were also calculated for outcome variables. Hedges' d effect sizes were calculated by dividing the difference between the baseline and post-intervention changes in the exercise training and control treatments by the pooled baseline standard deviation, then correcting for small sample-size bias.32 According to convention, effect sizes of d = 0.2, d = 0.5, and d = 0.8 were considered small, medium, and large in magnitude, respectively.32
Because increased OSA severity has been associated with increased sleepiness and fatigue and reductions in vigor,1,18 basic mediational analyses were conducted to evaluate whether exercise training improved daytime functioning through reductions in OSA severity. Proposed mediators included AHI, ODI, and TST90. Mediation analysis was performed using MacKinnon's product of coefficients test.33 Linear regression models were constructed to obtain coefficients associated with (1) the effect of the intervention on the proposed mediator (i.e., α coefficient) and (2) the effect of the proposed mediator on the outcome measure (i.e., β coefficient). The product of coefficients was obtained by multiplying the α and β coefficients, and asymmetric 95% confidence limits based on the distribution of the product of coefficients were created using the PRODCLIN program.33 Confidence intervals that did not include zero indicated a statistically significant mediation effect.
RESULTS
Participant and Intervention Summary
A summary of participant recruitment is provided in Figure 1. Forty-three participants were randomized to either an exercise training (n = 27) or a stretching control treatment (n = 16). Five individuals discontinued participation before study completion.
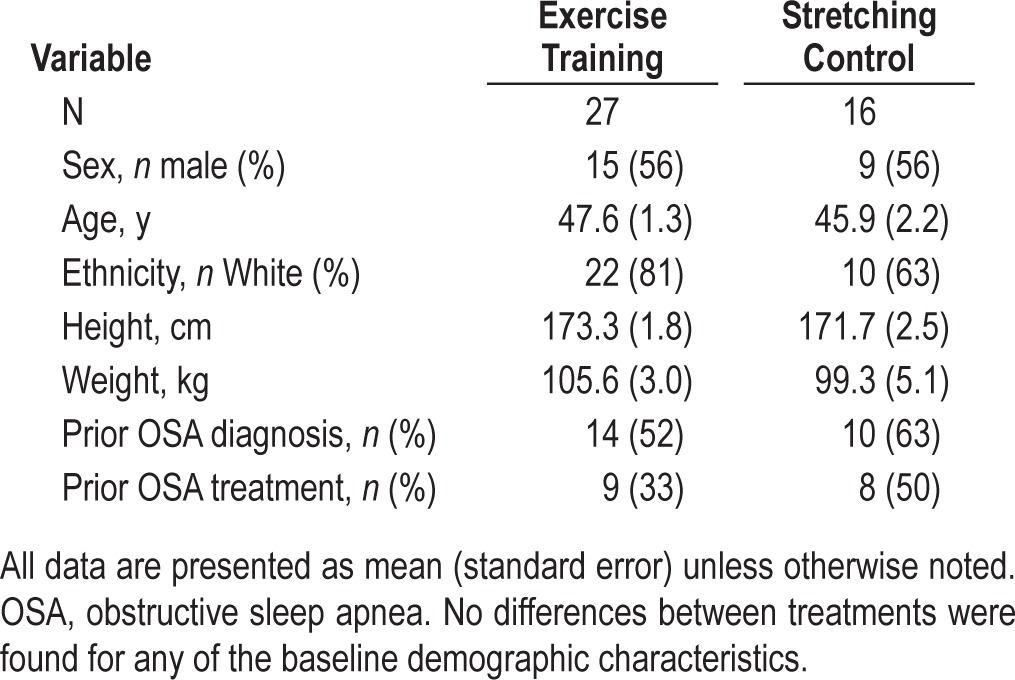
Baseline participant characteristics are summarized in Table 2. No statistically significant differences between treatments were found for any of the demographic characteristics. Exercise training participants had significantly higher ESS scores than stretching participants at baseline (t41 = −2.74, p = 0.01). No other baseline differences in daytime functioning were found between treatments. In addition, participants reported similar expectancy for improvements in mood, sleepiness, and overall health between treatments. Adherence, defined as the rate of attendance to prescribed sessions, did not differ between treatments (exercise: 87.0 ± 3.7%, control: 79.7 ± 5.3%).9
Table 2.
Baseline participant characteristics
Effect of Exercise Training on OSA Severity
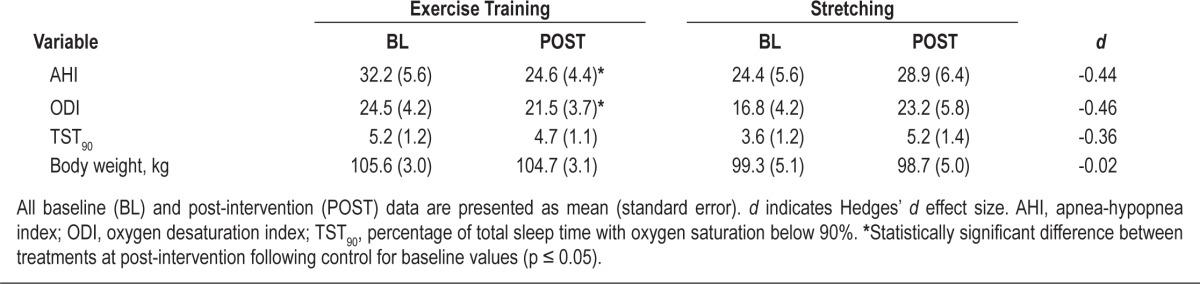
Table 3 summarizes the effects of exercise training on OSA severity and body weight. As previously reported,9 exercise training resulted in a 25% reduction in AHI that was significantly greater than that following the stretching control (F1,40 = 9.54, p < 0.01). There was also a reduction in ODI following exercise training compared to control (F1,40 = 5.05, p = 0.03), but not for TST90. The change in body weight did not significantly differ between the two treatments.9
Table 3.
OSA severity and body weight prior to and following treatment
Effect of Exercise Training on Mood
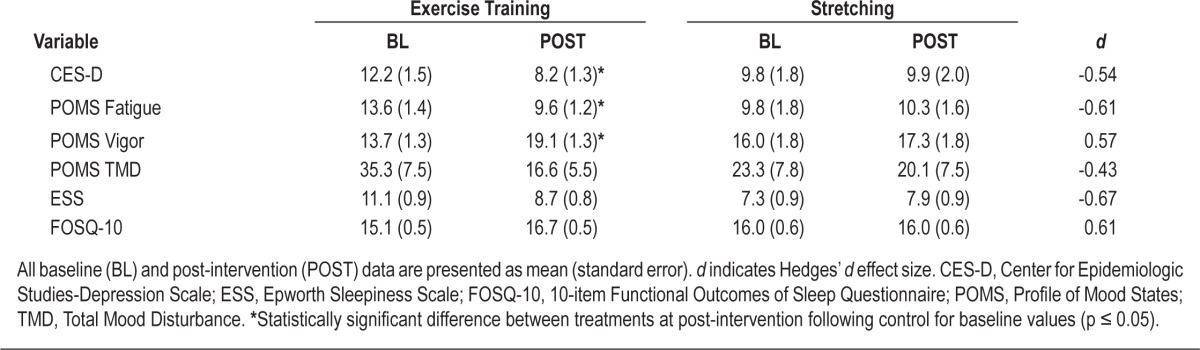
Table 4 provides a summary of the changes in mood between treatments following the intervention. Exercise training resulted in a significantly greater decrease in depressive symptoms compared with the stretching control treatment, as measured by the CES-D (F1,40 = 5.33, p = 0.03). This corresponded with a moderate effect size improvement when compared to control (d = −0.54).
Table 4.
Depressive symptoms, mood, and sleepiness prior to and following treatment
A summary of changes in POMS-related mood measures is provided in Table 4. Exercise training significantly decreased fatigue (F1,40 = 3.83, p = 0.05) and increased vigor (F1,40 = 5.91, p = 0.02) compared to control. These findings were associated with moderate-sized improvements for both fatigue (d = −0.61) and vigor (d = 0.57). Although the global measure of mood disturbance, TMD, was not statistically significantly decreased following exercise training compared to control, a moderate-sized decrease was noted (d = −0.42).
Effect of Exercise Training on Sleepiness
A summary of the changes in sleepiness following the treatments is presented in Table 4. Exercise training produced a moderate-sized reduction (d = −0.67) in ESS compared to control, though this reduction was not statistically significant. Similarly, exercise training resulted in a moderate-sized improvement in FOSQ-10 score (d = 0.61), despite the increase not being statistically significant.
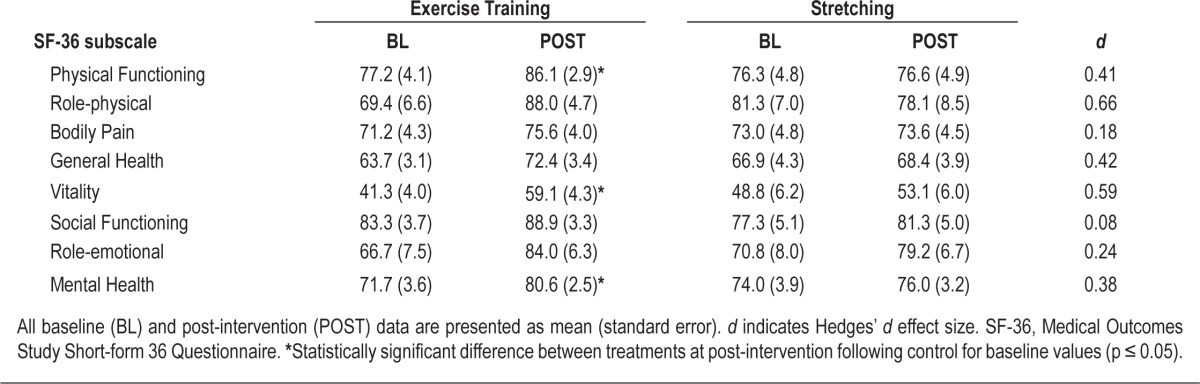
Effect of Exercise Training on Quality of Life
A summary of changes in QOL is provided in Table 5. Compared to control, exercise training resulted in significant improvements in physical functioning (F1,40 = 4.57, p = 0.04), vitality (F1,40 = 5.00, p = 0.03), and mental health (F1,40 = 4.11, p = 0.04). Small- to moderate-sized improvements (d > 0.35) following exercise training compared to control were noted for all SF-36 subscales except for bodily pain, social functioning, and role limitations due to emotional health.
Table 5.
Health-related quality of life prior to and following treatment
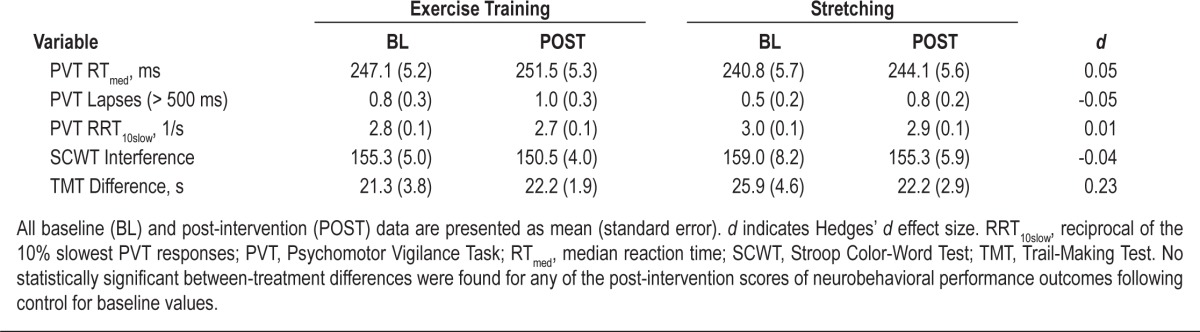
Effect of Exercise Training on Neurobehavioral Performance
Table 6 summarizes the results relating to neurobehavioral performance. When compared with control, no significant changes between treatments were noted for any of the performance measures following exercise training. In addition, effect size calculations revealed no notable post-intervention difference in any neurobehavioral performance parameters except for a slightly greater reduction in TMT Difference score in the stretching control treatment compared to exercise training (d = 0.23).
Table 6.
Neurobehavioral performance prior to and following treatment
Associations of Reduction in OSA Severity with Improvement in Daytime Functioning
The intervention produced significant improvements in AHI and ODI, as previously reported (AHI: α̂ = 10.41 [3.61], p < 0.01; ODI: α̂ = 8.52 [3.79], p = 0.03).9 Changes in AHI and ODI were significant predictors of reduction in POMS fatigue (AHI: β̂ = −0.15 [0.07], p = 0.03; ODI: β̂ = −0.17 [0.06], p < 0.01), and reduced TST90 predicted improvements in SF-36 physical function (β̂ = 1.15 [0.43], p = 0.01). Furthermore, reduction in ODI was a significant predictor of reduced PVT lapses (β̂ = −0.03 [0.01], p = 0.04). Mediation analyses indicated that the effect of exercise training on POMS fatigue was mediated by reductions in AHI and ODI (α̂β̂ 95% confidence limits: AHI [0.21, 3.57], ODI [0.16, 3.25]). No other significant mediation effects were noted for any of the other daytime functioning variables.
DISCUSSION
As secondary outcomes of a randomized trial that evaluated the efficacy of exercise training on OSA severity, the aim of the current analysis was to investigate the effect of exercise training on the daytime functioning of individuals with OSA. Exercise training resulted in moderate effect-size improvements in depressive symptoms, vigor, fatigue, physical and mental health aspects of QOL, and sleepiness. Of all the variables that were improved following exercise training, only reduction in fatigue was found to be mediated by exercise-induced reductions in OSA severity.
Exercise training resulted in significant improvements in fatigue and vigor, with corresponding moderate-sized effects (d > 0.55). These findings are consistent with an extensive literature showing improvements in fatigue and vigor following exercise training in other populations.13 Moreover, the results of the present study extend the findings of prior uncontrolled research that indicated significant improvements in fatigue and vigor following exercise training in individuals with OSA,8 as well as strong epidemiologic associations of levels of fatigue and vigor with physical activity levels in individuals with OSA.17 The observed improvements in vigor and fatigue are similar to those achieved by CPAP. For example, a recent study found that three weeks of therapeutic CPAP produced moderate-sized improvements (d ∼ 0.50) in fatigue and vigor compared to placebo CPAP.34
Exercise training also elicited significant improvements in depressive symptoms and some aspects of QOL. A higher level in the vitality dimension of QOL has been associated with a higher level of physical activity in previous cross-sectional research of individuals with OSA,16,17 and a significant improvement in role limitations due to physical problems following exercise training was previously noted in a small uncontrolled study of apneics.8 Exercise training improved most of the QOL components that are most detrimentally affected by OSA, including physical functioning, general health perception, and vitality.35 The QOL improvements achieved with exercise training in the current study are comparable to those achieved with CPAP therapy.35
The antidepressant effects of exercise have been well-documented in other populations.14 However, to our knowledge, no previous exercise training trials have investigated the effect of exercise training on depressive symptoms in adults with OSA. Given that depression is the most prevalent mental health condition observed in adults with OSA4 and the effects of CPAP on attenuating depressive symptoms are inconsistent,36 the results of the current study suggest that exercise training may be a useful nonpharmacologic therapy for reducing symptoms of depression in OSA patients.
In contrast to most other aspects of daytime functioning, there is minimal evidence that exercise significantly reduces sleepiness.37 In adults with OSA, epidemiologic research has associated higher daytime sleepiness with lack of exercise,18 and experimental research has documented a moderate reduction in ESS score (d ≥ −0.55) following 3 and 6 months of exercise training, respectively.8,10 The magnitude of improvement noted in the present study for ESS and FOSQ-10 scores following exercise training compared to control (d > 0.60) is consistent with prior research involving adults with OSA. Moreover, the ∼2.5 point ESS reduction following exercise training in the present study is comparable to the effects of CPAP therapy.38 However, due to the lack of statistically significant improvement in these parameters of sleepiness, the results should be interpreted with caution.
Neurobehavioral performance was the only dimension of daytime functioning that did not appear to be at least modestly improved with exercise training. However, given the moderate efficacy of exercise at reducing AHI and ODI9 and the apparent lack of impairment in neurobehavioral performance in our study sample compared to normative values and previously published reports of individuals with OSA,27,29,31 it is not surprising that improvements in neurobehavioral performance were not observed.
Because this study relied upon effect sizes to demonstrate improvements in many aspects of daytime functioning following exercise training, the results should be considered preliminary. The lack of detection of statistical significance for many of these measures can be attributed to the sample size (N = 43), which was powered to detect expected reductions in AHI and not necessarily the secondary outcomes of daytime functioning.9 However, it is noteworthy that effect sizes, which are independent of sample size limitations, revealed that exercise training produced moderate-sized improvements in most aspects of daytime functioning compared to the control treatment.
It has been hypothesized that individuals with OSA may be disinclined to exercise due to the impaired daytime functioning that is associated with the disorder, especially excessive sleepiness and fatigue.18 Thus, individuals who would be willing to exercise, such as the participants in the current study, may not be representative of the general OSA population. Conversely, individuals with significant daytime functioning impairment may be more likely to seek treatment than individuals with milder or absent daytime dysfunction. Our results suggest that participants were of similar impairment when compared to the overall OSA population, as the baseline daytime functioning of participants in the current study was similar to previously published OSA population samples (e.g., sleepiness,2 depressive symptoms4).
Similarly, as with any intervention study, the self-selection of participants introduces a potential bias toward those who may benefit from the intervention being studied. Furthermore, with a study in which participants could not be blinded to their treatment, expectancy effects could plausibly have driven the improvements in subjective daytime functioning. However, in the present study, both interventions were presented as active treatments, and no difference in expectancy for mood, sleepiness, or health improvements was noted between treatments following randomization. Therefore, that exercise training resulted in improved daytime functioning compared to a control condition of similar baseline expectancy suggests that the observed results were not driven by expectancy effects. Nevertheless, to reduce expectancy concerns, it would be prudent in future studies to include objective measures of sleepiness, such as the Multiple Sleep Latency Test or Maintenance of Wakefulness Test.
The different time commitments imposed by the two treatments is another potential limitation, as differing amounts of interaction with study staff could have theoretically influenced the subjective ratings of daytime functioning. However, we do not believe this factor significantly biased the outcomes of the present study. The treatment durations were chosen after carefully considering the advantages and disadvantages of having equal treatment durations. The stretching control and exercise training conditions were presented as “low intensity” and “moderate intensity” physical activity interventions, with the dosages of these activities in accordance with established guidelines.39 Compared with participants in the exercise training treatment, stretching treatment participants had a greater opportunity for interpersonal interaction with study staff per hour of treatment while they were individually led through their flexibility program, such that total interaction was comparable or perhaps greater for the stretching group. Moreover, in our view, 150 minutes/week of flexibility exercises might have seemed excessive. We surmised that reduced treatment duration for the stretching intervention would provide an appropriate balance between controlling for behavioral confounds and having credible active treatments for which there were no differences in any of the expectancy measures.
Intermediate assessments of vigor, fatigue, and sleepiness were not performed in the current study. It is possible that initiation of an exercise program may produce temporary increases in fatigue and sleepiness along with decreased energy, and may discourage continuation of exercise training in participants who already are tired and sleepy from the underlying OSA. There were no anecdotal reports of this phenomenon in the current study, which might be explained by the gradual progression of exercise dose during the initial four weeks of the study. Such a progression is important for beginning any exercise program, but may be even more essential for individuals with OSA. Future research should employ more frequent assessment of sleepiness, fatigue/energy, mood, and QOL changes with exercise training in order to develop a better understanding of the time course of these changes.
It is unknown how exercise training would affect the daytime functioning of individuals currently being treated with CPAP therapy or oral appliances. Although the moderate efficacy of exercise training on OSA severity8–10 indicates that it is likely insufficient as a stand-alone therapy for OSA, this trial demonstrates the potential utility of exercise training to reduce daytime functioning decrements in OSA. Because CPAP or oral appliance use fails to completely normalize daytime functioning in many cases6,40 and most of the daytime functioning improvements following exercise training were independent of OSA improvement in the current study, combining exercise training with CPAP or oral appliance therapy may be an effective treatment option for augmenting improvements in sleepiness, fatigue, and QOL. Future trials involving combinations of exercise training with CPAP or oral appliance therapy are needed to determine whether exercise training results in additive improvements in daytime functioning relative to CPAP or oral appliances alone.
In conclusion, our results indicated that exercise training produced moderate improvements in selected aspects of daytime functioning that are impaired in individuals with OSA, including depressive symptoms, vigor and fatigue, and aspects of QOL. Because of the reliance of effect sizes to demonstrate improvement in some aspects of daytime functioning and the small sample size of the study, the results need to be verified by larger trials. Nevertheless, these data provide preliminary evidence that exercise may be a valuable therapy for improving aspects of daytime functioning in adults with OSA, regardless of changes in OSA severity.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Blair receives book royalties from Human Kinetics and has received honoraria from Alere, Technogym, Santec, Clarity, and Jenny Craig. He has also received honoraria for lectures and consultations from scientific, educational, and lay groups. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Public Health Dissertation Grant 1R36CD000695-1 from the Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. Additional funding support for this work was provided by NHLBI T32 HL082610.
The authors gratefully acknowledge SleepMed of South Carolina and the WJB Dorn VA Sleep Laboratory for their assistance with recruitment and polysomnographic sleep assessments, respectively. The authors also are indebted to Shannon Crowley, Morgan Porter, Elizabeth Rose and Megan Wallner for their assistance with data collection. The work was performed in Dr. Youngstedt's Chronobiology Laboratory, the Clinical Exercise Research Center at the University of South Carolina, and the WJB Dorn VA Medical Center Sleep Laboratory.
REFERENCES
- 1.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest. 2000;118:372–9. doi: 10.1378/chest.118.2.372. [DOI] [PubMed] [Google Scholar]
- 3.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 5.Lacasse Y, Godbout C, Series F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J. 2002;19:499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- 6.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34:111–9. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 8.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–9. [PubMed] [Google Scholar]
- 9.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–40. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath. 2011;15:49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 11.Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–30. doi: 10.1016/j.brainresrev.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Med. 2010;11:934–40. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puetz TW, Flowers SS, O'Connor PJ. A randomized controlled trial of the effect of aerobic exercise training on feelings of energy and fatigue in sedentary young adults with persistent fatigue. Psychother Psychosom. 2008;77:167–74. doi: 10.1159/000116610. [DOI] [PubMed] [Google Scholar]
- 14.Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–78. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes C, Esteves AM, Bittencourt LR, Tufik S, Mello MT. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz J Med Biol Res. 2008;41:908–13. doi: 10.1590/s0100-879x2008005000036. [DOI] [PubMed] [Google Scholar]
- 17.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35:1088–92. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 18.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 2011;1:385–401. [Google Scholar]
- 22.McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego: Educational and Industrial Testing Services; 1971. [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32:915–9. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware JE, Jr., Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2004. [Google Scholar]
- 26.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 27.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30:1309–16. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golden C, Freshwater S. A Manual for the Adult Stroop Color and Word Test. Chicago: Stoelting; 2002. [Google Scholar]
- 29.Verstraeten E, Cluydts R, Pevernagie D, Hoffmann G. Executive function in sleep apnea: controlling for attentional capacity in assessing executive attention. Sleep. 2004;27:685–93. [PubMed] [Google Scholar]
- 30.Reitan RM, Davison LA. Clinical neurophysiology: current status and applications. New York: John Wiley; 1974. [Google Scholar]
- 31.Bedard MA, Montplaisir J, Malo J, Richer F, Rouleau I. Persistent neuropsychological deficits and vigilance impairment in sleep apnea syndrome after treatment with continuous positive airways pressure (CPAP) J Clin Exp Neuropsychol. 1993;15:330–41. doi: 10.1080/01688639308402567. [DOI] [PubMed] [Google Scholar]
- 32.Hedges LV, Olkin I. Statistical methods for meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 33.Mackinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34:121–6. doi: 10.1093/sleep/34.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186:131–44. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- 36.Habukawa M, Uchimura N, Kakuma T, et al. Effect of CPAP treatment on residual depressive symptoms in patients with major depression and coexisting sleep apnea: contribution of daytime sleepiness to residual depressive symptoms. Sleep Med. 2010;11:552–7. doi: 10.1016/j.sleep.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Loprinzi PD, Cardinal BJ. Association between objectively-measured physical activity and sleep, NHANES 2005-2006. Ment Health Phys Act. 2011;4:65–9. [Google Scholar]
- 38.Marshall NS, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. 2006;61:430–4. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 40.Ghazal A, Sorichter S, Jonas I, Rose EC. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J Sleep Res. 2009;18:321–8. doi: 10.1111/j.1365-2869.2009.00738.x. [DOI] [PubMed] [Google Scholar]