Abstract
Objective:
Smoking is a known risk factor for snoring, and is reported to be associated with an increased prevalence of obstructive sleep apnea syndrome (OSAS). The purpose of this was to determine the relationship of smoking to the severity of OSAS and examine what local histological changes in the uvular mucosa of OSAS patients might influence this relationship.
Study Design and Methods:
Fifty-seven OSAS subjects were included and classified according to smoking history and OSAS severity. Twenty-eight subjects were heavy smokers and 29 were nonsmokers; these 57 patients were divided according to moderate or severe OSAS. Histologic changes in the uvular mucosa were evaluated in all subjects as well as smoking duration and OSAS severity.
Results:
Among smokers, moderate-to-severe OSAS was more common, and apnea, hypopnea, and oxygen desaturation indices were higher. Moreover, smoking duration and OSAS severity were significantly correlated. Increased thickness and edema of the uvular mucosa lamina propria were observed in moderate and severe OSAS patients, and only smokers had significant changes in uvular mucosa histology. Positive staining for calcitonin gene-related peptide (CGRP), a neuroinflammatory marker for peripheral nerves, was increased in the uvular mucosa of smokers.
Conclusions:
Our results suggest that smoking may worsen OSAS through exacerbation of upper airway collapse at the level of the uvula, and that histological changes of the uvular mucosa correlated with smoking might be due to increased CGRP-related neurogenic inflammation.
Citation:
Kim KS; Kim JH; Park SY; Won HR; Lee HJ; Yang HS; Kim HJ. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med 2012;8(4):367-374.
Keywords: Smoking, obstructive sleep apnea, upper airway
Obstructive sleep apnea syndrome (OSAS) is a complex, chronic disorder characterized by snoring, periodic apnea, hypoxemia during sleep, and daytime hypersomnolence.1 Its prevalence is 16% to 33% in men and 8% to 19% in women.2,3 OSAS has been shown to increase the risk of hypertension, stroke, and cardiovascular disease.4–6 In addition, clinical studies have reported that OSAS increases morbidity from crashes caused by daytime somnolence, and have focused on the diagnosis, treatment, and prevention of OSAS.2
Obesity, male sex, older age, alcohol use, genetic factors, and a narrowed upper airway are well-known risk factors for OSAS, and smoking may be a risk factor for sleep apnea or snoring.3,7–9 Epidemiologic studies have confirmed the influence of smoking on OSAS and found that the morbidity of sleep related respiratory disorders is increased in smokers, depending on their smoking history, and that habitual smoking appears to be associated with OSAS.7–10 The correlation between smoking and OSAS has not been studied in detail. In particular, the direct influence of smoking on OSAS and the correlation between the amount of cigarette smoking and the severity of OSAS have not been adequately demonstrated.
OSAS is characterized by repetitive upper airway collapse during sleep. Narrowing of the upper airway can be accompanied by breathing difficulty during sleep and is a predisposing anatomic factor for OSAS.11–13 Pathological conditions such as a redundant or long uvula and enlarged tonsils are thought to be the predominant causes of upper airway collapse during sleep and are indications for surgery to decrease snoring, apnea, and snoring-related cardinal symptoms. The upper airway mucosa can be easily affected by smoking, and longer exposure to smoking may cause abnormal histological changes to the upper airway mucosa. Therefore, evaluation of the uvular changes after exposure to smoking is important for understanding how smoking directly affects the uvular mucosa.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The fact that the morbidity of sleep-related respiratory disorders is increased in smokers has been confirmed through epidemiologic studies. However the direct influence of smoking on OSAS at upper airway has not been adequately demonstrated.
Study Impact: We propose that longer exposure to smoking results in a higher prevalence of moderate or severe OSAS and cessation of smoking may be required to prevent upper airway narrowing that worsen OSAS.
This study was designed to determine the relationship between smoking and OSAS. We reviewed the sleep studies of smokers and nonsmokers who were diagnosed with OSAS to examine the relationship of smoking to OSAS, especially the severity of OSAS. To better understand the influence of smoking on OSAS severity, we obtained uvular mucosa from OSAS subjects during snoring surgery and investigated smoking-induced histological changes in the upper airway mucosa resulting in narrowing of the upper airway.
MATERIAL AND METHODS
Subjects
This study included 122 patients referred to the Sleep Disorder Clinic in the Department of Otolaryngology and Head – Neck Surgery of Chung-Ang University College of Medicine (Seoul, Korea) between March 2005 and February 2008 with a diagnosis of OSAS due to a narrowed oropharynx, who underwent uvulopalatopharyngoplasty (UPPP). All patients gave their informed consent to participate. Surgical samples for histologic study were obtained from all patients who met the criteria for UPPP, the diagnosis of OSAS was established by overnight respisomnography, and a narrowed oropharynx was confirmed via the Müuller maneuver using a flexible endoscope and lateral cephalometric roentgenogram. The records of 57 male patients with a clear smoking history and a normal anatomical cephalometric index except for a narrowed oropharynx were reviewed retrospectively, and the histological reports of their uvular specimens after UPPP were examined. Thirty-five patients treated with continuous positive airway pressure, 29 patients with hypertension, diabetes, or atherosclerotic vascular disease, and 11 patients who had an unclear smoking history were excluded.
Before UPPP, all patients underwent a pulmonary function test using forced spirometry. No patients had chronic respiratory diseases such as asthma or COPD. Patients with an active smoking history > 2 years before undergoing UPPP were considered current smokers, and patients with no smoking history were nonsmokers. Smoking history was quantified in number of pack years (PYs) as (packs smoked per day) × (years as a smoker), defined as 20 cigarettes smoked every day for one year. Smokers were divided into 2 groups: > 10 PY and ≤ 10 PY.
Sleep Study
All patients were examined prior to surgery by respisomnography ≥ 6 h using an Embletta PDS (Embla System, Reykjavik, Iceland) consisting of a pressure transducer system (pressure cannula), a thermistor sensor for oral and nasal airflow, a piezoelectric belt for thoracic and abdominal impedance, and a pulse oximeter (finger flex sensor) for O2 saturation and pulse. Sensors were attached in the evening and recording was initiated immediately. Respisomnographic recordings were analyzed using Somnoligica Studio (PSG software; Embla Systems, Bloomfield, CO, USA).
Based on the respisomnographic results, apnea was defined as complete cessation of airflow ≥ 10 s, and hypopnea was defined as a reduction in airflow associated with ≥ 4% drop in O2 saturation. Subjects with an apnea-hypopnea index (AHI) ≥ 5 were considered to have OSAS, and OSAS severity was classified as mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30), or severe (AHI ≥ 30). The oxygen desaturation index (ODI) measuring the number of oxygen desaturations ≥ 3% per sleeping hour was determined. Body mass index (BMI) and ODI were measured for each group in order to analyze the relationship between these variables and OSAS severity.14,15
Surgical Specimens and Histological Study
Surgical specimens were obtained from the uvulas of the 57 patients who underwent UPPP and fixed in 10% paraformaldehyde. Histological studies were performed using morphometric and qualitative methods. One pathologist measured each factor within one window of a light microscope and read, interpreted, and classified the histological findings.
1. Morphometric study
We measured the following 6 factors with a light microscope (×100): (1) thickness of the epithelium, (2) thickness of the lamina propria, (3) degree of vascular dilation (0, no vascular dilatation; 1, mild dilatation; 2, moderate dilation; 3, severe dilatation), (4) degree of fibrosis (0, no fibrosis; 1, mild fibrosis; 2, moderate fibrosis; 3, severe fibrosis), (5) amount of inflammatory cell infiltration, and (6) degree of submucosal gland proliferation. The number of submucosal glands was counted under a light microscope and graded according to the following scale: 1, number of submucosal glands < 3; 2, number of submucosal glands 3-10; 3, number of submucosal glands 11-30; and 4, number of submucosal glands > 30.
2. Qualitative Study
We noted the following histological findings in specimens under the light microscope (×100): hyperkeratosis, acanthosis in the epithelium, edema, vascular congestion in the lamina propria, hyperplasia in the submucosal glands, and atrophy of muscle tissues.
3. Immunohistochemistry
Immunohistochemical analysis was performed using the neuroendocrinological markers protein gene product (PGP) 9.5, substance P (SP), and calcitonin gene-related peptide (CGRP), to determine the expression of peripheral sensory nerves in patient uvulas or soft palates. The monoclonal antibodies used were PGP 9.5 (1:50; DAKO, Glostrup, Denmark), SP (1:100; Invitrogen, Carlsbad, CA, USA), and CGRP (1:200; Chemicon International, Inc., Billerica, MA, USA).
Briefly, 5-μm sections were fixed in acetone for 10 min at room temperature (RT). Nonspecific protein staining was blocked with goat serum. Slides were treated with 0.5% hydrogen peroxidase to eliminate endogenous peroxidase for 10 min at RT and incubated with primary antibody overnight at RT. After washing with Tris-buffered saline (TBS, pH 7.5), slides were incubated with horseradish peroxidase-conjugated secondary antibody (Thermo, Asheville, NC, USA) for 30 min at RT. Chromogen (3-amino-9-ethylcarbazole) was applied to the specimens, followed by Mayer's hematoxylin for counterstaining.
Statistical Analysis
ANOVA, independent sample t-tests, and Fisher exact test were used to assess the association between patient factors and respisomnography results. Statistical correlations were calculated using the Statistical Package for the Social Sciences (SPSS, version 12.0) and a p-value < 0.05 was considered statistically significant.
RESULTS
Characteristics of Patients
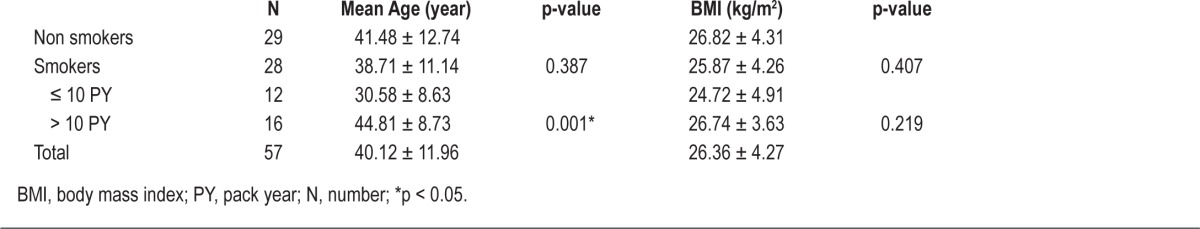
We recruited 57 men who fulfilled the inclusion criteria: 28 with a smoking history and 29 nonsmokers. The mean age was 40.1 years (17-72 years). We classified the smokers into 2 groups on the basis of a 10-PY smoking history, with 12 in the ≤ 10 PY group and 16 in the > 10 PY group. The ages of smokers and nonsmokers were not significantly different, but patients with a > 10 PY smoking history were older than patients with a ≤ 10 PY smoking history (Table 1).
Table 1.
Mean age and BMI according to smoking history and exposure to smoke
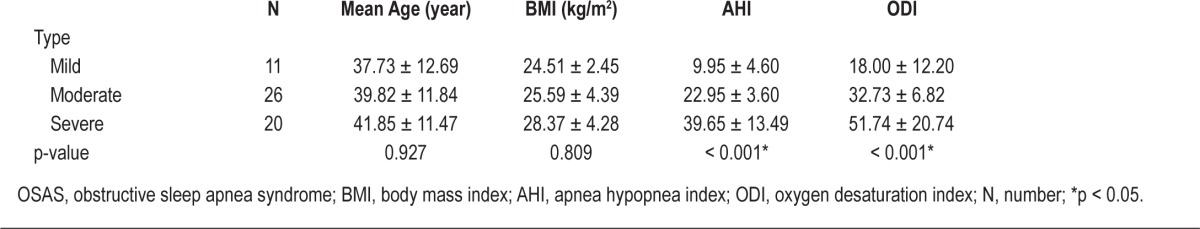
We classified OSAS severity as mild, moderate, or severe based on respisomnography results. The mild group had 11 patients (19.3%), the moderate group had 26 (45.6%), and the severe group had 20 (35.1%). Significant differences were not noted in mean age or BMI according to OSAS severity, contrary to AHI and ODI (Table 2).
Table 2.
Mean age, BMI, and AHI of patients by severity of OSAS
The classification of patients according to smoking history or PY was independent of influences of other OSAS risk factors such as cardiovascular diseases, obesity, male sex, and history of chronic medication use. It has been known that old age may be one of the risk factors of OSAS and the age of smokers in the > 10 PY and ≤ 10 PY groups was significantly different in our data. However, no difference of mean age was observed according to severity of OSAS and smoking history (smokers vs nonsmokers). We found that nonsmokers had relatively less severe OSAS than with smokers, even though mean age of nonsmokers was higher than that of smokers (Table 1). Therefore, the influence of age on OSAS severity would be excluded in our study and increased severity of OSAS in smokers with > 10 PY might be due to longer exposure to smoke.
Smoking and OSAS Severity
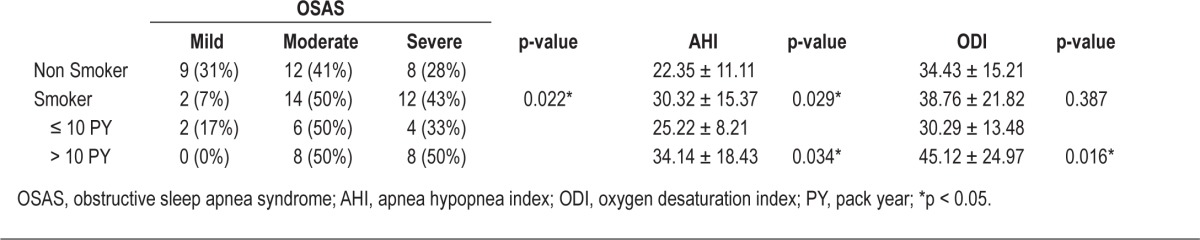
Next, we investigated the relationship between smoking history and OSAS severity. For nonsmokers, the number of subjects in each OSAS severity group was evenly distributed. In contrast, more smokers were in the moderate (50%) and severe OSAS groups (43%) than in the mild OSAS group (7%), and the difference was significant (Table 3). Thus, smoking negatively affected patients, resulting in increased OSAS severity.
Table 3.
Results of the statistical analysis of smoking history and severity of OSAS, AHI, and ODI
For patients with a > 10 PY smoking history, we observed an equal prevalence (50% each) of moderate and severe OSAS, and there were no patients with mild OSAS. The rate of moderate or severe OSAS in smokers with a > 10 PY smoking history was higher than in smokers with a ≤ 10 PY smoking history (Table 3). We also found that OSAS severity according to AHI was higher in smokers, especially those who had a longer period of exposure to smoking (Table 3).
Unlike the AHI, the ODI was not significantly different between smokers and non-smokers. However, it was significantly higher in smokers with a > 10 PY smoking history compared to nonsmokers and smokers with a ≤ 10 PY smoking history (Table 3).
Histological Changes in the Uvular Mucosa Due to Smoking in OSAS Patients
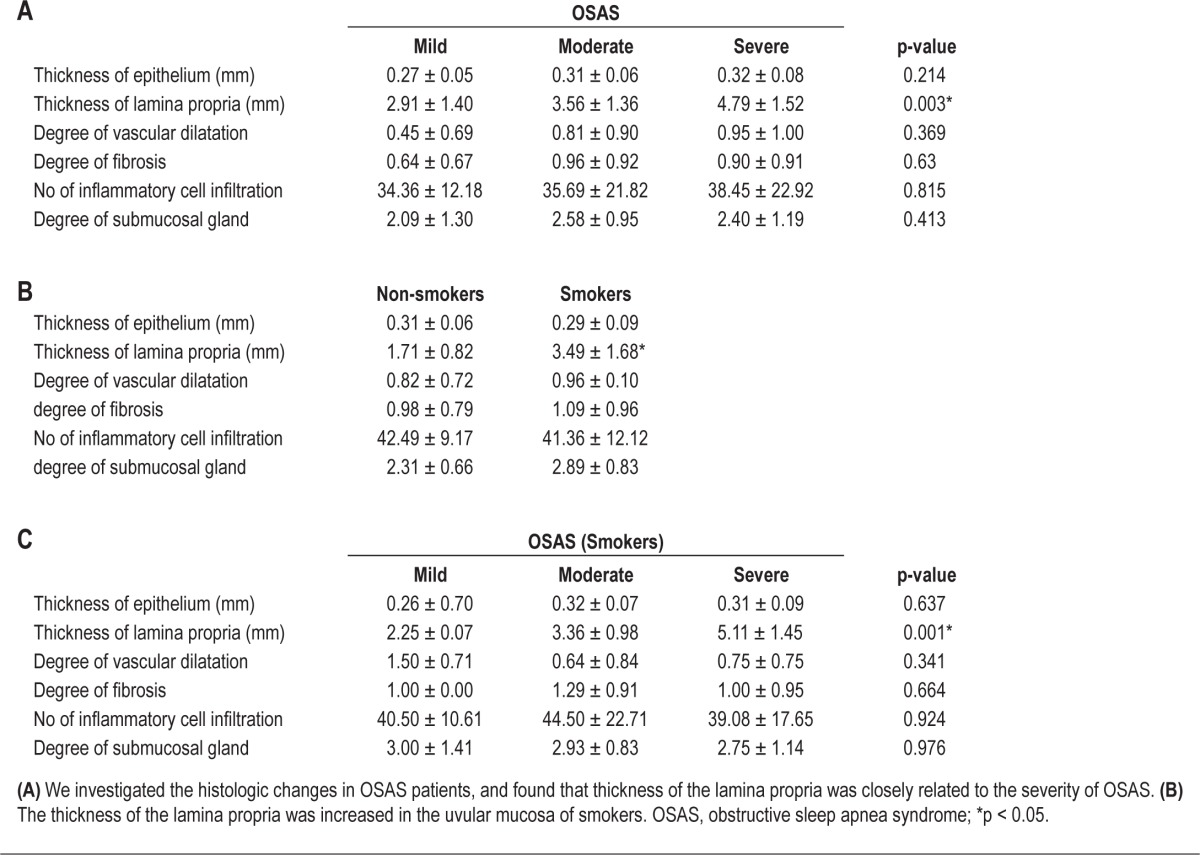
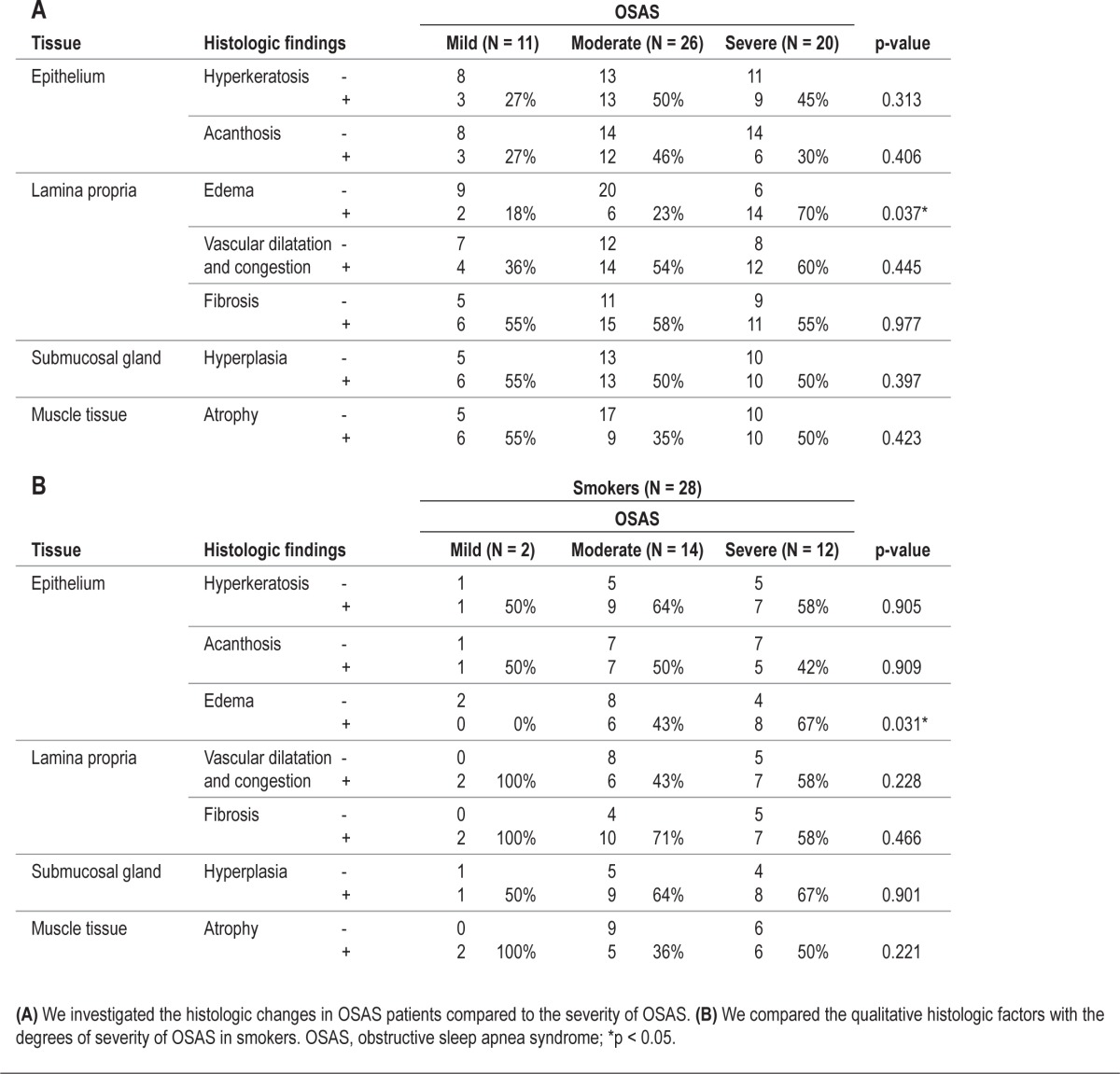
We examined the histological findings of the uvular mucosa and OSAS severity in nonsmokers and smokers to assess the effect of smoking on upper airway narrowing. First, we evaluated the histological findings of the uvular mucosa by morphometric analysis and compared our findings with OSAS severity, irrespective of smoking history. We found that the lamina propria was significantly thicker in the uvular mucosa with increased OSAS severity (Table 4A, Figure 1). No significant differences were observed in terms of thickness of the epithelium, degree of vascular dilation, degree of fibrosis, amount of inflammatory cell infiltration, or degree of proliferation of the submucosal glands (Table 4A). The lamina propria of the uvula was thicker in smokers than nonsmokers, and there was a significant correlation between the thickness of the lamina propria and OSAS severity in smokers (Table 4B, Figure 2).
Table 4.
Histologic changes in the uvula mucosa according to the severity of OSAS in nonsmokers and smokers (morphometric study)
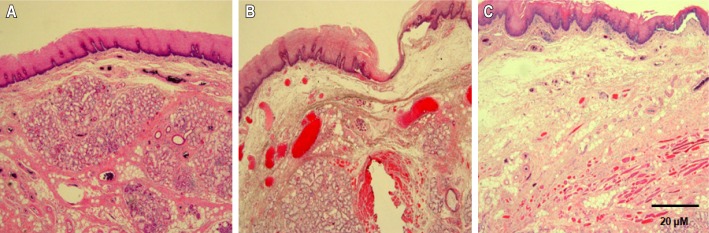
Figure 1. Histological changes in the lamina propria of the uvula mucosa in OSAS patients according to OSAS severity.
The thickness of the lamina propria increased in the uvula mucosa of moderate and severe OSAS patients. (A) mild OSAS, (B) moderate OSAS, (C) severe OSAS (H – E, × 40).
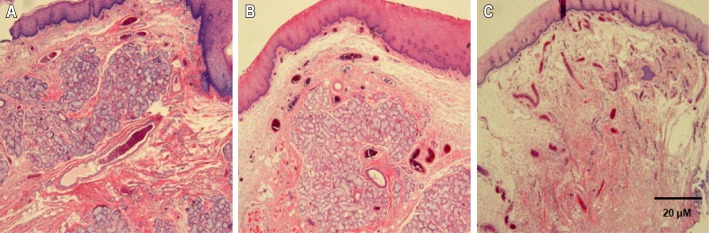
Figure 2. Histological changes in the lamina propria of the upper airway mucosa in OSAS patients according to smoking history.
Lamina propria thickness increased with longer smoking duration. The most severe thickening of the lamina propria was observed in the upper airway mucosa of smokers with a history of over 10 PY. (A) Nonsmoker, (B) < 10 pack years, (C) > 10 pack years (H – E, × 40).
Second, we carried out a qualitative investigation of the histological findings of the uvular mucosa. We classified findings according to OSAS severity and analyzed the correlation with smoking history. We found that edema of the lamina propria was increased in moderate and severe OSAS patients (Table 5A), while findings of hyperkeratosis or acanthosis of the epithelium, vascular congestion in the lamina propria, hyperplasia of the submucosal glands, and atrophy of the muscle tissue were not observed in the uvular mucosa of OSAS patients (Table 5A). Edema of the lamina propria was observed in the uvular mucosa of 14 smokers (n = 28, 50%) and was more definitive than the mucosal edema of nonsmokers (n = 29, 27%). In addition, the proportion of smokers with edematous lamina propria in the uvular mucosa was increased significantly according to OSAS severity, but the difference was minimal in nonsmokers (Table 5B).
Table 5.
Histologic changes in the upper airway according to OSAS severity in smokers and nonsmokers (qualitative study)
Changes in Neuroendocrine Parameters in the Smoking Group
Finally, we sought to determine why smoking results in a thicker and more edematous lamina propria in smokers. Stimulation through afferent peripheral nerves is reported to cause neuroendocrinological inflammatory changes, resulting in edema and vascular dilation in the tissue mucosa. CGRP, SP, and PGP 9.5 are proteins secreted from afferent peripheral nerves that are used as histological markers for the detection of afferent nerves. We examined the expression of these proteins in the uvular mucosa of smokers to determine the cause of increased edema and thickening of the lamina propria.
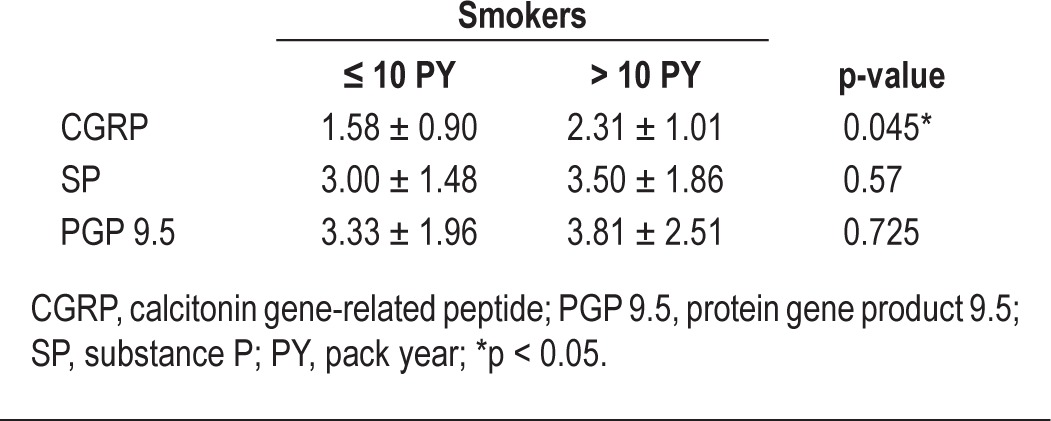
Immunohistochemistry revealed increased positive staining for CGRP in the uvular mucosa of smokers, and no significant differences were observed between smokers and nonsmokers in staining for SP or PGP 9.5 (Figure 3). We observed that expression of CGRP was significantly higher in the uvular mucosa of patients with a > 10 PY smoking history (Table 6). In nonsmokers, the mean value of CGRP expression was 0.52 ± 0.27 and significantly lower compared with smokers' CGRP expression (1.94 ± 0.95, p = 0.003).
Figure 3. Immunohistochemical staining for CGRP, PGP 9.5, and SP by smoking history.
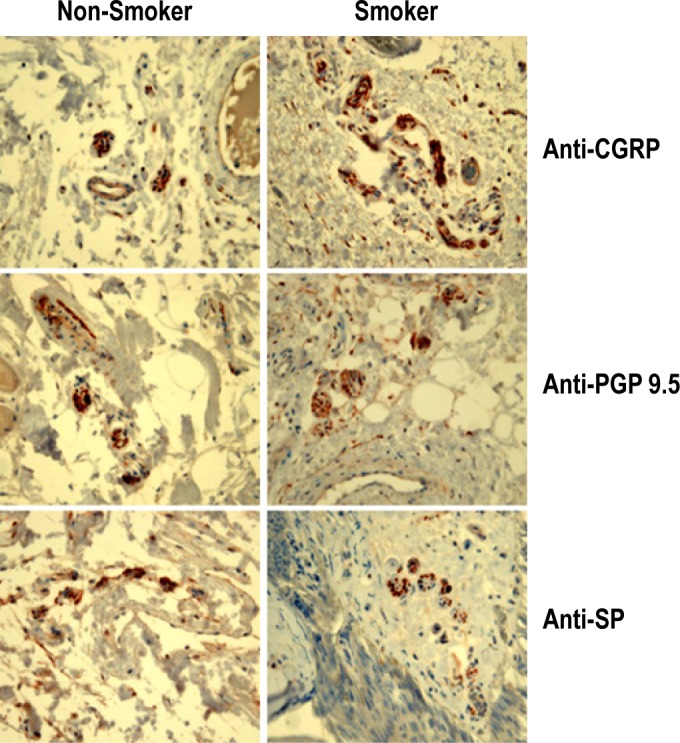
Table 6.
The results of immunohistochemistry for afferent peripheral nerves in the uvula mucosa according to smoking history
DISCUSSION
In this study, we found that moderate and severe OSAS were more prevalent in smokers, and that smoking might induce narrowing of the upper airway through an increase in edema and thickness in the uvular mucosa. The severity of OSAS and the narrowing of the upper airway were particularly increased in smokers with a > 10 PY history of smoking.
Although proving an independent effect of smoking on snoring is difficult, some studies have recognized that smoking might have an effect on sleep related disorders.8–10,14 Previous epidemiologic studies have suggested that smoking may be an independent risk factor for habitual snoring,7–9,10,14 and that snoring frequency increases with the number of cigarettes smoked or the duration of exposure to smoking.15–22 However, most studies compared patients' smoking history with OSAS prevalence or snoring-related diseases, and the relationship between smoking exposure and OSAS severity was not rigorously investigated or fully understood.
In this study, we attempted to verify the correlation between smoking and the severity of OSAS using medical records and patient specimens. We used consistent criteria to recruit patients in order to exclude the effects of other risk factors and to investigate the influence of smoking on OSAS. We excluded patients with systemic risk factors such as hypertension, diabetes, and cardiovascular or neurovascular diseases either currently or in the past. Age and BMI did not differ between smokers and nonsmokers; only male subjects were included to examine the influence of smoking more objectively.
We found that OSAS patients who were smokers had a higher AHI and ODI, and this increased with a longer smoking history, especially for smokers exposed to > 10 PY compared to nonsmokers. We determined that smoking increased the incidence of apnea and hypopnea and decreased oxygen desaturation during sleep in OSAS patients, and that longer exposure to smoking was a high-risk factor for severe OSAS.
Narrowing at the level of the oropharynx or palate is reportedly one of the most common predisposing factors for snoring and palatal surgeries, such as UPPP, uvuloplasty, and transpalatal advancement pharyngoplasty, which are popular modalities for correcting upper airway narrowing, resulting in decreased snoring at the level of the oropharynx.23,24 Anatomical variation has been less frequently reported at the uvula than at other oropharyngeal tissues25; redundant uvular mucosa directly induces upper airway narrowing independent of other systemic factors such as hypertension, obesity, and neurovascular diseases. We suggest that the uvular mucosa is the most suitable tissue of the oropharynx for comparing histological changes in OSAS subjects and for examining the local influence of smoking on upper airway narrowing and OSAS severity.
We found that increased thickness and edema of the lamina propria in OSAS subjects' uvular mucosa were predominant if OSAS subjects were smokers and that the histologic changes in the uvular mucosa were intensified according to OSAS severity. These histological changes were consistent with the findings of previous studies.25,26 In our limited study, we did not obtain uvular mucosa from nonsmoking healthy volunteers without snoring history and did not compare histologic changes in the uvular mucosa among healthy volunteers, nonsmoking OSAS patients, and smoking OSAS patients. However, the changes were more definitive in smokers with OSAS, and when the uvular mucosa was exposed to smoke for a long period of time, it was thicker, with a more edematous lamina propria. We examined only the local influence of smoking on airway narrowing or collapse. The severity of OSAS results from a cascade of multiple physiological processes interacting with features such as muscle tone, craniofacial structures, anatomical variations, mucosal edema of the airway, and total lung function. Although it was difficult to analyze the systemic effect of smoking using patients' clinical data, the patients included in this study were diagnosed with OSAS through radiographic methods, a pulmonary function test, flexible endoscopy, or respisomnography; and patients who had normal lung function and normal cephalometric structure, except redundant oropharyngeal mucosa, were recruited irrespective of smoking status in order to exclude the systemic effect of smoking. Therefore, we suggest that smoking may result in thickening and edematous changes of the uvular mucosa, thus leading to increased OSAS severity through upper airway narrowing.
We also investigated how smoking might induce histological changes of the uvular mucosa in OSAS patients. Mucosal edema is closely related to mucosal inflammation, and tobacco fumes may be involved in the induction of neuroendocrinologic inflammation.27,28 The afferent peripheral nerves in the airway mucosa respond to stimulation by fumes by secreting tachykinin, CGRP, and SP, which are required for neuroendocrinological inflammation.29–31 We found that CGRP-positive staining was substantially more prevalent in the uvular mucosa of smokers compared to PGP 9.5 and SP staining. In particular, CGRP staining was increased in the uvular mucosa of patients exposed to smoking for > 10 PY. CGRP-positive afferent peripheral nerves stimulate local vascular dilation, smooth muscle strength, and gland secretion; they also induce mucosal inflammation and edema.27,32–34 We determined that CGRP expression might be more specifically stimulated by smoke, and smoking may stimulate the secretion of CGRP from afferent nerves in the uvular mucosa, thereby increasing edema or thickness of the lamina propria in the uvulas of OSAS patients. In this study, we did not determine whether cessation of smoking can lead to the reversal or recovery of the histological changes seen in the uvular mucosa, and this remains unknown. Smoking is thought to lead to an increased risk of COPD, and patients with both COPD and OSAS suffer from more frequent episodes of oxygen desaturation and more total sleep time with hypoxemia and hypercapnia than OSAS patients without COPD.35 Therefore, we propose that smoking is an independent risk factor for OSAS subjects and that cessation of smoking may be required to prevent upper airway narrowing and systemic lung diseases that worsen OSAS.
In conclusion, we strongly suggest that smoking changes the uvular mucosa of OSAS subjects to become more thickened or edematous through CGRP-induced neurogenic inflammation, resulting in upper airway narrowing. Our findings suggest that longer exposure to smoking results in a higher prevalence of moderate or severe OSAS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;31(181):856–8. doi: 10.1126/science.181.4102.856. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998;21:27–36. doi: 10.1093/sleep/21.1.27. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg E, Gislason T. Epidemiology of sleep-related obstructive breathing. Sleep Med Rev. 2000;4:411–33. doi: 10.1053/smrv.2000.0118. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 5.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:1671–83. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 6.Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, Kramer MR. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med. 2007;101:1696–701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 8.Franklin KA, Gáislason T, Omenaas E, et al. The influence of active and passive smoking on habitual snoring. Am J Respir Crit Care Med. 2004;170:799–803. doi: 10.1164/rccm.200404-474OC. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg E, Taube A, Janson C, Gislason T, Svüardsudd K, Boman G. A 10-year follow-up of snoring in men. Chest. 1998;114:1048–55. doi: 10.1378/chest.114.4.1048. [DOI] [PubMed] [Google Scholar]
- 10.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–24. [PubMed] [Google Scholar]
- 11.Guilleminault C. Obstructive sleep apnea syndrome. A review. Psychiatr Clin North Am. 1987;10:607–21. [PubMed] [Google Scholar]
- 12.Hudgel DW. Variable site of airway narrowing among obstructive sleep apnea patients. J Appl Physiol. 1986;61:1403–9. doi: 10.1152/jappl.1986.61.4.1403. [DOI] [PubMed] [Google Scholar]
- 13.Remmers JE, Launois S, Feroah T, Whitelaw WA. Mechanics of the pharynx in patients with obstructive sleep apnea. Prog Clin Biol Res. 1990;345:261–8. [PubMed] [Google Scholar]
- 14.Bloom JW, Kaltenborn WT, Quan SF. Risk factors in a general population for snoring. Importance of cigarette smoking and obesity. Chest. 1988;93:678–83. doi: 10.1378/chest.93.4.678. [DOI] [PubMed] [Google Scholar]
- 15.Aksu K, Firat Güuven S, Aksu F, et al. Obstructive sleep apnoea, cigarette smoking and plasma orexin-A in a sleep clinic cohort. J Int Med Res. 2009;37:331–40. doi: 10.1177/147323000903700207. [DOI] [PubMed] [Google Scholar]
- 16.Sahlin C, Franklin KA, Stenlund H, Lindberg E. Sleep in women: Normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Med. 2009;10:1025–30. doi: 10.1016/j.sleep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Berg S. Obstructive sleep apnoea syndrome: current status. Clin Respir J. 2008;2:197–201. doi: 10.1111/j.1752-699X.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 18.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;15:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekici M, Ekici A, Keles H, et al. Risk factors and correlates of snoring and observed apnea. Sleep Med. 2008;9:290–6. doi: 10.1016/j.sleep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Lavie L, Lavie P. Smoking interacts with sleep apnea to increase cardiovascular risk. Sleep Med. 2008;9:247–53. doi: 10.1016/j.sleep.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Chervin RD, Guilleminault C. Obstructive sleep apnea and related disorders. Neurol Clin. 1996;14:583–609. doi: 10.1016/s0733-8619(05)70275-9. [DOI] [PubMed] [Google Scholar]
- 22.Theorell-Haglow J, Berne C, Janson C, Lindberg E. Obstructive sleep apnoea is associated with decreased insulin sensitivity in females. Eur Respir J. 2008;31:1054–60. doi: 10.1183/09031936.00074907. [DOI] [PubMed] [Google Scholar]
- 23.Metes A, Hoffstein V, Mateika S, Cole P, Haight JS. Site of airway obstruction in patients with obstructive sleep apnea before and after uvulopalatopharyngoplasty. Laryngoscope. 1991;101:1102–8. doi: 10.1288/00005537-199110000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Farmer WC, Giudici SC. Site of airway collapse in obstructive sleep apnea after uvulopalatopharyngoplasty. Ann Otol Rhinol Laryngol. 2000;109:581–4. doi: 10.1177/000348940010900609. [DOI] [PubMed] [Google Scholar]
- 25.Woodson BT, Garancis JC, Toohill RJ. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope. 1991;101:1318–22. doi: 10.1002/lary.5541011211. [DOI] [PubMed] [Google Scholar]
- 26.Sekosan M, Zakkar M, Wenig BL, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope. 1996;106:1018–20. doi: 10.1097/00005537-199608000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Lundberg JM, Martling CR, Lundblad L. Cigarette smoke-induced irritation in the airways in relation to peptide-containing, capsaicin-sensitive sensory neurons. Klin Wochenschr. 1988;11:151–60. [PubMed] [Google Scholar]
- 28.Lei YH, Barnes PJ, Rogers DF. Mechanisms and modulation of airway plasma exudation after direct inhalation of cigarette smoke. Am J Respir Crit Care Med. 1995;151:1752–62. doi: 10.1164/ajrccm.151.6.7767517. [DOI] [PubMed] [Google Scholar]
- 29.Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsson H, Carlsson-Nordlander B, Lindblad LE, Norbeck O, Svanborg E. Temperature thresholds in the oropharynx of patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1992;146:1246–9. doi: 10.1164/ajrccm/146.5_Pt_1.1246. [DOI] [PubMed] [Google Scholar]
- 31.Friberg D, Gazelius B, Lindblad LE, Nordlander B. Habitual snorers and sleep apnoeics have abnormal vascular reactions of the soft palatal mucosa on afferent nerve stimulation. Laryngoscope. 1998;108:431–6. doi: 10.1097/00005537-199803000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Uddman R, Luts A, Sundler F. Occurrence and distribution of calcitonin gene-related peptide in the mammalian respiratory tract and middle ear. Cell Tissue Res. 1985;241:551–5. doi: 10.1007/BF00214575. [DOI] [PubMed] [Google Scholar]
- 33.Di Maria GU, Bellofiore S, Geppetti P. Regulation of airway neurogenic inflammation by neutral endopeptidase. Eur Respir J. 1998;12:1454–62. doi: 10.1183/09031936.98.12061454. [DOI] [PubMed] [Google Scholar]
- 34.Zakkar M, Sekosan M, Wenig B, Olopade CO, Rubinstein I. Decrease in immunoreactive neutral endopeptidase in uvula epithelium of patients with obstructive sleep apnea. Ann Otol Rhinol Laryngol. 1997;106:474–7. doi: 10.1177/000348949710600606. [DOI] [PubMed] [Google Scholar]
- 35.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182:325–31. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]