Abstract
Study Objective:
The Berlin Questionnaire and Epworth Sleepiness Scale (ESS) are commonly used to screen for sleep apnea in non-pregnant populations. We sought to evaluate the Berlin and ESS in pregnancy and to determine whether an alternative screening approach could better detect sleep apnea in pregnant women.
Methods:
Pregnant women at high risk for sleep apnea (women with chronic hypertension, pre-gestational diabetes, obesity, and/or a prior history of preeclampsia) completed a sleep survey composed of the Berlin and ESS, and participated in an overnight sleep evaluation with the Watch-PAT100 (WP100), a wrist-mounted device designed to diagnose sleep apnea, defined as an apnea hypopnea index ≥ 5. Using multivariable statistics, demographic, clinical, and subjective symptoms that were independently associated with sleep apnea were determined and a prediction rule for the presence of sleep apnea was developed. The predictive capacity of this newly developed system was compared to that of the Berlin and ESS using receiver-operating curve (ROC) statistics.
Results:
Of the 114 women who participated and had a valid WP100 study, 100 completed the Berlin and 96 the ESS. The Berlin and ESS did not accurately predict sleep apnea in this high-risk pregnancy cohort, with ROC area under the curves (AUC) of 0.54 (p = 0.6) and 0.57 (p = 0.3), respectively. Conversely, a model incorporating frequent snoring, chronic hypertension, age, and body mass index performed significantly better (AUC 0.86, p > 0.001).
Conclusion:
The Berlin and ESS are not appropriate tools to screen for sleep apnea in high-risk pregnant women. Conversely, our four-variable model more accurately predicts sleep apnea in pregnancy.
Citation:
Facco FL; Ouyang DW; Zee PC; Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med 2012;8(4):389-394.
Keywords: Pregnancy, sleep apnea screening
Sleep disordered breathing (SDB) describes a group of disorders characterized by abnormalities of respiration (e.g., pauses in breathing) or the quality of ventilation during sleep. Obstructive sleep apnea (OSA), the most common such disorder, is characterized by the repetitive collapse or partial collapse of the upper airway during sleep and the need to arouse to resume normal ventilation. The cardinal clinical manifestations of sleep apnea are loud and persistent snoring, bed partner-observed pauses in breathing, and excessive daytime sleepiness. OSA has clearly been linked to poor sleep and impaired daytime function, but there are also data linking OSA to other health outcomes, principally cardiovascular and metabolic disease.1–7
Pregnancy has been associated with several alterations in sleep and a high incidence of sleep disturbances.8 With regard to OSA, pregnancy-associated weight gain and fluid retention can lead to airway edema and increased airway resistance, which can result in snoring and OSA. This is particularly true for women who are overweight or obese (pre-pregnancy body mass index [BMI] ≥ 25), experience excessive weight gain during pregnancy, or suffer from excessive fluid retention during pregnancy (e.g., preeclampsia).8–10 The determination of whether a woman has sleep apnea is important, as this condition during pregnancy may be associated with an increased risk of gestational hypertension, gestational diabetes, preterm birth, and intrauterine growth restriction.11–14
Brief Summary
Current Knowledge/Study Rationale: Pregnancy has been associated with several alterations in sleep and a high incidence of sleep disturbances. In this prospective study we evaluated the performance of the Berlin Questionnaire and Epworth Sleepiness Scale (ESS) as screening tools for sleep apnea in pregnancy. We also sought to determine if an alternative screening algorithm could better detect sleep apnea in pregnant women.
Study Impact: Our findings indicate that the Berlin and ESS are not reliable predictors of sleep apnea in high-risk pregnant women. Conversely, a simpler four-variable screening tool that includes self-reported frequent snoring, chronic hypertension, BMI, and age more accurately predicts sleep apnea in pregnancy. Further research to understand the impact of sleep apnea on maternal and fetal health, is needed before evidence based guidelines regarding screening for sleep apnea in pregnancy can be established.
Most studies examining sleep apnea in pregnancy have relied on a diagnosis made from subjective questionnaires (e.g., the Berlin Questionnaire and Epworth Sleepiness Scale [ESS]) that were designed for and validated in non-pregnant, predominately male, middle-aged, and elderly populations.15 Indeed, there are some data suggesting that these tools are not as accurate in pregnancy.16 The objective of this study was to evaluate the performance of the Berlin and ESS in pregnancy, and to determine if an alternative screening algorithm could better detect sleep apnea in pregnant women.
METHODS
Women with singleton pregnancies at high risk for sleep apnea who were between 6 and 20 weeks of gestation were recruited at 2 university-affiliated hospitals. Women who were considered at high risk for sleep apnea were those with chronic hypertension (diagnosed prior to pregnancy), pre-gestational diabetes (type 1 or type 2), obesity (pre-pregnancy BMI ≥ 30), and/or a prior history of preeclampsia.17–19 All women were asked to complete a sleep survey, comprised of the Berlin and ESS, and to participate in an overnight at-home sleep evaluation with the Watch-PAT100 (WP100), a wrist-mounted, ambulatory device designed to diagnose sleep apnea.
The Berlin Questionnaire uses 10 self-administered questions about known risk factors for sleep apnea. The questions are grouped into 3 categories: Category 1 questions assess snoring behavior; Category 2 questions assess wake time sleepiness; and Category 3 questions assess for the presence of obesity (BMI ≥ 30) or chronic hypertension.20 Data from non-pregnant populations has demonstrated that a high-risk Berlin score has a sensitivity ranging from 68% to 86% and a specificity ranging from 46% to 95% for sleep apnea.15
The ESS is used to assess daytime sleepiness symptoms, which are common among those with OSA. It consists of 8 questions regarding the tendency to fall asleep in certain situations (e.g., sitting and reading, sitting as a passenger in a car). ESS scores range from 0 to 24. Excessive daytime sleepiness is typically defined as a total score ≥ 10 or ≥ 12.21 Studies of sleep apnea in non-pregnant populations have shown that ESS scores are positively correlated with objective measures of sleep apnea.22
Sleep apnea is diagnosed by measuring the total number of apneas (cessations of airflow) and hypopneas (reductions in airflow) per hour of sleep. An apnea-hypopnea index (AHI) ≥ 5 is diagnostic for sleep apnea. We used the Watch-PAT100 (WP100, Itamar Medical Ltd., Israel) to objectively assess for sleep apnea in this pregnant cohort (Figure 1). Several studies have shown that there is a significant correlation between the WP100 AHI and in-laboratory polysomnography evaluation, the gold standard for diagnosing sleep apnea.23–25 The WP100 measures peripheral arterial tone (PAT), oxygen saturation, pulse rate, and sleep duration (via actigraphy) continuously throughout the night. Apneas and hypopneas typically result in an increase in sympathetic tone, an increase in heart rate, and an oxygen desaturation; therefore, analysis of the WP100 signals allows for the determination of the AHI. WP100 studies were uploaded and an automated analysis was performed using zzzPAT software scoring algorithms (version 4.0).
Figure 1.
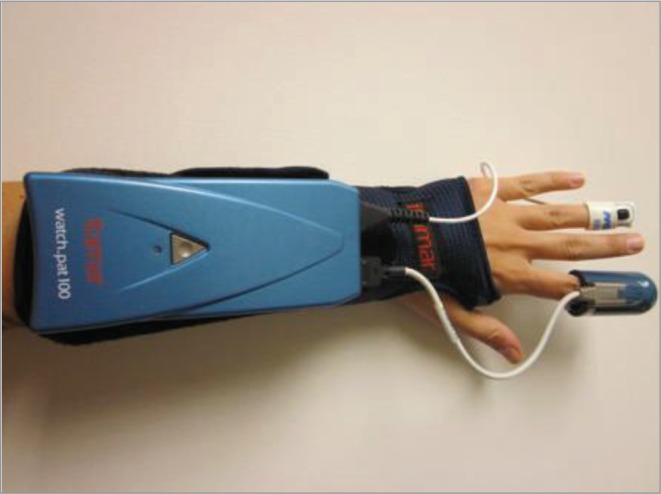
Watch-PAT100
After informed consent was obtained, subjects were shown a brief video instructing them on the proper use of the WP100. They were then asked to wear the device for one night of home sleep and to complete the 2 sleep surveys (Berlin and ESS) either the night of, or the day following the sleep study. Pertinent demographic and clinical data were abstracted from the prenatal record.
Demographic and clinical characteristics of those with and without sleep apnea as determined by the WP100 were compared using the t-test and χ2 test for continuous and categorical variables, respectively. The classification ability of the Berlin and the ESS with regard to the diagnosis of OSA were assessed with the area under the curve (AUC) derived from the receiver-operating characteristic curve (ROC). The questions of the Berlin were also individually analyzed to determine which, if any, accurately identified women with OSA. To develop a pregnancy specific prediction rule for sleep apnea, demographic, clinical, and subjective symptoms that were associated with sleep apnea with p values < 0.10 in univariable analysis were included as covariates in a multivariable logistic regression model, and those with p-values < 0.05 were retained in the final multivariable logistic regression model. A prediction model for the presence of OSA, using an integer-based score, was developed from the logistic regression model using a regression coefficient-based scoring method. Points were assigned to each predictor by multiplying the regression coefficient by 10 and rounding to the nearest integer. The area under the ROC of this scoring system was compared to that of the Berlin and ESS.
A test for screening or prediction should have an AUC ≥ 0.7.26,27 Therefore, using a β of 0.1, an α of 0.05, and assuming an estimated 30% prevalence of OSA in our high-risk population,17–19 100 women were required for a statistically significant AUC of at least 0.7 to be detectable.
All analyses were performed using PASW 18.0 statistical software (SPSS Inc, Chicago, IL). The study was approved by the institutional review boards of Northwestern University and NorthShore University HealthSystem.
RESULTS
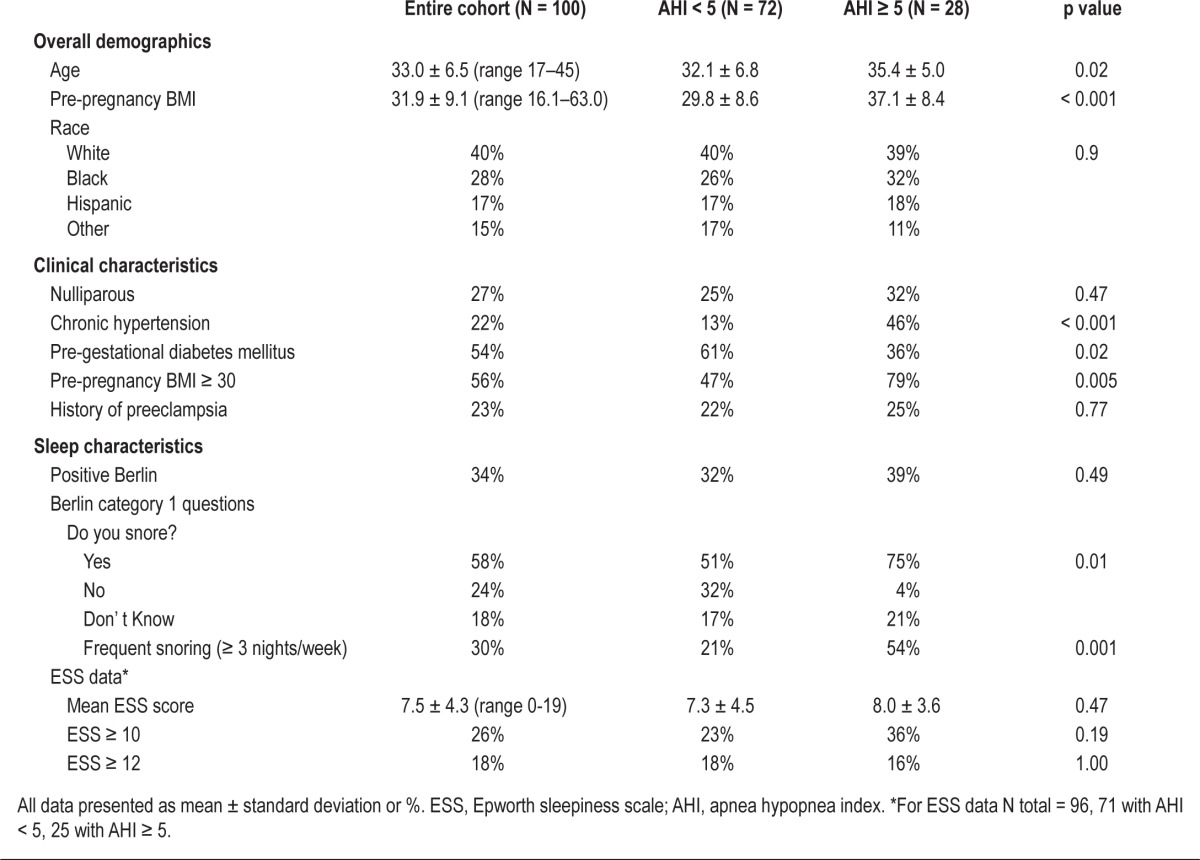
Of the 122 women who were recruited, 114 had a valid WP100 study; of these, 100 completed the Berlin and 96 completed the ESS. Mean (± standard deviation) gestational age at the time of the sleep study was 16.5 ± 3.7 weeks, and 28% of women had an AHI ≥ 5. The median AHI was 1.5 (interquartile range 0.5-6.0). Additional demographic and clinical characteristics of the 100 participants with a valid WP100 study and at least one screening survey, stratified by the diagnosis of OSA, are presented in Table 1. Women with OSA were older, had higher pre-pregnancy BMIs, were more likely to have chronic hypertension, and were less likely to have pre-gestational diabetes. Women with and without sleep apnea were equally as likely to have a positive Berlin score (39% vs. 32%, p = 0.49) and had similar ESS scores (Table 1). Both screening tests performed poorly with an AUC < 0.7: Berlin 0.54 (95% CI 0.41, 0.67, p = 0.6) and ESS 0.57 (95% CI 0.45, 0.70, p = 0.3). The Berlin had a sensitivity of only 39% (95% CI 22%, 59%) and a specificity of 68% (95% CI 56%, 78%). Similarly, an ESS score of ≥ 10 had 36% sensitivity (95% CI 19%, 57%) and 77% specificity (95% CI 66%, 86%). When we examined the individual Category 1 (snoring) and Category 2 (sleepiness) questions of the Berlin we found that only the snoring questions differentiated women with and without sleep apnea. Moreover, it was the frequency of snoring that was most differentiating. Women reporting frequent snoring (≥ 3 times per week) were > 4 times more likely to have an AHI ≥ 5 than those with less frequent or absent snoring (54% vs. 21%, OR = 4.4, 95% CI 1.4, 11.2).
Table 1.
Subject characteristics
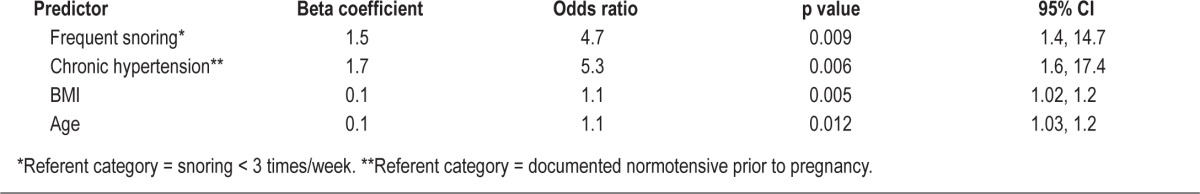
Using the results of the univariable analysis (Table 1), a multivariable logistic regression analysis was performed that revealed that pre-pregnancy BMI, age, chronic hypertension, and frequent snoring were independent significant factors in the identification of OSA in this cohort of high-risk pregnant women. As described in the methods, a four variable-based prediction rule for the presence of sleep apnea was developed from the logistic regression model using a regression coefficient-based scoring method (Table 2). The β coefficients for frequent snoring and chronic hypertension were very similar (1.5 vs. 1.7); thus we simplified the scoring system by the changing the score for chronic hypertension from 17 to 15, since this did not significantly alter the predictive capabilities of the model. Therefore, in this model, women receive 15 points if they report frequent snoring and another 15 points if they have chronic hypertension, and this sum is then added to the summation of their age and BMI. This model [(15 if frequent snoring) + (15 if chronic hypertension) + age + BMI] had an AUC of 0.850 (95% CI 0.77, 0.93, p value in comparison against null hypothesis (AUC = 0.5) = < 0.001). It performed significantly better than the Berlin (p < 0.001) and ESS (p < 0.001) screening evaluations for OSA detection (Figure 2). The best discriminatory point, identified by the part of the ROC graph that was closest to the upper left corner, was a score of 75 (Table 3). OSA was identified in women who had a score greater than or equal to this value with a sensitivity of 86% (95% CI 66%, 95%) and a specificity of 74% (95% CI 62%, 83%).
Table 2.
Results of the multivariable prediction model for sleep apnea
Figure 2.
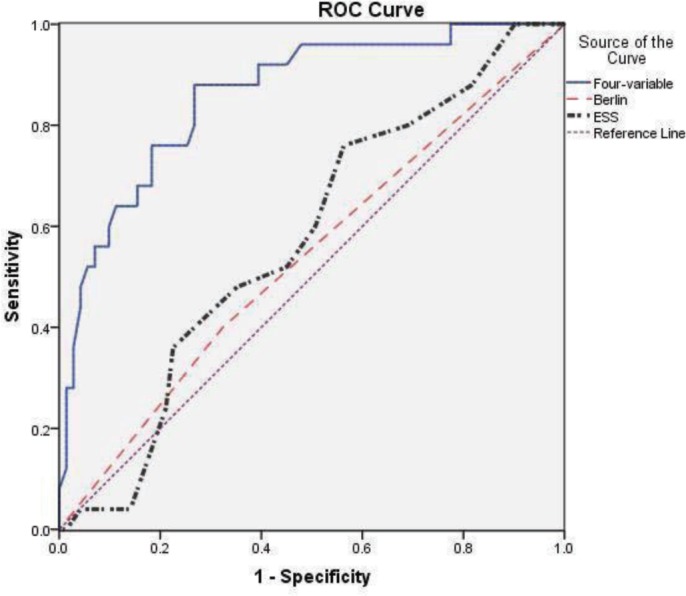
Receiver-operating characteristic curves for the Berlin, Epworth Sleepiness Scale, and for our four-variable model
Table 3.
Sensitivity, specificity, and positive and negative predictive values (PPV, NPV) for various cutoff values of our fourvariable prediction rule
Race did not meet the required p value in the univariate analysis and therefore was not included in our model; however, as a trial, it was added to the multivariate analysis. Race remained nonsignificant and did not alter the p values and odds ratios for the other 4 significant variables (data not shown).
DISCUSSION
In this prospective study we evaluated the performance of the Berlin and ESS as screening tools for sleep apnea in pregnancy. Our findings indicate that these screening tools are not reliable predictors of sleep apnea in high-risk pregnant women. Conversely, a simpler four-variable screening tool that includes self-reported frequent snoring, chronic hypertension, BMI, and age predicts sleep apnea with high sensitivity and specificity.
There are several possible reasons why the Berlin and ESS do not perform well in pregnancy. First, they both incorporate daytime sleepiness to differentiate individuals with and without OSA. While daytime sleepiness is a common symptom of sleep apnea in non-pregnant populations, it is a very common complaint during pregnancy even in women without OSA.12,19 Therefore, daytime sleepiness questions are not likely to be specific for sleep apnea in pregnancy. Second, the Berlin scoring algorithm uses BMI as a categorical variable (BMI ≥ 30), despite data that suggest a more linear relationship between BMI and OSA.28 For example, Young et al. found a four-fold increase in the prevalence of sleep apnea with each increase in the standard deviation (5.6 kg/m2) of the BMI.18 Our four-variable prediction model demonstrates that utilizing BMI as continuous variable significantly improves the ability to predict sleep apnea in pregnancy. Finally, age is not accounted for by either the Berlin or ESS. Yet epidemiological data demonstrate that sleep apnea prevalence increases steadily with age in midlife.17 Most studies that have evaluated the performance of the Berlin studied this instrument in older populations, which may lessen the importance of age as a relevant variable, given that the relationship between OSA and age attenuates for those older than 65.15,20
There are data that support our findings regarding the poor predictive capacity of the Berlin in high-risk women. Olivarez et al. studied a group of women admitted to an antepartum service (the majority of whom had either chronic hypertension, pregnancy induced hypertension, or pre-gestational or gestational diabetes mellitus) and reported a sensitivity (35%) and specificity (64%) for the Berlin that were nearly identical to those found in our pregnant population.16 However, we recognize that the results of our study may not be generalizable to all pregnant women. The reliability of the Berlin and of our four-variable model in a general or low-risk obstetrical population remains unknown and warrants further investigation. Regardless, the positive predictive values for all OSA screening tests will be significantly lower in such a population because there will be a lower prevalence of OSA.
We used an AHI 5 to define the presence of sleep apnea in our cohort. While this is the standard definition of sleep apnea, there remains a lack of consensus regarding which AHI value (i.e., what severity of sleep apnea) is most clinically relevant. In non-pregnant populations, an AHI ≥ 5 is considered abnormal, given the known association with cardiovascular and metabolic morbidity, although higher AHI levels are associated with an even greater frequency of adverse outcomes.4–5,29,30 On the other hand, even AHI values < 5 have been reported by some to be associated with the development of hypertension.4 Given the lack of full consensus and the existing data, an AHI ≥ 5 was chosen for this study, given that it is a commonly accepted diagnostic threshold. If in the future, this threshold were to be changed, all screening tools would need to be evaluated anew. In addition, we focused our study on identifying SDB in early pregnancy and our findings may not be applicable to new-onset, third trimester SDB. Many studies have reported that SDB symptoms increase and pregnancy progress.19,31 However, as we have yet to define what is clinically significant SDB in pregnancy, it remains unclear if SDB that is only present in late pregnancy (i.e., new-onset SDB) is as clinically relevant as SDB that is present in early pregnancy and persists or worsens as pregnancy progresses. Future studies are needed to understand the impact of and how to best assess for new-onset SDB in late pregnancy.
It should be noted that other approaches for the objective measurement of OSA exist. The gold standard for documenting OSA is in-laboratory polysomnography (PSG), and the lack of full PSG assessment of AHI in our cohort is certainly a limitation of this study. Unfortunately, the expense and burden of this testing limits its utility. The cost and complexity of in-laboratory PSG have led to the development of simpler diagnostic techniques for sleep apnea, such as the WP100 device. The WP100 does not measure ventilation during sleep directly, but instead generates an AHI by analyzing heart rate accelerations, increases in peripheral arterial tone, and decreases in oxygenation, all of which are associated with apneic and hypopneic events. The WP100 allows for home recordings, which, in addition to significantly lower costs, are less likely to be hampered by changes in environmental factors (i.e., bed comfort, noise, temperature) that are inevitably encountered when studying a patient in a sleep-laboratory setting. Studies in non-pregnant populations have shown that the respiratory indices, such as the AHI, derived from the WP100 are strongly correlated with those obtained from PSG (r = 0.90), and have also demonstrated that the WP100 is an accurate and reliable ambulatory method for the detection of sleep apnea.23–25,32 It is well established that certain biological parameters measured by the WP100 are altered in pregnancy. Specifically, resting heart rate is known to increase while systemic vascular resistance decreases. However, the algorithm for scoring WP100 events takes into consideration that every individual, pregnant or not, has a different baseline heart rate and peripheral arterial tone and therefore, events are scored when there is a change in baseline (increase in heart rate, increase in peripheral arterial tone).23 Pulse oximetry and actigraphy measures, also used by the Watch-PAT, should not be different when measured in pregnancy. Therefore we believe that there is no good biologic foundation to suggest that the objective measures of the WP100 will correlate less well with PSG measures just because a woman is pregnant. Moreover, O'Brien et al. recently presented data comparing Watch-PAT to full PSG in pregnant subjects. Their results indicate that among pregnant women, WP AHI correlated very well with PSG AHI(r = 0.76, p < 0.0001) and that the WP has excellent sensitivity (88%) and specificity (86%) for identification of SDB (AHI ≥ 5).33 Future studies, using alternative objective assessments of sleep apnea, AHI cutoff values, and patient populations, to reevaluate the Berlin and ESS and validate our four-variable screening tool should be performed and are planned.
In summary, our study suggests that the Berlin and ESS do not reliably screen for sleep apnea in high-risk pregnant women. Our four-variable prediction rule, which incorporates the presence of frequent snoring and chronic hypertension as well as age and BMI, more accurately predicts sleep apnea in pregnancy. Validation of our findings in a larger cohort of pregnant women, as well as further research to understand the impact of sleep apnea on maternal and fetal health, are needed before evidence based guidelines regarding screening for sleep apnea in pregnancy can be established.
DISCLOSURE STATEMENT
Presented as a poster at the 31st annual Society for Maternal-Fetal Medicine Annual Meeting, San Francisco, CA, February, 2011. This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Joseph Kang, Ph.D. for competent statistical assistance. Financial Support: NIH/NICHD 1K12HD050121, Preeclampsia Foundation Vision Grant, Northwestern Memorial Foundation Dixon Translational Research Initiative.
REFERENCES
- 1.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5:15–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AB, Nieto FJ, Guidry U, et al. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(Suppl 4):S122–6. [PubMed] [Google Scholar]
- 7.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–52. [PubMed] [Google Scholar]
- 8.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 9.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 10.Champagne KA, Kimoff RJ, Barriga PC, Schwartzman K. Sleep disordered breathing in women of childbearing age and during pregnancy. Indian J Med Res. 2010;131:285–301. [PubMed] [Google Scholar]
- 11.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:e261–5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 12.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 13.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203:142. doi: 10.1016/j.ajog.2010.03.041. e141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 16.Olivarez SA, Maheshwari B, McCarthy M, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202:552. doi: 10.1016/j.ajog.2009.12.008. e1-7. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 19.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2009;115:77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 20.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves MA, Paiva T, Ramos E, Guilleminault C. Obstructive sleep apnea syndrome, sleepiness, and quality of life. Chest. 2004;125:2091–6. doi: 10.1378/chest.125.6.2091. [DOI] [PubMed] [Google Scholar]
- 23.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003;4:435–42. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27:923–33. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–74. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 26.Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–51. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 27.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 28.Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30:455–65. doi: 10.1016/j.ccm.2009.05.012. vii. [DOI] [PubMed] [Google Scholar]
- 29.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 182:69–77. doi: 10.1164/rccm.200911-1746OC. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 32.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien LM, Bullough AS, Hewlett MM, et al. Relationship Between home polysomnography and watch-pat measures of sleep-disordered breathing in pregnancy (abstract 955) SLEEP, Associated Professional Sleep Societies Annual Meeting. 2011 Minneapolis. [Google Scholar]