Abstract
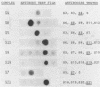
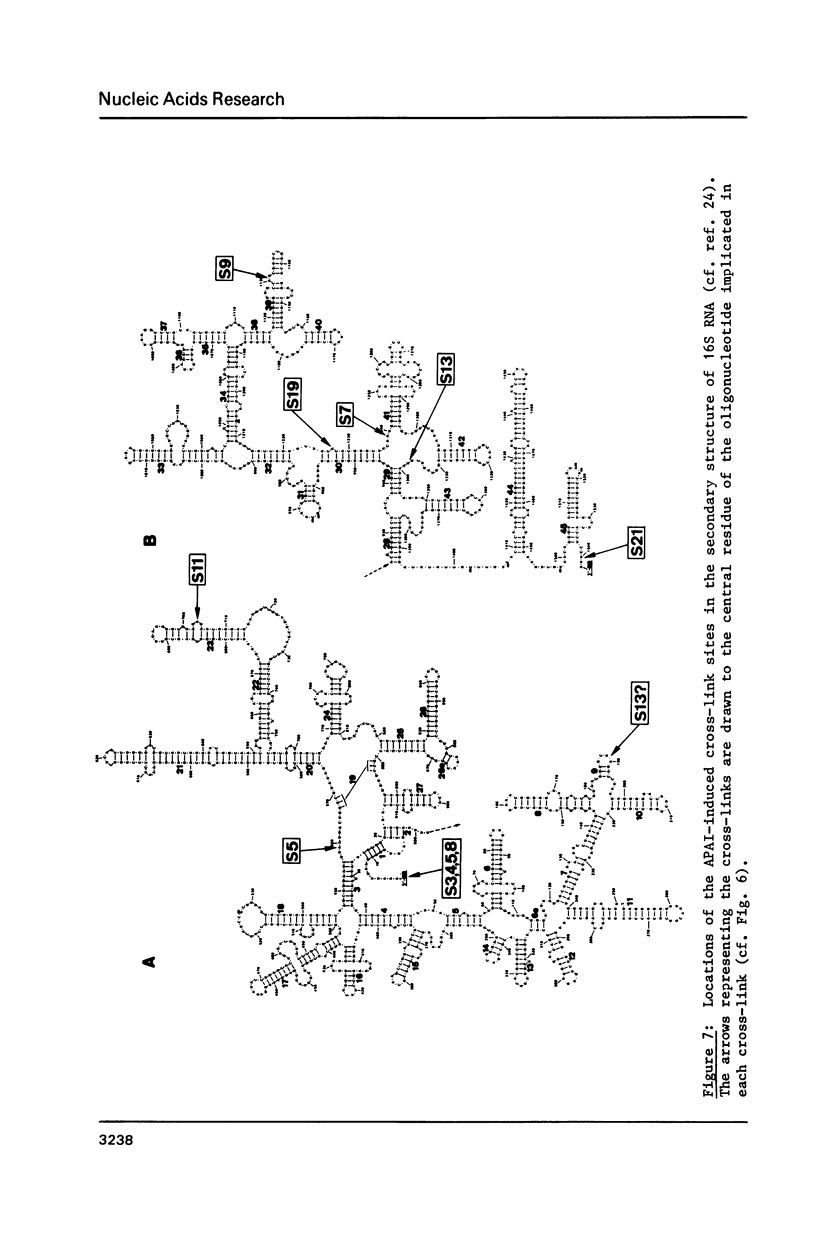
RNA-protein cross-links were introduced into E. coli 30S ribosomal subunits by treatment with methyl p-azidophenyl acetimidate. After partial nuclease digestion of the RNA moiety, a number of cross-linked RNA-protein complexes were isolated by a new three-step procedure. Protein and RNA analysis of the individual complexes gave the following results: Proteins S3, S4, S5 and S8 are cross-linked to the 5'-terminal tetranucleotide of 16S RNA. S5 is also cross-linked to the 16S RNA within an oligonucleotide encompassing positions 559-561. Proteins S11, S9, S19 and S7 are cross-linked to 16S RNA within oligonucleotides encompassing positions 702-705, 1130-1131, 1223-1231 and 1238-1240, respectively. Protein S13 is cross-linked to an oligonucleotide encompassing positions 1337-1338, and is also involved in an anomalous cross-link within positions 189-191. Protein S21 is cross-linked to the 3'-terminal dodecanucleotide of the 16S RNA.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmadja J., Stiege W., Zobawa M., Greuer B., Osswald M., Brimacombe R. The tertiary folding of Escherichia coli 16S RNA, as studied by in situ intra-RNA cross-linking of 30S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1986 Jan 24;14(2):659–673. doi: 10.1093/nar/14.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The complete nucleotide sequence of the ribosomal 16-S RNA from Excherichia coli. Experimental details and cistron heterogeneities. Eur J Biochem. 1979 Oct 15;100(2):399–410. doi: 10.1111/j.1432-1033.1979.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Fink G., Fasold H., Rommel W., Brimacombe R. Reagents suitable for the crosslinking of nucleic acids to proteins. Anal Biochem. 1980 Nov 1;108(2):394–401. doi: 10.1016/0003-2697(80)90604-1. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A., Isono K. An improved method for two-dimensional gel-electrophoresis: analysis of mutationally altered ribosomal proteins of Escherichia coli. Mol Gen Genet. 1981;181(3):309–312. doi: 10.1007/BF00425603. [DOI] [PubMed] [Google Scholar]
- Kyriatsoulis A., Maly P., Greuer B., Brimacombe R., Stöffler G., Frank R., Blöcker H. RNA-protein cross-linking in Escherichia coli ribosomal subunits: localization of sites on 16S RNA which are cross-linked to proteins S17 and S21 by treatment with 2-iminothiolane. Nucleic Acids Res. 1986 Feb 11;14(3):1171–1186. doi: 10.1093/nar/14.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P., Brimacombe R. Refined secondary structure models for the 16S and 23S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1983 Nov 11;11(21):7263–7286. doi: 10.1093/nar/11.21.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P., Rinke J., Ulmer E., Zwieb C., Brimacombe R. Precise localization of the site of cross-linking between protein L4 and 23S ribonucleic acid induced by mild ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Biochemistry. 1980 Sep 2;19(18):4179–4188. doi: 10.1021/bi00559a007. [DOI] [PubMed] [Google Scholar]
- Moazed D., Van Stolk B. J., Douthwaite S., Noller H. F. Interconversion of active and inactive 30 S ribosomal subunits is accompanied by a conformational change in the decoding region of 16 S rRNA. J Mol Biol. 1986 Oct 5;191(3):483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rinke J., Meinke M., Brimacombe R., Fink G., Rommel W., Fasold H. The use of azidoarylimidoesters in RNA-protein cross-linking studies with Escherichia coli ribosomes. J Mol Biol. 1980 Mar 5;137(3):301–304. doi: 10.1016/0022-2836(80)90318-6. [DOI] [PubMed] [Google Scholar]
- Stiege W., Zwieb C., Brimacombe R. Precise localisation of three intra-RNA cross-links in 23S RNA and one in 5S RNA, induced by treatment of Escherichia coli 50S ribosomal subunits with bis-(2-chloroethyl)-methylamine. Nucleic Acids Res. 1982 Nov 25;10(22):7211–7229. doi: 10.1093/nar/10.22.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. A., Jr, Saigo K., McLeod E., Ito J. The separation of DNA segments attached to proteins. Anal Biochem. 1979 Feb;93(1):158–166. [PubMed] [Google Scholar]
- Ulmer E., Meinke M., Ross A., Fink G., Brimacombe R. Chemical cross-linking of protein to RNA within intact ribosomal subunits from Escherichia coli. Mol Gen Genet. 1978 Apr 6;160(2):183–193. doi: 10.1007/BF00267480. [DOI] [PubMed] [Google Scholar]
- Vasilenko S. K., Ryte V. C. [Isolation of highly purified ribonuclease from cobra (Naja oxiana) venom]. Biokhimiia. 1975 May-Jun;40(3):578–583. [PubMed] [Google Scholar]
- Vassilenko S. K., Carbon P., Ebel J. P., Ehresmann C. Topography of 16 S RNA in 30 S subunits and 70 S ribosomes accessibility to cobra venom ribonuclease. J Mol Biol. 1981 Nov 15;152(4):699–721. doi: 10.1016/0022-2836(81)90123-6. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Wower I., Brimacombe R. The localization of multiple sites on 16S RNA which are cross-linked to proteins S7 and S8 in Escherichia coli 30S ribosomal subunits by treatment with 2-iminothiolane. Nucleic Acids Res. 1983 Mar 11;11(5):1419–1437. doi: 10.1093/nar/11.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower I., Wower J., Meinke M., Brimacombe R. The use of 2-iminothiolane as an RNA-protein cross-linking agent in Escherichia coli ribosomes, and the localisation on 23S RNA of sites cross-linked to proteins L4, L6, L21, L23, L27 and L29. Nucleic Acids Res. 1981 Sep 11;9(17):4285–4302. doi: 10.1093/nar/9.17.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir A., Miskin R., Elson D. Inactivation and reactivation of ribosomal subunits: amino acyl-transfer RNA binding activity of the 30 s subunit of Escherichia coli. J Mol Biol. 1971 Sep 14;60(2):347–364. doi: 10.1016/0022-2836(71)90299-3. [DOI] [PubMed] [Google Scholar]