Abstract
Study Objectives:
The present study aimed at further investigating trait aspects of sleep-related cognitive arousal and general cognitive arousal and their association with both objective and subjective sleep parameters in primary insomnia patients.
Methods:
A clinical sample of 182 primary insomnia patients and 54 healthy controls was investigated using 2 nights of polysomnography, subjective sleep variables, and a questionnaire on sleep-related and general cognitive arousal.
Results:
Compared to healthy controls, primary insomnia patients showed both more sleep-related and general cognitive arousal. Furthermore, sleep-related cognitive arousal was closely associated with measures of sleep-onset and sleep-maintenance problems, while general cognitive arousal was not.
Conclusions:
Cognitive-behavioral treatment for insomnia might benefit from dedicating more effort to psychological interventions that are able to reduce sleep-related cognitive arousal.
Citation:
Spiegelhalder K; Regen W; Feige B; Hirscher V; Unbehaun T; Nissen C; Riemann D; Baglioni C. Sleep-related arousal versus general cognitive arousal in primary insomnia. J Clin Sleep Med 2012;8(4):431-437.
Keywords: Cognitive arousal, subjective sleep parameters, polysomnography, primary insomnia
Insomnia is one of the most prevalent health complaints worldwide. It is defined by difficulties initiating or maintaining sleep or non-restorative sleep, accompanied by significant daytime impairments.1 Chronic insomnia affects up to 22% of the population2 and commonly occurs as a comorbid condition in other medical or mental disorders. Primary insomnia, an exclusionary diagnosis of poor sleep, ruling out psychiatric, medical, and additional sleep-related pathology,3 is estimated to affect up to 3% of the population.4
Current models of primary insomnia highlight the role of cognitive, emotional, and physiological hyperarousal for the development and maintenance of the disorder.5–9 With respect to cognitive processes, patients' cognitions are often dominated by worries and ruminations that are associated with a level of arousal that is incompatible with restorative sleep. Accordingly, previous studies have shown that cognitive arousal is positively correlated with prolonged sleep onset latency in insomnia patients.10–15Furthermore, some studies included an experimental increase of pre-sleep cognitive arousal levels that resulted in difficulties falling asleep as well as in distorted sleep perception.16,17 Focusing on the content of these intrusive thoughts revealed that thinking about sleep and the anticipated consequences of poor sleep is very common in poor sleepers.11,18,19 In line with this, the attentional system of patients with primary insomnia has been described as particularly sensitive to sleep-related information. According to the attention-intention effort model,6 this focus on sleep-related information leads to a cascade of processes inducing the poor sleeper to direct effort and intention towards sleeping which is incompatible with the ability to obtain restorative sleep. Several studies have investigated attentional preference for sleep-related cues in primary insomnia using computerized tasks.20–26 The majority of this work supports the notion that poor sleepers show an attentional bias for sleep-related stimuli relative to good sleeper controls. Consistent with these results, altered emotional responses to sleep-related stimuli have been reported in people with insomnia as compared to good sleepers.27 Of note, studies on anxiety suggest a direct causal link from facilitated selective attention to worry.28
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cognitive arousal is positively correlated with prolonged sleep onset latency in insomnia. There is a lack of data how cognitive arousal is associated with sleep maintenance parameters and how sleep is differentially influenced by sleep-related cognitive arousal versus general cognitive arousal.
Study Impact: Habitual sleep-related cognitive arousal was associated with subjective and objective measures of disturbed sleep initiation and disturbed sleep maintenance, while habitual general cognitive arousal was not. This might encourage the investigation of interventions that specifically target sleep-related cognitive arousal.
Although an enhanced attentional focus towards sleep-related cues may be involved in the development and maintenance of chronic insomnia, several important questions have not been answered up to now. First, little effort has been made to investigate the relationship between cognitive arousal and objectively determined sleep parameters, and the studies that have been carried out have reported inconsistent results. For example, van Egeren et al.14 found no significant correlation between pre-sleep cognitive activity and polysomnographically determined sleep onset latency. However, Wicklow and Espie19 reported that objective sleep onset latency, as measured by actigraphy, was positively correlated with pre-sleep cognitive activity. Second, existing studies restricted their analysis of sleep parameters to the sleep onset latency. However, cognitive arousal may also have an impact on sleep maintenance parameters, and the investigation of these may deepen the understanding of the interaction between rumination and sleep. Third, as suggested by Carney et al.,18 the distinction between general cognitive arousal and sleep-related cognitive arousal might be important because their impact on sleep parameters might substantially differ. More specifically, the authors suggested that sleep-related cognitive arousal might be more closely related to impaired sleep quality. Finally, only one study has evaluated the relationship between cognitive variables and sleep parameters in a clinically referred sample of primary insomnia patients.10 However, this investigation focused primarily on the distinction between worry and rumination as independent constructs and not on the distinction between sleep-related cognitive arousal and general cognitive arousal. Additionally, objectively determined sleep parameters were not used in this study.
The current study aimed at investigating sleep-related arousal and general cognitive arousal and their association with objective and subjective sleep parameters in patients with primary insomnia and healthy good sleepers. The first aim was to compare cognitive arousal levels in insomnia patients and good sleepers. Based on the above mentioned presumed role of hyperarousal in the development and maintenance of insomnia, we hypothesized that sleep-related and general cognitive arousal levels would be higher in patients with primary insomnia than in good sleeper controls. The second aim of the current study was to investigate the association between cognitive arousal levels and sleep parameters with the hypothesis that higher levels of sleep-related and general cognitive arousal would be associated with indicators of poor sleep.
METHODS
Participants
In the current investigation, 182 patients with primary insomnia according to DSM-IV criteria29 and 54 healthy controls according to the Research Diagnostic Criteria (RDC) for normal sleepers3 were investigated. The study sample is overlapping with the one that was investigated by Feige et al.30 and was obtained by reviewing the data of the archival database of the Sleep Center at the Department of Psychiatry and Psychotherapy Sleep Center, University of Freiburg Medical Center, for the period 1995-2008. Altogether, 4173 individuals were investigated in this period. Of these, 3937 individuals did not meet the following inclusion/exclusion criteria. All participants were either primary insomnia patients or healthy good sleepers. All participants were free of any psychoactive medication known to affect sleep at least one week prior to the sleep laboratory examination and did not receive cognitive-behavioral therapy for insomnia before taking part in the study. Participants with a PLMS (periodic leg movements during sleep) arousal index > 5.0 or an apnea index > 5.0 were excluded from the current analyses. Written informed consent was obtained from all subjects before participating in the investigation.
Procedure
Insomnia patients were referred to our sleep disorders clinic by their primary care provider or medical specialist. Healthy controls were recruited through local advertisements. Before entering the protocol, all participants underwent our standard physical and psychiatric examination, as well as a clinical interview by an experienced physician, excluding those with psychiatric or occult sleep disorder pathology (including hypersomnias, parasomnias, sleep-related breathing disorders, sleep-related movement disorders, and circadian rhythm sleep disorders). Additionally, all participants underwent a standard physical examination, including electrocardiogram (ECG), electroencephalogram (EEG) and routine laboratory investigations (blood cell count, liver, renal and thyroid function) to exclude those with serious medical conditions. All participants underwent 2 consecutive nights of PSG sleep monitoring at the sleep center. During these 2 nights, participants had to refrain from alcohol and caffeine. On the first day of their visit, all participants were asked to complete a self-rating questionnaire measuring sleep-related and general cognitive arousal (the FEPS-II questionnaire, described in detail below) and the German version of the Pittsburgh Sleep Quality Index (PSQI). In the morning after each sleep recording some minutes after lights on, all participants were asked to complete a self-rating measure of subjective aspects of sleep (the SF-A questionnaire, described in detail below). After completion of the study, all patients received treatment according to current guidelines.
Questionnaires
All participants completed a questionnaire that was specifically designed to measure trait aspects of sleep-related and general cognitive arousal in patients with sleep disorders (“Fragebogen zur Erfassung allgemeiner und spezifischer Persönlichkeitsmerkmale Schlafgestörter,” FEPS-II,31 see Table 1 for the item list). Respondents were instructed to indicate for each item whether it was consistent with their habitual experience. The FEPS-II consists of 23 items presented on a 5-point Likert-type scale (“not at all” to “completely”) divided into 2 subscales, as yielded by factor analysis. “Sleep-related cognitive arousal” (originally named “focusing,” 11 items resulting in an overall value from 11 to 55) refers to the tendency to excessively think about difficulties in initiating or maintaining sleep or about the anticipated daytime consequences of poor sleep (e.g., “When I sleep poorly on one night I know that it will interfere with my work performance on the next day”). The dimension “general cognitive arousal” (12 items resulting in an overall value from 12 to 60) captures the tendency to repetitively think about unresolved problems (e.g., “I often see problems that others don't see”). The underlying rationale for the questionnaire is that dysfunctional, negative cognitions such as continually ruminating or worrying about not being able to sleep or about unresolved problems are important factors for the development and maintenance of insomnia. Internal consistency of the measure was demonstrated in 402 patients with sleep disorders and 346 healthy good sleepers for both sleep-related cognitive arousal (α = 0.90) and general cognitive arousal (α = 0.91).31 In the same investigation, test-retest reliability (1 to 4 months interval) was r = 0.82 for sleep-related cognitive arousal and r = 0.92 for general cognitive arousal.
Table 1.
Items of the FEPS-II (English translation by the authors of the current investigation)
The “Schlaffragebogen A” (SF-A)32 captures subjective aspects of sleep in the preceding night. The questionnaire includes subjective estimates of wake times (sleep onset latency and wake time after sleep onset [WASO]). Subjective sleep efficiency was calculated using SF-A wake times and PSG-documented bedtimes because bedtimes are not estimated by subjects on the SF-A. The German version33 of the Pittsburgh Sleep Quality Index (PSQI)34 was used to assess sleep habits and sleep quality in the preceding 2 weeks.
Polysomnography
All participants underwent 2 consecutive nights of PSG sleep monitoring. A standard laboratory procedure and PSG montage were followed. Nocturnal sleep was recorded on 24-channel EEG-polysomnographs for 8 h from lights out (23:00) until lights on (07:00). All recordings included EEG (C3-A2; C4-A1), EOG (horizontal and vertical), and EMG (submental), and were scored visually by experienced raters according to the criteria of Rechtschaffen and Kales.35 During the first night, all participants were screened for apneas and periodic leg movements by monitoring abdominal and thoracic effort, nasal airflow, oximetry, and bilateral tibialis anterior EMG. Sleep recordings were evaluated for the following parameters of sleep continuity: total sleep time (TST); sleep efficiency: ratio of TST to time in bed * 100 %; wake after sleep onset (WASO): difference between TST and sleep period time (time from sleep onset until final awakening); sleep onset latency: time from lights out until sleep onset (defined as first epoch of stage 2); arousal index: number of arousals per hour; number of awakenings. Sleep architecture parameters were amounts of stages Wake, 1, 2, slow wave sleep (SWS), and REM sleep as percentage of sleep period time.
Statistical Analysis
Descriptive presentation of the data includes mean values and standard deviations. Independent t-tests were used to assess demographic and clinical differences between primary insomnia patients and healthy controls. To test for associations between sleep parameters and FEPS-II subscales, 12 general linear model analyses were performed for the full sample (including primary insomnia patients and healthy controls). The 12 outcome variables of these analyses were the SF-A- and PSG-derived sleep efficiency, WASO, and sleep onset latency for the first and second night. For each of these general linear model analyses, 5 predictor variables were used; the 2 FEPS-II subscales, the diagnosis (primary insomnia vs. healthy controls), age, and gender. For the overall effects, the level of significance was set at p < 0.004 (2-tailed) after applying Bonferroni α adjustment for multiple parallel tests (12 analyses). For single tests, the level of significance was set at p < 0.05 (2-tailed).
Due to adaptation effects, it is a common practice to exclude the first sleep laboratory night from the analysis.36 However, in the current study, we analyzed both nights because the adaptation to the lab environment might be associated with increased arousal levels, possibly emphasizing our effects of interest.
RESULTS
Sample Description
The group of primary insomnia patients consisted of 106 women and 76 men (age: 42.7 ± 11.9 years). They had a mean score of 11.1 ± 3.2 on the PSQI. Sixteen patients suffered from sleep onset insomnia, 66 from sleep maintenance insomnia, and 97 from mixed insomnia. Three patients had a complaint of non-restorative sleep in the absence of difficulty initiating and maintaining sleep. Average duration of primary insomnia was 10.9 ± 9.5 years. The control group consisted of 38 women and 16 men (age: 40.0 ± 13.2 years). Mean PSQI score was 3.1 ± 1.7. There were no significant group differences for sex distribution (χ2(1,234) = 2.09, p = 0.15) or age (t234 = 1.35, p = 0.18). The patient group had significantly higher PSQI values than the control group (t234 = 23.73, p < 0.001).
Group Comparison of Sleep Parameters
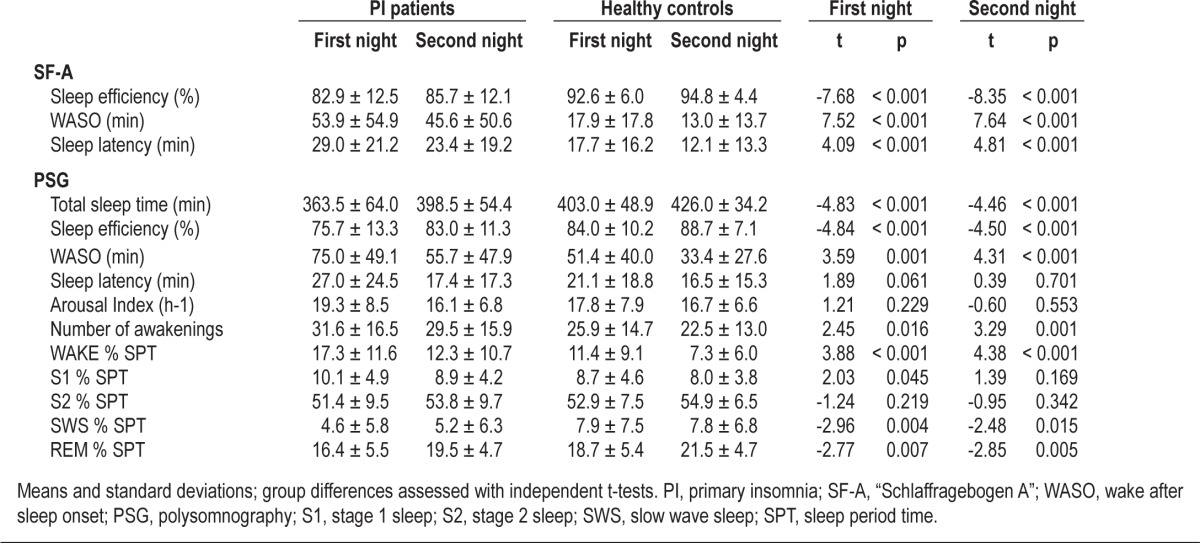
Polysomnographic and subjective sleep data of the 2 groups are presented in Table 2. With respect to polysomnography, insomnia patients had a significantly lower sleep efficiency and total sleep time than healthy controls on both nights. Additionally, SWS % and REM % were decreased in primary insomnia patients. WASO, Wake %, and the number of awakenings were significantly increased. Furthermore, in the first night, S1 % was higher in insomnia patients. With respect to subjective data, insomnia patients had significantly lower sleep efficiency as well as significantly increased WASO and sleep onset latency in both nights.
Table 2.
SF-A values and polysomnographic data of primary insomnia (PI) patients and healthy controls
Group Comparison of Cognitive Arousal Levels
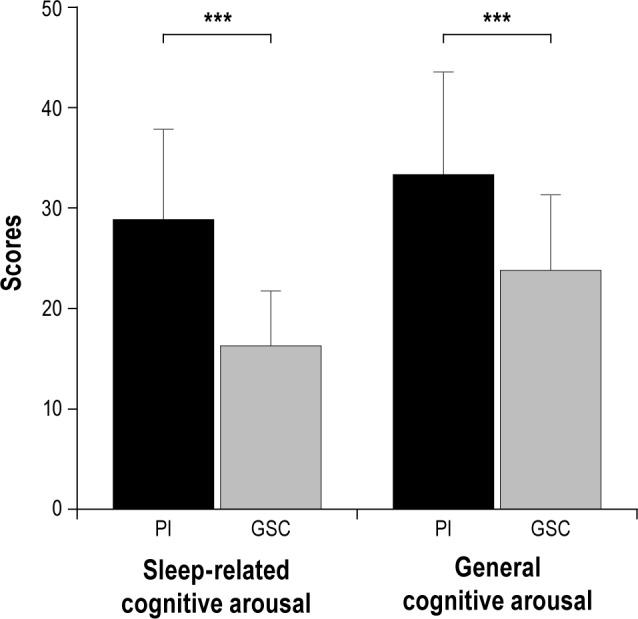
With respect to the FEPS-II subscales, primary insomnia patients had significantly higher values than the control group for both sleep-related cognitive arousal (t234 = 12.65, p < 0.001) and general cognitive arousal (t234 = 7.49, p < 0.001; see Figure 1). The correlation between the 2 FEPS-II subscales across the whole group was r = 0.62.
Figure 1. Mean FEPS subscale scores (with standard deviations) of 182 primary insomnia (PI) patients and 54 good sleeper controls (GSC).
Association between Cognitive Arousal and Sleep Parameters
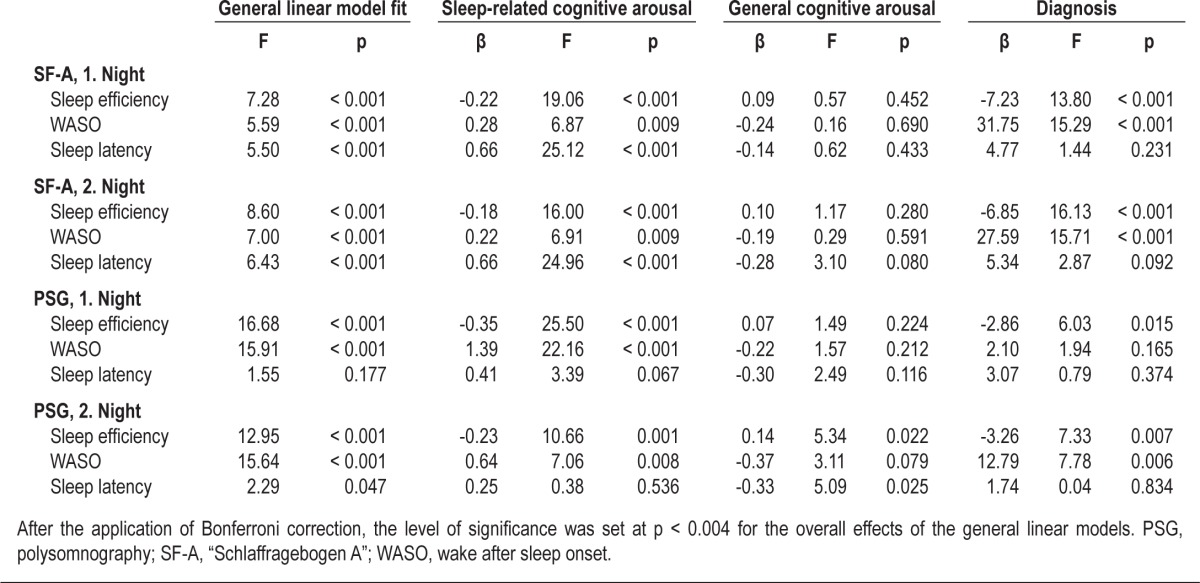
The results of the general linear model analyses with the sleep parameters as dependent variables and the 2 FEPS-II subscales as well as the diagnosis as independent variables are presented in Table 3.
Table 3.
Results of the 12 general linear model analyses with sleep-related parameters as dependent variables and the FEPS-II subscales, the diagnosis, age, and gender as independent variables
Sleep-related cognitive arousal was significantly related to all sleep parameters apart from objectively determined sleep latency. More specifically, sleep-related cognitive arousal was negatively associated with sleep efficiency and positively with WASO and subjectively determined sleep latency. This indicates that increased sleep-related cognitive arousal is associated with poor sleep quality, even when controlling for general cognitive arousal and the diagnosis of the participants.
General cognitive arousal was only related to objectively determined sleep efficiency in the second night, indicating that increased general cognitive arousal is associated with increased sleep efficiency when controlling for sleep-related cognitive arousal and the diagnosis of the participants. While there seems to be an association between general cognitive arousal and sleep latency of the second night, the corresponding overall effect of the general linear model did not reach statistical significance after applying Bonferroni correction.
Association between Cognitive Arousal and Sleep Parameters for Insomnia Subgroups
Additional analyses were conducted by restricting the insomnia patients group to (a) sleep-maintenance insomnia patients and (b) insomnia patients with objective short sleep duration. Concerning the differentiation in insomnia with and without short sleep duration, we split up the insomnia group using an 85% sleep efficiency criterion for the PSG data of the second night.3 For both sleep-maintenance insomnia patients and insomnia patients with objective short sleep duration, there were no major differences in the association between cognitive arousal and sleep parameters to the results reported in Table 3.
DISCUSSION
In the current study, we investigated the relationship between trait aspects of sleep-related and general cognitive arousal levels and both subjectively and objectively determined sleep parameters of two consecutive sleep lab nights. In line with our first hypothesis, primary insomnia patients reported both increased sleep-related arousal and increased general cognitive arousal compared to healthy controls. In line with our second hypothesis, increased sleep-related cognitive arousal levels were significantly related to measures of poor sleep. However, contrary to expectations, our analyses revealed that general cognitive arousal was not independently related to disturbed sleep.
Previous studies have primarily focused on the sleep onset period when investigating the association between cognitive arousal and sleep parameters in poor sleepers. Concerning this, we replicated previous findings of an association between cognitive arousal and subjectively reported sleep latency.10–17 However, there has been a lack of information about the association between cognitive arousal and sleep maintenance parameters. The current study filled this gap by investigating sleep efficiency and wake after sleep onset and their relationship to sleep-related and general cognitive arousal. The results showed that both increased WASO and decreased sleep efficiency are related to sleep-related cognitive arousal. This is in line with the clinical experience that patients with sleep maintenance disorders report a cognitive hyperactivity during the night similarly to patients with sleep onset insomnia.
Furthermore, our data support the assumption that sleep-related cognitive arousal is more closely associated with sleep disturbances than general cognitive arousal. In the current study, general cognitive arousal did not show any significant relationship to poor sleep that was independent of sleep-related cognitive arousal. This could explain, at least partially, the conflicting results reported by van Egeren et al.14 and Wicklow and Espie.19 While the first study focused on increased cognitive activity in terms of general cognitive arousal, the second study investigated pre-sleep cognitive activity mainly characterized by thinking about sleep loss and its possible consequences. Consistent with our results, the first study did not find a significant relationship with sleep onset latency, while the second study reported significant correlations.
With respect to the clinical significance of our findings, it has to be noted that the explained variance is generally low for cognitive arousal levels. However, the mean group difference in the FEPS-II subscale “sleep-related cognitive arousal” (12.4 points) corresponds to a sleep efficiency difference of 3.5% to 5.1%, a WASO difference of 10.2 to 17.7 minutes, and a difference in subjective sleep onset latency of 9.4 to 9.5 minutes (please refer to the β-values of Table 3). These data support the hypothesis that sleep-related cognitive arousal is a clinically relevant factor for sleep disturbances.
Several limitations of the present study have to be addressed. First, the observations in the current study are based on cross-sectional data; thus no causal relationship between cognitive arousal and sleep parameters can be inferred from this design. Second, previous studies showed that more than two nights are needed to derive stable estimates of both polysomnographic and diary-rated sleep in insomnia patients.37 Therefore, future studies are needed to investigate the association between cognitive arousal and sleep parameters across multiple nights. Third, the clinically referred patients in the current study are a heterogeneous group, including different insomnia subtypes and lifetime histories of medication use. However, this makes the patient group more representative of the population of clinically referred primary insomnia patients in general; this is especially important as some previous investigations in this field have recruited nonclinical samples, and the results of the current study were stable across different insomnia subgroups. Furthermore, in the current analyses, we did not discriminate between worry and rumination, although there is some evidence that these two cognitive processes are separate constructs with respect to their impact on sleep disturbances.10 Additionally, since we did not include a state arousal measure, conclusions of the current analysis are restricted to trait aspects of arousal. Moreover, standardized bedtimes of 8 hours duration have been used in the current study resulting in differences to participants' habitual sleep patterns. However, most sleep parameters are more comparable between subjects when standardized bedtimes are used. Last, both insomnia patients and healthy controls tended to overestimate their PSG-determined total sleep time. With respect to insomnia patients, this result was unexpected, and we cannot rule out that this might have affected our results on trait aspects of cognitive arousal. However, previous studies conducted both by our group30 and others38 also reported that overestimating total sleep time is not as uncommon in insomnia patients as generally assumed.
Despite these limitations, the current study provides novel data suggesting that cognitive arousal plays an important role for both sleep-onset and sleep-maintenance problems and that sleep-related cognitive arousal is more strongly related to sleep disturbances than general cognitive arousal. Although the current study design does not permit inferences of causality, future studies are worthwhile to investigate the effects of cognitive techniques that specifically target sleep-related cognitive arousal. Concerning this, Harvey et al.38 suggested (a) identifying and evaluating patients' sleep-related worrisome thoughts, and (b) reducing monitoring for sleep-related threat by actively directing attention away from it. Dedicating more effort to these psychological interventions might improve the treatment efficacy of cognitive-behavioral therapy for insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and related health problems, tenth revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, second edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14:227–38. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10:215–45. doi: 10.1016/j.smrv.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 8.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Carney CE, Harris AL, Moss TG, Edinger JD. Distinguishing rumination from worry in clinical insomnia. Behav Res Ther. 2010;48:540–6. doi: 10.1016/j.brat.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey AG. Pre-sleep cognitive activity: a comparison of sleep-onset insomniacs and good sleepers. Br J Clin Psychol. 2000;39:275–86. doi: 10.1348/014466500163284. [DOI] [PubMed] [Google Scholar]
- 12.Nelson J, Harvey AG. An exploration of pre-sleep cognitive activity in insomnia: imagery and verbal thought. Br J Clin Psychol. 2003;42:271–88. doi: 10.1348/01446650360703384. [DOI] [PubMed] [Google Scholar]
- 13.Robertson JA, Broomfield NM, Espie CA. Prospective comparison of subjective arousal during the pre-sleep period in primary sleep-onset insomnia and normal sleepers. J Sleep Res. 2007;16:230–8. doi: 10.1111/j.1365-2869.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 14.van Egeren L, Haynes SN, Franzen M, Hamilton J. Presleep cognitions and attributions in sleep-onset insomnia. J Behav Med. 1983;6:217–32. doi: 10.1007/BF00845382. [DOI] [PubMed] [Google Scholar]
- 15.Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med. 2009;71:771–5. doi: 10.1097/PSY.0b013e3181ae58e8. [DOI] [PubMed] [Google Scholar]
- 16.Tang NK, Anne Schmidt D, Harvey AG. Sleeping with the enemy: clock monitoring in the maintenance of insomnia. J Behav Ther Exp Psychiatry. 2007;38:40–4. doi: 10.1016/j.jbtep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Tang NK, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep. 2004;27:69–78. doi: 10.1093/sleep/27.1.69. [DOI] [PubMed] [Google Scholar]
- 18.Carney CE, Edinger JD, Meyer B, Lindman L, Istre T. Symptom-focused rumination and sleep disturbance. Behav Sleep Med. 2006;4:228–41. doi: 10.1207/s15402010bsm0404_3. [DOI] [PubMed] [Google Scholar]
- 19.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38:679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 20.Jones BT, Macphee LM, Broomfield NM, Jones BC, Espie CA. Sleep-related attentional bias in good, moderate, and poor (primary insomnia) sleepers. J Abnorm Psychol. 2005;114:249–58. doi: 10.1037/0021-843X.114.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Lundh LG, Fröding A, Gyllenhammar L, Broman JE, Hetta J. Cognitive bias and memory performance in patients with persistent insomnia. Cogn Behav Ther. 1997;26:27–35. [Google Scholar]
- 22.MacMahon KM, Broomfield NM, Espie CA. Attention bias for sleep-related stimuli in primary insomnia and delayed sleep phase syndrome using the dot-probe task. Sleep. 2006;29:1420–7. doi: 10.1093/sleep/29.11.1420. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelhalder K, Espie C, Nissen C, Riemann D. Sleep-related attentional bias in patients with primary insomnia compared with sleep experts and healthy controls. J Sleep Res. 2008;17:191–6. doi: 10.1111/j.1365-2869.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelhalder K, Espie C, Riemann D. Is sleep-related attentional bias due to sleepiness or sleeplessness? Cogn Emot. 2009;23:541–50. [Google Scholar]
- 25.Spiegelhalder K, Kyle SD, Feige B, et al. The impact of sleep-related attentional bias on polysomnographically measured sleep in primary insomnia. Sleep. 2010;33:107–12. doi: 10.1093/sleep/33.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods H, Marchetti LM, Biello SM, Espie CA. The clock as a focus of selective attention in those with primary insomnia: an experimental study using a modified Posner paradigm. Behav Res Ther. 2009;47:231–6. doi: 10.1016/j.brat.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Baglioni C, Lombardo C, Bux E, et al. Psychophysiological reactivity to sleep-related emotional stimuli in primary insomnia. Behav Res Ther. 2010;48:467–75. doi: 10.1016/j.brat.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch CR, MacLeod C, Mathews A, Sandher O, Sivani A, Hayes S. The contribution of attentional bias to worry: distinguishing the roles of selective engagement and disengagement. J Anxiety Disord. 2011;25:272–7. doi: 10.1016/j.janxdis.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 30.Feige B, Al-Shajlawi A, Nissen C, et al. Does REM sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–90. doi: 10.1111/j.1365-2869.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann MR, Schneider G, Rasch T, et al. Fragebogen zur Erfassung spezifischer Persönlichkeitseigenschaften Schlafgestörter II (FEPS II) Göttingen: Hogrefe; 1996. [Google Scholar]
- 32.Görtelmeyer R. Internationale Skalen für Psychiatrie. Weinheim: Beltz; 1981. Schlaffragebogen SF-A und SF-B. [Google Scholar]
- 33.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington: DC: NIH; 1968. [DOI] [PubMed] [Google Scholar]
- 36.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18:463–9. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanable PA, Aikens JE, Tadimenti L, et al. Sleep latency and duration estimates among sleep disorder patients: Variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:1–9. [PubMed] [Google Scholar]
- 39.Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behav Res Ther. 2007;45:2491–501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]