Abstract
Study Objectives:
To assess the efficacy and safety of sodium oxybate (SXB) in narcolepsy-cataplexy patients.
Design:
Systematic review and meta-analysis.
Patients:
Adults with narcolepsy-cataplexy.
Interventions:
SXB.
Measurements and Results:
Electronic databases (e.g., MEDLINE) and references of included studies were searched to identify randomized controlled trials (RCTs) assessing the efficacy and safety of SXB for patients with narcolepsy-cataplexy. Risk of bias was appraised using the Cochrane risk of bias tool. Meta-analysis was conducted in Review Manager Version 5.
Six RCTs and 5 companion reports were included after screening 14 full-text articles and 483 citations. All were private-industry funded. SXB (usually 9 g/night) was superior to placebo for reducing mean weekly cataplexy attacks (n = 2 RCTs, mean difference [MD]: −8.5, 95% CI: −15.3, −1.6), increasing maintenance wakefulness test (MWT) (n = 2, MD: 5.18, 95% CI: 2.59-7.78), reducing sleep attacks (n = 2, MD: −9.65, 95% CI: −17.72, −1.59), and increasing Clinical Global Impression scores (n = 3, relative risk, RR: 2.42, 95% CI: 1.77-3.32). SXB did not significantly increase REM sleep versus placebo (n = 2, MD: −0.49, 95% CI: −3.90, 2.92). Patients receiving SXB had statistically more adverse events versus placebo, including nausea (n = 3, relative risk [RR]: 7.74, 95% CI: 3.2, 19.2), vomiting (n = 2, RR: 11.8, 95% CI: 1.6, 89.4), and dizziness (n = 3, RR: 4.3, 95% CI: 1.1, 16.4). Enuresis was not significantly different from placebo (n = 2, RR: 2.6, 95% CI: 0.8, 9.8). All meta-analyses had minimal statistical heterogeneity (p-value > 0.1).
Conclusion:
Narcolepsy patients on SXB have significant reductions in cataplexy and daytime sleepiness. SXB is well tolerated in patients with narcolepsy, and most adverse events were mild to moderate in severity.
Citation:
Alshaikh MK; Tricco AC; Tashkandi M; Mamdani M; Straus SE; BaHammam AS. Sodium oxybate for narcolepsy with cataplexy: systematic review and meta-analysis. J Clin Sleep Med 2012;8(4):451-458.
Keywords: Sodium oxybate, Xyrem, randomized controlled trial, systematic review, meta-analysis, narcolepsy, cataplexy
Narcolepsy is a sleep disorder characterized by excessive daytime sleeping (EDS) associated with irresistible attacks of sleep, sudden loss of muscle tone (cataplexy), disrupted nocturnal sleep, hypnagogic/hypnopompic hallucinations, and sleep paralysis.1 Cataplexy is specific to narcolepsy and is the most accurate diagnostic marker of the disease. It is characterized by a sudden, usually bilateral, partial or complete loss of muscle tone that is provoked by emotional stimuli. Studies have shown that 65% to 75% of patients with narcolepsy have cataplexy.2,3 The prevalence of narcolepsy with cataplexy is approximately 25 to 50 per 100,000 people, with an incidence of approximately 0.74 per 100,000 person-years.2,3 It is often extremely incapacitating, interfering with every aspect of life, including work and social settings.1,4,5
Currently there is no cure for narcolepsy, with treatment focusing on symptom control. Pharmacological management of EDS commonly involves medications that increase wakefulness, including non-sympathomimetic stimulants, (e.g., modafinil) and sympathomimetic stimulants (e.g., amphetamine, methamphetamine, dexamphetamine, and methylphenidate. Several drugs have been used to treat cataplexy, such as tricyclic antidepressants and serotonin norepinephrine reuptake inhibitors; however, none of these medications are Food and Drug Administration (FDA) approved for cataplexy treatment.
SXB was recently approved by the FDA to treat patients diagnosed with narcolepsy and symptoms of cataplexy. It is currently authorized by the European Medicines Agency to treat narcolepsy with cataplexy as a whole disease in adults, and by the FDA to treat cataplexy in patients with narcolepsy, with an “expanded indication” for the treatment of excessive daytime sleepiness.6 It is the sodium salt of γ-hydroxybutyrate (GHB), an endogenous cerebral inhibitory neurotransmitter.7 Its mode of action is uncertain, but it may involve stimulation of γ-aminobutyric acid B (GABA [B]) receptors.8,9 SXB is rapidly absorbed and eliminated, having a mean elimination half-life of 30-60 minutes.10 Strict regulations have been established with regard to the prescription and dispensing of the drug and patients usually receive extensive education on its use.
In this article, we aimed to systemically review the efficacy and safety of SXB on EDS, cataplexy, quality of life, and the associated side effects among people with narcolepsy and cataplexy through a systematic review and meta-analysis.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was used to guide the reporting of this review.11
Eligibility Criteria
The inclusion criteria were randomized controlled trials (RCTs) of participants with narcolepsy and cataplexy, which examined the use of SXB. We did not limit inclusion by comparator, language, publication status (i.e., unpublished reports could be included), or year of publication. When multiple study publications reported data from the same population (i.e., companion reports), the trial reporting the primary outcome of interest was considered the major publication and the other report(s) was used for supplementary data.
The primary outcome was elimination of excessive daytime sleepiness (EDS) according to subjective or objective indicators. Objective laboratory tests included the multiple sleep latency test (MSLT), which is a validated objective measure of the ability or tendency to fall asleep.12,13 In addition, it allows documentation of sleep onset rapid eye movement sleep (SOREM). Another objective laboratory test is the maintenance of wakefulness test (MWT), which is a validated objective measure of the ability to stay awake for a defined time that measures the mean time latency of falling asleep during 4 to 5 sessions of trying to stay awake.12 Subjective validated scales included the Epworth Sleepiness Scale (ESS), which is a specialized, validated sleep questionnaire containing 8 items that ask for self-reported disclosure of the expectation of dozing in a variety of situations. Scores ≥ 10 indicate an abnormal result.14 The subjective outcome of elimination of cataplexy or reduction of the symptoms by > 50% from patient diaries was also included.
Secondary outcomes included quality of life using the short-form (SF-36) scale, Clinical Global Impression of change (CGI-C), and harms, including the type of adverse event and number of adverse events per treatment group.
Information Sources
Medical Subject Headings and text words related to SXB for narcolepsy with cataplexy were used to search MEDLINE (OVID interface, 1950 to October 2010), EMBASE (OVID interface, 1980 to October 2010), CINAHL (EBSCOhost interface, 1997 to October 2010), PsycInfo (Scholar's Portal interface, 1806 to October 2010), and the Cochrane Central Register of Controlled Trials (Wiley interface, inception to October 2010). To supplement the search, we searched a clinical trial registry (www.clinicaltrials.gov), scanned the reference lists of included studies, searched the authors' personal files, and contacted narcolepsy experts via email to identify further studies to be included, as well as the manufacturer of SXB (Jazz Pharmaceuticals).
Search
An experienced information specialist conducted all of the literature searches. The search strategy for the main electronic search (MEDLINE) is presented in the appendix; details on the other searches are available from the authors on request.
Study Selection
To ensure reliability, a training exercise was conducted prior to commencing the screening process. Two independent reviewers screened the search results for inclusion using a pre-defined relevance criteria form and obtained the full text of potentially relevant articles and screened them to determine inclusion, independently. Discrepancies at any stage were resolved by discussion or the involvement of a third reviewer. The level of agreement during screening was assessed using a κ statistic.15 We determined a priori that an acceptable level of agreement would be > 60%.15
Data Collection Process
A draft data extraction form was developed, piloted, and modified as necessary. Two reviewers assessed study quality and extracted all of the data using the standardized data extraction form, independently. Discrepancies were resolved by discussion or the involvement of a third reviewer.
Data Items
The extracted data included study characteristics (e.g., study period, sample size, geographic location, setting), participant characteristics (e.g., population, narcolepsy diagnosis, mean age, gender), and results from the primary and secondary outcomes.
Risk of Bias in Individual Studies and Across Studies
The risk of bias in individual studies was assessed using the Cochrane risk of bias tool.16 This tool consists of 6 items pertaining to sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. The risk of bias across studies was assessed using the outcome reporting bias criterion from the Cochrane risk of bias tool. Publication bias was to be assessed using funnel plots,17 but there were too few studies included in each meta-analysis to assess publication bias sufficiently.
Summary Measures
The summary measures were the relative risk (RR) and the mean difference (MD).
Synthesis of Results
The studies were plotted in a forest plot to examine heterogeneity visually. Statistical heterogeneity was examined using the I2 and χ2 statistics.18 Pooled estimates were derived using a random-effects model, and 95% CIs were derived based on a normal distribution.19 All analyses were conducted in Review Manager Version 5 (The Cochrane Collaboration, available from http://ims.cochrane.org/revman/download).
RESULTS
Study Selection
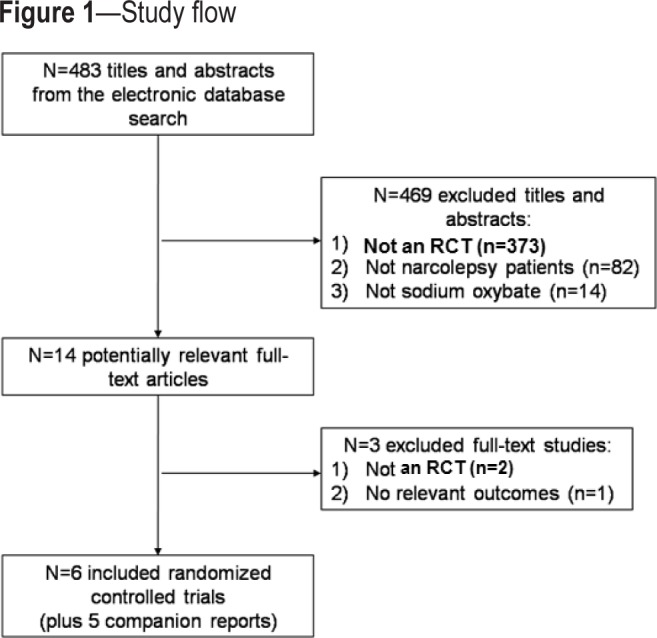
The literature search resulted in a total of 483 citations (i.e., titles and abstracts; Figure 1). We excluded 469 of these because they were not RCTs (n = 373), they did not include patients with narcolepsy and cataplexy (n = 82), or they did not examine the effects of SXB (n = 14). Fourteen full-text articles were retrieved and examined for relevance, and 6 RCTs fulfilled the inclusion criteria,20–25 along with 5 companion reports (Figure 1).25–28 Two articles were excluded at the full-text level of screening because they were not RCTs,29,30 and one study was excluded because it did not report any relevant outcomes.31 There was excellent agreement between reviewers at level 1 screening (κ = 0.92, 95% CI: 0.81 to 1.03), and lower agreement at level 2 screening, due to the small number of studies included at this level (κ = 0.46, 95% CI: −0.08 to 1.00).
Figure 1. Study flow.
Study and Patient Characteristics
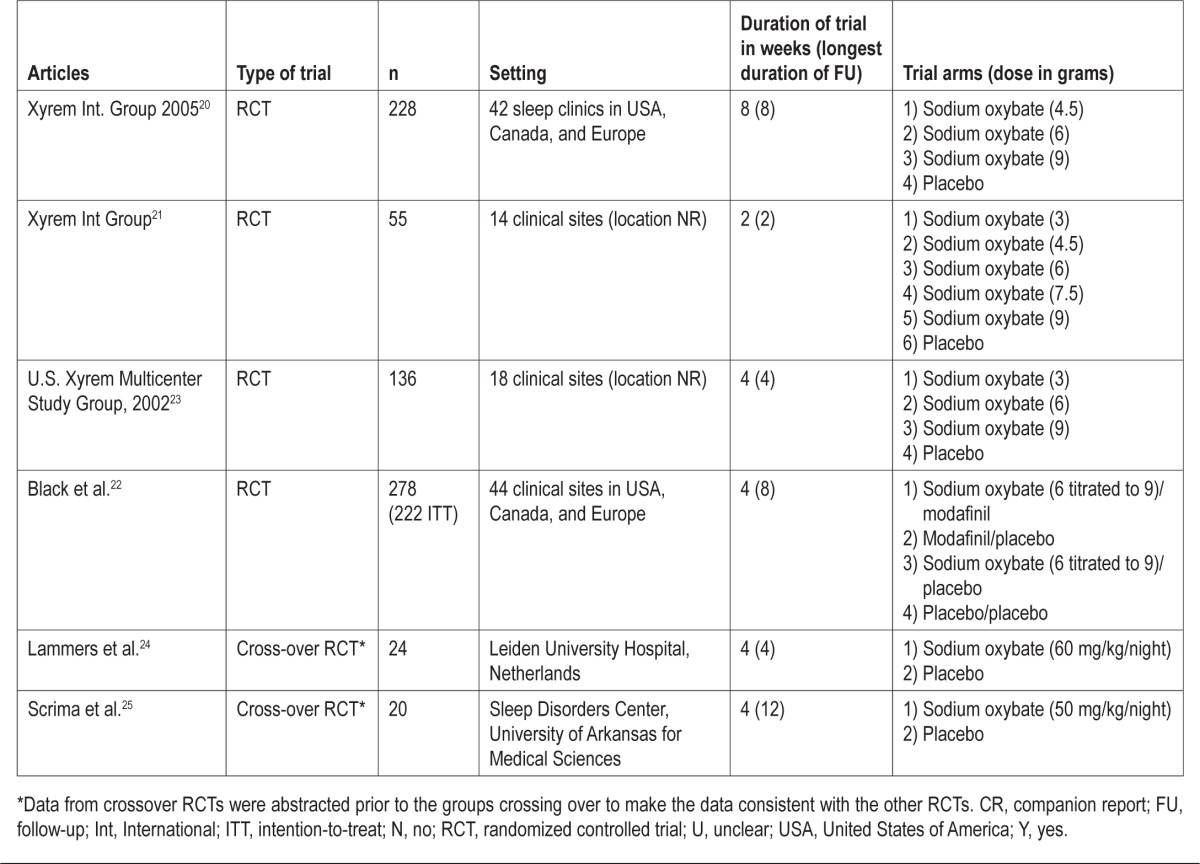
Except for 2 studies,24,25 all studies were published after 2002 (Table 1). Most of the studies were conducted in clinics in the USA, Canada, and Europe. Duration of the RCTs ranged from 4-8 weeks, except for one study25 that lasted for 12 weeks. SXB at a dose range between 4.5 to 9 g/night was the dose examined in most of the studies.24,25
Table 1.
Study characteristics
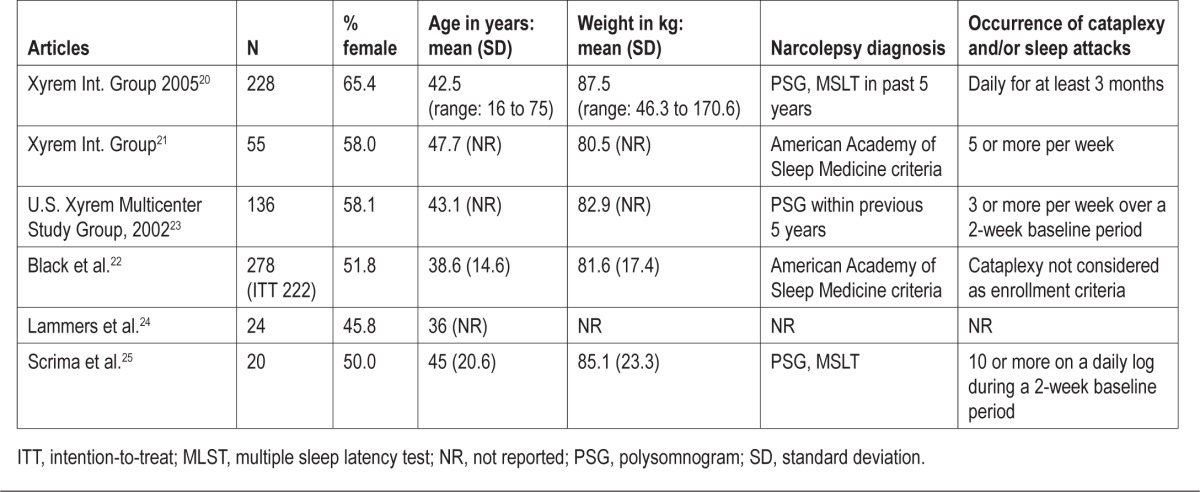
The number of patients ranged from 20 to 228, and the percentage of females ranged from 50% to 65% (Table 2). The diagnosis of narcolepsy was based on classical symptoms of narcolepsy and an MSLT showing ≥ 2 SOREM periods. MSLT in one study was conducted at home.24 All studies excluded patients with other sleep disorders. One study that assessed the effect of SXB on excessive daytime sleepiness did not include cataplexy as an enrollment criterion.22 No paper analyzed all of the clinical features of narcolepsy or performed all diagnostic tests.
Table 2.
Patient characteristics
Risk of Bias
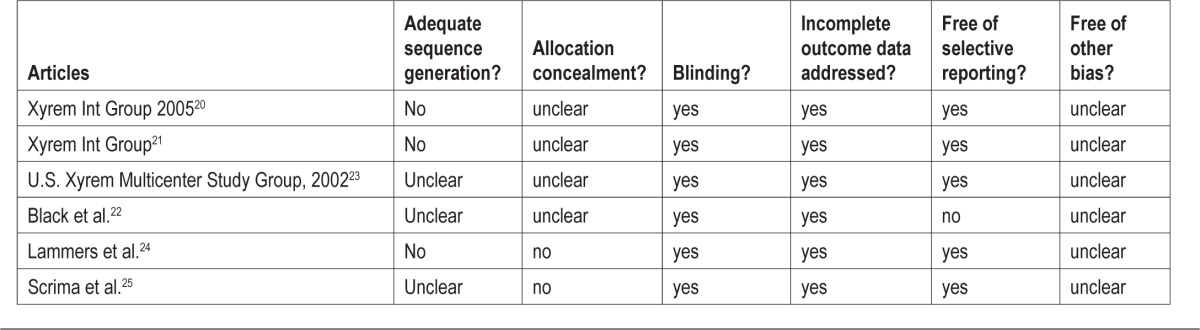
None of the included RCTs were assessed as having adequate sequence generation or allocation concealment (Table 3). All of the studies adequately blinded participants and addressed incomplete outcome data. Five of the 6 studies were free from selective outcome reporting.22 All of the studies scored unclear on other biases, as they involved private-industry funding. Four of the included studies were sponsored by the manufacturing company.20–23
Table 3.
Risk of bias results
Efficacy Meta-Analysis Results
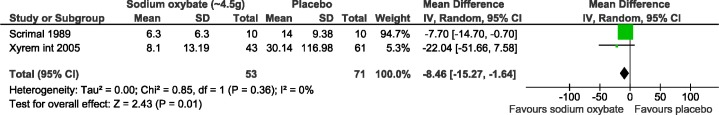
Four studies reported cataplexy attacks,21,23,25,26 and 2 were included in the meta-analysis (Figure 2).25,26 The 2 other studies could not be included in the meta-analysis because the standard error was not reported and these data could not be obtained from the SXB manufacturer.21,23 The first study25 comprised 20 subjects, and the other study26 included 104 subjects. Compared with placebo, cataplexy attacks were statistically significantly decreased with 4.5 g/night of SXB (pooled results: MD: −8.5, 95% CI: −15.3 to −1.6). No statistical heterogeneity was observed, with an I2 of 0% and a χ2 p-value of 0.36 on the test.
Figure 2. Mean weekly cataplexy attacks meta-analysis.
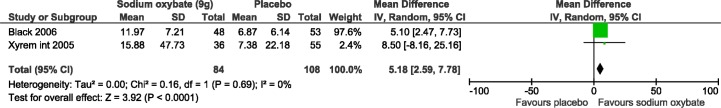
Two studies reported the benefit of SXB on excessive daytime sleepiness which was measured by MWT and both were included in the meta-analysis (n = 101 and 91 subjects; Figure 3).20,22 At a dose of 9 g/night, SXB increased sleep latency significantly in the MWT compared to placebo (pooled results: MD: 5.18, 95% confidence interval, CI: 2.59-7.78). No statistical heterogeneity was observed, with an I2 of 0% (χ2 p-value = 0.69). The above 2 studies used different protocols for the MWT. While the Xyrem International Study group used the 40-min version of the protocol,32 Black and Houghton used the 20-min version of the protocol.22 Theoretically, this may influence the sleep latencies obtained, particularly in patients who do not fall asleep very fast.
Figure 3. Maintenance of wakefulness test meta-analysis.
One of the included trials examined combination therapy trial of SXB and modafinil.22 The mean MWT values were statistically different in the SXB monotherapy and modafinil monotherapy and combination therapy (SXB + modafinil) compared to placebo.22 The median ESS scores decreased significantly in a dose-related manner in the international trial in patients receiving SXB (4.5 g, 6 g, and 9 g/night) compared to placebo.20
Black and Houghton (combination-therapy trial) reported a significant reduction by 20% and 27% in the median ESS scores in the SXB monotherapy and SXB + modafinil combined therapy recipients, respectively.22 On the other hand, the ESS scores did not change significantly in the placebo or modafinil monotherapy recipients.22 However, we could not conduct a meta-analysis on the ESS score because the standard error was not reported, and these data could not be obtained from the SXB manufacturer.
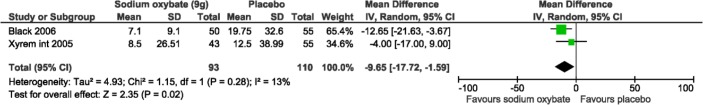
Two studies reported the number of weekly sleep attacks and both were included in the meta-analysis with a total of 105 and 98 participants (Figure 4).20,22 Sleep attacks were statistically significantly decreased with 9 g/night of SXB compared to placebo (pooled results: mean difference, MD: −9.65, 95% confidence interval, CI: −17.72, −1.59); low heterogeneity was observed, with an I2 of 13% (χ2 p-value = 0.28).
Figure 4. Mean sleep attacks meta-analysis.
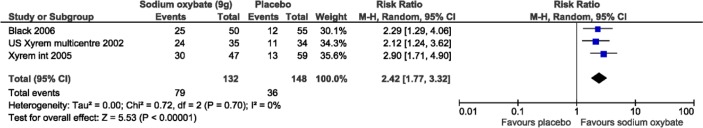
Three studies reported the Clinical Global Impression of severity and Change (CGI) of “very much improved” and all were included in the meta-analysis (n = 106, 69, and 105; Figure 5).20,22,23 CGI scores significantly increased with 9 g/night of SXB (pooled results: mean difference, MD: 2.42, 95% confidence interval, CI: 1.77-3.32). No statistical heterogeneity was observed, with an I2 of 0% and a p-value of 0.7 on the χ2 test. In the combination-therapy trial “very much improved” was seen only in the arms SXB monotherapy and the combination therapy (SXB + modafinil), while the modafinil monotherapy arm did not differ from that in the placebo arm.22
Figure 5. Proportion of patients “much improved” or “very much improved” on the Clinical Global Impression of Change versus all other categories meta-analysis.
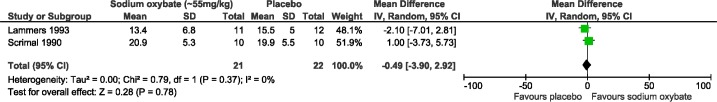
Two studies reported the percentage of REM sleep before and after SXB and were included in the meta-analysis with a sample size of 22 subjects24 and 20 subjects,25 but there were no significant differences between groups (pooled results: MD −0.49, 95% confidence interval, CI: −3.90, 2.92; Figure 6). No statistical heterogeneity was observed, with an I2 of 0% (χ2 p-value = 0.37).
Figure 6. Polysomnographic data for nocturnal sleep meta-analysis (REM).
Harms Meta-Analysis Results
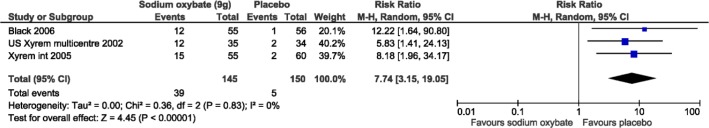
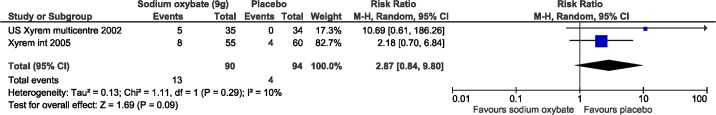
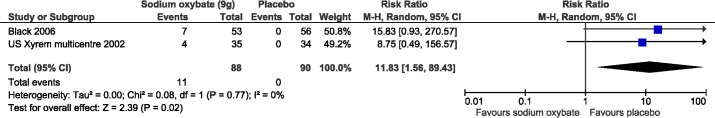
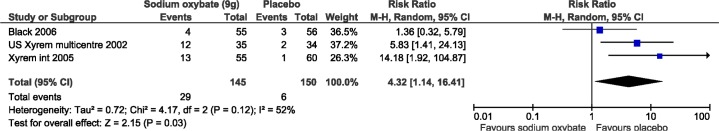
All harms meta-analyses included SXB at 9 g/night versus placebo. Patients receiving SXB had statistically more adverse events versus placebo, including nausea (3 studies, relative risk [RR]: 7.74, 95% CI: 3.2, 19.2; Figure 7), vomiting (2 studies, RR: 11.8, 95% CI: 1.6, 89.4; Figure 8), and dizziness (3 studies, RR: 4.3, 95% CI: 1.1, 16.4; Figure 9). Enuresis was not significantly different from placebo (2 studies, RR: 2.6, 95% CI: 0.8, 9.8; Figure 10), yet there was a trend towards favoring placebo versus SXB. In the US Xyrem study, 10 patients (7.4%) withdrew because of adverse events.23 Side effects were significantly more common in the SXB recipients compared to placebo and included nausea, vomiting, dizziness, and urinary incontinence.23 In the international Xyrem trial, 21 (9.2%) patients withdrew due to adverse events; however, in that trial, there was no difference in the incidence of urinary incontinence between SXB and placebo recipients.32 In the combination therapy trial, adverse events were reported in 70% of placebo, 54% of modafinil monotherapy, 60% of SXB monotherapy, and 79% of SXB + modafinil combination therapy.22 In this latter trial 1, 2, 4, and 6 patients withdrew from the placebo, modafinil monotherapy, SXB monotherapy, and SXB + modafinil combination therapy, respectively, due to adverse events.22 Serious side effects were reported infrequently. Acute confusion was reported in one patient in the US trial at a dose of SXB 6 g/night.23 In the combination therapy trial, one patient developed a serious psychotic disorder related to narcissistic personality disorder.22
Figure 7. Adverse events: gastrointestinal/nausea.
Figure 8. Adverse events: vomiting.
Figure 9. Adverse events: dizziness.
Figure 10. Adverse events: enuresis.
DISCUSSION
This systematic review assessed the efficacy and safety of SXB in narcolepsy patients and included six randomized controlled trials. All patients were diagnosed as having narcolepsy based on established criteria. This is the first meta-analysis that we are aware of to assess the effect of SXB in narcolepsy patients. Due to the short follow-up (2 to 12 weeks), we were unable to investigate long-term efficacy and harms.
We found that SXB in all trials resulted in significant reduction in cataplexy attacks and EDS. SXB reduced the frequency of cataplexy in a dose-related manner compared to placebo. Two studies have demonstrated beneficial effects on cataplexy attacks even with a smaller dose of SXB (4.5 g/night).20,25 EDS was reduced and sleep latency increased when evaluated by MWT in the SXB arm compared to placebo. The improvement was dose-related; however, the benefits documented in the MWT were statistically significant only in the higher dose (9 g/night). The improvement in the EDS appeared after 8 weeks of treatment.22,26 Although SXB reduces the frequency of sleep attacks, it did not eliminate them completely. Surprisingly, in the study by Black and Houghton, modafinil monotherapy had no significant effect on sleep latency in the MWT and or on the ESS scores.22 These results contradict previous studies, which demonstrated that modafinil in comparison with placebo results in significant improvement in daytime sleepiness when assessed by ESS, MSLT, and MWT.33
In a recent report, four patients with narcolepsy treated with sodium oxybate were followed for approximately 2 years.34 The beneficial effect on cataplexy and daytime sleepiness persisted during the follow-up period.34
The Clinical Global Impression of Change (CGI) scores, which are commonly used measures of symptom severity, treatment response, and the efficacy of treatments were dichotomized to responders as “very much improved” or “much improved.” “Much improved” and “very much improved” were statistically significant in all the doses (4.5, 6, and 9 g/night) compared to placebo.20 It was interesting to note that in the combination-therapy trial, “very much improved” was seen only in the arms SXB monotherapy and the combination therapy (SXB + modafinil) versus modafinil alone.22
Only one study examined quality of life indicators.27 Improved quality of life was observed for SXB at 9 g/night versus placebo in all subscales of the Functional Outcomes of Sleep Questionnaire, except for intimacy and sexual relationships. A statistically significant and clinically relevant result was found for SXB at 9 g/night versus placebo.
Sleep is frequently disturbed in patients with narcolepsy. Polysomnographic studies have documented several changes in patients with narcolepsy including prolonged awakening after sleep onset, increased stage N1, increased stage shift, and reduced stage N3.35 Several studies have shown that nightly SXB improves subjective and objective measures of nocturnal sleep and sleep architecture, with robust increases in stage N3 and delta power.24,25,28,34,36–41 Two recent randomized trials have shown that SXB resulted in significant dose-related improvements in slow wave sleep, total sleep time, and a decrease in stage N1, wake after sleep onset, and nighttime awakenings.28,41
SXB is generally well tolerated, with mild-to-moderate side effects that are dose-related. There is a concern about a narrow margin between efficacy and toxicity of SXB.42 In general, the incidence of side effects increases with dose, and most side effects subside upon reducing the dose.
Long-term follow-up data on adverse events are limited. A 12-month extension study reported adverse events in 93% of patients, including dizziness, headache, nausea, urinary incontinence, viral infection, somnolence, and pain.21 Dizziness was the only adverse event that was statistically more common in the SXB group.21
All of the trials included in this systematic review excluded patients with sleep disordered breathing (SDB). Therefore, caution is advised when treating narcoleptics with concurrent SDB, and physicians should confirm that patients with concurrent obstructive sleep apnea are compliant with positive airway pressure therapy before starting SXB.
We identified two review articles assessing the efficacy and tolerability of SXB in patients with narcolepsy. The authors did not conduct a systematic literature search or meta-analysis and included fewer trials than we do here. The findings of both reviews were consistent with the findings of this systematic review.10,42 Both reported improvement in cataplexy and daytime sleepiness and good tolerability of SXB. Evidence-based practice parameters of the American Academy of Sleep Medicine for the treatment of narcolepsy and other hypersomnias of central origin considered SXB as an acceptable patient-care strategy that reflects a high degree of clinical certainty for cataplexy, daytime sleepiness, and disrupted sleep due to narcolepsy; our review supports this recommendation.43 For the treatment of hypnagogic hallucination and sleep paralysis, they considered the evidence as uncertain.
There are a number of limitations of this report. One limitation is the fact that the included studies have a relatively short follow-up period. Nevertheless, the effect of SXB in the assessed outcomes was obvious during the follow-up periods. Another limitation is the small sample sizes of the included trials. Limitations in the systematic review process include that it was limited to the English language; no unpublished trials were identified (although we did contact trial authors and searched for unpublished material), we could not obtain data for all the outcomes from the SXB manufacturer, and we were unable to formally assess for publication bias because too few trials were included in the meta-analyses.
On the basis of this review, it can be concluded that patients with narcolepsy on SXB have a significant reduction in cataplexy based on diaries and significant improvement in daytime sleepiness based on objective (MWT) and validated subjective (ESS) assessment methods. Reviewed data suggest that SXB is well tolerated in patients with narcolepsy, and most adverse events were mild to moderate in severity. The study raises further questions that need to be explored in the future including; the long-term efficacy and tolerability of SXB, the effect of SXB in patients with concurrent sleep disordered breathing, and the effect of different dosages on patients with milder form of narcolepsy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Laure Perrier for the literature searches and Kevin Thorpe along with Maggie Hong Chen for their statistical analysis consultation and the University Sleep Disorders Center, King Saud University for logistic support. This review was conducted as part of a systematic review course taught Drs. Tricco and Straus through the Li Ka Shing Knowledge Institute of St Michael's Hospital. This systematic review was funded, in part, by the National Plan for Science and Technology, King Abdulaziz City for Science and Technology at King Saud University.
REFERENCES
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369:499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.BaHammam AS, Alenezi AM. Narcolepsy in Saudi Arabia. Demographic and clinical perspective of an under-recognized disorder. Saudi Med J. 2006;27:1352–7. [PubMed] [Google Scholar]
- 3.Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 4.Dauvilliers Y, Billiard M, Montplaisir J. Clinical aspects and pathophysiology of narcolepsy. Clin Neurophysiol. 2003;114:2000–17. doi: 10.1016/s1388-2457(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 5.Longstreth WT, Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Zaharna M, Dimitriu A, Guilleminault C. Expert opinion on pharmacotherapy of narcolepsy. Expert Opin Pharmacother. 2010;11:1633–45. doi: 10.1517/14656566.2010.484021. [DOI] [PubMed] [Google Scholar]
- 7.Keam S, Walker MC. Therapies for narcolepsy with or without cataplexy: evidence-based review. Curr Opin Neurol. 2007;20:699–703. doi: 10.1097/WCO.0b013e3282f22ad9. [DOI] [PubMed] [Google Scholar]
- 8.Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–61. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 9.Madden TE, Johnson SW. Gamma-hydroxybutyrate is a GABAB receptor agonist that increases a potassium conductance in rat ventral tegmental dopamine neurons. J Pharmacol Exp Ther. 1998;287:261–5. [PubMed] [Google Scholar]
- 10.Owen RT. Sodium oxybate: efficacy, safety and tolerability in the treatment of narcolepsy with or without cataplexy. Drugs Today (Barc) 2008;44:197–204. doi: 10.1358/dot.2008.44.3.1162240. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 13.Chervin RD, Kraemer HC, Guilleminault C. Correlates of sleep latency on the multiple sleep latency test in a clinical population. Electroencephalogr Clin Neurophysiol. 1995;95:147–53. doi: 10.1016/0013-4694(95)00075-a. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 16.Hartling L, Ospina M, Liang Y, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ. 2009;339:b4012. doi: 10.1136/bmj.b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed] [Google Scholar]
- 21.U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5:119–23. doi: 10.1016/j.sleep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29:939–46. doi: 10.1093/sleep/29.7.939. [DOI] [PubMed] [Google Scholar]
- 23.The U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25:42–9. [PubMed] [Google Scholar]
- 24.Lammers GJ, Arends J, Declerck AC, Ferrari MD, Schouwink G, Troost J. Gammahydroxybutyrate and narcolepsy: a double-blind placebo-controlled study. Sleep. 1993;16:216–20. doi: 10.1093/sleep/16.3.216. [DOI] [PubMed] [Google Scholar]
- 25.Scrima L, Hartman PG, Johnson FH, Jr, Hiller FC. Efficacy of gamma-hydroxybutyrate versus placebo in treating narcolepsy-cataplexy: double-blind subjective measures. Biol Psychiatry. 1989;26:331–43. doi: 10.1016/0006-3223(89)90048-6. [DOI] [PubMed] [Google Scholar]
- 26.Xyrem International Study Group. Further evidence supporting the use of sodium oxybate for the treatment of cataplexy: a double-blind, placebo-controlled study in 228 patients. Sleep Med. 2005;6:415–21. doi: 10.1016/j.sleep.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Weaver TE, Cuellar N. A randomized trial evaluating the effectiveness of sodium oxybate therapy on quality of life in narcolepsy. Sleep. 2006;29:1189–94. doi: 10.1093/sleep/29.9.1189. [DOI] [PubMed] [Google Scholar]
- 28.Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly administration of sodium oxybate results in significant reduction in the nocturnal sleep disruption of patients with narcolepsy. Sleep Med. 2009;10:829–35. doi: 10.1016/j.sleep.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Borgen LA, Okerholm RA, Lai A, Scharf MB. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J Clin Pharmacol. 2004;44:253–7. doi: 10.1177/0091270003262795. [DOI] [PubMed] [Google Scholar]
- 30.A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26:31–5. [PubMed] [Google Scholar]
- 31.Ristanovic RK, Liang H, Hornfeldt CS, Lai C. Exacerbation of cataplexy following gradual withdrawal of antidepressants: manifestation of probable protracted rebound cataplexy. Sleep Med. 2009;10:416–21. doi: 10.1016/j.sleep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 32.A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed] [Google Scholar]
- 33.Golicki D, Bala MM, Niewada M, Wierzbicka A. Modafinil for narcolepsy: systematic review and meta-analysis. Med Sci Monit. 2010;16:RA177–86. [PubMed] [Google Scholar]
- 34.Alshaikh MK, Gacuan D, George S, Sharif M, Bahammam AS. Long-term follow-up of patients with narcolepsy-cataplexy treated with sodium oxybate (Xyrem) Clin Neuropharmacol. 2011;34:1–4. doi: 10.1097/WNF.0b013e318203d415. [DOI] [PubMed] [Google Scholar]
- 35.Baker TL, Guilleminault C, Nino-Murcia G, Dement WC. Comparative polysomnographic study of narcolepsy and idiopathic central nervous system hypersomnia. Sleep. 1986;9(1 Pt 2):232–42. doi: 10.1093/sleep/9.1.232. [DOI] [PubMed] [Google Scholar]
- 36.Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6:1–6. doi: 10.1017/s0317167100119304. [DOI] [PubMed] [Google Scholar]
- 37.Broughton R, Mamelak M. Effects of nocturnal gamma-hydroxybutyrate on sleep/waking patterns in narcolepsy-cataplexy. Can J Neurol Sci. 1980;7:23–31. [PubMed] [Google Scholar]
- 38.Scharf MB, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry. 1985;46:222–5. [PubMed] [Google Scholar]
- 39.Scrima L, Hartman PG, Johnson FH, Jr, Thomas EE, Hiller FC. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13:479–90. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- 40.Mamelak M, Black J, Montplaisir J, Ristanovic R. A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep. 2004;27:1327–34. doi: 10.1093/sleep/27.7.1327. [DOI] [PubMed] [Google Scholar]
- 41.Black J, Pardi D, Hornfeldt CS, Inhaber N. The nightly use of sodium oxybate is associated with a reduction in nocturnal sleep disruption: a double-blind, placebo-controlled study in patients with narcolepsy. J Clin Sleep Med. 2010;6:596–602. [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson DM, Keating GM. Sodium oxybate: a review of its use in the management of narcolepsy. CNS Drugs. 2007;21:337–54. doi: 10.2165/00023210-200721040-00007. [DOI] [PubMed] [Google Scholar]
- 43.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1705–11. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]