Abstract
Objective
Exercise is known to improve physical functioning and health status in Chronic Obstructive Pulmonary Disease (COPD). Recently, disturbances in protein turnover and amino acid kinetics have been observed after exercise in COPD. The objective was to investigate which dairy protein is able to positively influence the protein metabolic response to exercise in COPD.
Materials and Methods
8 COPD patients and 8 healthy subjects performed a cycle test on two days while ingesting casein or whey protein. Whole body protein breakdown (WbPB), synthesis (WbPS), splanchnic amino acid extraction (SPE), and NetWbPS (=WbPS–WbPB) were measured using stable isotope methodology during 20 minutes of exercise (at 50% peak work load of COPD group). The controls performed a second exercise test at the same relative workload. Exercise was followed by 1 hour of recovery.
Results
In the healthy group, WbPS, SPE, and NetPS were higher during casein than during whey feeding (p<0.01). WbPS and NetPS were higher during exercise, independent of exercise intensity (p<0.01). NetPS was higher during casein feeding in COPD due to lower WbPB (p<0.05). Higher SPE was found during exercise during casein and whey feeding in COPD (p<0.05). Lactate levels during exercise were higher in COPD (p<0.05) independent of the protein. Post-exercise, lower NetPS values were found independent of protein type in both groups.
Conclusion
Casein resulted in more protein anabolism than whey protein which was maintained during and following exercise in COPD. Optimizing protein intake might be of importance for muscle maintenance during daily physical activities in COPD.
Keywords: exercise, COPD, whole body protein metabolism, casein, whey
Introduction
Physical exercise is an important element in pulmonary rehabilitation of patients with Chronic Obstructive Pulmonary Disease (COPD). Exercise training, often combined with nutritional support, has been shown to increase muscle function and exercise capacity (1, 2). We previously showed that differences exist in the acute response in protein metabolism to exercise between patients with COPD and healthy elderly (3). Low intensity constant work rate cycle exercise initiated a significant decrease in muscle and elevated plasma amino acid levels in normal weight COPD, suggesting enhanced amino acid efflux from muscle. In addition, we observed reduced protein turnover and urea synthesis rates up to 1 hour after a short bout of low intensity exercise in patients with emphysema (4). This suggests that low intensity exercise is able to produce profound disturbances in protein metabolism in COPD. In order to optimize the metabolic response to exercise, insight in the effects of protein feeding on protein metabolism during and following exercise is warranted in these patients.
Several studies in healthy subjects found that consumption of different intact proteins can modulate the anabolic response to feeding at rest and during exercise. We observed that muscle concentrations of several amino acids including the branched-chain amino acids (BCAA) changed differently after casein and soy protein feeding in healthy controls (5). Furthermore, leg amino acid uptake was different without acutely changing muscle protein kinetics (5). Whey and casein protein, known of its high levels of BCAA, were able to promote higher whole body nitrogen retention at rest (6, 7) and larger skeletal muscle accretion after (resistance) exercise than soy protein in healthy subjects (8). BCAA are known as essential substrates and regulators of synthesis of body proteins (9). Intake of a single dose of whey protein after resistance exercise resulted in higher muscle protein synthesis rates than casein protein in young men (8). Studies on the acute of chronic effects of different proteins during or after exercise in COPD patients are scarce (10).
The anabolic differences found between casein, whey and soy protein in healthy subjects may largely be related to how quickly the proteins are digested (i.e., fast vs. slow) instead of the quality of the amino acid composition of the proteins. To eliminate the effect of a difference in absorption rate, we previously performed studies in COPD in which proteins were given in a “continuous” way by intake of frequent small meals. We observed that casein was more anabolic than soy protein in COPD (11, 12). The anabolic response during casein intake was even higher than during soy feeding to which BCAA were added to reach similar levels as present in casein (12). This suggests that other factors besides the high BCAA content are responsible for the high anabolic capacity of casein protein in patients with COPD. Interestingly, casein feeding was able to induce a higher level of protein anabolism in COPD than in control subjects (11), which was associated with a significantly lower splanchnic extraction of amino acids in the COPD group. It is still unclear whether casein proteinis also more favorable than whey protein in COPD suggesting a disease related effect as whey protein is often considered as the more favorable protein for increasing muscle protein synthesis in healthy elderly (8, 13, 14).
In the present study, we examined whether casein is preferable over whey protein in optimizing the response in protein metabolism during and following 20 min of constant cycle exercise in patients with COPD. We chose to provide the proteins via sip feeding instead of bolus feeding as this method is able to evaluate the effects of the quality of the amino acid composition of casein and whey protein by eliminating their differences in digestion rate. Although we realize that this pattern of sip feeding would more reflect an enteral feeding model, it will give important insight in whether use of casein hydrolysates might be an option to improve protein metabolism during physical activity in COPD in future studies.
Methods
Study population
A group of 8 COPD patients with moderate airflow obstruction and 8 healthy age-matched volunteers were studied. All subjects were men. All patients were in clinically stable condition and had moderate COPD GOLD stage 2 + 3 (15). The patients were not suffering from respiratory tract infection or exacerbation of their disease at least 4 weeks prior to the study. Exclusion criteria were malignancy, cardiac failure, distal arteriopathy, recent surgery, severe endocrine, hepatic or renal disorder, and use of oral corticosteroids 3 months preceding the study. The maintenance treatment of the studied COPD patients consisted of inhaled β2-agonists, inhaled anticholinergics, inhaled corticosteroids and/or oral theophylline. Written informed consent was obtained from all subjects and the study was approved by the medical ethics committee of the University Hospital Maastricht.
Study protocol
The protocol started in the early morning after an overnight fast for at least 8 hours. After insertion of a catheter into an antecubital vein, the first blood sample was taken for baseline measurements. A primed-constant continuous intravenous infusion of stable isotopes (80mL/h) was started with the use of a calibrated pump (IVAC Corporation, San Diego, CA) (L-[ring-2H5]-Phenylalanine: infusion rate= 0.053 μmol/kgFFM/min, priming dose= 2.19 μmol/kgBW; L-[ring-2H2]-Tyrosine: infusion rate= 0.018 μmol/kgFFM/min, priming dose= 0.95 μmol/kgBW). Primed infusion of L-[ring-2H4]-Tyrosine: priming dose= 0.31 μmol/kgBW was given in addition through the same catheter. L-[1-13C]-Phenylalanine was given orally with the protein meal in the prandial state every 20 min (prime: 0.88 μmol/kgBW; infusion: 0.055 μmol/kgFFM* min). The tracers were purchased from Cambridge Isotopic Laboratories (Woburn, MA, USA).
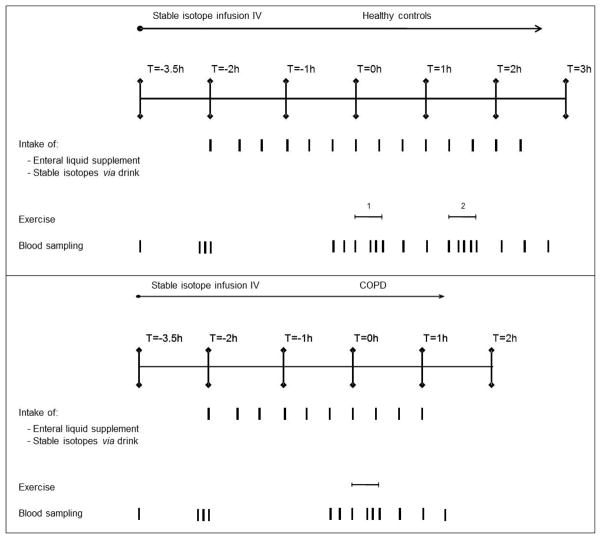
A second catheter was placed in a superficial dorsal vein of the hand of the contralateral arm, using the heated box technique (16). After 1.5 h of stable isotope infusion, enteral nutrition was started by sip feeding every 20 min. Three arterialized venous blood samples were taken just before start of the exercise bout (at t=−20, −10 and 0) to assure an isotopic steady state. At 220 min into infusion (t=0 for all groups) all subjects performed a submaximal exercise test on an electronically braked cycle ergometer (Cornival 400, Lode, Groningen, The Netherlands) for 20 minutes. Work rate for the COPD group was calculated as 50% of their own peak workload, as obtained during an incremental cycle exercise test, and the control group cycled at the average workload used in the COPD group. Pedaling frequency was between 60 and 70 rpm and held constant throughout the test. An infrared electrode was placed on a finger to measure oxygen saturation (Fasttrac, Sensor Medics Co., Anaheim, California). Heart rate was measured using a sport tester (PE3000, Polar Electro company, Kempele, Finland). After a recovery period of one hour, the healthy controls performed an additional cycle ergometer test at the same relative workload (50% of their own peak workload), followed by 60 minutes of recovery. In this way the control group had to cycle at two different workloads: one at the same mean absolute workload as the COPD group and the second at the same relative workload. The same relative exercise intensity was chosen as it is places a comparable metabolic strain on COPD patients and control subjects. The same absolute work rate was done because in real-life situations, subjects perform absolute work. Arterialized-venous blood was sampled at 10, 15, 20, 40, 60, and 80 minutes to measure kinetics during and after the exercise bout. In addition, blood was sampled at 85, 90, 95, 100, 120, 140 and 160 min in the healthy control group to measure kinetics during and after the second exercise bout (see Figure 1).
Figure 1.
Overview of study design for the healthy controls and the COPD group. The COPD group (lower panel) performed one exercise bout during 20 minutes at 50% of their own peak workload, followed by 60 min recovery. To get the same absolute workload for the control group, the control group (upper panel) cycled at a work rate corresponding to the average of the workload used in the COPD group (exercise bout 1). After a recovery period of one hour, the healthy controls performed an additional cycle ergometer test during 20 minutes at the same relative workload as the COPD subjects (50% of their own peak workload, exercise bout 2) followed by 60 minutes of recovery.
Body weight was measured using an electronic beam scale with digital readout to the nearest 0.1 kg (model 708; Seca) with the subjects standing barefoot and wearing light indoor clothing. Body height was measured to the nearest 0.1 cm (model 220, Seca). Body composition was measured using Bioelectrical Impedance Spectroscopy (BIS Xitron 4000B, Xitron Technologies, San Diego, USA) in order to express protein metabolism data per kg of fat-free mass (FFM). FFM was calculated using the regression equation described by Dey et al. (17). Between-group comparisons were done by adjusting body weight and FFM for differences in body height. For this purpose, these parameters were divided by squared height (kg/m2), as suggested by VanItallie (18) to obtain BMI and FFMI. Physical activity level was assessed by the Physical Activity Scale for the Elderly (PASE). This scale measures the level of physical activity in elderly individuals. PASE comprises measures of self-reported occupational, household, and leisure activities during a one week period (19).
Enteral protein meals
To avoid metabolic changes due to recent modifications of the diet, the subjects were instructed to eat their usual diet during at least 3 days preceding the study. The casein test meal on the experimental day contained 29.5 g sodium caseinate (4.0 g N, DMV International Veghel, The Netherlands) and 68.5g of maltodextrin (Glucodry 210, Amylum Europe, Aalst, The Netherlands), dissolved in ultrapure water to 1000 ml fluid at 60°C. The whey test meal on the experimental day contained 29.5 g whey protein isolate: 4.0 g N, DMV Campina The Netherlands) and 68.5 g of maltodextrin (Glucodry 210, Amylum Europe, Aalst, The Netherlands), dissolved in ultrapure water to 1000 ml fluid at 60°C. The nitrogen content was identical in both meals (4g). Furthermore, NaCl, KCl, CaCl2.H2O and NaH2OO4.H2O were added to obtain equal amounts of sodium, potassium, calcium and phosphor in both test meals if necessary. Both the casein and whey protein intakes consisted of a fluid ingestion of 0.67 mL/kg BW which contained 18 mg protein/kg BW and 46 mg maltodextrin/kg BW per 20 min. In total, about 553 ml enteral nutrition and 13.5 g protein (based on 10 ingestions and a 75 kg subject) was supplied to the COPD patients during the 5 h study, and 704 ml enteral nutrition and 18.9 g protein (based on 14 ingestions and a 75 kg subject) during the 6h 20 min study in the control subjects. When extrapolating to 24h, this would correspond to 0.9 g protein/kg bw * day which is equal to the previously reported habitual protein intake in COPD (11). All meals were prepared at least 1 hour before the start of the experiment and kept at 4°C until use. For amino acid composition of the meals, see Table 1.
Table 1.
Amino acid composition of the casein (Na-Caseinate) and whey protein (WPI) meal
| Casein protein | Whey protein | |
|---|---|---|
| g/100 g protein | ||
| ASP | 2.91 | 5.76 |
| ASN | 4.07 | 3.50 |
| GLU | 12.43 | 8.87 |
| GLN | 10.46 | 4.78 |
| SER | 6.88 | 3.24 |
| GLY | 1.82 | 1.53 |
| THR | 4.43 | 4.09 |
| HIS | 2.92 | 1.72 |
| ALA | 3.16 | 4.39 |
| ARG | 3.73 | 1.99 |
| TYR | 5.98 | 3.27 |
| VAL | 6.96 | 4.18 |
| MET | 2.98 | 1.89 |
| ILE | 6.06 | 5.17 |
| PHE | 5.24 | 3.08 |
| TRP | 1.21 | 2.25 |
| LEU | 9.33 | 11.11 |
| LYS | 7.98 | 9.15 |
| CYS | 0.27 | 3.31 |
| PRO | 11.23 | 3.13 |
| Sum BCAA | 22.36 | 20.46 |
| Sum EAA | 38.96 | 37.85 |
ASP: aspartate, ASN: asparagine, GLU: glutamate, GLN: glutamine, SER: serine, GLY: glycine, THR: threonine, HIS: histidine, ALA: alanine, ARG: arginine, TYR: tyrosine, VAL: valine, MET: methionine, ILE: isoleucine, PHE: phenylalanine, TRP: tryptophan, LEU: leucine, LYS: lysine, CYS: cysteine, PRO: proline. Sum BCAA: sum of LEU, VAL, and ILE. Sum EAA: sum of all essential amino acids.
Sample processing
Analysis of arterialized venous blood
Promptly after sampling, blood was distributed in pre-chilled, heparinized tubes (Becton Dickinson Vacutainer system, Franklin Lakes, New Jersey, USA) and kept on ice to minimize enzymatic reactions. All analyses were performed in plasma, obtained by centrifugation of whole blood at 4°C for 10 min at 3120 g. Plasma was deproteinized by mixing it with 20 mg dry sulfosalicylic acid, or with 90 μl of a 500 g/L trichloroacetic acid solution. All samples were stored at −80°C until further analysis.
Biochemical analysis
The enrichments (tracer-to-tracee ratios (TTR)) of the amino acids PHE and TYR in arterialized-venous plasma were analyzed by liquid chromatography mass spectrometry system (Thermoquest LCQ, Veenendaal, The Netherlands) (20). Plasma concentrations of amino acids were determined with the use of a fully automated High Performance Liquid Chromatography (HPLC, Pharmacia, Woerden, The Netherlands (21). Plasma glucose, lactate, urea and ammonia were analyzed spectrophotometrically on a COBAS Mira S (Roche Diagnostica, Hoffman-La Roche, Basel, Switserland) by standard enzymatic methods (22). Plasma insulin concentration was analysed with a commercially available electrochemiluminescence immunoassay (Hitachi Modular Analyzer; Roche, Mannheim, Germany).
Intra- and inter individual coefficients of variance (CV%) for isotope ratio measurements with the used LC-MS system were between 2 and 5% (21). For the measurements of the plasma amino acids, we reported CV% between 2 and 7% (23). For urea, glucose and lactate with approved, standardized methods, reported analytical CV% of 1.6% and 2.4%, respectively (24). For plasma ammonia, we used the technique of Rodger (25) with reported CV% of 5% and 8%, resp. The biological variation that we observed was larger than the reported CV% of the measured substances in plasma.
Calculations
All the metabolic data were determined under steady-state conditions. The sum of BCAA represents the sum of VAL, LEU and ILE. Tracer-tracee ratio of PHE and TYR reached an isotopic steady state within 1.5 hours of infusion in the post absorptive state and within 2 hours of feeding (data not shown) in both groups.
-
(1)
Whole body protein synthesis was calculated by subtracting hydroxylation of PHE to TYR from whole body rate of disappearance (=whole body Ra under steady-state) of PHE (=infusion rate/TTR-PHE5 in plasma) (26).
Splanchnic extraction (SPEPHE) represents the fraction (in %) of ingested phenylalanine, taken up by the gut and liver during its first pass and metabolized via oxidation or protein synthesis, calculated as (27):
-
(2)
Ra2H5-PHE and Ra13C-PHE represents whole body rate of appearances of phenylalanine calculated from I.V. 2H5-PHE en I.G. 13C-PHE isotopes, respectively.
Whole body rate of appearance of phenylalanine, not coming from PHE in protein given by the diet (endogenous phenylalanine (RaPHE endo)), is calculated by subtracting the corrected PHE intake from whole body phenylalanine rate of appearance, as represented in formulas (3) and (4).
-
(3)
-
(4)
-
(5)
-
(6)
During exercise and postexercise, whole body Ra PHEendo, the rate of disappearance of PHE (RdPHE) were calculated using the one-pool model non-steady state equations of Steele (28), modified for use with stable isotopes.
-
(7)
-
(8)
-
(9)
P: correction factor of the pool size for instant mixing (p=0.25 for PHE (plasma pool). Vd is the volume of distribution (total body water pool = 0.5 l/kgbody weight). (TTR2−TTR1)/(t2−t1) and (C2−C1)/(t2−t1) is the change in tracer to tracee ratio, and the change in plasma concentration of the tracee, respectively, between two time points.
WbPB and (net) WbPS were expressed in nmol kg FFM−1 min−1
Statistical analysis
Results are expressed as mean±standard error (SE). The mean value of the measures of protein kinetics and the concentrations of amino acids at the time points −20,−10, and 0 min was used as the fed state (at t=0). If data failed the normality or equal variance test, they were log-transformed where appropriate. The unpaired Student’s t test was used to determine differences in general characteristics between the COPD and control group. Subsequently, a repeated measures (mixed model) analysis of variance (ANOVA) was performed with a time effect for the two different phases during the experiment (exercise (t=0–20 min) and recovery (t=20–80 min)) for the COPD group and four different phases in the control group (exercise low intensity (t=0–20 min), recovery 1 (t=20–80 min), exercise high intensity (t=80–100 min) and recovery 2 (t=100–160 min). The level of significance was set at p<0.05, and P values are given for the time effect, protein effect, and the time × protein interaction.
Results
Age, height, body weight and BMI did not differ significantly between the COPD and control group and no recent involuntary weight loss was present in any of the subjects (Table 2). FFMI was significantly lower in the COPD group (p<0.05). The COPD patients were characterized by moderate airflow obstruction and mildly reduced diffusing capacity for CO. Furthermore reduced levels were found in the COPD group for FVC, and increased levels for ITGV and RV (p<0.05). In the control group, all lung function values were within the normal range. Physical activity level was significantly lower in the COPD than in the control group (118±18 vs. 148±17, p<0.01) as assessed by PASE questionnaire.
Table 2.
General characteristics of the COPD and control group
| Control (n=8) | COPD (n=8) | P values | ||
|---|---|---|---|---|
| Age | y | 63.1 ± 2.8 | 68.1 ± 2.2 | 0.186 |
| Height | m | 1.74 ± 0.02 | 1.74 ± 0.03 | 0.812 |
| Weight | kg | 77.5 ± 3.4 | 81.8 ± 3.3 | 0.376 |
| BMI | kg/m2 | 25.4 ± 0.8 | 27.2 ± 0.7 | 0.141 |
| FFMI | kg/m2 | 19.1 ± 0.5 | 17.1 ± 0.3 | 0.011 |
| FEV1 | % pred | 110 ± 4 | 50 ± 4 | 0.000 |
| Dlco | % pred | 104 ± 6 | 78 ± 6 | 0.010 |
| FVC | % pred | 116 ± 4 | 93 ± 8 | 0.026 |
| ITGV | % pred | 111 ± 7 | 146 ± 11 | 0.018 |
| TLC | % pred | 106 ± 5 | 115 ± 6 | 0.281 |
| RV | % pred | 98 ± 5 | 145 ± 19 | 0.047 |
Values are means ± SEM, n = 8/group. COPD: Chronic Obstructive Pulmonary Disease, BMI: body mass index, FFMI: Fat-free mass index, FEV1: forced expiratory volume in one second, Dlco: diffusing capacity for carbon monoxide, FVC: forced vital capacity; ITGV: intrathoracic gas volume; TLC: total lung capacity. There were no significant differences between the groups.
Work rate of the COPD group was set at 50% of the peak work load (Wpeak: 108±12 Watt). The mean absolute work rate of the COPD group (54 Watt) was used as the workload for the first exercise bout of the control group and corresponded to 25% of its own peak workload of the control group (Wpeak: 212±22 Watt). After 1 hour of recovery, the control subjects performed a second exercise bout at a workload corresponding to 50% of their own peak workload. Heart rate was significantly higher during exercise in both groups (p<0.01), independent of the type of protein ingested (data not shown). Heart rate was higher in the COPD than in the control group during the first exercise bout (p<0.05) and comparable to that during the second exercise bout. Transcutaneous O2 saturation was not significantly different during exercise and between both proteins (data not shown).
Protein turnover and splanchnic extraction
Prandial pre-exercise state
The COPD group had significantly higher values for WbPS and WbPB (p<0.001) and lower values for SPE (p<0.01) than the control group during both casein and whey feeding (Figure 2–5). NetPS was higher in the COPD than in the control during casein (p<0.01) but not during whey feeding. Furthermore, NetPS was higher during casein than whey feeding in the COPD (p<0.01) and the control group (p<0.05) but no significant differences were found in WbPB or WbPS.
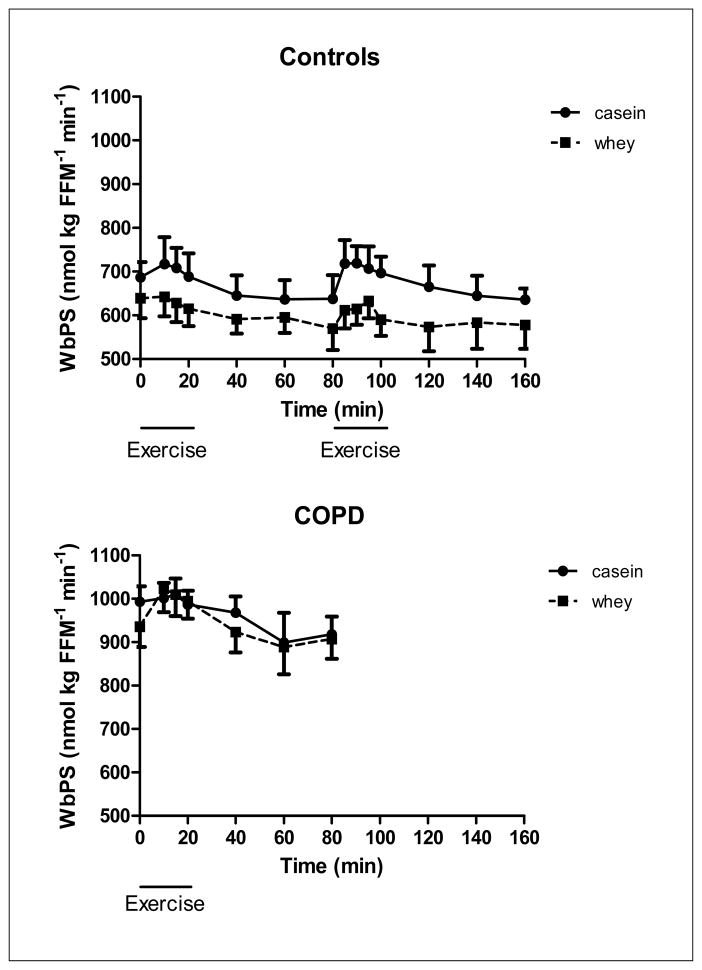
Figure 2.
Changes in whole body protein synthesis of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant protein effect during exercise and recovery in the healthy control group (p<0.05), independent of the work intensity, but not in the COPD group. There was no significant time effect and no significant protein by time interaction during exercise and recovery in the COPD and control group.
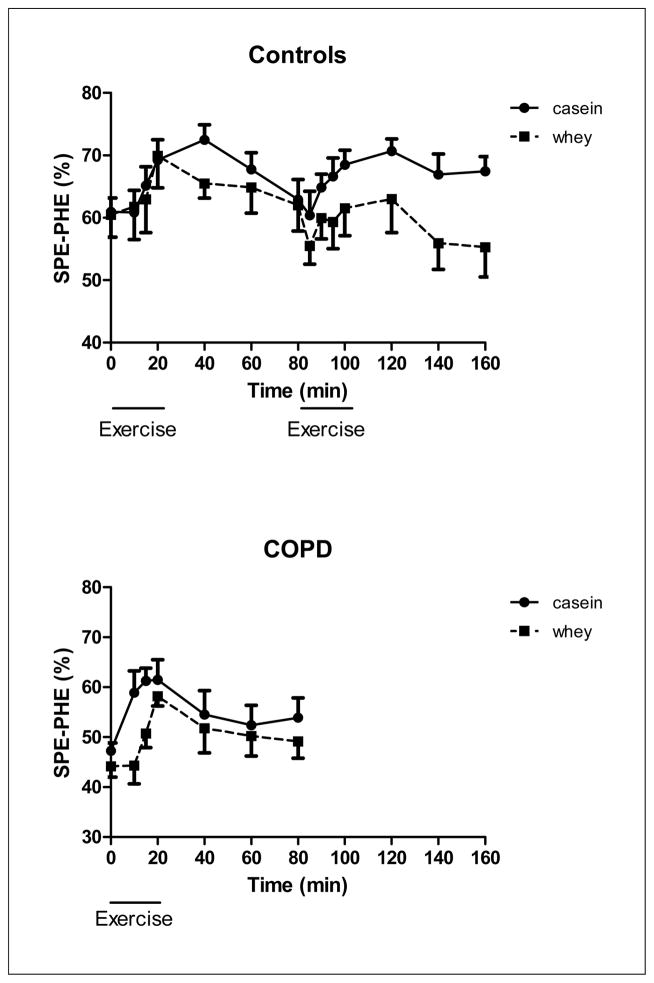
Figure 5.
Changes in splanchnic extraction of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant protein effect during exercise in the COPD group (p<0.001) and during high intensity exercise and recovery (p<0.05) in the healthy control group. Furthermore, there was a significant time effect (p<0.001) during exercise but not in recovery in the COPD group. There was no significant time effect in the control group and no significant protein by time interaction in the COPD and control group.
Exercise and recovery
Control subjects
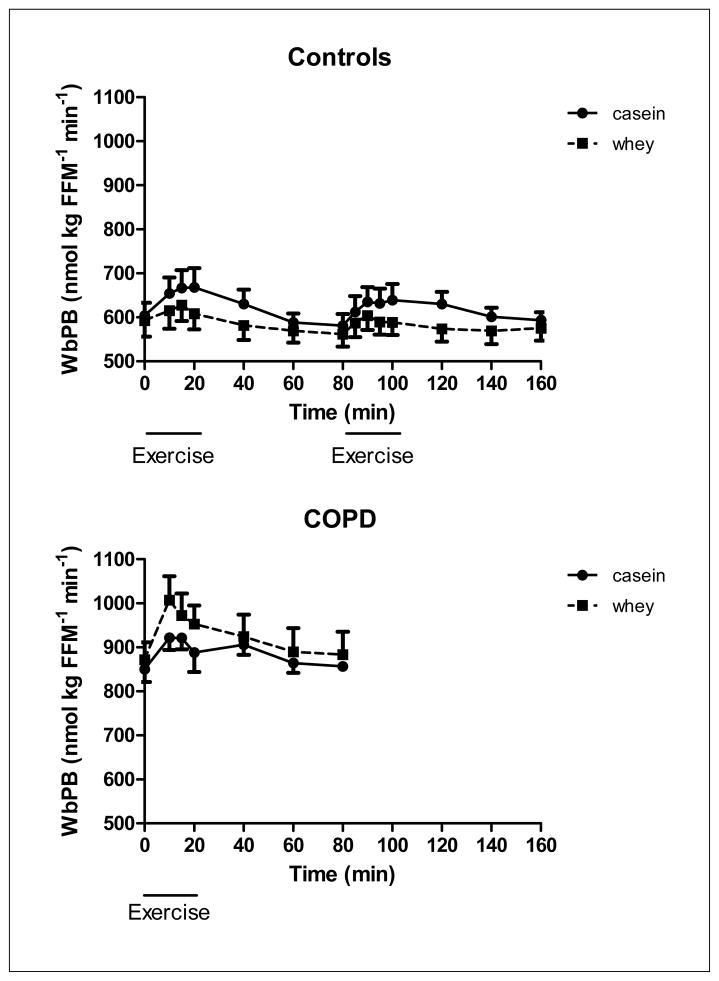
In the healthy control group, WbPS (Figure 2) was significantly higher during casein feeding throughout the study (p<0.05) and independent of the exercise intensity and recovery phase. There was a protein effect for WbPB (Figure 3) in recovery from high intensity exercise (p<0.05) indicating that higher values were obtained during casein feeding. No significant protein by time interactions were observed for WbPS or WbPB. Higher values for NetPS (p<0.05, Figure 4) during casein feeding were also present in the healthy control group during and after high intensity exercise. Although no significant time effect was observed, the average NetPS values were lower at the end of the recovery period (60 min after exercise) as compared to pre-exercise levels during intake of both protein meals (casein:−48%, whey: −95%). Due to the fact that pre-exercise levels were lower during whey as compared to casein feeding, NetPS during whey feeding was not different from zero during recovery from high intensity exercise. Higher levels for SPE (Figure 5a) were found during casein feeding only during high intensity exercise (p<0.05) and recovery from high intensity exercise (p<0.001).
Figure 3.
Changes in whole body protein breakdown of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant protein effect during exercise (p<0.05) in the COPD group and a tendency towards a significant time effect during exercise (P=0.06). In the control group, there was a protein effect during recovery from heavy exercise (P<0.05) and a tendency towards a protein effect during heavy exercise (p=0.09). There was no time effect in the healthy control group. There was no significant protein by time interaction during exercise and recovery in the COPD and control group.
Figure 4.
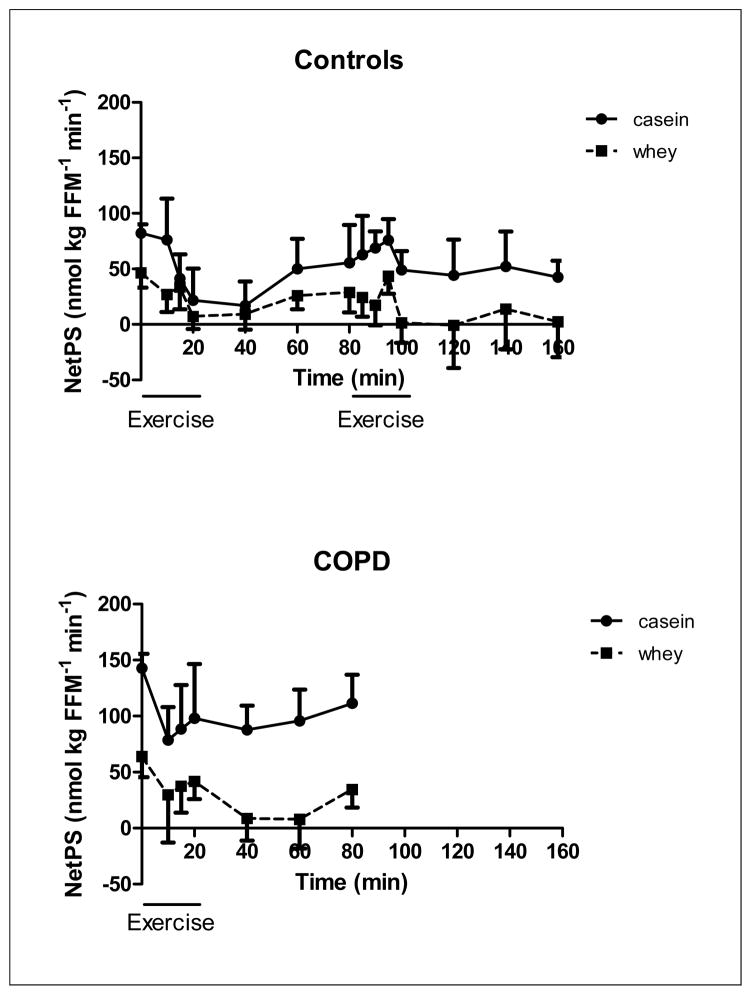
Changes in Net whole body protein synthesis of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant protein effect during exercise and recovery in the COPD and control group (p<0.01). There was a significant time effect during both exercise and recovery in the COPD group (p<0.001) but during heavy exercise and recovery in the control group (p<0.05). There was no significant protein by time interaction during exercise and recovery in the COPD and control group. The absolute response in Net PS during intake of the whey protein meal was different from 0 (NetPS=0) during exercise and recovery in the COPD group (P<0.05) and during the whole study period in the healthy control group (P<0.01) with the exception of recovery from heavy exercise.
COPD
WbPS during exercise and recovery was not different between casein and whey protein feeding, and there was a tendency towards a time effect (p=0.07) (Figure 2b). A protein effect (P<0.05) and a tendency towards a time effect (P=0.06) was present in WbPB during exercise (Figure 3b) but not in recovery, indicating that exercise increased WbPB in the COPD group, and higher values were present during whey feeding during exercise.
There was a protein (p<0.001) but no time effect in NetPS (Figure 4b) in the COPD group during exercise and recovery. Although no significant time effect was observed in the COPD group, the average NetPS values were lower at the end of the recovery period (60 min after exercise) as compared to pre-exercise levels during intake of both protein meals (casein: −22%, whey:−46%).
There was a protein (p<0.001) and time (p<0.001) effect for SPE in the COPD group (Figure 5b) during exercise but not in recovery indicating that exercise increased SPE and higher values in SPE were found during casein than during whey feeding.
Plasma amino acids and metabolites
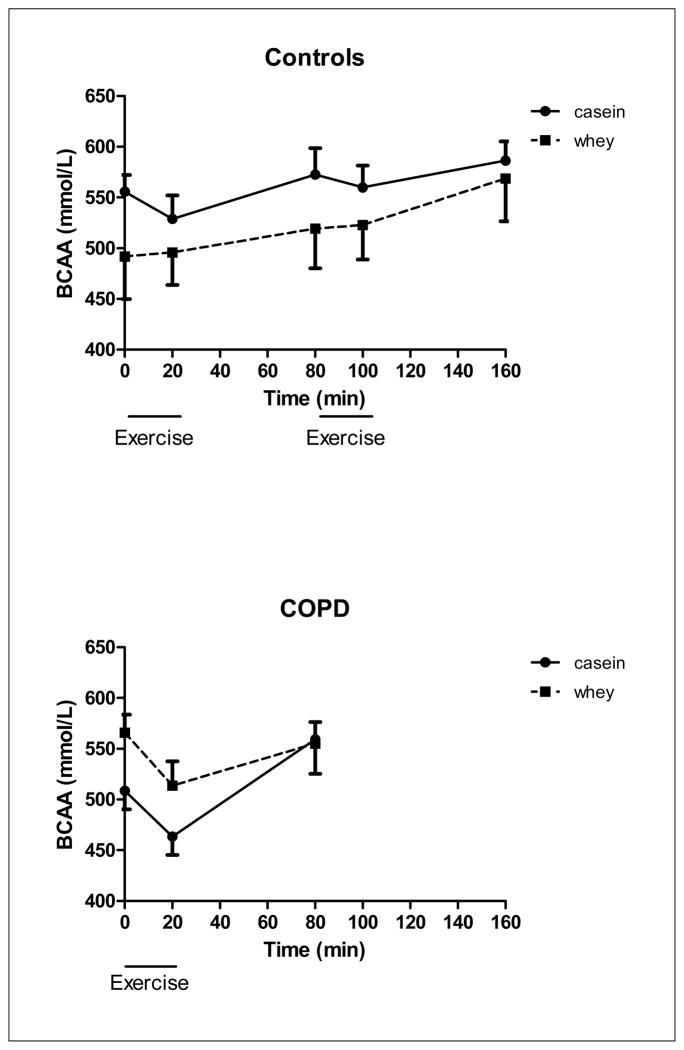
There was a protein effect for plasma sum BCAA (Figure 6b), LEU and PHE (data not shown), indicating higher values for PHE and lower values for sum BCAA and LEU after casein feeding. Furthermore, there was a time effect for plasma sum BCAA and LEU (p<0.05), indicating that reduced values were found during exercise which normalized in recovery in the COPD group. In the control group, there was no protein and time effect for PHE and Sum BCAA (Figure 6a).
Figure 6.
Changes in BCAA concentration of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant protein effect during exercise in the COPD group (p<0.05). Furthermore, there was a significant time effect (p<0.05) during exercise and recovery in the COPD group. There was no significant protein or time effect during exercise and recovery in the control group, and there was no significant protein by time interaction in the COPD and control group.
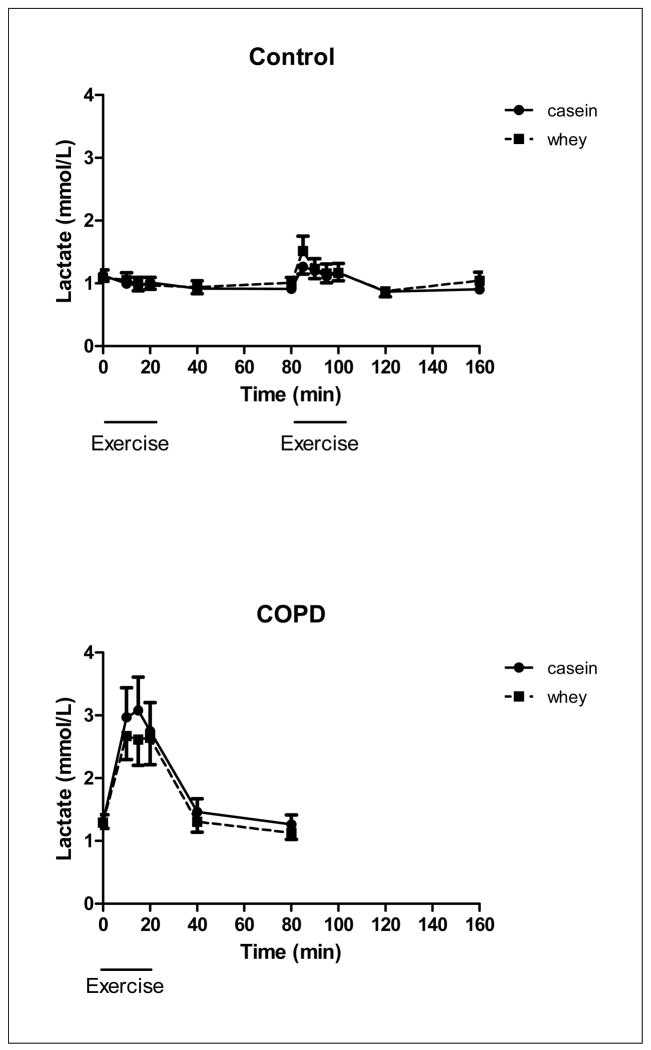
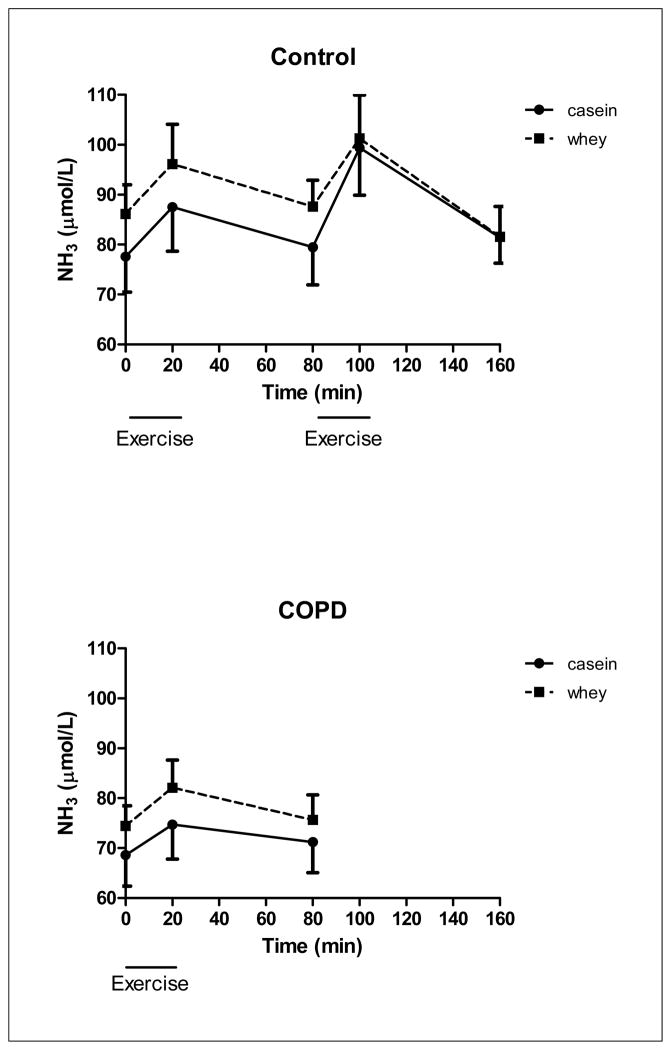
There was a time but no protein effect for lactate (Figure 7) during exercise and recovery in the COPD group (p<0.001) and during recovery from high intensity exercise in the control group indicating that higher lactate levels independent of the protein ingested. However lactate during exercise was significantly higher in the COPD group than obtained during the low and high intensity exercise in the control group (p<0.05). There was no protein and time effect for NH3 (Figure 8) in the COPD group. There was no protein effect for NH3 in the control group but a time effect during high intensity exercise and recovery from high intensity exercise (p<0.05), indicating that higher NH3 levels during high intensity exercise and recovery.
Figure 7.
Changes in lactate concentration of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Repeated measures mixed model ANOVA: there was a significant time effect during exercise and recovery in the COPD group (p<0.001) and during recovery from high intensity exercise in the control group (p<0.05). There was no significant protein or protein by time interaction in the COPD and control group.
Figure 8.
Changes in NH3 concentration of the healthy control group (upper panel, n=8) during 20 minutes of low and high intensity exercise both followed by 1 hour of recovery, and the COPD group (lower panel, n=8) during 20 minutes of exercise and 1 hour of recovery, during intake of a casein protein meal (circles) vs. a whey protein meal (squares) provided every 20 min until the end of the test day. Mean values ± 1 SE are shown. Simple factorial ANOVA: there was a significant time effect during exercise and recovery during high intensity exercise in the control group (p<0.05). There was no significant protein or protein by time interaction in the COPD and control group.
Discussion
The present study shows that net whole body protein anabolism was higher during casein than during whey protein feeding in both the COPD and control group which remained during and following exercise. The proteins were provided via sip feeding to evaluate the effects of the quality of the amino acid composition between casein and whey protein. The study indicates that the amino acid composition of casein is preferable over whey protein in inducing and maintaining protein anabolism during and following exercise in COPD.
The COPD patients had higher pre-exercise levels for protein turnover during both casein and whey protein feeding than the healthy elderly subjects. Enhanced protein turnover in COPD is an energy consuming process as it is associated with higher levels for resting energy expenditure (29). This enhanced energy expenditure can be diminished by regular physical activity as it is able to counteract the increased protein turnover in COPD (30). The enhanced anabolic response to casein intake in COPD is in line with our previous study also showing higher levels after intake of soy and a soy meal enriched with BCAAs (12) in COPD than in healthy subjects. The increase in net protein synthesis after whey intake was lower than after casein (despite a similar protein and nitrogen load) in the COPD and healthy groups. This difference in anabolism might be related to differences in BCAA distribution as LEU level is higher in whey than in casein protein, whereas the concentrations of ILE and VAL are lower.
Previously, we observed an increase in whole body protein breakdown during a comparable exercise study but without feeding in COPD and healthy subjects (3). Furthermore, amino acid levels were reduced in muscle and elevated in plasma after exercise in the COPD group (3), suggesting an increased amino acid efflux from muscle. To avoid exercise induced catabolism, insight in the effects of protein feeding during exercise in COPD is therefore warranted. In the present study, an exercise-induced increase in whole body protein breakdown was observed in COPD during both casein and whey protein feeding. Evidence exists that besides muscle, the gut is a site of protein catabolism and acts as a source of the increased rate of appearance during exercise (31, 32) via releasing α-amino nitrogen as a result of increased gut protein degradation (31). BCAAs are released from splanchnic tissues at an elevated rate during exercise (32) and serve as energy source for the contracting skeletal muscle. Furthermore, breakdown of labile gut protein will provide essential amino acids that may also be used for the attenuation of muscle protein catabolism at a later stage. In this way the gut sacrifices itself so that skeletal muscle protein may be spared (33). The increased gut proteolysis during exercise is likely related to the change in the distribution of the blood flow from the splanchnic bed to skeletal muscle and decreased splanchnic perfusion (34). Nicotine use and certain medications are factors known to reduce splanchnic blood flow (35, 36). It is unlikely that the presence of hypercapnia or hypoxia during exercise in COPD will significantly alter splanchnic blood flow or indices of perfusion (37, 38). The increased SPE confirms the important role of the gut during exercise and suggests that the exercise induced proteolysis in the gut may be diminished by supplementing the most optimal amino acid composition to the gut. In line, we observed that the type of protein actually mattered as SPE was higher during casein than during whey feeding in the COPD group during exercise, suggesting that casein protein stimulates gut anabolism more than whey protein. It could well be that the amino acid requirements of the gut during exercise are better matched by casein than by whey protein in COPD.
Whey as well as casein protein did not modify the previously observed exercise induced response in protein synthesis in COPD (4). The elevated protein synthesis during casein feeding in the control group could be explained by the elevated free intracellular amino acid pool of amino acids coming from protein intake, protein breakdown in the gut (31), and possibly from skeletal muscle proteolysis immediately after exercise (39, 40). The importance of the transfer of amino acids from gut to muscle and vice versa remains unclear. Immediately after exercise, the gut will restore its prior exercise-induced losses by using a portion of the protein meal for its own anabolism before releasing whatever is left to the peripheral tissues. If the high SPE of casein indicates that this protein is a more anabolic protein for the gut (11) and thus can restore gut protein pools quicker after exercise, it is likely that more essential amino acids will become available for muscle anabolism early in recovery.
The average NetPS values were lower at the end of the recovery period as compared to pre-exercise levels during intake of both protein meals. As pre-exercise levels were already lower during whey feeding, NetPS levels during recovery from high intensity exercise did not reach positive values during whey feeding anymore in the control group, reflecting absence of protein anabolism, and less protein anabolism in the COPD group at 60 minutes postexercise. These data suggest that casein is able to better maintain protein anabolism than whey protein after exercise.
Recently it has been shown that more of the ingested protein-derived amino acids were incorporated in newly synthesized muscle protein when exercise was performed before food intake (14). Another study showed that consumption of a protein drink after aerobic exercise (at 50% VO2max) increased whole body protein turnover in older adults during the third and fourth hour of postexercise recovery (41). The several fold increase in the uptake of LEU across muscle during exercise remained elevated hours after exercise, which likely is due to an accelerated amino acid transport into cells and increase blood flow which increases substrate availability of free amino acids for protein synthesis (42, 43). Unraveling the optimal combination of exercise, type of protein feeding, and timing of feeding in relation to exercise is therefore important when trying to achievemaximal protein anabolism in COPD.
In the present study, higher lactate levels were found during exercise in COPD, indicating early lactic acidosis (44). This and the recently observed enhanced glucose turnover (45), indicates an increased exercise induced metabolic stress in COPD. Epiphenomena of COPD such as reduced skeletal muscle oxidative capacity, relative hypoxemia, and sympathetic activation may contribute to this observation (46). The lactate levels during exercise were comparable during casein and whey feeding, suggesting no difference in aerobic power. Also NH3, which accumulates when there is excessive anaerobic and reduced oxidative energy production during exercise, was not different between both proteins. Acute differences in physical capacity after casein and whey intake are therefore not expected. Interestingly, a recent study showed that supplementation of pressured whey but not casein protein, in combination with 8 weeks of exercise training was able to increase endurance tolerance in COPD but did not improve quadriceps strength and muscle mass or the occurrence of quadriceps contractile fatigue (10). However, highly enriched pressured whey (47) was compared to regular casein protein on a per gram basis (10), but not given isonitrogenously as in our study, and significantly more men were studied during whey than casein feeding. The increased tolerance after pressured whey feeding was attributed to reduced central but not contractile fatigue.
Conclusions
Physical activity plays an important role in daily life and during pulmonary rehabilitation in patients with COPD. This study in a relatively small number of COPD patients shows that casein protein is able to enhance protein anabolism at rest and maintain it during and following exercise in COPD. This suggests that optimization of dietary habits during a physical active lifestyle in COPD might have an important impact on muscle maintenance in daily life of these patients. In the present study, the casein and whey protein supplements were provided as small sip feeds to adjust for a digestion difference between the proteins. Although we realize that this pattern of protein feeding represents more an enteral feeding model instead of a daily life bolus meal, it shows that use of protein hydrolysates might be an option to improve protein anabolism in COPD. Future studies are needed in a larger group of COPD patients to examine whether chronic bolus supplementation of casein hydrolysates in combination with exercise training will increase muscle mass, induce functional changes and enhance physical capacity in COPD.
Acknowledgments
Funding
The project described was supported in part by a grant from the European Diary Association, Brussels, Belgium (unrestricted grant), and by Award number R-01HL095903 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent official views of the NHLB Institute or the National Institutes of Health.
List of abbreviations
- COPD
Chronic Obstructive Pulmonary Disease
- WBPB
Whole body protein breakdown
- WBPS
Whole body protein synthesis
- NetPS
Net Protein synthesis
- PS
Protein synthesis
- PASE
Physical Activity Scale for the Elderly
- SPE
splanchnic extraction
- BCAA
branched-chain amino acids
- FEV1
forced expiratory volume in one second
- BW
Body weight
- FFM
Fat-free mass
- NH3
Ammonia
- LEU
Leucine
- ILE
Isoleucine
- VAL
Valine
- PHE
phenylalanine
- TYR
Tyrosine Sum
- AA
Sum amino acids
- Ra
Rate of appearance
- Rd
Rate of disappearance
- SE
Standard error
Footnotes
Disclosure statement
The authors declare that they have no competing interests.
Authors’ contributions
ME was responsible for the study design, data collection, data analysis and writing of the manuscript; EW and AS for the study design and review of manuscript; ND for the study design, data analysis and writing the manuscript, ER and CDC performed the data collection.
Clinical Trial Registration Nr: NCT01418469
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- 2.Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1268–74. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 3.Engelen MP, Wouters EF, Deutz NE, Does JD, Schols AM. Effects of exercise on amino acid metabolism in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(4):859–64. doi: 10.1164/ajrccm.163.4.2006137. [DOI] [PubMed] [Google Scholar]
- 4.Engelen MP, Deutz NE, Mostert R, Wouters EF, Schols AM. Response of whole-body protein and urea turnover to exercise differs between patients with chronic obstructive pulmonary disease with and without emphysema. Am J Clin Nutr. 2003;77(4):868–74. doi: 10.1093/ajcn/77.4.868. [DOI] [PubMed] [Google Scholar]
- 5.Luiking YC, Engelen MP, Soeters PB, Boirie Y, Deutz NE. Differential metabolic effects of casein and soy protein meals on skeletal muscle in healthy volunteers. Clinical nutrition (Edinburgh, Scotland) 30(1):65–72. doi: 10.1016/j.clnu.2010.06.012. Epub 2010/08/06. [DOI] [PubMed] [Google Scholar]
- 6.Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, et al. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr. 2003;133(5):1308–15. doi: 10.1093/jn/133.5.1308. Epub 2003/05/06. [DOI] [PubMed] [Google Scholar]
- 7.Luiking YC, Deutz NE, Jakel M, Soeters PB. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J Nutr. 2005;135(5):1080–7. doi: 10.1093/jn/135.5.1080. [DOI] [PubMed] [Google Scholar]
- 8.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107(3):987–92. doi: 10.1152/japplphysiol.00076.2009. Epub 2009/07/11. [DOI] [PubMed] [Google Scholar]
- 9.Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care. 2001;4(1):39–43. doi: 10.1097/00075197-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Laviolette L, Lands LC, Dauletbaev N, Saey D, Milot J, Provencher S, et al. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. Journal of medicinal food. 2010;13(3):589–98. doi: 10.1089/jmf.2009.0142. Epub 2010/06/05. [DOI] [PubMed] [Google Scholar]
- 11.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Altered interorgan response to feeding in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(2):366–72. doi: 10.1093/ajcn.82.2.366. [DOI] [PubMed] [Google Scholar]
- 12.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85(2):431–9. doi: 10.1093/ajcn/85.2.431. [DOI] [PubMed] [Google Scholar]
- 13.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, et al. The rate of protein digestion affects protein gain differently during aging in humans. The Journal of physiology. 2003;549(Pt 2):635–44. doi: 10.1113/jphysiol.2002.036897. Epub 2003/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr. 2011;93(2):322–31. doi: 10.3945/ajcn.2010.29649. Epub 2010/11/19. [DOI] [PubMed] [Google Scholar]
- 15.Fabbri LM, Hurd SS. Global Strategy for the Diagnosis, Management and Prevention of COPD: 2003 update. Eur Respir J. 2003;22(1):1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 16.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism: clinical and experimental. 1981;30(9):936–40. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 17.Dey DK, Bosaeus I, Lissner L, Steen B. Body composition estimated by bioelectrical impedance in the Swedish elderly. Development of population-based prediction equation and reference values of fat-free mass and body fat for 70- and 75-y olds. European journal of clinical nutrition. 2003;57(8):909–16. doi: 10.1038/sj.ejcn.1601625. [DOI] [PubMed] [Google Scholar]
- 18.VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–9. doi: 10.1093/ajcn/52.6.953. Epub 1990/12/01. [DOI] [PubMed] [Google Scholar]
- 19.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. Journal of clinical epidemiology. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 20.van Eijk HM, Deutz NE. The liquid chromatography mass spectrometry approach to measure amino acid isotope ratios. Curr Opin Clin Nutr Metab Care. 2004;7(5):557–63. doi: 10.1097/00075197-200409000-00008. Epub 2004/08/06. [DOI] [PubMed] [Google Scholar]
- 21.van Eijk HM, Suylen DP, Dejong CH, Luiking YC, Deutz NE. Measurement of amino acid isotope enrichment by liquid chromatography mass spectroscopy after derivatization with 9-fluorenylmethylchloroformate. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1–2):48–56. doi: 10.1016/j.jchromb.2007.05.020. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 22.Neeley WE, Phillipson J. Automated enzymatic method for determining ammonia in plasma, with 14-day reagent stability. Clinical chemistry. 1988;34(9):1868–9. [PubMed] [Google Scholar]
- 23.van Eijk HM, Rooyakkers DR, Deutz NE. Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2–3 microns Spherisorb ODS II column. Journal of chromatography. 1993;620(1):143–8. doi: 10.1016/0378-4347(93)80062-9. [DOI] [PubMed] [Google Scholar]
- 24.Widjaja A, Morris RJ, Levy JC, Frayn KN, Manley SE, Turner RC. Within- and Between-Subject Variation in Commonly Measured Anthropometric and Biochemical Variables. Clinical chemistry. 1999;45(4):561–6. [PubMed] [Google Scholar]
- 25.Rodger MR, Jenkins P. Enzymic fluorometric assay of plasma ammonia with a centrifugal analyzer. Clinical chemistry. 1984;30(10):1670–2. [PubMed] [Google Scholar]
- 26.Engelen MP, Deutz NE, Wouters EF, Schols AM. Enhanced levels of whole-body protein turnover in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1488–92. doi: 10.1164/ajrccm.162.4.2002045. [DOI] [PubMed] [Google Scholar]
- 27.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. The American journal of physiology. 1999;277(3 Pt 1):E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 28.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–30. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 29.Kao CC, Hsu JW, Bandi V, Hanania NA, Kheradmand F, Jahoor F. Resting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary disease. Metabolism: clinical and experimental. 2011 doi: 10.1016/j.metabol.2011.02.013. Epub 2011/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen AM, Mittendorfer B, Magkos F, Iversen M, Pedersen BK. Physical activity counteracts increased whole-body protein breakdown in chronic obstructive pulmonary disease patients. Scandinavian journal of medicine & science in sports. 2008;18(5):557–64. doi: 10.1111/j.1600-0838.2007.00727.x. Epub 2007/12/11. [DOI] [PubMed] [Google Scholar]
- 31.Williams BD, Wolfe RR, Bracy DP, Wasserman DH. Gut proteolysis contributes essential amino acids during exercise. The American journal of physiology. 1996;270(1 Pt 1):E85–90. doi: 10.1152/ajpendo.1996.270.1.E85. [DOI] [PubMed] [Google Scholar]
- 32.Hamada K, Matsumoto K, Okamura K, Doi T, Minehira K, Shimizu S. Effect of amino acids and glucose on exercise-induced gut and skeletal muscle proteolysis in dogs. Metabolism: clinical and experimental. 1999;48(2):161–6. doi: 10.1016/s0026-0495(99)90027-6. Epub 1999/02/19. [DOI] [PubMed] [Google Scholar]
- 33.Soeters PB, de Jong CH, Deutz NE. The protein sparing function of the gut and the quality of food protein. Clinical nutrition (Edinburgh, Scotland) 2001;20(2):97–9. doi: 10.1054/clnu.2000.0376. [DOI] [PubMed] [Google Scholar]
- 34.Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, et al. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63(6):2269–77. doi: 10.1152/jappl.1987.63.6.2269. Epub 1987/12/01. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto T, Yoneda M, Shimada T, Kurosawa M, Terano A. Intraportal nicotine infusion in rats decreases hepatic blood flow through endothelin-1 and both endothelin A and endothelin B receptors. Toxicology and applied pharmacology. 2004;196(1):1–10. doi: 10.1016/j.taap.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Wennmalm A, Carlsson I, Edlund A, Eriksson S, Kaijser L, Nowak J. Central and peripheral haemodynamic effects of non-steroidal anti-inflammatory drugs in man. Archives of toxicology Supplement = Archiv fur Toxikologie. 1984;7:350–9. doi: 10.1007/978-3-642-69132-4_58. [DOI] [PubMed] [Google Scholar]
- 37.Kiefer P, Nunes S, Kosonen P, Takala J. Effect of an acute increase in PCO2 on splanchnic perfusion and metabolism. Intensive care medicine. 2001;27(4):775–8. doi: 10.1007/s001340100898. [DOI] [PubMed] [Google Scholar]
- 38.Rowell LB, Blackmon JR. Human cardiovascular adjustments to acute hypoxaemia. Clinical physiology (Oxford, England) 1987;7(5):349–76. doi: 10.1111/j.1475-097x.1987.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 39.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. The American journal of physiology. 1995;268(3 Pt 1):E514–20. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. The American journal of physiology. 1997;273(1 Pt 1):E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 41.Murphy C, Miller BF. Protein consumption following aerobic exercise increases whole-body protein turnover in older adults. Appl Physiol Nutr Metab. 35(5):583–90. doi: 10.1139/H10-047. Epub 2010/10/22. [DOI] [PubMed] [Google Scholar]
- 42.Clark MG, Rattigan S, Barrett EJ. Nutritive blood flow as an essential element supporting muscle anabolism. Curr Opin Clin Nutr Metab Care. 2006;9(3):185–9. doi: 10.1097/01.mco.0000222097.90890.c2. Epub 2006/04/12. [DOI] [PubMed] [Google Scholar]
- 43.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. The American journal of physiology. 1997;273(1 Pt 1):E122–9. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 44.Engelen MP, Schols AM, Does JD, Gosker HR, Deutz NE, Wouters EF. Exercise-induced lactate increase in relation to muscle substrates in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(5):1697–704. doi: 10.1164/ajrccm.162.5.9910066. [DOI] [PubMed] [Google Scholar]
- 45.Franssen FM, Sauerwein HP, Ackermans MT, Rutten EP, Wouters EF, Schols AM. Increased postabsorptive and exercise-induced whole-body glucose production in patients with chronic obstructive pulmonary disease. Metabolism: clinical and experimental. doi: 10.1016/j.metabol.2010.09.004. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 46.Gosker HR, van Mameren H, van Dijk PJ, Engelen MP, van der Vusse GJ, Wouters EF, et al. Skeletal muscle fibre-type shifting and metabolic profile in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;19(4):617–25. doi: 10.1183/09031936.02.00762001. [DOI] [PubMed] [Google Scholar]
- 47.Vilela RM, Lands LC, Chan HM, Azadi B, Kubow S. High hydrostatic pressure enhances whey protein digestibility to generate whey peptides that improve glutathione status in CFTR-deficient lung epithelial cells. Molecular nutrition & food research. 2006;50(11):1013–29. doi: 10.1002/mnfr.200600074. Epub 2006/10/21. [DOI] [PubMed] [Google Scholar]