Abstract
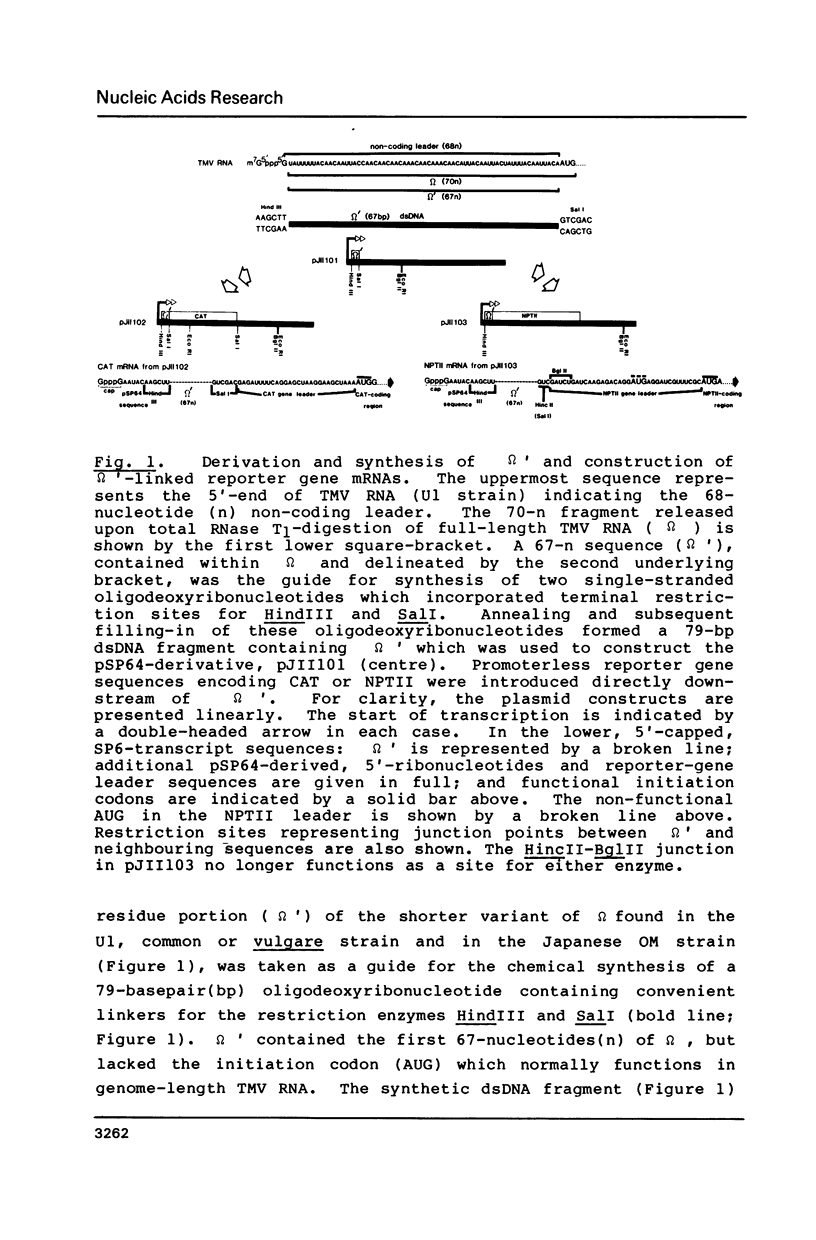
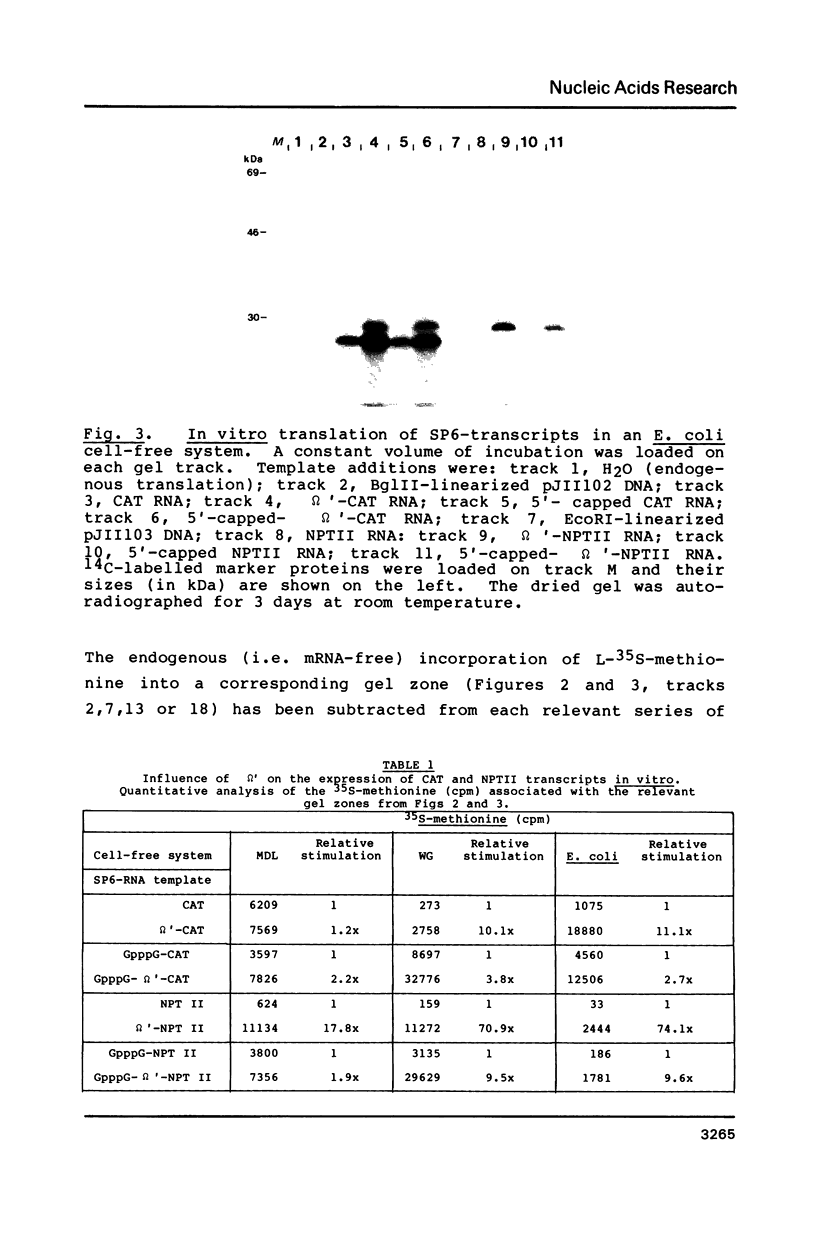
A 67-nucleotide portion of the non-coding, 5'-leader sequence of tobacco mosaic virus RNA [defined as omega' (Gr. omega prime)] has been shown to enhance the translation of contiguous foreign gene transcripts both in vitro and in vivo. Chemically-synthesized omega', containing convenient linker sequences, was inserted into derivatives of an in vitro transcription plasmid (pSP64) between the bacteriophage-SP6 promoter and sequences coding for either chloramphenicol acetyltransferase (CAT) or neomycin phosphotransferase (NPTII). Run-off in vitro transcripts, with or without a 5'-cap structure (G(5')ppp(5')G) and/or the omega' sequence, were tested in mRNA-dependent cell-free translation systems derived from rabbit reticulocyte lysate, wheat germ extract or Escherichia coli (MRE 600). In all cases, the presence of omega' increased the translational expression of both reporter genes, typically between 2- to 10-fold. Electroporation of isolated mesophyll protoplasts from Nicotiana tabacum cv. Xanthi, or microinjection of oocytes from Xenopus laevis, with SP6-transcripts containing the CAT-coding region confirmed and extended the value of omega' as a potential translational enhancer of gene expression in vivo.
Full text
PDF














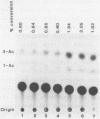
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Close T. J., Rodriguez R. L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982 Dec;20(2):305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Coleman J., Inouye M., Nakamura K. Mutations upstream of the ribosome-binding site affect translational efficiency. J Mol Biol. 1985 Jan 5;181(1):139–143. doi: 10.1016/0022-2836(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond D. R., Armstrong J., Colman A. The effect of capping and polyadenylation on the stability, movement and translation of synthetic messenger RNAs in Xenopus oocytes. Nucleic Acids Res. 1985 Oct 25;13(20):7375–7394. doi: 10.1093/nar/13.20.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron D., Marcus A. Translation of TMV-RNA in a cell-free wheat embryo system. Virology. 1973 Jun;53(2):343–348. doi: 10.1016/0042-6822(73)90212-2. [DOI] [PubMed] [Google Scholar]
- Gallie D. R., Hagiya M., Kado C. I. Analysis of Agrobacterium tumefaciens plasmid pTiC58 replication region with a novel high-copy-number derivative. J Bacteriol. 1985 Mar;161(3):1034–1041. doi: 10.1128/jb.161.3.1034-1041.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfin D. E., Mandeles S. Sequences of oligonucleotides prepared from tobacco mosaic virus ribonucleic acid. Virology. 1975 Apr;64(2):388–399. doi: 10.1016/0042-6822(75)90115-4. [DOI] [PubMed] [Google Scholar]
- Glover J. F., Wilson T. M. Efficient translation of the coat protein cistron of tobacco mosaic virus in a cell-free system from Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):485–492. doi: 10.1111/j.1432-1033.1982.tb06463.x. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harland R., Weintraub H. Translation of mRNA injected into Xenopus oocytes is specifically inhibited by antisense RNA. J Cell Biol. 1985 Sep;101(3):1094–1099. doi: 10.1083/jcb.101.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. False starts in translational control of gene expression. Nature. 1985 Aug 15;316(6029):580–581. doi: 10.1038/316580a0. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowland J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics. 1974 Sep;78(1):383–394. doi: 10.1093/genetics/78.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Binding of ribosomes to linear and circular forms of the 5'-terminal leader fragment of tobacco-mosaic-virus RNA. Eur J Biochem. 1981 Feb;114(2):221–227. doi: 10.1111/j.1432-1033.1981.tb05139.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandeles S. Location of unique sequences in tobacco mosaic virus ribonucleic acid. J Biol Chem. 1968 Jul 10;243(13):3671–3674. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Takamatsu N., Ohno T., Okada Y. The 5'-terminal sequence of TMV RNA. Question on the polymorphism found in vulgare strain. FEBS Lett. 1983 Oct 17;162(2):282–285. doi: 10.1016/0014-5793(83)80772-8. [DOI] [PubMed] [Google Scholar]
- Mundry K. W. A model of the coat protein cistron of tobacco mosaic virus and its biochemical investigation: the model the experimental approach, and the isolation of a long oligonucleotide from TMV-RNA. Z Vererbungsl. 1965;97(3):281–296. doi: 10.1007/BF02035933. [DOI] [PubMed] [Google Scholar]
- Ohno T., Okada Y., Shimotohno K., Miura K., Shinshi H. Enzymatic removal of the 5'-terminal methylated blocked structure of tobacco mosaic virus RNA and its effects on infectivity and reconstitution with coat protein. FEBS Lett. 1976 Aug 15;67(2):209–213. doi: 10.1016/0014-5793(76)80368-7. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K., Guilley H., Jonard G., Hirth L. Nucleotide sequence at the 5' extremity of tobacco-mosaic-virus RNA. 1. The noncoding region (nucleotides 1-68). Eur J Biochem. 1978 Mar 15;84(2):513–519. doi: 10.1111/j.1432-1033.1978.tb12194.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Lundquist R. C., Kado C. I. Genetic map of the crown gall suppressive IncW plasmid pSa. Mol Gen Genet. 1982;186(1):10–15. doi: 10.1007/BF00422905. [DOI] [PubMed] [Google Scholar]
- Tyc K., Konarska M., Gross H. J., Filipowicz W. Multiple ribosome binding to the 5'-terminal leader sequence of tobacco mosaic virus RNA. Assembly of an 80S ribosome X mRNA complex at the AUU codon. Eur J Biochem. 1984 May 2;140(3):503–511. doi: 10.1111/j.1432-1033.1984.tb08131.x. [DOI] [PubMed] [Google Scholar]