Abstract
Background
Alcohol is the most widely consumed substance of abuse, and its use during pregnancy can lead to serious disorders of brain development. The precise molecular action of alcohol on human brain development, however, is still unknown. We previously enriched multipotent progenitor cells, radial glia cells, from human fetal forebrain and demonstrated that they express transcription factor Pax6 which is necessary for their neurogenic fate.
Methods
Enriched human fetal radial glia cells were maintained in vitro as either control or Pax6-expressing retrovirus infected cells. Cultures were treated with increasing doses of alcohol to evaluate Pax6 expression, proliferation and differentiation of radial glia cells by immunocytochemistry, Western Blot and RT-PCR methods.
Results
In vitro treatment with alcohol reduced the expression of transcription factor Pax6 and proliferation of radial glia cells, which decreased neurogenesis. Consistent with this finding, the over-expression of Pax6 in radial glia cells under alcohol treatment rescued cell proliferation and restored the generation of neurons. In contrast to this effect on neurogenesis, the over-expression of Pax6 inhibits the generation of astroglia regardless of alcohol treatment, implying lineage specific effects.
Conclusions
These findings suggest that the effect of alcohol on human neurogenesis is partially due to the reduced expression of transcription factor Pax6 in radial glia cells.
Keywords: cerebral cortex development, human cortical progenitors, human fetus, LeX immunopanning, retrovirus
Introduction
Alcohol abuse is a major public health problem in the United States. Maternal alcohol abuse leads to broad abnormalities of central nervous system development described as Fetal Alcohol Syndrome (FAS) (Jones and Smith, 1973). Prenatal exposure to alcohol reduces significantly the total number of neurons in the cerebral cortex resulting in microcephaly with various degrees of mental retardation (Miller and Potempa, 1990, Mooney and Miller, 1999). In rodents, treatment with ethanol induces neuronal cell death (Mooney and Miller, 2003), inhibits neural cell proliferation both in vivo (Miller, 1989) and in vitro (Kennedy et al., 1986), and inhibits neuronal migration (Miller, 1993). Others have found that ethanol can induce cell proliferation and depletion of mouse cortical neuroepithelial cells in vitro (Santillano et al., 2005).
Majority of studies on cellular effects of ethanol are done in rodents, whereas the precise molecular and cellular action of ethanol on human brain development is still not well understood. Studies on postmortem material of children with FAS show ectopic clusters of cells in the brain that indicates impaired cell proliferation and migration during brain development (Guerri and Renau-Piqueras, 1997, Peiffer et al., 1979, Riley et al., 1995, Swayze et al., 1997). Few in vitro studies reported that treatment with ethanol increases fetal human neurosphere size and changes the fate of neural progenitor cells (Vangipuram et al., 2008, Vangipuram and Lyman, 2010). In addition, gene expression was also changed after ethanol treatment of human fetal brain slices (Hashimoto-Torii et al., 2011).
Here, we studied how ethanol treatment affects proliferation and differentiation of human cortical radial glia (RG) cells, and whether these effects depend on the expression of transcription factor Pax6 (paired box 6). In the mouse dorsal telencephalon, Pax6 has a role in determining neurogenetic fate of RG cells (Gotz et al., 1998). This finding has been confirmed in a mutant Sey mouse where a non-functional Pax6 molecule results in a 50% reduction of the number of both RG cells and cortical neurons (Heins et al., 2002). Accordingly, the over-expression of Pax6 in vitro can instruct the neuronal fate of all progenitors in either embryonic or adult neurosphere preparations (Hack et al., 2004).
In both rodents and human, RG cells differentiate into the intermediate progenitors specified by the transcription factor Tbr-2 (T-brain 2), and subsequently into young neurons of the cortical plate (Englund et al., 2005, Mo et al., 2007, Hansen et al., 2010). We have shown that this process depends on the expression of Pax6, since knocking down the expression of Pax6 with shRNA resulted in decreased neurogenesis from enriched human fetal RG cells (Mo and Zecevic, 2008).
Here, we demonstrate that ethanol treatment reduced proliferation and neuronal differentiation of radial glia cells through a decrease of Pax6 expression. These effects can be partially reversed by over-expression of Pax6 in vitro.
Materials and Methods
Human Fetal Brain Tissue
Human fetuses (n=3), ranging in age from 16–21 gestational weeks, were obtained from the Tissue Repository of The Albert Einstein College of Medicine (Bronx, NY). Tissue was collected with proper parental consent and the approval of the Ethics Committees of the University of Connecticut and The Albert Einstein College of Medicine. Ultrasonic and neuropathological examinations found no evidence of disease or abnormalities. The postmortem delay was on average 15 minutes. Brain tissue was collected in oxygenized Hank’s Balanced Salt Solution (HBSS) containing 0.75% D-glucose, and transported on ice to our lab. All procedures were performed under sterile conditions. Dissociated cell cultures were prepared from the ventricular/subventricular zone (VZ/SVZ) of the fetal forebrain and dissected from the frontally cut hemispheres as a tissue band approximately 2000 μm high from the ventricular surface as reported before (Zecevic et al., 2005, Mo et al., 2007). Tissue was dissociated with 0.05% trypsin-0.02% EDTA (Invitrogen, Carlsbad, CA) and triturated through a fire-polished pipette. Cells were resuspended in DMEM/F12 (Invitrogen) and subjected to immunopanning.
Real time Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen) according to the protocol provided by the company. Real time RT-PCR analysis was performed starting with 1μg of reverse transcribed total RNA, with a 200 nM concentration of both forward and reverse primers in a final volume of 25 μl, using the SYBR Green PCR core reagents and the BioRad iCycler detection System (Bio-rad, Hercules, CA). mRNA levels were normalized to GAPDH mRNA. The sequence of primers is: Pax6: forward: 5′-gaatcagagaagacaggcca3′, reverse: 5′gtgtaggtatcataactccg3′; GAPDH: forward, 5′ggtgaaggtcggagtcaacgga3′, reverse: 5′tcttccaggagcgagatccctc3′. Experiments were repeated three times. The data were presented as means± SEMs and analyzed using Student’s t tests. The criterion for significance was set at p≤0.05.
Immunopanning and cell culture
To isolate neural progenitor cells, we used immunopanning with the surface marker LeX, according to a procedure described earlier (Mo et al., 2007). LeX (fucose N-acetyl lactosamine), is an extracellular matrix-associated carbohydrate, also known as SSEA1 or CD15, used to enrich mouse embryonic stem cells (Kim and Morshead, 2003, Imura et al., 2006, Liour et al., 2006, Capela and Temple, 2006). We have shown that in human fetal cultures majority (95%) of cells obtained after immunopanning with LeX antibody were radial glia cells based on their immunoreactions with various radial glia markers (Mo et al., 2007). LeX+ cells were first cultured in DMEM/F12/B27 supplemented with 10 ng/ml FGF2, which increased their proliferation (Capela and Temple, 2006). In the further text we refer to this medium as the proliferation medium. Subsequently, the amount of FGF2 was reduced to 1ng/ml, which facilitated cell differentiation, hence we refer to this medium as differentiation medium. Immunolabeling with LeX antibody (1:100, Lab Vision, Fremont, CA) determined that the purity of immunopanned cells was 95%.
Retrovirus production and LeX+ cell infection
To over express Pax6 in LeX+ cells, we infected them with a retrovirus expressing Pax6 linked to GFP. Cortical cells from fetal brains were used for this experimental approach. Pax6-expressing retrovirus was produced from a stably transfected packaging cell line (293gpg, gift from Dr. Götz). 293gpg cells were transfected at ~90% confluence with pMIX-Pax6 using lipofectamine (Invitrogen). Supernatant was harvested 48 h after transfection, filtered through 0.45 μm low-protein binding filters (Millipore, Billerica, MA), and stored at −80°C. LeX+ cells were cultured in proliferation medium supplemented with 1 μg/ml polybrene. Volumes of retrovirus sufficient to infect 500 cells were added and incubated for 24 hours at 37°C. Then the infection medium was replaced with proliferation medium, in which the cells were cultured for another 48 hours. The cells were evaluated with immunocytochemistry following the treatment as indicated.
Proliferation assay
Bromodeoxyuridine (BrdU; 20 μM, Sigma, St. Louis, MO) was added to the cell cultures for the last 6 h before immunostaining. A thymidine analog BrdU, incorporates into DNA of dividing cells and can then be detected by immunocytochemistry. Briefly, cells were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 10 min, treated with 2 N HCl for 10 min at room temperature, neutralized by rinsing in 100 mM boric acid (pH 8.5, Sigma) for 10 min, followed by incubation in a blocking solution containing 3% bovine serum albumin (BSA; Sigma) in Tris buffered saline (TBS; 10mM Tris; 150mM NaCl; 2 mM MgCl2) for 1 h. Primary antibody, monoclonal anti-BrdU (1:100; Sigma) was applied overnight at 4° C. After washing in TBS for 5 minutes three times, the cells were incubated with the secondary antibody.
Immunostaining
Cell cultures were fixed with 4% paraformaldehyde for 10 minutes, washed with PBS at room temperature, and incubated with primary and secondary antibodies, as described previously (Mo, et al., 2007). The following primary antibodies were used for specific cell types: anti β-III-tubulin (TuJ1), anti Tbr2 and anti GFAP. β-III-tubulin is a tubulin isotype specifically expressed in neuronal progenitors and immature neurons (Sullivan, 1988, Memberg and Hall, 1995, Zecevic, 2004). Antibody to transcription factor Tbr2 (rabbit IgG, 1:1000; gift from Dr. Hevner, Univ. Washington Sch. of Med.) was used to label intermediate progenitor cells in the subventricular zone. The Tbr2 (T-brain 2) gene encodes a T-box containing transcriptional regulator and is commonly used as a marker of intermediate progenitor cells (IPCs) because its expression is specific to cortical IPCs. (Englund et al., 2005, Arnold et al., 2008). We used this antibody previously on human fetal brain tissue (Mo and Zecevic, 2008).
Glial fibrillary acid protein (GFAP) is an intermediate filament protein that is highly expressed in cells of astroglial lineage. Its antibody has been used to label human radial glia cells and astrocytes (Choi, 1986, Zecevic, 2004).
The specificity of primary antibodies was tested with corresponding isotype controls (mouse IgG1, IgG2a or IgG2b, or rabbit serum), whereas the specificity of secondary antibodies was tested by omitting the primary antibodies from the protocol. Both tests resulted in a lack of immune reaction.
Ethanol treatment
Cell cultures were treated with ethanol in a range of concentrations from 13 (60 mg/dl), 70 (320 mg/dl) to 100mM (460mg/dl). To keep the concentration of ethanol in the medium stable, we placed the culture dishes on racks in sealable plastic containers (Adickes et al., 1988, Luo and Miller, 1997). A water bath containing 0 to 100mM ethanol was used in the bottom of the containers and CO2 was injected into the sealed container to keep concentrations at about 5.0%. The water bath was changed every day. Cultures were maintained for up to 5 days in the indicated culture medium.
Cell counting and statistical analysis
Cells stained with nuclear stain bisbenzamide and various cellular markers were visualized with a Zeiss Axiovision fluorescence microscope and photographed with a digital camera. Before quantification, ten pre-designated adjacent optical fields of view were selected in each culture and examined at magnification 10x (one field = surface area 0.5 mm2). The percentage of immunolabeled cells of total bisbenzamide or GFP positive cells was calculated. Statistical analyses were performed using SPSS software (version 15 SPSS Inc., Chicago, IL). A one way analysis of variance (ANOVA) was used to analyze the effect of ethanol on cell proliferation, Pax6 mRNA and Pax6 protein levels. Each measure was treated as the dependent variable and alcohol concentration as the between group factor. Tukey’s post hoc test was used to clarify main effects and interactions. Student’s t tests were used to analyze the effects of Pax6 over expression on radial glia cells and their progeny. The data were expressed as means ± SEMs and the criterion for significance was set at p≤0.05.
Results
The effect of ethanol on cell proliferation is dose dependent
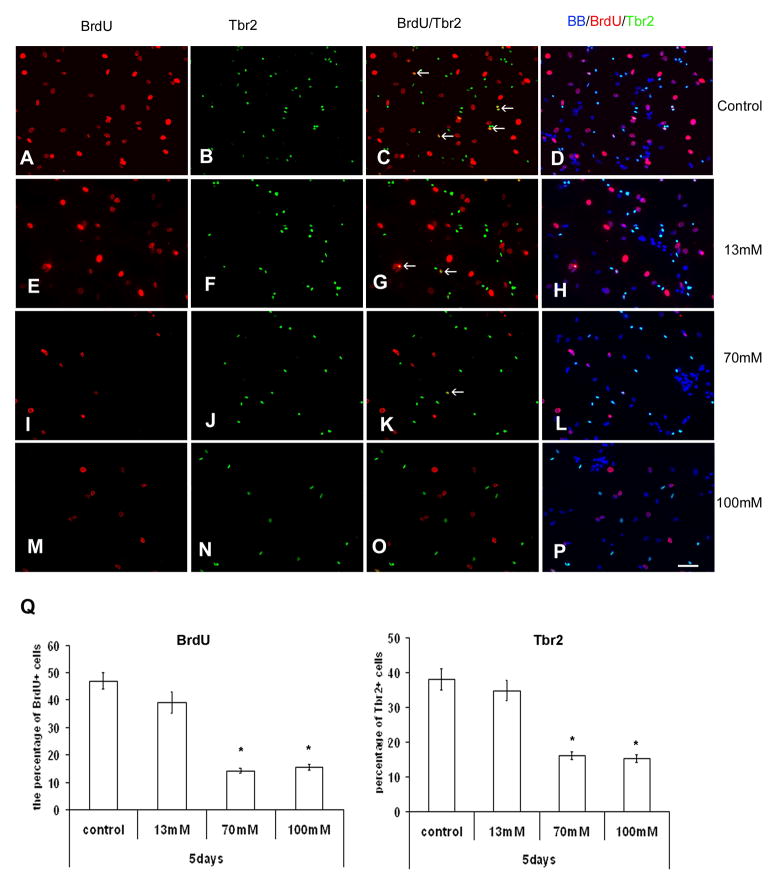
As shown in Fig. 1, the effect of ethanol on cell cultures of the human fetal cerebral cortex was dose-dependent. Cell proliferation (BrdU+ cells) was decreased with ethanol. A one way ANOVA with BrDU+ staining as the dependent variable and alcohol concentration as the between group factor revealed a significant main effect of alcohol [F(3,36)=196.6; p<0.000] on cell proliferation. Tukey’s post hoc test showed that all three ethanol doses [13 mM (p=0.006), 70 mM (p<0.000), and 100 mM (p<0.000)] decreased cell proliferation. On the other hand, the number of Tbr2+ intermediate progenitors was decreased by 50% compared to control with doses of 70 or 100mM of ethanol. A one way ANOVA with Tbr2+ cells as the dependent variable and ethanol concentration as the between group factor revealed a significant effect of ethanol [F(3,36)=72.2; p<0.000], where ethanol reduced the number of Tbr2+ cells. Tukey’s post hoc test revealed a dose effect of ethanol, where the 13 mM concentration of ethanol did not have an effect on number of Tbr2+ cells (p=0.988), but the higher doses of ethanol significantly decreased their number [70 mM (p<0.000), 100 mM (p<0.000)]. In addition, the proliferation of Tbr2+ intermediate progenitors (BrdU+/Tbr2+ cells) was also significantly inhibited by increasing concentrations of ethanol, as confirmed by a one way ANOVA [F(3,36)=6.04; p=0.002]. Tukey’s post hoc test showed that the 13 mM had no effect (p=0.547), whereas the 70 mM (p=0.01) and 100 mM (p=0.003) ethanol concentrations decreased proliferation of Tbr2+ intermediate progenitors..
Figure 1.
The effect of ethanol on the cell proliferation. The cells from VZ/SVZ were cultured in proliferation medium for 5 days, with addition of BrdU in last 6 hrs. Immnunostaining with BrdU (red) and Tbr2 (green). A–D) Control cultures –arrows point to proliferating, double labeled cells; Gradual decrease of double-labeled cells is seen with the increase of ethanol dosage. E–H) treatment with 13mM ethanol; I–L) 70mM of ethanol; and M–P), 100mM of ethanol Q) Percentages of BrdU+ and Tbr2+ cells from all cells stained with nuclear marker bisbemzamid (BB-blue) are reduced with increase of ethanol concentration. Scale bar: 50μm.
Ethanol acts through reduction of Pax6 expression in RG cells
Our previous study demonstrated that human RG cells are highly prolific and can give rise to both astrocytes and neurons (Mo et al., 2007) and that transcription factor Pax6 is very important for neurogenic capacity of RG cells (Mo and Zecevic, 2008). Here, we studied how ethanol treatment in vitro affects Pax6 expression in human RG cells. We first used real time RT-PCR to detect levels of Pax6 mRNA in cultured human cortical cells. The specificity of the real-time RT-PCR product following gel electrophoreses was confirmed by the presence of a single product of the expected length (302 bp, not shown).
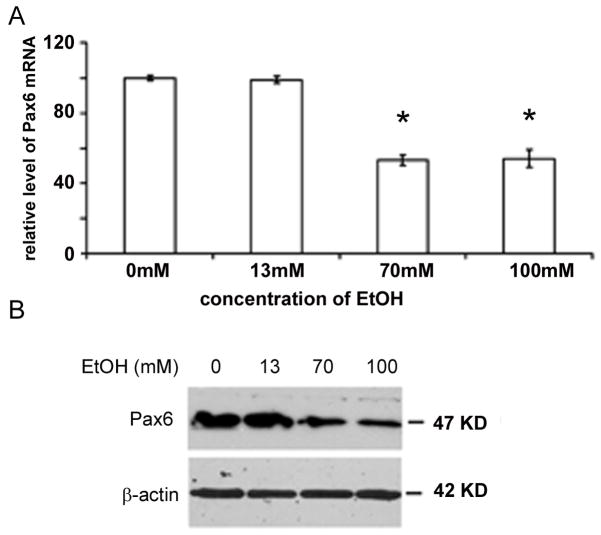
As shown in Fig. 2, similar levels of Pax6 mRNA expression were seen in control and cell cultures treated with 13mM of ethanol. In contrast, Pax6 mRNA expression was almost 2 fold lower with the treatment of 70 and 100mM of ethanol (Fig. 2A.). A one way ANOVA with Pax6 mRNA levels as the dependent variable and ethanol concentration as the between group factor revealed a significant main effect of ethanol [F(3,32)=330.1; p<0.000]. Tukey’s post hoc test revealed an ethanol dosage effect, where small ethanol doses (13 mM) had no effect on Pax6 mRNA levels (p=0.907), but both 70 mM (p<0.000) and 100 mM (p<0.000) ethanol concentrations decreased Pax6 mRNA levels. Accordingly, levels of Pax6 protein were decreased after treatments with 70 and 100mM of ethanol, as seen with Western blot analysis (Fig. 2B). Since 100mM ethanol in human blood is almost lethal dose, whereas 70mM ethanol corresponds to ranges found in chronic drinkers (320 mg/dl) (Adachi et al., 1991, Sathyan et al., 2007), concentration of 70mM was applied in subsequent experiments.
Figure 2.
The amount of both A) mRNA and B) Pax6 protein is reduced in relation to treatment with increasing ethanol concentration as seen with real time RT- PCR and Western blot respectively. β-actin- protein loading control (n=4) * significantly different than control (p<0.05)
Over-expression of Pax6 in RG cells
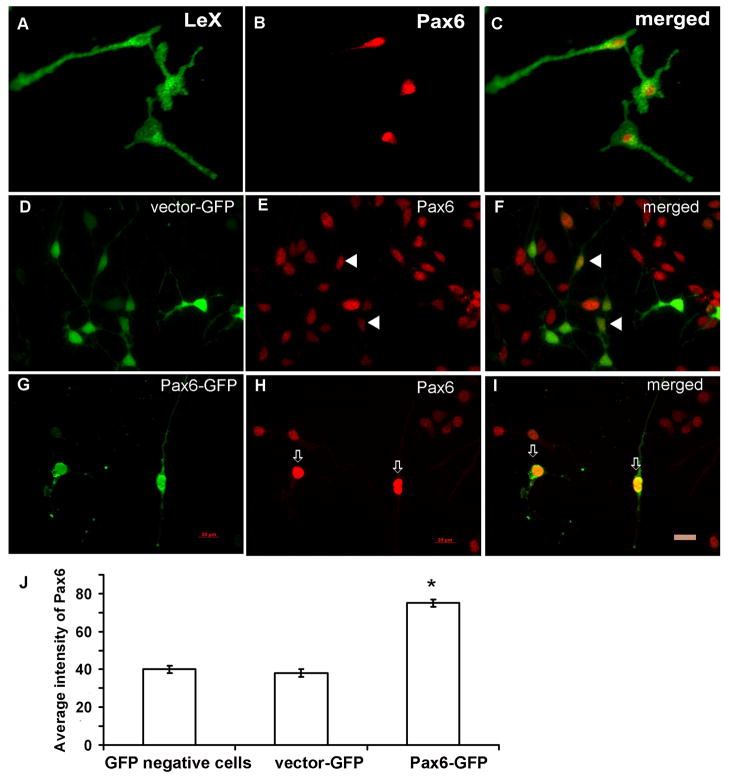
We enriched RG cells in vitro by immunopanning with LeX antibody and demonstrated that RG cells express Pax6 (Fig. 3A–C). In control cultures which were infected with control virus (vector-GFP), the infection efficacy was somewhat higher, resulting in around 35% of cultured cells showing a green signal (Fig. 3D–F). After infection with Pax6 expressing retrovirus (Pax6-GFP), approximately one quarter of cells were infected and were green (GFP+) (Fig. 3G–I). Moreover, the immunostaining demonstrates the higher level of Pax6 protein (Fig. 3G–I). The expression level, presented by average density, was quantified by Metamorph software (Molecular Devices, Inc., Sunnyvale, CA) and showed a two fold increase in Pax6 immunofluorescence in cells that were transduced by Pax6-GFP in comparison to either non-infected cells or control virus infected cells (Fig. 3J). A one way ANOVA with Pax6 protein as the dependent variable and infection method as the between group factor revealed a significant effect of infection method on Pax6 protein levels [F(2,87)=494.78; p<0.000]. Tukey’s post hoc test showed that, compared to control, the vector-GFP method had no effect (p=0.447), whereas the Pax6-GFP significantly increased Pax6 protein levels (p<0.000). This result confirmed that the infection method itself did not affect the expression level of Pax6, and that the increased immunofluorescence was specific to cells that over-expressed Pax6.
Figure 3.
The over-expression of Pax6 in LeX+ radial glia cells. A–C) LeX+ cells (green) isolated by immunopenning co-express transcription factor Pax6 (red). D–F) The cells infected with control retrovirus (vector-GFP, arrowheads) have similar level of Pax6 expression compared to non-infected cells; G–I) The cells infected with Pax6 retrovirus have significantly higher level of Pax6 expression than non-infected cells (arrows); J) Quantification (n=500) of immunoflurescence intensity showed two-times stronger signal in Pax6 infected cells than in either non-infected cells or control virus infected cells (Metamorph program). Scale bar 20 μm.
Over-expression of Pax6 in RG cells rescues ethanol-induced decrease of cell proliferation
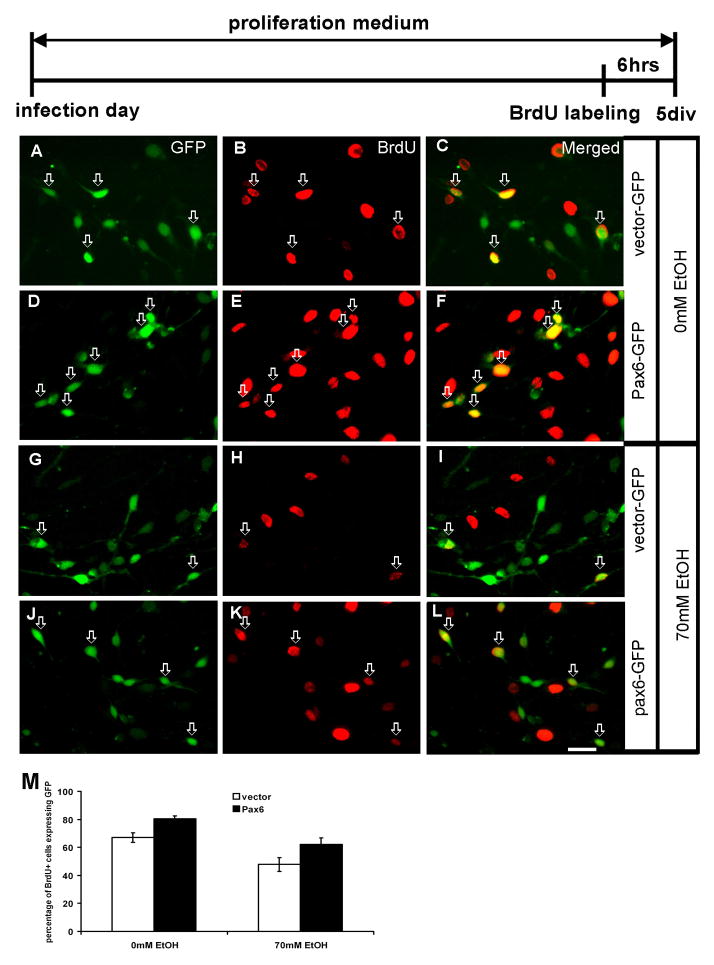
To determine the effect of ethanol on RG cell proliferation, we estimated the percentage of BrdU+ cells from all transduced (GFP+) cells. As shown in Fig. 4, in control cultures kept in proliferation medium, two thirds (67%) of GFP+ cells were co-labeled with BrdU. This percentage further increased to 80% in the cells with Pax6 over-expression (p<0.05) (Fig. 4A–F, M). This is consistent with the idea that the over-expression of Pax6 increases cell proliferation in control conditions. After treatment with 70mM ethanol for 5 days, the percentage of GFP+/BrdU+ cells was reduced to 47% from all cells with control vector. In contrast, cells with Pax6-GFP showed an increase in BrdU labeling (62%, P<0.05) (Fig. 4G–L, M). Thus, our results demonstrate that the over-expression of Pax6 can partially rescue proliferation of RG cells compared to the cells infected with vector-GFP (Fig. 4M).
Figure 4.
The effect of ethanol treatment on proliferation of transduced cells in the proliferation medium with BrdU added for the last 6 hours in culture (time-line diagram on top). Control cultures (0mM ethanol) (A–F). A–C) Cells with control vector (green) are proliferating as seen by their co-labeling (red, arrows, n=235). D–F) Transduction of Pax6-GFP (green) results in increased proliferation - more cells are co-labeled with BrdU (yellow, arrows, n=246). Cultures treated with 70 mM ethanol (G–L). G–I) Proliferation of cells infected with control vector is reduced (n=228), but J–L) the cells with Pax6-GFP vector (n=225) still proliferate more than control vector infected cells (M).
Progeny of RG cells that over-express Pax6
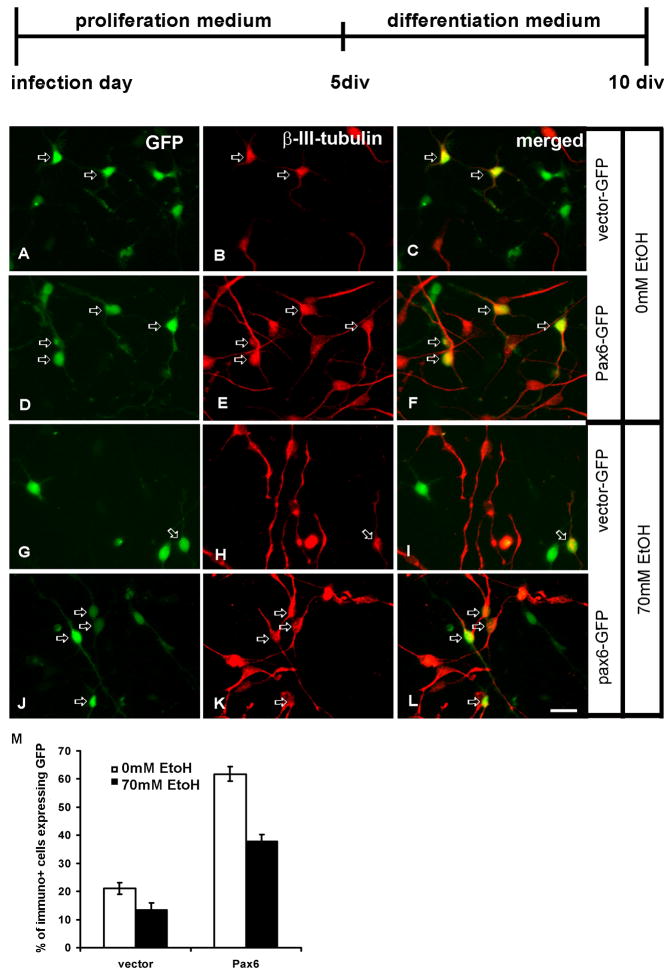
We have shown so far that ethanol treatment reduced the amount of Pax6 in RG cell cultures (Fig. 2) and that it prompted a decrease in proliferation of RG cells (Fig. 4). We also established that the over-expression of Pax6 in RG cells could partially rescue ethanol-induced decrease of cell proliferation (Fig. 4). Next, we examined the effect of Pax6 over-expression on cell differentiation both in control conditions and after ethanol treatment. As in the previous experiment, LeX+ radial glia cells were infected with either vector-GFP or Pax6-GFP, and cultured for 5 days in proliferation medium and for an additional 5 days in differentiation medium. Thereafter, their progeny was studied by cell-type specific immunolabeling. We first examined the progeny of infected cells in control medium, without ethanol treatment. As shown in Fig. 5, while vector-GFP infected cells generated 21% of β-III-tubulin+ neurons (Fig. 5A–C, M), Pax6 over-expressing cells generated three times more β-III-tubulin+ neurons (61%) (Fig. 5G–L, M).
Figure 5.
Neurons generated in differentiation medium from LeX+ radial glia cells. The top line shows the time scale for in vitro experiments with proliferation (10 ng/ml bFGF) and differentiation (without bFGF) media. Control (0mM ethanol) cultures (A–F). A–C) Cells with control vector-GFP differentiate into β-III-tubulin+ cells (red, arrows, n=241). D–F) Pax6-GFP transduced cells (n=237) generate three times more β-III-tubulin+ cells. Cultures treated with 70mM ethanol (G–L). G–I) The number of generated β-III-tubulin+ cells from either vector-GFP (n=225) (G–H) or Pax6-GFP (n=227) (J–L) is reduced compared to their controls. Quantification graph shows that the over- expression of Pax6 tripled neurogenesis in control conditions, and had relative protective effect on neurogenesis after the ethanol treatment (M). Scale Bar- 20μm.
In 70mM ethanol treated cultures, the number of β-III-tubulin+ cells generated from vector-GFP cells was reduced from 21% to 13% (p<0.05, Fig. 5G–I, M). The reduction of generated β-III-tubulin+ cells from Pax6-over-expressing cells was also pronounced (from 61 to 37%, p<0.01, Fig. 5J–L, M). However, when compared to control cultures, the over-expression of Pax6 had a considerable protective effect on RG differentiation into neurons (Fig. 5M). Thus, over-expression of Pax6, even after ethanol treatment, resulted in almost three times more neurons being generated in comparison to cells with control vector-GFP.
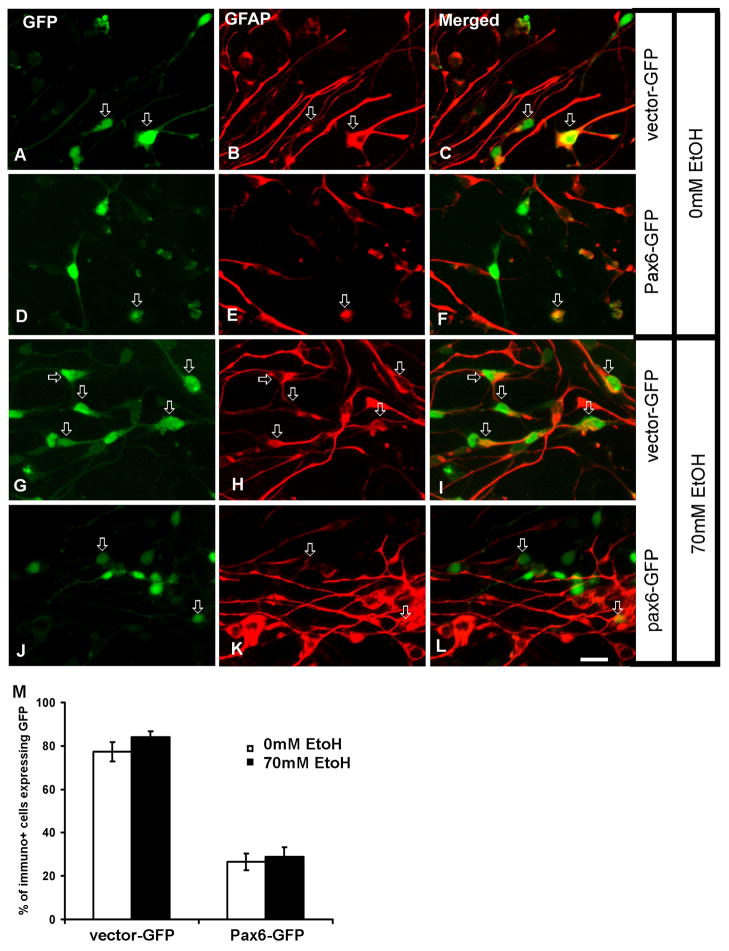
The effect of Pax6 dosage on differentiation to astroglia cells was, however, different. As shown in Fig. 6, in control cultures the percentage of GFAP+ astroglia generated from vector-GFP cells was 77% (Fig. 6A–C, M), whereas this percentage was significantly reduced in Pax6-GFP infected cells (26%) (Fig. 6D–F, M). Ethanol treatment did not significantly change the relative percentages of generated GFAP+ astroglia cells compared to cultures not treated with ethanol. Thus, the percentage of GFAP+ cells generated from vector-GFP cells in ethanol-treated cultures was 84% (Fig. 6G–I, M) vs. 77% in non-treated cultures (Fig. 6A–C, M). Similarly, although Pax6-overexpressing cells generated much less GFAP+ cells, their percentage was comparable in control (28%) and ethanol treated cultures (26%) (Fig. 6D–F, M).
Figure 6.
Astroglia generation in differentiation medium. Control (0mM ethanol) cultures (A–F). A–C) Larger number of GFAP+ astroglia cells is generated from vector-GFP transduced cells (arrows, n=268), than from D–F) Pax6 over-expressing cells (n=276). In cultures treated with 70mM ethanol G–L) the number of GFAP+ astroglia generated from vector-GFP (n=275) cells is similar in ethanol treated (G–I) and non-treated cultures (AC), whereas the number of GFAP+ cells generated from Pax6-GFP cells (n=282) is reduced both in ethanol treated (J–L) and non-treated cultures (D–F). M) Quantification of GFAP+ cells in different conditions shows that there is similar reduction of GFAP+ cells when Pax6 is over expressed regardless of ethanol treatment. Scale bar-20μm.
Taken together, these results speak in favor of a selective effect of ethanol on RG-neurogenetic potential by two mechanisms. First, by reducing proliferation of both multipotent RG progenitors and more restricted intermediate progenitors (Tbr2+ cells). And second, by reducing RG differentiation to neurons through reduction of Pax6 expression.
Discussion
We have demonstrated that in vitro treatment of human multipotent RG cells with ethanol reduces their proliferation. This treatment also affects Tbr2+ intermediate progenitors derived from RG cells, which are neuronal restricted progenitors. Human cortical neurons originate from the ventricular/subventricular zone (VZ/SVZ), a proliferative zone around lateral ventricles. Multipotent progenitors located in the VZ/SVZ, the radial glia (RG) cells, have a dual role: generate neurons and glia and provide the migratory scaffolding for migrating cortical neurons (Howard et al., 2008, Zecevic, 2004, Rakic, 2003, Hansen et al., 2010).
Expression of Pax6 is reduced after ethanol exposure
In rodents, the expression of Pax6 is critical for the neurogenetic fate of RG cells. It has been reported that the absence of Pax6 results in a significant reduction of cortical neurons (Heins et al., 2002). Similar to the reports in rodents, we had shown that knocking down Pax6 expression in human fetal cortical RG cultures results in decreased proliferation of RG cells and a subsequent decrease in neurogenesis (Mo and Zecevic, 2008). Here, we demonstrate that ethanol can reduce the Pax6 expression in human fetal RG cells, which in turn reduces generation of both the intermediate progenitors (Tbr2+ cells) and young neurons (β-III-Tubulin+ cells). This is in accord with a recent report on fetal human and mouse cortical slice cultures treated for 24 hours with ethanol that showed, among other changes in gene transcripts, decreased Tbr2 immunoreactivity in the cortical VZ/SVZ (Hashimoto-Torii et al., 2011).
We further demonstrate in this study that the detrimental effects of ethanol on neurogenesis can be partially rescued by over-expression of Pax6 in cortical RG cells. This finding suggests that the function of Pax6 is probably involved in the ethanol effect on brain development. Similarly, in vivo experiments on various animal models, from rodents (Aronne et al., 2008) to Xenopus (Peng et al., 2004), have demonstrated that treatment with ethanol reduces Pax6 expression. Notably, in Xenopus, this treatment also produces microcephaly (Peng et al., 2004).
Pax6 dosage affects RG cell proliferation
We used a short exposure to BrdU in human RG cell cultures and demonstrated that in control cultures kept in proliferation medium two thirds (67%) of GFP+ cells were co-labeled with BrdU. When the cells were transduced with Pax6 (over-expression), the percentage of proliferating cells that over-expressed Pax6 increased to 80%. Thus, Pax6 regulates proliferation of RG cells in control conditions. As expected, ethanol treatment (70mM ethanol, 5 days) of cells with the control vector reduced cell proliferation to 47%. However, when cells that over-expressed Pax6 were treated in the same way, their proliferation was less affected and 62% were proliferating. Although that percentage of RG proliferation is not as high as in the cells transduced with Pax6-GFP and without ethanol treatment (80%), our results demonstrate that the over-expression of Pax6 can partially rescue RG proliferation under ethanol treatment. Why the over-expression of Pax6 cannot fully rescue the cell proliferation, is not clear. Several reasons, including the reduction of endogenous Pax6, should be considered.
Cell fate of RG changes after ethanol treatment
Our study demonstrates that ethanol exposure changes RG cell fate in the human fetal brain cultures. We found that neurogenesis was reduced, whereas gliogenesis was somewhat enhanced, similar to what has been reported for neural stem cells isolated from human fetal brains. This may explain why individuals with FAS, although do not have lethal brain abnormalities, have a range of cognitive impairments (Mattson et al., 2011, Miller and Spear, 2006).
Lastly, we found that the effects of ethanol on neurogenesis are dose dependent. Importantly, a low dose (13mM) and short exposure to ethanol did not have significant detrimental effects on neurogenesis compared to control cultures as modeled in vitro in human fetal forebrain cells.
The effect of ethanol on subtypes of cortical neurons
An important unresolved issue is whether ethanol can selectively affect different neuronal cell types, such as projection and interneurons, or neurons positioned in different cortical layers. This has obvious functional relevance since various neuronal subtypes have different roles in building complex cortical circuits. Different neuronal subtypes also have distinct sites and time of origin during the long development of the human cerebral cortex. Our results are obtained from fetal brains ranging from 16–21 gestational weeks, which coincides with the time when upper cortical layers (II–IV) are formed (Rakic, 1974, Rakic, 2009). Neurons from upper cortical layers establish cortico-cortical connections important for the appearance of higher brain functions in humans (Hill and Walsh, 2005). Thus, excessive consumption of ethanol at that time of upper cortical layers generation can deregulate their cell populations. These cellular changes could not be diagnosed easily with classical methods of neuropathology, but new methodology, however, demonstrates subtle molecular changes in gene expression (Hashimoto-Torii et al., 2011) or DNA methylation (Garro et al., 1991) after ethanol exposure. These changes caused by ethanol can be reflected in complex cognitive deficits related to FAS (for review see Mattson et al., 2011).
Here, we have shown that the number of Tbr2+ intermediate progenitors was reduced after ethanol treatment. These progenitors are destined to supply projection neurons to upper cortical layers (Arnold et al., 2008, Englund et al., 2005). Our previous study has demonstrated that knocking down Pax6 in human cortical progenitors reduced the number of both projection and interneurons (Mo and Zecevic, 2008). Our preliminary results, which need to be further repeated, suggest that the over-expression of Pax6 after ethanol treatment rescued some of the glutaminergic neurons, whereas GABAergic interneurons did not change significantly in these experiments(not shown).
In monkey, the percentage of glutaminergic and GABAergic neurons in the somatosensory cortex did not change after in-utero exposure to ethanol, but the overall number of cortical neurons was significantly reduced, suggesting that the primary detrimental effect of ethanol is on neuronal precursor (Miller and Spear, 2006). Studies in rodents with a short interval of ethanol exposure at various embryonic days showed that the effect was less pronounced on the generation of neurons and their survival, but cell lineage could be switched during terminal division and even some time after that (Miller and Hu, 2009). In another report, ethanol was found to negatively affect proliferation of interneuron progenitors in medial ganglionic eminence (MGE), whereas differentiation into GABAergic cells and their tangential migration were increased, resulting in more GABAergic cells in embryonic cortex (Cuzon et al., 2008). However, since at the same time sensitivity to GABA was elevated and the amount of ambient GABA was increased, the balance of cortical development was impaired which might result in cell death and reduce cortical neuron number in adults (Cuzon et al., 2008).
In conclusion, this study demonstrates that in vitro treatment with ethanol is dose dependent and results in decreased proliferation of RG cells, reduced expression of Pax6, and reduced neurogenesis. Furthermore, the over-expression of Pax6 in RG cells in ethanol-treated human cell cultures effectively rescued RG cells proliferation and their differentiation into neurons (neurogenesis).
Acknowledgments
This study was supported by NIH grant NS41489 and Connecticut Innovation Stem Cell Grant 2008-013 (NZ) and NIH 5T32 NS041224 (VM). We thank Dr. B. Poulos (The Tissue Repository, Albert Einstein College of Medicine, Bronx, NY) for providing autopsy specimens of human fetal brain tissue.
References
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–53. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- Adickes ED, Mollner TJ, Lockwood SK. Closed chamber system for delivery of ethanol to cell cultures. Alcohol Alcohol. 1988;23:377–81. doi: 10.1093/oxfordjournals.alcalc.a044832. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–84. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronne MP, Evrard SG, Mirochnic S, Brusco A. Prenatal ethanol exposure reduces the expression of the transcriptional factor Pax6 in the developing rat brain. Ann N Y Acad Sci. 2008;1139:478–98. doi: 10.1196/annals.1432.006. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev Biol. 2006;291:300–13. doi: 10.1016/j.ydbio.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Choi BH. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J Neuropathol Exp Neurol. 1986;45:408–18. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Cuzon VC, Yeh PW, Yanagawa Y, Obata K, Yeh HH. Ethanol consumption during early pregnancy alters the disposition of tangentially migrating GABAergic interneurons in the fetal cortex. J Neurosci. 2008;28:1854–64. doi: 10.1523/JNEUROSCI.5110-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–51. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro AJ, McBeth DL, Lima V, Lieber CS. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin Exp Res. 1991;15:395–8. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Gotz M, Stoykova A, Gruss P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron. 1998;21:1031–44. doi: 10.1016/s0896-6273(00)80621-2. [DOI] [PubMed] [Google Scholar]
- Guerri C, Renau-Piqueras J. Alcohol, astroglia, and brain development. Mol Neurobiol. 1997;15:65–81. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–78. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Kawasawa YI, Kuhn A, Rakic P. Combined transcriptome analysis of fetal human and mouse cerebral cortex exposed to alcohol. Proc Natl Acad Sci U S A. 2011;108:4212–7. doi: 10.1073/pnas.1100903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci. 2002;5:308–15. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–7. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- Howard BM, Zhicheng M, Filipovic R, Moore AR, Antic SD, Zecevic N. Radial glia cells in the developing human brain. Neuroscientist. 2008;14:459–73. doi: 10.1177/1073858407313512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–93. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kennedy LA, Sheppard MS, Bhaumick B, Laverty WH. Reduced binding of basic somatomedin by mouse placental membranes following maternal alcohol administration. Dev Pharmacol Ther. 1986;9:132–44. doi: 10.1159/000457085. [DOI] [PubMed] [Google Scholar]
- Kim M, Morshead CM. Distinct populations of forebrain neural stem and progenitor cells can be isolated using side-population analysis. J Neurosci. 2003;23:10703–9. doi: 10.1523/JNEUROSCI.23-33-10703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liour SS, Kraemer SA, Dinkins MB, Su CY, Yanagisawa M, Yu RK. Further characterization of embryonic stem cell-derived radial glial cells. Glia. 2006;53:43–56. doi: 10.1002/glia.20257. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21:1186–94. [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal Alcohol Spectrum Disorders: Neuropsychological and Behavioral Features. Neuropsychol Rev. 2011 doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memberg SP, Hall AK. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 1995;27:26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of prenatal exposure to ethanol on neocortical development: II. Cell proliferation in the ventricular and subventricular zones of the rat. J Comp Neurol. 1989;287:326–38. doi: 10.1002/cne.902870305. [DOI] [PubMed] [Google Scholar]
- Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17:304–14. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Hu H. Lability of neuronal lineage decisions is revealed by acute exposures to ethanol. Dev Neurosci. 2009;31:50–7. doi: 10.1159/000207493. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Miller MW, Spear LP. The alcoholism generator. Alcohol Clin Exp Res. 2006;30:1466–9. doi: 10.1111/j.1530-0277.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Mo Z, Moore AR, Filipovic R, Ogawa Y, Kazuhiro I, Antic SD, Zecevic N. Human cortical neurons originate from radial glia and neuron-restricted progenitors. J Neurosci. 2007;27:4132–45. doi: 10.1523/JNEUROSCI.0111-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Is Pax6 critical for neurogenesis in the human fetal brain? Cereb Cortex. 2008;18:1455–65. doi: 10.1093/cercor/bhm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Effects of prenatal exposure to ethanol on systems matching: the number of neurons in the ventrobasal thalamic nucleus of the mature rat. Brain Res Dev Brain Res. 1999;117:121–5. doi: 10.1016/s0165-3806(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Ethanol-induced neuronal death in organotypic cultures of rat cerebral cortex. Brain Res Dev Brain Res. 2003;147:135–41. doi: 10.1016/j.devbrainres.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Peiffer J, Majewski F, Fischbach H, Bierich JR, Volk B. Alcohol embryo-and fetopathy. Neuropathology of 3 children and 3 fetuses. J Neurol Sci. 1979;41:125–37. doi: 10.1016/0022-510x(79)90033-9. [DOI] [PubMed] [Google Scholar]
- Peng Y, Yang PH, Ng SS, Wong OG, Liu J, He ML, Kung HF, Lin MC. A critical role of Pax6 in alcohol-induced fetal microcephaly. Neurobiol Dis. 2004;16:370–6. doi: 10.1016/j.nbd.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–7. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–9. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–35. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 1995;19:1198–202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–57. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KF. Structure and utilization of tubulin isotypes. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–40. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Grever WE, Parker GC, Lyman WD. Ethanol increases fetal human neurosphere size and alters adhesion molecule gene expression. Alcohol Clin Exp Res. 2008;32:339–47. doi: 10.1111/j.1530-0277.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Vangipuram SD, Lyman WD. Ethanol alters cell fate of fetal human brain- derived stem and progenitor cells. Alcohol Clin Exp Res. 2010;34:1574–83. doi: 10.1111/j.1530-0277.2010.01242.x. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristic of radial glia in the human fetal telencephalon. Glia. 2004;48:27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–22. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]