Abstract
The immune system has evolved into two main arms, the primitive innate arm that is the first line of defense but relatively short-lived and broad acting, and the advanced adaptive arm that generates immunologic “memory” allowing rapid, specific recall responses. T cell-independent type-2 (TI-2) antigens (Ags) invoke innate immune responses. However, due to its “at the ready” nature, how the innate arm of the immune system maintains tolerance to potentially abundant host TI-2 Ags remains elusive. Therefore, it is important to define the mechanisms that establish innate immune tolerance. This review highlights recent insights into B cell tolerance to theoretical self TI-2 Ags, and examines how the B cell-restricted Siglecs, CD22 and Siglec-G, might contribute to this process.
Keywords: B cells, TI-2 Ag, CD22, Siglec-G
B cell tolerance checkpoints that prevent autoimmune disease
During lymphocyte development, central tolerance mechanisms eliminate autoreactive T cells in the thymus and B cells in the bone marrow. This prevents the vast majority of these potentially destructive lymphocytes from inhabiting the periphery where they would promote autoimmune disease. Nevertheless, a significant percentage of lymphocytes, primarily B cells, escape negative selection and reside in peripheral lymphoid tissues [1, 2]. These B cells are generally characterized by a quiescent state of antigen (Ag) non-responsiveness called anergy , have a shortened lifespan, and are found primarily within the transitional B cell subsets that inhabit the spleen. It has been proposed that up to half of the B cells generated in wild type mice undergo anergy once exposed to self Ags in the periphery [3].
Maintenance of B cell anergy requires constant exposure to self Ag, as major B cell phenotypic characteristics of anergy including shortened lifespan, enhanced basal intracellular calcium levels, reduced IgM surface expression, and impaired trafficking of internalized IgM are reversed when self Ag exposure is removed [1, 4–6]. B cell anergy appears to be maintained through perpetual B cell Ag receptor (BCR) desensitization, whereby the BCR signals poorly upon additional Ag stimulation and is partitioned away from endocytic compartments required for Ag processing and stimulation of intracellular innate molecules such as toll-like receptors [6]. Thereby, anergic B cells specific for T cell-dependent Ags cannot process and present Ag to helper T cells properly. These B cells thus fail to receive necessary co-stimulatory signals from T cells and are rapidly turned over due to accelerated apoptosis. The constant competition for B cell survival factors, including BAFF (BLyS), are also likely to regulate homeostasis of the mature, transitional, and anergic B cell pools [7]. Supporting this concept, transgenic mice overexpressing BAFF develop autoimmune symptoms characteristic of lupus [8].
Persistence of the anergic B cell pool may function to fill “holes” in the peripheral B cell repertoire, whereby subsequent BCR mutations or editing enables a shift in Ag specificity and the participation of these cells in immune responses to pathogens. CD22 and Siglec-G, B cell-restricted molecules, may serve as rheostats to maintain the proper balance of transmembrane signals in this process. For example, CD22 negatively regulates B cell signaling through the co-receptor CD40 [9–11], and B cells from Cd22−/− mice transgenic for CD40 ligand that is constitutively expressed in the B lineage (CD154TGCd22−/−) proliferate spontaneously ex vivo [11]. Although both Cd22−/− and CD154TG mice develop autoimmunity, the hallmark characteristic of CD154TGCd22−/− mice is the abrogation of isotype switching and IgG autoantibody production [11]. This is explained by a dramatic expansion of the IL-10-producing regulatory B10 B cell subset in the spleens of these mice. Selective therapeutic depletion of this B cell subset in normal mice leads to heightened IgG responses to T cell-dependent Ags administered without adjuvant. Therefore, in the absence of CD22 negative regulation, important compensatory mechanisms including expansion of the regulatory B10 cell subset may maintain B cell tolerance by limiting pathogenic autoantibody production and the severity of autoimmune disease [12].
This review highlights recent insights into another important aspect of B cell tolerance: how non-responsiveness to potentially-abundant host TI-2 Ags is achieved. Bacterial TI-2 Ags such as capsular polysaccharides stimulate B cell activation and antibody (Ab) production independent of T cell help. However, these bacterial TI-2 Ags may be structurally similar to molecules ubiquitously expressed by host cells, which would become accessible to Ag-specific B cells by expression at tissue surfaces or when released from dead or dying cells. Herein we describe how CD22 and Siglec-G appear to regulate tolerance to self-TI-2 Ags by distinguishing these molecules from foreign TI-2 Ags, resulting in the suppression of autoreactive B cell activation.
TI-2 Ags require unique tolerance mechanisms
Immune responses to TI-2 Ags are crucial for generating protective immunity to encapsulated extracellular bacteria such as Streptococcus pneumoniae. These high molecular weight Ags possess repetitive B cell epitopes and degrade slowly in vivo, but do not require associated T cell help or additional microbial products such as TLR agonists, and do not have T cell epitopes that associate with MHC molecules [13, 14]. Elegant studies performed over 20 years ago showed that TI-2 Ags must exceed a specific molecular mass and epitope (e.g. hapten) valency to activate B cells [13]. TI-2 Ags exceeding these threshold requirements produce an acute amount of BCR clustering, called an “immunon”, which is capable of inducing B cell proliferation in vivo. Multivalent BCR crosslinking by TI-2 Ags induces rapid humoral immune responses, with IgG3 representing the major IgG isotype produced in response to TI-2 Ags in mice [15, 16].
B cells responding to TI-2 Ags do not require a second co-stimulatory signal from helper T cells for full activation in order to prevent autoimmunity. Therefore, unique tolerance mechanisms may have evolved in order to optimize B cell responses to foreign TI-2 Ags while suppressing B cell responses to self TI-2 Ags with similar activating potential. Among these possible mechanisms, humoral responses against pathogen TI-2 Ags are primarily restricted to CD5+ and marginal zone B cells [17–19]. These B cell subsets produce relatively low affinity IgM Abs and have a limited capacity to become isotype-switched memory B cells or long-lived plasma cells, suggesting that TI-2 Ags have a limited potential for promoting pathogenic autoimmunity [19]. This likely explains why marginal zone B cells display a diverse repertoire of BCR specificities that bind T cell-independent and – dependent Ags, and some self-Ags [20]. Similarly, regulatory B10 cells share overlapping phenotypic markers with CD5+ and marginal zone B cells, and are significant source of “natural” Abs [21, 22]. B10 cells also display a diverse repertoire of BCR specificities that bind T cell-independent and – dependent Ags, and some self-Ags, which may allow them to suppress humoral, cellular and innate immune responses to frequently encountered Ags [21].
Siglecs: Transmembrane receptors that modulate signal transduction
Siglecs are type-I transmembrane proteins that belong to the immunoglobulin (Ig) superfamily and function as mammalian lectins [23]. Their membrane-distal (N-terminal) Ig-like domains bind species-specific sialic acid (Sia) motifs presented on protein and lipid scaffolds. Siglecs regulate intracellular signaling pathways via conserved cytoplasmic tyrosine motifs that become phosphorylated and recruit effector molecules in response to intrinsic activation signals or ligand binding [23]. Siglecs that have been well described include sialoadhesin (Siglec-1), CD22 (Siglec-2), and CD33 (Siglec-3). CD22 is a B cell-restricted surface protein that modulates BCR signal transduction in mature B cells [24]. The more recently described Siglec-G is also expressed by mature B cells, but primarily influences signaling in the CD5+ B1a cell subset [25–27]. Here we discuss how Siglecs contribute to control of B cell function. We also examine recent data suggesting that CD22 and Siglec-G control B cell tolerance to self TI-2 Ags [28]. The proposed models suggest that CD22 and Siglec-G evolved to inhibit the generation of humoral immune responses to self TI-2 Ags that are decorated with Sia ligands, while allowing for humoral responses to foreign TI-2 Ags that lack these modifications.
CD22
Structure and expression
Human and mouse CD22 have seven extracellular Ig-like domains [24]. The highest amount of conservation occurs between the amino-terminal V-set Ig-like domain involved in ligand binding, as well as the ~140 amino acid cytoplasmic domain which harbors conserved tyrosine motifs, including immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that recruit the potent phosphotyrosine and phosphoinositide phosphatases SHP-1 and SHIP [24, 29, 30] (Figure 1a). CD22 expression is B cell-specific and developmentally regulated in both mice and humans, with the vast majority of mature peripheral B cells expressing CD22 [24]. In the spleen CD22 is expressed at high levels on follicular, mantle, and marginal zone B cells, while germinal center B cells express CD22 weakly (Figure 1a). CD22 expression is lost during B cell differentiation into plasma cells.
Figure 1. CD22 and Siglec-G structure and expression.
The extracellular Ig-like domains and cytoplasmic tyrosine signaling domains of CD22 (a) and Siglec-G (b) are indicated, along with their relative expression levels within lymphoid compartments. Red indicates a high expression within specific anatomic structures or within B cell lineages relative to low (pink) or lack of expression (white). NK, natural killer cells; Mφ, myelomonocytic lineage cells; PMN, neutrophils; DC, dendritic cells.
Ligands
CD22 interacts with diverse Sia-bearing molecules expressed on various cell types, including B and T cells, neutrophils, monocytes, and erythrocytes [31–33]. CD22 mediates these cell-cell interactions through the recognition of N-linked oligosaccharides possessing Sia residues [31]. Mouse CD22 binds ligands contain α2,6-linked N-glycolylneuraminic acid (NeuGc), whereas humans only express CD22 ligands in the form of N-acetylneuraminic acid (NeuAc) [34–36]. β-galactoside α2,6-sialyltransferase is a golgi enzyme necessary for the generation of CD22 ligands [37], and ligand binding and intercellular adhesion are eliminated by treating lymphoid and non-lymphoid target cells with neuraminidase [31]. Proposed CD22 ligands identified in vitro include CD22 itself, isoforms of CD45, soluble IgM pentamers, haptoglobin, and Ly-6 proteins [38–40]. CD22 is also reported to associate with the BCR [41], although it was recently suggested that cis interactions between neighboring CD22 molecules have the greatest relevance in situ [42].
Transmembrane signaling
Proposed CD22 functions include regulation of transmembrane signaling, a sensing mechanism for neighboring leukocytes, and B cell tissue localization [24]. The simultaneous addition of CD22 monoclonal antibodies (mAbs) and anti-BCR Abs to human B cell cultures results in more potent B cell proliferation than anti-BCR Abs alone [43], as does pre-treatment of B cells with a solid-phase CD22 mAb prior to BCR stimulation [29]. Based on this it was proposed that the physical sequestration of CD22 away from the BCR results in “de-repression” of BCR signaling [44]. Supporting this, B cells from Cd22−/− mice generate augmented intracellular [Ca2+]i responses after BCR engagement [29, 45–48], in agreement with the identification of a CD19-CD22 regulatory loop that establishes B cell signaling thresholds [49, 50]. Additional features of Cd22−/− mice are shorter B cell lifespans, enhanced BCR-mediated apoptosis, and reduced numbers of circulating blood and bone marrow B cells, but normal tissue migration patterns [9, 45, 47, 48].
CD22 ligand-binding domain interactions in cis with other cell surface Sia-bearing glycoproteins on B cells reportedly “masks” CD22 on the cell surface [51–53]. CD22 masking may provide continuous tonic suppressive signals, preventing B cell hyper-activation through the BCR. CD22 masking is a readily reversible process, allowing CD22 to be redistributed to sites of cell-cell contact [31, 54]. CD22 is “unmasked” after B cell co-stimulation via CD40, which may relieve CD22 negative regulation of BCR signaling within germinal centers [53], where CD22 expression is also downregulated (Figure 1a).
Knock-in mice expressing either the CD22Δ1-2 or CD22AA CD22 mutants confirm the importance of ligand-binding in maintaining B cell homeostasis [9]. CD22Δ1-2 mice express a truncated CD22 protein that lacks both amino-terminal Ig-like domains, while CD22AA mice express CD22 containing 2 point mutations in the first Ig-like domain that abrogate ligand binding activity [55]. As in Cd22−/− mice, mature peripheral B cells and recirculating bone marrow B cells in CD22Δ1-2 and CD22AA mice have high in vivo turnover rates [9]. Altered B cell homeostasis in these models suggests that CD22 ligand binding serves as a sensing mechanism for endogenous Sia-decorated ligands (Figure 2a), without which B cells become chronically stimulated and prematurely undergo apoptosis [10]. Cell surface CD22 expression on mature B cells is also reduced in both CD22Δ1-2 and CD22AA mice [9], indicating that ligand- binding activity retains optimal protein levels at the cell surface. Nevertheless, [Ca2+]i responses, CD22 phosphorylation, and CD22/SHP-1 interactions following BCR stimulation ex vivo are normal in B cells from CD22Δ1-2 and CD22AA mice, demonstrating that CD22 regulation of some key intracellular signaling pathways through its cytoplasmic domain does not require ligand binding. This duality of function for CD22 may serve as an example whereby a single molecule has evolved to have multiple roles in transmembrane signaling.
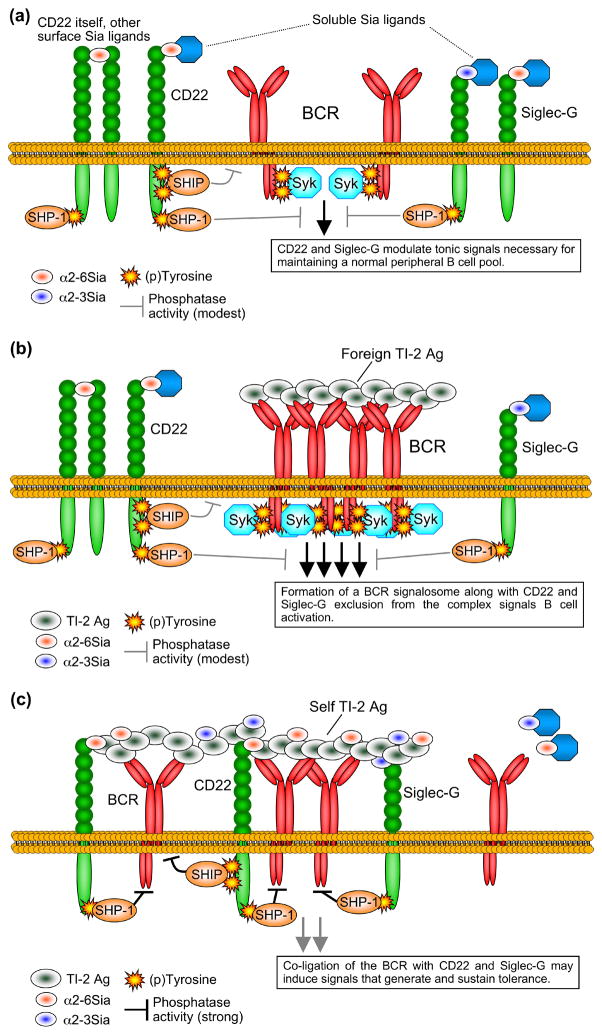
Figure 2. Potential models for B cell tolerance regulation by CD22 and Siglec-G.
(a) Basal phosphorylation of CD22 and Siglec-G and interactions with endogenous Sia ligands maintains B cell homeostasis, likely by balancing tonic signals generated through the BCR on resting B cells. Recruited SHP-1 may dephosphorylate tyrosines of the BCR ITAMs and downstream substrates such as CD19, while SHIP acts to dephosphorylate specific inositol phospholipids involved in the generation of important second messengers. A balance between these signals maintains B cell quiescence, optimizing peripheral longevity. (b) During immune responses to foreign TI-2 Ags, the BCR of Ag-specific B cells is crosslinked, while CD22 and Siglec-G are largely excluded from the BCR signalosome. This leads to robust signaling by Syk recruited to the phosphorlated ITAMs of CD79, and also to optimal phosphorylation of CD19 (not shown), culminating in robust downstream positive signals for B cell activation. CD22 and Siglec-G continue to balance signals generated through the signalosome via SHP-1 and SHIP, preventing B cell hyper-stimulation that may otherwise lead to apoptosis. (c) Self TI-2 Ags modified with Sia ligands recognized by the BCR, CD22 and Siglec-G suppress B cell activation by co-clustering these receptors. The phosphorylated ITIMs and other tyrosine-based signaling sites of CD22 and Siglec-G recruit the inhibitory phosphatases SHP-1 and SHIP into the BCR complex, preventing BCR immunon formation and the resulting positive signals that induce proliferation and Ab production. These conditions are proposed to induce sustained tolerizing signals through currently undefined mechanisms.
A role in autoimmune disease?
A role for CD22 in tolerance was first suggested in an in vitro study. B cell activation by Ag- expressing target cells that co-expressed α2-6-Sia glycoconjugates was suppressed when the B cells expressed CD22 [56]. The conclusion was that B cell negative regulation by CD22 dampens reactivity to self-Ags, preventing autoreactive B cell activation. CD22 polymorphisms may also contribute to autoimmunity. At least three alleles of the Cd22 gene have been identified [57, 58]. The Cd22a allele is uniquely found in some autoimmune-prone strains of mice, including DBA/1, DBA/2J, NZB, NZW, NZC, PL/J and AKR/J [59]. Likewise, a Cd22c/Lyb-8c allele was identified in BXSB mice that are lupus-prone [59]. Most polymorphisms of these alleles occur within the ligand-binding domain, suggesting that alterations in CD22 ligand binding may contribute to autoimmunity. NZW mice also synthesize aberrant Cd22 mRNAs that interfere with upregulated cell surface CD22 expression after LPS exposure [58]. Moreover, two independent Cd22−/− mouse lines have increased autoantibody production against double-stranded DNA, single-stranded DNA, and histone complexes [11, 46, 60].
CD22 is the primary phosphoprotein associated with intracellular SHP-1 phosphatase in B cells [49]. Motheaten (me/me) mice that express no SHP-1 and motheaten viable (mev/mev) mice that express catalytically compromised SHP-1 have elevated levels of spontaneous autoantibodies [61, 62]. B cells from both SHP-1-defective and Cd22−/− mice are hyper-responsive to activation signals and have an IgMlo MHC class IIhi phenotype [48, 63]. CD22 also negatively regulates CD19 function [49, 50], whereby CD22 deficiency coupled with heightened CD19 signaling augments autoantibody production in mice [64, 65]. Thus, impaired CD22 expression or function can alter B cell signaling thresholds and potentially contribute to autoimmunity.
Siglec-G
Structure and ligands
Like CD22, Siglec-G is B cell-restricted, and broadly expressed by mature B cell subsets [25]. Siglec-G is the mouse ortholog of human Siglec-10, having 5 extracellular Ig-like domains and a cytoplasmic domain with 3 conserved tyrosine motifs, including one within a consensus ITIM (Figure 1b). As with the multiple ITIMs of CD22, the Siglec-G ITIM likely recruits SHP-1 to negatively regulate signaling, as already described for Siglec-10 [66]. Like CD22, Siglec-G recognizes α2-6 Sia residues of N-linked oligosaccharides [27]. However, Siglec-G is unique in that it recognizes Sia ligands carrying both α2-3- and α2-6-linkages with similar affinities (Figure 2, [28]). The diverse ligand binding activity of Siglec-G along with its structural differences from CD22 suggests that it has unique functions in regulating B cell homeostasis and activation.
Function
The CD5+ B1a B cell population is enriched within the peritoneal and pleural cavities of normal mice [67]. CD5+ cells are uniquely expanded in Siglecg−/− mice, including within the spleen [25], suggesting a more dominant role for Siglec G within this B cell subset than in other B cell compartments. Supporting this, CD5+ B cells from Siglecg−/− mice generate augmented intracellular [Ca2+]i responses following BCR engagement [25]. CD22 and Siglec-G also appear to have non-redundant functions as revealed in CD22 and Siglec-G double-deficient (Cd22−/− Siglecg−/−) mice. B cells from Cd22−/−Siglecg−/− mice are hyper-proliferative to TLR agonists, and these animals develop augmented levels of anti-DNA and anti-nuclear autoantibodies along with mild glomerulonephritis, all beyond that in mice with single molecule deficiencies [68]. Siglec-G and Siglec-10 have also been shown to play a role in the suppression of immune responses to danger-associated molecular patterns (DAMPs) through their interactions with CD24, a process that appears to prevent tissue damage by distinguishing DAMPs from pathogen-associated molecular patterns (PAMPs) [69].
CD22 and Siglec-G may regulate B cell tolerance to self TI-2 Ags
Although foreign TI-2 Ags generally activate the marginal zone and CD5+ B cell subsets which produce relatively low affinity Abs, self TI-2 Ags may nevertheless evoke tolerance mechanisms to suppress cognate B cell activation. Without such mechanisms in place, unencumbered activation of self-reactive B cells even within these largely innate-acting B cell subsets would no doubt lead to autoimmune disease over time. While Sia-bearing glycans are largely absent from most microbes, with the exception of some pathogenic strains that may have evolved Sia expression as a means to subvert innate immune responses [70], these glycans are ubiquitously expressed in nearly all animal tissues. This difference in Sia expression between pathogen and host has led to the suggestion that B cell-restricted Siglecs play a role in self/non-self discrimination to TI-2 Ags. It should be noted that specific self TI-2 Ags have not been identified, but have been hypothesized to include derivatives of living or dead host cells, such as cell membranes or membrane fragments, which would theoretically harbor repetitive elements that are similar in structure to TI-2 Ags derived from encapsulated bacterial surfaces [28].
It was recently shown that both CD22 and Siglec-G are able to recognize a model synthetic TI-2 Ag harboring Sia motifs that have uniquely evolved in mammals [28]. In that study, polyacrylamide (PA) served as the core multivalent backbone for Sia and non-Sia TI-2 Ag conjugates. Using this model system, a TI-2 Ag composed of the “prototypic” mouse CD22 ligand NeuGcα2-6Galβ1-4GlcNAc (NeuGc) conjugated to PA (PA-NeuGc) bound well to B cells from wild type mice. However, PA-NeuGc binding was reduced on B cells from either Cd22−/− or Siglecg−/− mice, and was undetectable on B cells from Cd22−/−Siglecg−/− mice, indicating that both Siglecs are involved in PA-NeuGc recognition. Thus, CD22 and Siglec-G cooperatively mediate the binding of native Sia ligands that may modify currently undefined self TI-2 Ags.
To test the hypothesis that self TI-2 Ags induce B cell tolerance via CD22 and Siglec-G, the hapten nitrophenol (NP) was conjugated to synthetic TI-2 Ags. These conjugates included PA alone (NP-PA), PA-NeuGc (NP-PA-NeuGc), and PA-NeuGc carrying an additional modification of the molecule biphenyl-acetyl which appears to selectively augment CD22-binding activity (NP-PA-bNeuGc). When immune responses to these TI-2 Ags were measured in wild type mice, hapten-specific IgM and IgG3 responses were robust for NP-PA, but negligible for NP-PA-bNeuGc, and significantly reduced for NP-PA-NeuGc [28]. That NP-PA-bNeuGc more effectively inhibited humoral immunity than NP-PA-NeuGc was attributed to its higher affinity for CD22. Remarkably, Siglecg−/− mice generated similar, robust IgM and IgG3 anti-NP responses to both NP-PA and NP-PA-NeuGc. In wild type and Cd22−/− mice however, IgM and IgG3 responses to NP-PA-NeuGc immunization were reduced relative to NP-PA, suggesting that Siglec-G played a dominant inhibitory role over CD22 upon primary immunization with this model NeuGc ligand.
Additional experiments suggested that Sia TI-2 Ags do in fact induce B cell tolerance. Immunization of wild type mice with NP-PA-NeuGc resulted in suppressed IgM and IgG3 anti-NP responses upon secondary challenge with NP-PA. This effect was even more striking when the NP-PA-bNeuGc conjugate was used, whereby mice were unresponsive to secondary challenge with NP-PA for > 1 month. Primary IgM and IgG3 anti-NP responses were even suppressed when NP-PA-bNeuGc was administered in Ribi adjuvant, which contains TLR agonists and other proinflammatory agents, suggesting that self TI-2 Ags can even prevent B cell activation during ongoing inflammatory responses. Importantly, the expression of both CD22 and Siglec-G was required for optimal tolerance induction by NP-PA-bNeuGc, as Cd22−/− and Siglecg−/− mice each elicited significant IgM anti-NP responses during secondary challenge with NP-PA. However, anti-NP IgG3 responses remained muted in Cd22−/−, Siglecg−/−, and Cd22−/−Siglecg−/− mice, which the authors proposed may reflect the activity of an unidentified B cell Siglec bound by the high affinity NP-PA-bNeuGc. Regardless, these studies provide compelling evidence that appropriately sialylated NP-PA Ags are effective inducers of B cell tolerance, which may apply to natural self TI-2 Ags as well. The eventual identification of specific endogenous self TI-2 Ags will allow this to be tested in future studies.
Models for CD22 and Siglec-G regulation of B cell tolerance to self Ags
CD22 and Siglec-G ligand binding might serve as global, Ag-independent desensitizing mechanisms for the multitude of endogenous Sia-decorated self molecules, which regulates tonic B cell signaling, subset development, and homeostasis (Figure 2a). In response to foreign and self TI-2 Ags, the current data suggest a two step model regarding Siglecs and B cell tolerance. First, tolerizing signals are muted when the BCR is heavily crosslinked by foreign TI-2 Ags, as the BCR immunon is sequestered away from CD22/Siglec-G and their inhibitory phosphatases (Figure 2b). By contrast, self TI-2 Ags suppress B cell activation because they co-ligate the BCR and CD22/Siglec-G, bringing inhibitory phosphatases in close proximity to the BCR complex, preventing immunon formation and positive activating signals (Figure 2c). The latter scenario likely results in the apoptosis of some self TI-2 Ag-specific B cells, although it appears that other B cells survive and are truly tolerized due to alterations in important BCR-associated signaling pathways dependent on CD19 signaling [28]. Whether this model applies to all TI-2 Ags as well as to self and foreign T cell-dependent Ags is not clear.
Despite significant progress, gaps remain in our understanding of CD22 and Siglec-G function. Also, the complex function of CD22 in B cell signal transduction adds complexity to the interpretation of the new data suggesting a role in establishing tolerance. For example, CD22 regulates some BCR-mediated signaling events through ligand-independent mechanisms, and mice lacking CD22 ligand-binding activity have a reduction in marginal zone B cells [9]. It remains to be explored whether Siglec-G molecules deficient in ligand-binding activity function similarly. Thus, normal tolerance mechanisms may be altered in unappreciated ways in Cd22−/− and Siglecg−/− mice, potentially resulting in the clonal deletion of TI-2 Ag-specific B cells from the periphery. Furthermore, while most B cells express CD22 and Siglec-G (Figure 1), only a small fraction of B cells would be specific for any given TI-2 Ag. Given this fact, coupled with the propensity of CD22 to rapidly internalize from the cell surface [71, 72], it is reasonable to postulate that a major fraction of self TI-2 Ags are bound and cleared by B cells independent of BCR ligation. From a therapeutic standpoint, it also remains to be seen how efficiently an administered Sia-bearing TI-2 Ag would compete for CD22 and Siglec-G binding given the preponderance of endogenous self TI-2 Ags. Additional characterization of these processes should help clarify operable tolerance mechanisms and rule out trivial explanations for the importance of CD22 and Siglec-G in both T cell-independent and – dependent immune responses.
Concluding remarks
CD22 and Siglec-G may maintain peripheral B cell tolerance through diverse mechanisms, including ligand-mediated recognition of self TI-2 Ags, and the regulation and maintenance of B cell subsets such as regulatory B10 cells, B1a cells, and marginal zone B cells [9–11, 25–28, 45–48, 60, 68, 73]. Thereby, modulating B cell homeostasis and activation by altering the regulatory activities of CD22 and Siglec-G may be effective future strategies in treating autoimmune disease. In support of this concept, the therapeutic efficacy of intravenous Ig (IVIg) for autoimmune disease occurs at least partially through the suppression of B cell activation via CD22, whereby the Sia-bearing fraction of IVIg inhibits BCR signaling and promotes apoptosis in human B cells [74]. A similar function for Sia-bearing IgM complexed with Ag has been demonstrated utilizing primary mouse B cells [75]. Furthermore, marginal zone B cells and CD5+ B cells, each having a possible role in the pathogenesis of some humoral autoimmune syndromes, are particularly susceptible to alterations in the expression and/or ligand-binding activities of CD22 and Siglec-G, respectively [7, 9, 25, 26, 48, 68, 71, 73, 76]. Thus, targeting the ligand binding domains of CD22 and Siglec-G with mAbs or ligand mimetics may be effective therapeutic agents affecting B cell tolerance and subset distribution.
Acknowledgments
This work was supported by National Institutes of Health grants 5U19AI056363 (from the National Institute of Allergy and Infectious Diseases) and 5U54AI057157 (from the National Institute of Allergy and Infectious Diseases and the Southeast Regional Center of Excellence for Emerging Infections and Biodefense), and a grant from the Lymphoma Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodnow CC, et al. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 2.Klinman NR. The "clonal selection hypothesis" and current concepts of B cell tolerance. Immunity. 1996;5:189–195. doi: 10.1016/s1074-7613(00)80314-3. [DOI] [PubMed] [Google Scholar]
- 3.Merrell KT, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Gauld SB, et al. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 5.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill SK, et al. Endocytic sequestration of the B cell antigen receptor and toll-like receptor 9 in anergic cells. Proc Natl Acad Sci, USA. 2009;106:6262–6267. doi: 10.1073/pnas.0812922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SH, et al. B-cell homeostasis requires complementary CD22 and BLyS/BR3 survival signals. Int Immunol. 2010;22:681–691. doi: 10.1093/intimm/dxq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stadanlick JE, Cancro MP. BAFF and the plasticity of peripheral B cell tolerance. Curr Opin Immunol. 2008;20:158–161. doi: 10.1016/j.coi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poe JC, et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 10.Poe JC, et al. Severely-impaired B lymphocyte proliferation, survival and induction of the c-Myc:Cullin 1 ubiquitin ligase pathway resulting from CD22 deficiency on the C57BL/6 genetic background. J Immunol. 2004;172:2100–2110. doi: 10.4049/jimmunol.172.4.2100. [DOI] [PubMed] [Google Scholar]
- 11.Poe JC, et al. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS ONE. 2011;6:e22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiLillo DJ, et al. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 13.Dintzis RZ, et al. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989;143:1239–1244. [PubMed] [Google Scholar]
- 14.Mond JJ, et al. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 15.Perlmutter RM, et al. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 16.Haas KM, et al. Complement receptors CD21/35 link innate and protective immunity during Streptococcus pneumoniae infection by regulating IgG3 antibody responses. Immunity. 2002;17:713–723. doi: 10.1016/s1074-7613(02)00483-1. [DOI] [PubMed] [Google Scholar]
- 17.Haas KM, et al. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Martin F, et al. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. 19. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 20.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 21.Maseda D, et al. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol. 2012;188:1836–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanaba K, et al. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crocker PR, et al. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 24.Tedder TF, et al. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 26.Jellusova J, et al. Siglec-G regulates B1 cell survival and selection. J Immunol. 2010;185:3277–3284. doi: 10.4049/jimmunol.1001792. [DOI] [PubMed] [Google Scholar]
- 27.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–143. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 28.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2009;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doody GM, et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 30.Poe JC, et al. CD22 forms a quaternary complex with SHIP, Grb2 and Shc. A pathway for regulation of B lymphocyte antigen receptor-induced calcium flux. J Biol Chem. 2000;275:17420–17427. doi: 10.1074/jbc.M001892200. [DOI] [PubMed] [Google Scholar]
- 31.Engel P, et al. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils and monocytes. J Immunol. 1993;150:4719–4732. [PubMed] [Google Scholar]
- 32.Engel P, et al. Identification of the ligand binding domains of CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp Med. 1995;181:1581–1586. doi: 10.1084/jem.181.4.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamenkovic I, Seed B. The B cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990;344:74–77. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- 34.Kelm S, et al. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 35.Powell LD, et al. Natural ligands of the B cell adhesion molecule CD22β carry N-linked oligosaccharides with α-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268:7019–7027. [PubMed] [Google Scholar]
- 36.Powell LD, Varki A. The oligosaccharide binding specificities of CD22-β, a sialic acid-specific lectin of B-cells. J Biol Chem. 1994;269:10628–10636. [PubMed] [Google Scholar]
- 37.Munro S, et al. B lymphocyte surface antigen CD75 is not α-2,6-sialyltransferase but is a carbohydrate antigen modified by the enzyme. Cell. 1992;68:1003–1004. doi: 10.1016/0092-8674(92)90070-s. [DOI] [PubMed] [Google Scholar]
- 38.Hanasaki K, et al. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22: selective recognition of immunoglobulin M and haptoglobin. J Biol Chem. 1995;270:7543–7550. doi: 10.1074/jbc.270.13.7543. [DOI] [PubMed] [Google Scholar]
- 39.Stamenkovic I, et al. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and α2,6 sialyltransferase, CD75, on B cells. Cell. 1991;66:1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 40.Pflugh DL, et al. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J Immunol. 2002;169:5130–5136. doi: 10.4049/jimmunol.169.9.5130. [DOI] [PubMed] [Google Scholar]
- 41.Leprince C, et al. CD22 associates with the human surface IgM-B cell antigen receptor complex. Proc Natl Acad Sci USA. 1993;90:3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han S, et al. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 43.Pezzutto A, et al. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp130/140. J Immunol. 1987;138:98–103. [PubMed] [Google Scholar]
- 44.Tooze RM, et al. Counterregulation by the coreceptors CD19 and CD22 of MAP kinase activation by membrane immunoglobulin. Immunity. 1997;7:59–67. doi: 10.1016/s1074-7613(00)80510-5. [DOI] [PubMed] [Google Scholar]
- 45.Nitschke L, et al. CD22 is a negative regulator of B-cell receptor signaling. Curr Biol. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 46.O'Keefe TL, et al. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274:798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 47.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 48.Sato S, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 49.Sato S, et al. CD19 and CD22 expression reciprocally regulates tyrosine phosphorylation of Vav protein during B lymphocyte signaling. Proc Natl Acad Sci, USA. 1997;94:13158–13162. doi: 10.1073/pnas.94.24.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujimoto M, et al. Modulation of B lymphocyte antigen receptor signal transduction by a CD19/CD22 regulatory loop. Immunity. 1999;11:191–200. doi: 10.1016/s1074-7613(00)80094-1. [DOI] [PubMed] [Google Scholar]
- 51.Jin L, et al. Sialic acid binding domains of CD22 are required for negative regulation of B cell receptor signaling. J Exp Med. 2002;195:1199–1205. doi: 10.1084/jem.20011796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelm S, et al. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J Exp Med. 2002;195:1207–1213. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 1998;95:7469–7474. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins BE, et al. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci USA. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Merwe PA, et al. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271:9273–9280. [PubMed] [Google Scholar]
- 56.Lanoue A, et al. Interaction of CD22 with a2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur J Immunol. 2002;32:348–355. doi: 10.1002/1521-4141(200202)32:2<348::AID-IMMU348>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Law CL, et al. Organization of the murine Cd22 locus. Mapping to chromosome 7 and characterization of two alleles. J Immunol. 1993;151:175–187. [PubMed] [Google Scholar]
- 58.Mary C, et al. Dysregulated expression of the Cd22 gene as a results of a short interspersed nucleotide element insertion in Cd22a lupus-prone mice. J Immunol. 2000;165:2987–2996. doi: 10.4049/jimmunol.165.6.2987. [DOI] [PubMed] [Google Scholar]
- 59.Lajaunias F, et al. Polymorphisms in the Cd22 gene of inbred mouse strains. Immunogenetics. 1999;49:991–995. doi: 10.1007/s002510050584. [DOI] [PubMed] [Google Scholar]
- 60.O'Keefe TL, et al. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 62.Kozlowski M, et al. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med. 1993;178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cyster JG, Goodnow CC. Protein tyrosine phosphatase 1C negatively regulates antigen receptor signaling in B lymphocytes and determines thresholds for negative selection. Immunity. 1995;2:13–24. doi: 10.1016/1074-7613(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 64.Sato S, et al. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 65.Asano N, et al. B lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight-skin mouse. Am J Pathol. 2004;165:641–650. doi: 10.1016/S0002-9440(10)63328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitney G, et al. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 67.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 68.Jellusova J, et al. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 69.Chen GY, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haas KM, et al. CD22 ligand binding regulates normal and malignant B lymphocyte survival in vivo. J Immunol. 2006;177:3063–3073. doi: 10.4049/jimmunol.177.5.3063. [DOI] [PubMed] [Google Scholar]
- 72.O'Reilly MK, et al. CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells. J Immunol. 2011;186:1554–1563. doi: 10.4049/jimmunol.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poe JC, et al. CD19, CD21 and CD22: multifaceted response regulators of B lymphocyte signal transduction. Int Rev Immunol. 2001;20:739–762. doi: 10.3109/08830180109045588. [DOI] [PubMed] [Google Scholar]
- 74.Seite JF, et al. IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood. 2010;116:1698–1704. doi: 10.1182/blood-2009-12-261461. [DOI] [PubMed] [Google Scholar]
- 75.Adachi T, et al. CD22 serves as a receptor for soluble IgM. Eur J Immunol. 2012;42:241–247. doi: 10.1002/eji.201141899. [DOI] [PubMed] [Google Scholar]
- 76.Samardzic T, et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur J Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]