Abstract
Stimulation of the offspring immune response during development is known to influence growth and behavioral phenotype. However, the potential for maternal antibodies to block the behavioral effects of immune activation during the neonatal period has not been assessed. We challenged female zebra finches (Taeniopygia guttata) prior to egg laying and then challenged offspring during the nestling and juvenile periods with one of two antigens (keyhole limpet hemocyanin (KLH) or lipopolysaccharide (LPS)). We then tested the effects of maternal and neonatal immune challenges on offspring growth rates and neophobia and learning ability of offspring during adulthood. Neonatal immune challenge depressed growth rates. Neophobia of adult offspring was influenced by a combination of maternal treatment, offspring treatment, and offspring sex. Males challenged with LPS during the nestling and juvenile periods had reduced learning performance in a novel foraging task; however, female learning was not impacted. Offspring challenged with the same antigen as mothers exhibited similar growth suppression and behavioral changes as offspring challenged with a novel antigen. Thus, developmental immune challenges have long-term effects on the growth and behavioral phenotype of offspring. We found limited evidence that matching of maternal and offspring challenges reduces the effects of immune challenge in the altricial zebra finch. This may be a result of rapid catabolism of maternal antibodies in altricial birds. Our results emphasize the need to address sex differences in the long-term effects of developmental immune challenge and suggest neonatal immune activation may be one proximate mechanism underlying differences in adult behavior.
Keywords: Bird, developmental stress, growth, learning, maternal antibodies, maternal effects, maternal immune challenge, neonatal immune challenge, neophobia, zebra finch
Introduction
Activation of the maternal immune response during pregnancy and activation of the offspring immune response during early development have both been demonstrated to influence the behavioral phenotype of offspring (Bilbo and Schwarz, 2009; Meyer et al., 2009). In particular, learning and memory impairments have been demonstrated after perinatal infection or immune activation (Bilbo et al., 2005; Harre et al., 2008; Kohman et al., 2008). Furthermore, the “immune origins of neurodevelopmental disorders hypothesis” proposes that in at least some individuals, propensity to develop neuropsychiatric disorders such as schizophrenia and autism is influenced by neonatal infection (Meyer et al., 2005; Meyer et al., 2011).
Maternal and perinatal infections both induce production of elevated levels of pro-inflammatory cytokines, which have been demonstrated to adversely affect brain development and increase the risk of neurodevelopmental disorders (Bilbo and Schwarz, 2009). The effects of infection and immune challenge on personality, especially fear-related behaviors such as neophobia, may also be mediated by altered sensitivity of the neuroendocrine response to stress through the hypothalamic-pituitary-adrenal (HPA) axis (de Kloet et al., 2002; Spencer and Verhulst, 2007). Under homeostatic conditions, immune system activity enhances learning, memory, neural plasticity, and neurogenesis and the nervous system activates brain immune cells during learning (Yirmiya and Goshen, 2011). Conversely, when the immune system is strongly activated either due to infection or injury, the immune response suppresses learning, memory, neural plasticity, and neurogenesis (Yirmiya and Goshen, 2011).
Despite a growing literature isolating sensitive developmental periods for effects of immune activation on behavior and mechanisms of action translating immune activation into altered behavioral responses, previous research has not assessed how maternal and neonatal infections may interact to influence the behavioral phenotype of offspring. One mechanism through which maternal immune activation may modulate the offspring response to infection is the transmission of antibodies from mothers to offspring. Young vertebrates have limited ability to endogenously produce antibodies and are dependent on maternally derived antibodies as a source of humoral immunity (Grindstaff et al., 2003). Maternal antibodies reflect maternal infection history and there is good evidence from domesticated animals that maternal antibodies provide protection against infection and increase the likelihood of offspring survival after infection (Heller et al., 1990; Lemke and Lange, 1999). In the absence of maternal antibodies, young are primarily dependent on innate immune defenses (Davison et al., 2008). The inflammatory response associated with an induced innate immune response invokes both higher energetic costs and greater risk of autoimmune damage than an induced antibody response (Lee, 2006). Furthermore, behavioral effects of immune system stimulation during development are expected to be most profound when an inflammatory response is triggered, rather than a predominantly antibody-mediated response (Yirmiya and Goshen, 2011). However, if mothers and offspring are exposed to the same antigens and mothers transmit protective levels of maternal antibodies to offspring, then the altered learning and memory that would otherwise be induced by an inflammatory response in naïve offspring may be reduced after immune challenge or infection. A similar process was demonstrated to occur and provided partial protection of offspring from the growth suppressive effects of immune challenge during development (Grindstaff, 2008). In this study, immune challenged Japanese quail (Coturnix japonica) chicks had reduced growth rates in comparison to control, non-challenged offspring. However, within the immune challenged group, young challenged with the same antigen as mothers (maternal group) had higher growth rates than offspring challenged with a different antigen than mothers (novel group) (Grindstaff, 2008). The presence of antigen-specific maternal antibodies likely provided partial protection to young from the growth suppressive effects of immune challenge during development. The potential interactive effects between maternal and offspring antigen exposure on behavior have not been previously addressed.
To test whether matching of maternal and offspring immune challenges could block the growth suppressive and behavioral effects of developmental immune challenge and to characterize the behavioral effects of maternal and developmental immune challenges, we challenged female zebra finches (Taeniopygia guttata) and their newly hatched, cross-fostered young with lipopolysaccharide (LPS) or keyhole limpet hemocyanin (KLH) or administered a control injection. We then measured growth rates, neophobia, and learning ability of offspring. LPS is a component of the outer membranes of Gram-negative bacteria. It induces fever, inflammation, and immediate behavioral changes (e.g., anorexia, somnolence) (Hart, 1988). In birds, it also elicits an antibody response by the immunized individual and the antibodies are transmitted to egg yolks (Grindstaff, 2008). KLH is a copper containing respiratory protein derived from the keyhole limpet (Megathura crenulata) (Dixon et al., 1966). It stimulates a strong antibody response but does not trigger as severe an inflammatory or pyrogenic response as many other immunogens (Jurincic-Winkler et al., 1995). Additionally low and moderate doses of KLH do not cause stimulation of the HPA axis (Stenzel-Poore et al., 1993), whereas LPS stimulates the HPA axis (Karrow, 2006). The potential for maternal and neonatal immune activation to exert persistent effects on offspring learning has not been previously assessed outside of rodent systems.
We predicted that both KLH and LPS immune challenge of offspring would reduce their growth rates. However, we expected that LPS challenged offspring would have the slowest growth rates due to stimulation of an inflammatory response. We also predicted that maternal antibodies might block some of the growth-suppressive effects of immune challenge with KLH and LPS (Grindstaff, 2008). LPS challenge during development was predicted to impair learning in birds as described previously in rodents and was predicted to exert stronger effects on behavior (both neophobia and learning) than KLH due to stimulation of a pro-inflammatory response and stronger activation of the HPA axis. Again we predicted that maternal antibodies might block some of the effects of developmental immune challenge with either KLH or LPS on behavior.
Methods
Study population
All research was approved by the Oklahoma State University Institutional Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 1985). Adult domesticated zebra finches were obtained from two breeders (Magnolia Bird Farms-CA or Quality Pets-OK). Birds were housed as breeding pairs in 45W×45D×40H cm wire cages. Adjacent cages were separated by visual barriers. Room lighting was maintained at 16 L:8 D. During breeding, all cages were equipped with nest boxes and nesting material. Pairs were assigned at random; however, birds that failed to breed within three weeks were assigned to new partners.
Birds were provisioned ad libitum with a commercial exotic finch seed mix (SunSeed Economy Finch Mix), water, and cuttlebone. All breeding pairs received ¼ teaspoon per day of a breeding supplement (a mixture of commercial egg food ABBA 92A with seed, RedBird Products; ground, hard-boiled chicken egg with shell; and vitamins, Zoo Med Laboratories, Avian Plus). Non-breeding adults were provided with spinach once a week or the breeding supplement once a week.
Nest boxes were checked 1–2 times daily during breeding for the presence of eggs and/or young. Eggs were weighed to the nearest 0.01 g on the day of laying and individually labeled. Clutches were defined as complete when two days passed without egg-laying. To facilitate synchronization of egg laying for cross-fostering, first clutches were removed during incubation, which stimulated production of a replacement clutch. More specifically, first clutch eggs were collected within 24 hours of laying and replaced with dummy eggs. Once a sufficient number of females from each of the treatment groups had laid a first clutch, birds were given a booster immune challenge (see below) and dummy eggs were removed seven days later. All described analyses are based on offspring resulting from the replacement clutch.
Maternal treatment
Sixty females were randomly assigned to one of two antigen treatments or the control group. The two antigen treatment groups were: LPS derived from Salmonella typhimurium and KLH. Birds in the control group were injected with an equal volume of sterile phosphate-buffered saline (PBS). The KLH group was immunized with 50 μg of KLH (Calbiochem 374817) in 50 μl of autoclaved PBS (Sigma P5368) (n=20) (Hasselquist et al., 1999). The LPS group was immunized with 1.0 mg LPS/kg body weight (Sigma, L7261) in 50 μl of PBS (n=20) (Owen-Ashley et al., 2006). The control group was injected with PBS only (n=20). All treatments were injected intra-abdominally after cleaning the area with a 70% isopropyl alcohol swab. Females were immunized for the first time prior to the production of the first clutch. A booster immunization was then given at least 35 days after the initial challenge, shortly before production of the replacement clutch. Nest boxes and nest materials were provided 20 days after the primary immunization.
Hatching and cross-fostering
Daily hatch checks began 13 days after the first egg in the replacement clutch was laid. Whenever possible, hatching order was assigned to newly hatched young. Young were weighed to the nearest 0.01 g and the natal down on the body was colored with non-toxic markers to individually identify nestlings on the day of hatching (day 0). Young were cross-fostered within 72 hours of hatching. Within a natal nest, young were equally divided into the three treatment groups. Young within a foster nest did not differ by more than 60 hours in age. This is representative of natural variation in hatching asynchrony within the colony. Clutch and brood sizes were matched during cross-fostering so that the foster brood size was ±1 of the clutch size laid by the female. Within a foster nest, each nestling had a unique combination of colors applied to the natal down on the head and body for individual identification. Young were banded with individually numbered leg bands beginning on day 10 post-hatch. There were 134 young in total that survived until at least 11 days post-hatch. These young originated from 44 different females (13 KLH, 14 LPS, 17 PBS).
Offspring treatment
On day 5, nestlings received a primary immunization. All young within a foster nest received the same treatment as the foster mother. Young within a foster nest differed in whether they received the same treatment as their natal mother or one of the other two treatments. Control offspring received an intra-abdominal injection of 25 μl of sterile PBS. LPS challenged offspring received an intra-abdominal injection of 0.5 mg LPS/kg body weight in 25 μl sterile PBS. KLH challenged offspring received an intra-abdominal injection of 12.5 μg KLH in 25 μl sterile PBS. On day 28, young received a secondary immunization with the adult female doses.
Offspring growth
Offspring body mass was measured to the nearest 0.01 g on days 0, 5, 10, 17, 28, 36, 50, and 65. Tarsus length and wing chord were measured on days 10, 17, 28, 36, 50, and 65. Tarsus length was measured to the nearest 0.01 mm with digital calipers and wing chord was measured with a wing rule to the nearest 0.5 mm.
Neophobia
When birds were approximately one year post-hatch (346–426 days), their responses to novel objects and ability to solve a novel foraging task were tested. The responses to novel objects were used as an index of neophobia (Martins et al., 2007). Twenty-four hours prior to the novel objects trials, birds were moved from group-housed cages (n=2–10 birds/cage) and transferred to individual cages that were visually isolated from other birds. Six birds were tested at a time. Birds were allowed to acclimate for 24 hours before trials commenced (day 1). Tripods used for videotaping were left in place throughout the trials to allow the birds to acclimate to the presence of the tripods. The sequence of birds used in the trials was assigned on the basis of a random number generator. Birds were weighed to the nearest 0.01g both prior to the start of trials and at the completion of trials.
On the morning of day 2 after the acclimation period had ended, videocameras (Sony DCR-SX40) were started and the first novel object, an AA battery was placed in the middle of the cage floor (Martins et al., 2007). The person running the trial then left the aviary. The first and last 10 seconds of trials were not scored and the total trial length was 2 minutes. Four to six hours after the first novel object trial, the same procedures were repeated with the second novel object, a blue plastic frog (42.3 mm × 47.4 mm × 28.8 mm). Neophobic responses were later scored with Sony PMB software on a scale from 0–4 based on the closest contact to the novel object during the 2-minute trial. Birds that directly contacted the novel object received a score of 0 and were considered to be least neophobic. Birds that landed on the floor of the cage in the middle section (central 20 cm) received a score of 1. Birds that landed on the floor of the cage in the left or right outer sections (outer 12.5 cm) received a score of 2. Birds that remained on the feeder or perches in the middle of the cage received a score of 3 and birds that remained on the outer sections of the perches received a score of 4.
Learning
The ability of birds to learn how to solve a novel foraging task was assessed after completion of novel object trials according to methods described in Boogert et al. (2008). At the end of day 2 (after completion of novel object trials), food was removed from cages and foraging boards (see below) were placed in cages 2.5 hours before the lights were turned out for the day. On day 3, trials began within 30 minutes of lights on. The period of food deprivation served to increase the motivation of the birds to solve the foraging task and the overnight exposure to the foraging boards provided an acclimation period to the novel object. Learning trials were recorded as described above for novel object trials.
The novel foraging task consisted of a foraging board (30 cm × 15.3 cm × 1.95 cm) with 10 wells (diameter=10.5 mm, 50 mm apart). Each well contained three white millet seeds. Lids for the wells were made from opaque, thick plastic between a washer and a felt circle that fit snugly into the well. Lids weighed 0.9–1.0 grams. Following Boogert et al. (2008), a systematic shaping procedure was used to progressively train the birds on the four levels of the task. In the first level, wells were completely exposed and lids were beside the wells. In the second level, wells were half-covered by the lids. In the third level, lids were rested on top of wells to obscure the opening. In the fourth level, lids were fitted securely into wells. In order to complete the task, a bird had to successfully complete level four of the task twice in consecutive trials. To reach the next level of the task, the bird had to successfully eat all of the millet out of at least two of the ten wells during the fifteen-minute trial period. If a bird did not successfully complete a level, in the next trial, it returned to the previous level of the task. After each trial, all wells were uncovered to allow free access to seed. If a bird did not feed from any wells during two consecutive trials and did not feed during the inter-trial period, the cage feeder was placed in the cage during the next inter-trial period to give the bird an opportunity to feed. Trials were 15 minutes in duration and one hour elapsed between trials. Immediately before the next trial, foraging boards were removed, wiped clean, wells were refilled with seed, and lids were placed on wells as necessary.
Each bird was given a maximum of six trials per day over a period of three consecutive days. Thus birds had a maximum of 18 trials to solve the task. Birds had free access to food in cage feeders for at least two hours before feeders were removed for the 2.5-hour period before lights out. After completion of the task, birds were returned to their home cages. The number of trials required to complete the task was recorded for each bird. Birds that were at level four of the task at the end of 18 trials, but had not yet completed level four twice in succession, were assigned a score of 19. Birds that were at level three of the task at the end of 18 trials were assigned a score of 20. Similarly birds at levels two or one of the task at the end of the 18 trials were assigned scores of 21 and 22, respectively.
Statistical analyses
Statistical analyses were performed using SAS (v. 9.1, SAS Institute 2008). All variables were checked for normality of residuals and homogeneity of variance prior to analyses. Data were analyzed using mixed models (Proc Mixed) in which maternal identity and day by maternal identity were included as random factors. The covariance structures between repeated measurements of the same individuals were explicitly modeled. A spatial power law covariance model was used to account for unequally spaced longitudinal growth measurements in which correlations were expected to decline as a function of time (Littell et al., 2006). The spatial power law covariance model is a generalization of an autoregressive error model (Littell et al., 2006), which allows for accurate assessment of within subject slopes (Schielzeth and Forstmeier, 2009). Denominator degrees of freedom were approximated using the Kenward-Rodgers method (Littell et al., 2006). Relevant covariates such as foster female identity and the latency between maternal immune challenge and production of the replacement clutch were included in initial models, but did not contribute significantly in any case and were removed from final models. Hatching order contributed significantly (p<0.05) to growth models and was retained in final models.
The effects of maternal and offspring immune treatments on offspring growth and learning were tested through two approaches. First, all three maternal and offspring treatments were included in analyses to test the effects of each of the treatments on offspring development. Second, to test the effect of the presence of antigen-specific maternal antibodies on offspring development, offspring were categorized on the basis of whether they were predicted to have maternal antibodies specific for the challenge they received, no maternal antibodies for the treatment they received, or were not challenged (control group). This second approach tests the effect of receiving a novel immune challenge on offspring development (sensu Grindstaff, 2008). Growth data were analyzed separately for three periods of the experiment reflecting growth before the offspring primary immunization (day 0–day 5), between the primary and secondary immunizations (day 5–day 28), and from the secondary immunization to the completion of size measurements (day 28–day 65). Maternal antibodies were anticipated to have the greatest effect on offspring growth after the primary immunization, whereas any negative effects of maternal immune challenge on offspring size were expected to be greatest immediately after hatch.
Results
Effects of maternal treatment on reproductive investment and early offspring size The latency to produce a replacement clutch after maternal challenge was not significantly impacted by treatment (Range: 7–59 days; F2,12.5=2.98, p=0.087). However, control females tended to have a shorter latency between treatment and production of the replacement clutch than LPS or KLH treated females. Clutch size and average clutch mass also were not significantly impacted by maternal treatment (Clutch size: F2,38.5=0.58, p=0.56; Average mass: F2,42.6=0.23, p=0.80). Hatching success (F2, 31=0.23, p=0.79) and offspring hatching mass (F2,40.6=0.35, p=0.71) were not affected by maternal treatment. Finally, maternal treatment did not affect offspring mass gain prior to immune challenge from day 0 to day 5 (maternal treatment*day: F2,34.9=0.98, p=0.38).
Effects of maternal and offspring treatments on offspring growth
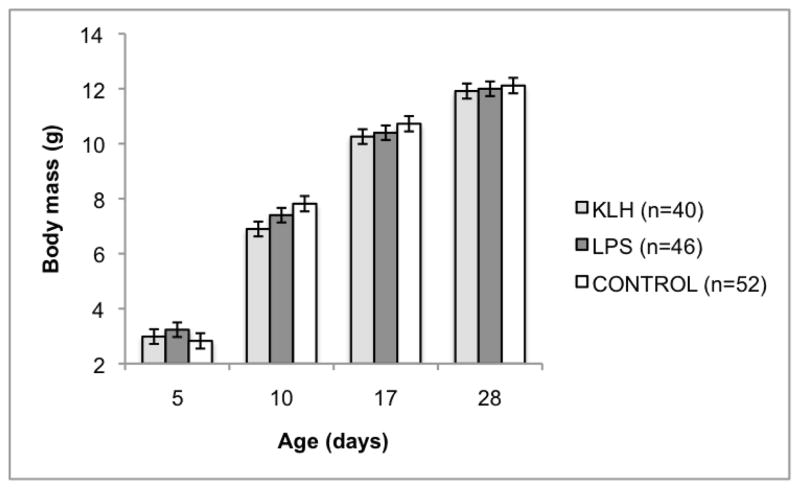
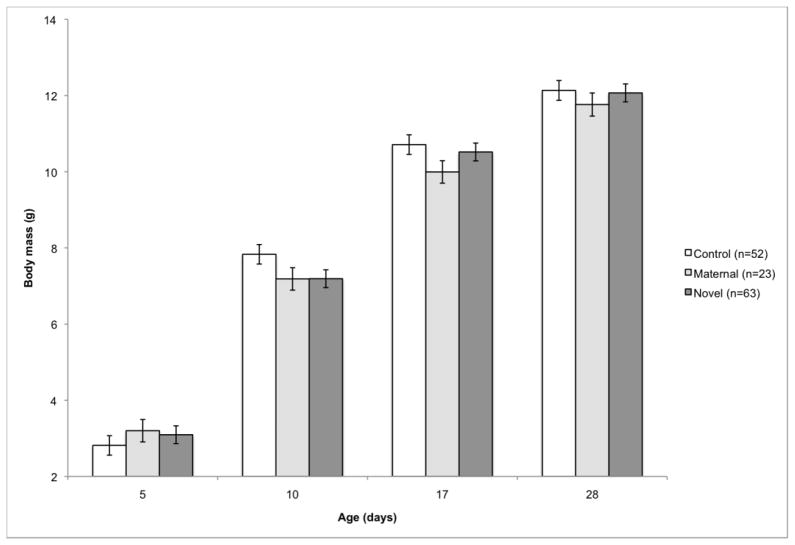
Offspring body mass gain from day 5 (primary immunization) to day 28 (secondary immunization) was significantly impacted by offspring treatment (F6, 294=3.62, p=0.0018; Fig. 1), but was not affected by maternal treatment (F6, 115=0.35, p=0.91; Supp. Table 1). Offspring body mass was not significantly different among offspring treatment groups on day 5 immediately before challenge, but on day 10 post-hatch, KLH challenged young were smaller than control young (p=0.0011). By day 28 post-hatch (immediately prior to secondary immunization), all offspring were again of equivalent body mass. There was also a significant effect of matching between maternal and offspring challenges on offspring body mass gain (F6, 341=4.44, p=0.0002; Fig. 2; Supp. Table 2). Control offspring had the highest growth rate, followed by offspring challenged with a novel antigen (day 10: control vs. maternal: p=0.033, control vs. novel: p=0.0059; day 17: control vs. maternal: p=0.018, control vs. novel: p=0.41). Offspring challenged with the same antigen as mothers had the slowest growth rates. Body mass gain from day 28 (secondary immunization) to day 65 was not impacted by maternal treatment (F6,111=0.47, p=0.83), offspring treatment (F6,276=1.02, p=0.41) or the interaction between maternal and offspring treatments (F12,278=1.22, p=0.27; Supp. Table 3). Mass gain also was not impacted by matching of maternal and offspring challenges (F6,308=1.77, p=0.10; Supp. Table 4).
Fig. 1.
Effect of offspring immune challenge on body mass gain from day 5 to day 28 post-hatch. The offspring immune challenge was administered on day 5. Offspring body mass gain from day 5 to day 28 was significantly impacted by offspring treatment (F6, 294=3.62, p=0.0018). Sample sizes are presented in the legend. KLH=keyhole limpet hemocyanin, LPS=lipopolysaccharide, Control=phosphate buffered saline injection.
Fig. 2.
Effect of offspring group on body mass gain from day 5 to day 28 post-hatch. The offspring immune challenge was administered on day 5. Matching between maternal and offspring challenges significantly affected offspring body mass gain (F6, 341=4.44, p=0.0002). Sample sizes are presented in the legend. Offspring in the control group were injected with phosphate buffered saline, offspring in the maternal group were challenged with the same antigen as their mothers, and offspring in the novel group were challenged with an antigen to which their mothers had not been exposed.
Tarsus growth from day 10 (after primary immunization) to day 28 (secondary immunization) was not significantly impacted by maternal treatment (F4, 71.6=0.76, p=0.56), offspring treatment (F4, 178=0.22, p=0.93), or the interaction between maternal and offspring treatments (F8, 180=0.24, p=0.98; Supp. Table 1). Offspring tarsus growth also was not impacted by matching of maternal and offspring challenges (F4, 200=0.31, p=0.87; Supp. Table 2). Final tarsus length was significantly impacted by a three-way interaction between maternal treatment, offspring treatment, and offspring sex (F4, 66.5=4.04, p=0.0055; Supp. Table 3) such that sons and daughters responded differently to treatment based on maternal immune challenge. KLH challenged sons of KLH challenged mothers and KLH challenged daughters of control mothers had, on average, shorter tarsi. Challenge with a novel antigen did not significantly affect tarsus length (F2, 81.9=1.34, p=0.27; Supp. Table 4).
Wing growth from day 10 to day 28 was not significantly affected by maternal treatment (F4, 68,7=1.46, p=0.23), offspring treatment (F4, 206=2.23, p=0.067), or their interaction (F8, 207=0.74, p=0.65; Supp. Table 1). Early wing growth also was not significantly affected by challenge with a novel antigen (F4, 229=2.18, p=0.073; Supp. Table 2). Offspring wing growth from day 28 to day 65 was not impacted by maternal treatment (F6,106=0.04, p=1.00), offspring treatment (F6,288=0.44, p=0.85) or their interaction (F12,290=0.82, p=0.63; Supp. Table 3). Late wing growth also was not impacted by challenge with a novel antigen (F6,317=1.17, p=0.32; Supp. Table 4).
Effects of maternal and offspring treatments on offspring neophobia
Neophobic responses did not differ significantly between males and females (Battery: F1,110=0.96, p=0.33; Frog: F1,107=0.05, p=0.83). There was a tendency for birds that were heavier at the start of trials to approach the battery more closely (F1,103=3.64, p=0.059), but this effect was not present for the frog (F1,100=0.30, p=0.59). There was also no effect of mass loss during the trials (Battery: F1,99=0.77, p=0.38; Frog: F1,96=0.00, p=0.96) or mass at the completion of the trials (Battery: F1,105=1.54, p=0.22; Frog: F1,102=0.09, p=0.77) on neophobic behavior. Responses to the two novel objects were positively correlated (F1,107=5.58, p=0.02). However, responses to the two novel objects were not related to learning ability (Battery: F1,101=0.54, p=0.46; Frog: F1,101=0.93, p=0.34).
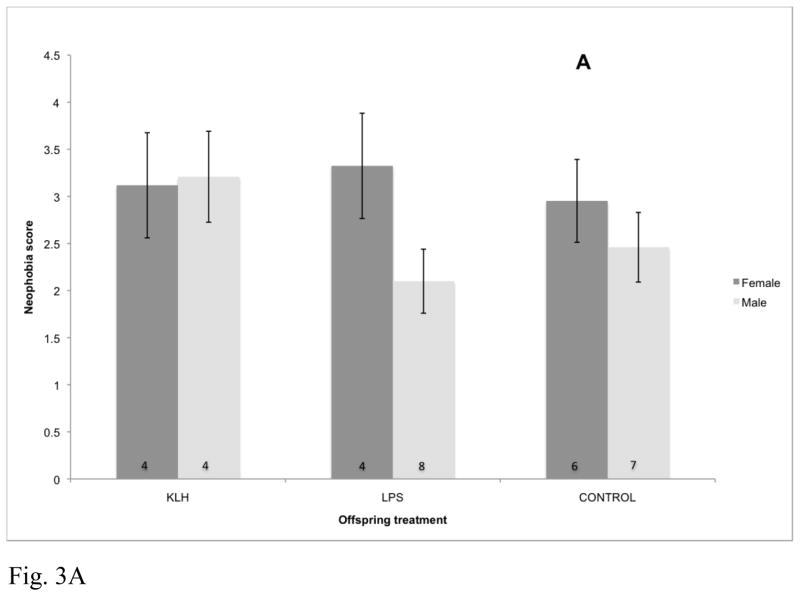
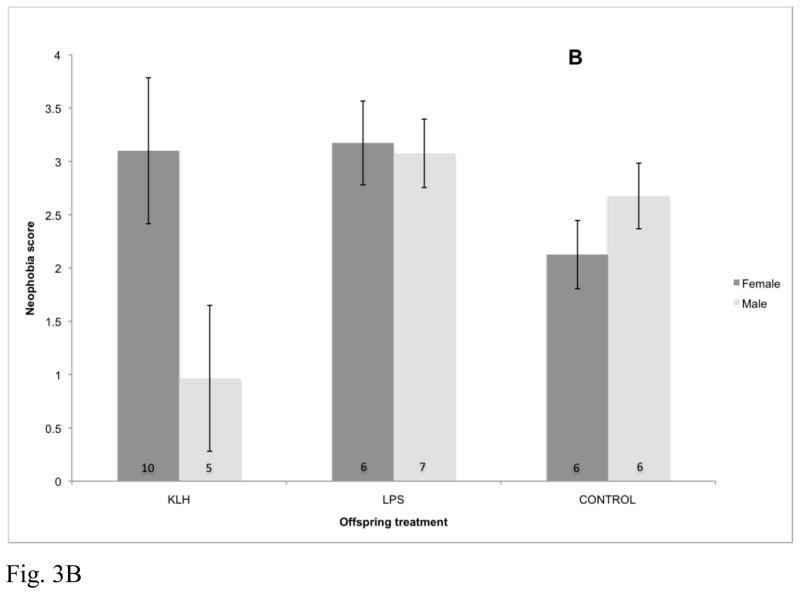
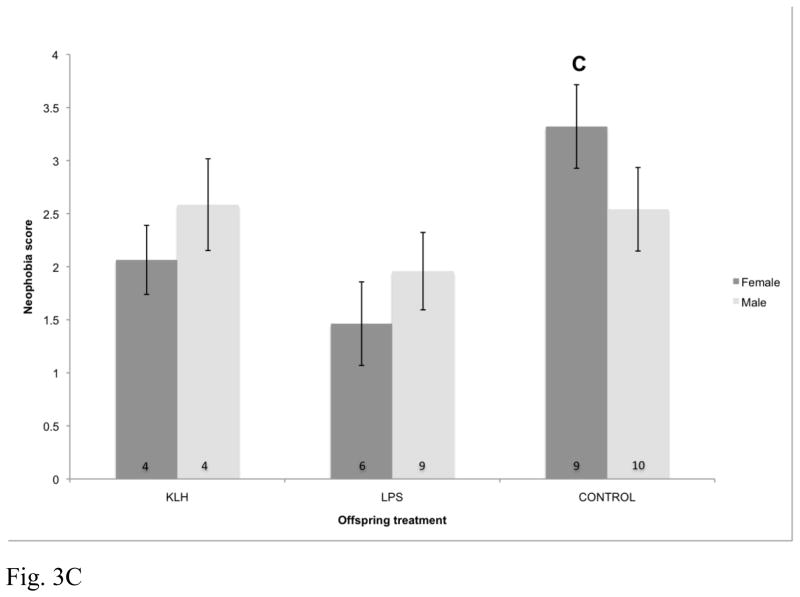
Neophobic response to the frog novel object was significantly impacted by the interaction between maternal treatment, offspring treatment, and offspring sex (F4,83.9=3.01, p=0.023; Fig. 3). Conversely, response to the battery was not impacted by maternal treatment (F2,42.1=2.01, p=0.15), offspring treatment (F2,95.7=0.24, p=0.79) or the interactions between maternal and offspring treatments (F4,94.6=1.06, p=0.38). Behavioral responses were not impacted by the matching of maternal and offspring treatments for either the frog (F2,105=0.95, p=0.39) or the battery (F2,99.6=1.42, p=0.25).
Fig. 3.
Effects of maternal immune challenge, offspring developmental immune challenge, and offspring sex on offspring neophobic behavior assessed at approximately 1 year post-hatch (F4,83.9=3.01, p=0.023). Birds that received a score of 0 were considered to be least neophobic and birds that received a score of 4 were considered to be most neophobic. Numbers within bars represent sample sizes. KLH=keyhole limpet hemocyanin, LPS=lipopolysaccharide, Control=phosphate buffered saline injection. (A) Effects of offspring immune challenge on neophobic behavior for offspring of KLH challenged mothers. (B) Effects of offspring immune challenge on neophobic behavior for offspring of LPS challenged mothers. (C) Effects of offspring immune challenge on neophobic behavior for offspring of control mothers.
Effects of maternal and offspring treatments on offspring learning ability
Males were significantly slower to learn the novel foraging task than females across treatment groups (F1,105=4.57, p=0.035). Birds that were lighter at the start of trials solved the foraging task more quickly (F1,100=6.48, p=0.012). Mass loss during the trials (F1,99=3.46, p=0.066) and mass at the completion of the trials (F1,104=3.44, p=0.066) both tended to impact learning ability with birds that lost the most mass and lighter birds solving the task more quickly than other birds. Males and females did not differ significantly in mass before (F1, 103=1.60, p=0.21) or after (F1, 105=1.45, p=0.23) the trials or mass loss during the trials (F1, 99=0.04, p=0.83).
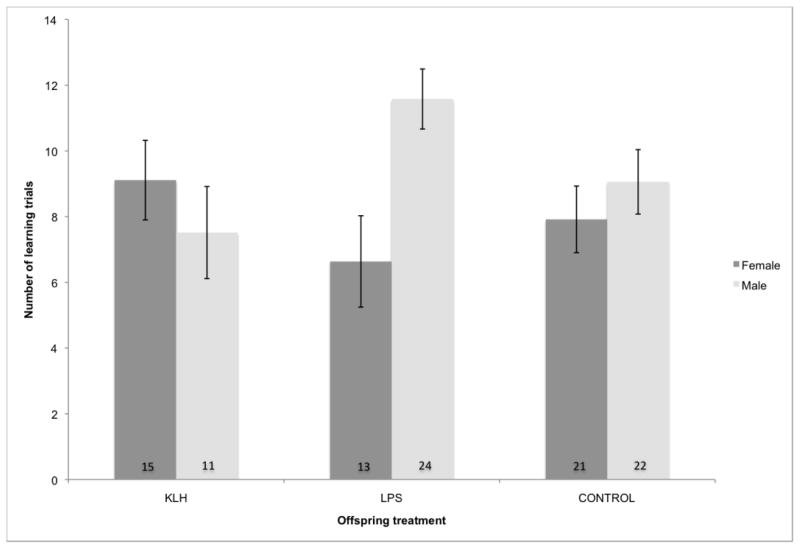
After accounting for the effect of mass at the beginning of trials on learning ability (F1,82=8.60, p=0.0044), there was a significant interaction effect between offspring treatment and offspring sex on learning ability (F2,82=3.55, p=0.033; Fig. 4). LPS-challenged males had significantly reduced performance in the learning task compared to LPS-challenged females and offspring in the other groups. Matching of maternal and offspring treatments did not significantly impact learning ability (F2,90.2=1.07, p=0.35).
Fig. 4.
Effects of offspring immune challenge and offspring sex on number of learning trials required to solve a foraging task (F2,82=3.55, p=0.033). Offspring immune challenges were administered on days 5 and 28 post-hatch and learning ability was assessed at approximately 1 year post-hatch. Numbers within bars represent sample sizes. KLH=keyhole limpet hemocyanin, LPS=lipopolysaccharide, Control=phosphate buffered saline injection.
Discussion
Maternal immune challenge did not significantly affect reproductive investment or offspring body size prior to offspring immunization. Thus, it is unlikely that the subsequent effects of maternal treatment on offspring size and behavior were mediated through indirect effects of maternal condition or egg size. The ability of zebra finches to learn a novel task was significantly affected by current body condition such that heavier birds learned the task more slowly than lighter birds. This is likely linked to motivation because the task involved access to food and lighter birds were likely more food-motivated than heavier birds. Furthermore, males learned the task significantly more slowly than females across treatment groups. Males and females did not differ significantly in body mass. After accounting for both of these effects, offspring treatment significantly affected adult learning such that males challenged with LPS had the slowest learning rates. Females challenged with LPS did not have reduced learning rates. Thus as predicted, LPS challenge during development altered learning in adult offspring (Bilbo and Schwarz, 2009), but only in males. A majority of the previous studies addressing the effects of neonatal infection on adult learning have focused solely on males, thus little is known about potential sex differences. In two previous studies that did address sex differences, neonatal LPS challenge increased expression of hypothalamic cyclooxygenase-2 in male rats, but not females (Kentner et al., 2010) and male mice exposed to prenatal stress increased HPA axis sensitivity and anhedonia in adulthood, but female mice did not (Mueller and Bale, 2008). Studies of sex differences in response to stressors or to direct elevation of glucocorticoids in rodents have demonstrated that the hippocampus of females is generally more resistant to both morphological and physiological effects of chronic stress (Andreano and Cahill, 2009). In males, chronic stress reduces glucocorticoid receptor density and performance in a spatial memory task, but chronic stress increases glucocorticoid receptor density and spatial memory in females (Kitraki et al., 2004). Furthermore, neonatal infection has been demonstrated to alter gene expression in neuroendocrine and immune pathways in a sex-specific manner. Male neonatal infection is associated with upregulation of genes involved in cellular development and nervous system development and function. Conversely, female neonatal infection is associated with T-helper 2 immune bias (Wynne et al., 2011).
Contrary to our predictions, maternal antibodies did not ameliorate the effects of developmental immune challenge on adult learning because there was no effect of the matching of maternal and offspring treatments. We predicted that offspring challenged with the same antigens as mothers would not have reduced learning ability. Instead, offspring treatment alone impacted offspring learning and matching of maternal and offspring treatments did not provide neuroprotective effects. In mammals, maternal immune activation induces elevated levels of the pro-inflammatory cytokine interleukin-6 (IL-6) in the placenta that may then be transmitted to the developing fetus (Hsiao and Patterson, 2011). IL-6 has also been demonstrated to induce the same behavioral deficits as challenge with a viral mimic (Hsiao and Patterson, 2011). Thus, in mammals, the negative effects of elevated cytokine levels induced by maternal immune activation may outweigh any potential protective effects of maternal antibodies. Production of pro-inflammatory cytokines in the oviduct after maternal immune activation has recently been documented in chickens (Nii et al., 2011), but it is unknown if, and how, oviductal cytokines might impact avian embryonic development.
Learning performance in the novel foraging task was not correlated with neophobic response to two novel objects. Thus, it is unlikely that slower learners were simply more neophobic in response to the novel foraging board used for the learning task. Learning performance by male zebra finches in this task has previously been linked to song complexity such that males that were faster at learning the task also sang songs with more elements (Boogert et al., 2008). Female zebra finches prefer to mate with males with more syllables in their songs (Spencer et al., 2005; Holveck and Riebel, 2007). However, it is unclear if females also prefer males with more song elements (Boogert et al., 2008). In our study, the secondary immunization was administered on day 28 post-hatch during the subsong period (Zann, 1996) when male song development is particularly sensitive to the effects of developmental stressors (Nowicki et al., 2002). Thus, challenge with LPS may have impacted male learning of both a novel foraging task and song learning. Both of these behaviors have important fitness consequences. Behavioral flexibility and the ability to learn how to exploit novel resources are expected to be particularly advantageous in novel environments (Sol, 2009).
Offspring neophobia was influenced by an interaction between maternal and offspring treatments and offspring sex. Thus, adult behavior was determined by the maternal and developmental environments in a sex-dependent manner. Models of parasite dependent and immune system dependent personality differences have suggested that parasite presence and immune system activation should favor resource-accruing personalities such as higher activity levels (Kortet et al., 2010) to balance the associated costs. A previous study of mallards (Anas platyrhynchos) found partial support for this hypothesis as mallards that were challenged with sheep red blood cells during development exhibited greater activity as adults in novel environments (Butler et al., 2012). However, although maternal and developmental immune challenges affected response to a novel object in zebra finches, challenged offspring were not uniformly less neophobic. Instead, the effects of immunization were antigen and sex-specific suggesting that the effects of parasitism and immune system activation on behavior may be more complicated than current models propose. Neither did the matching of maternal and offspring treatments in the current study impact neophobia. Thus, it was not simply that maternal antibodies blocked negative effects of developmental immune challenge. In fact, LPS challenged offspring with mothers that had also been challenged with LPS were on average the least neophobic and this was especially true for daughters. In contrast, KLH challenged offspring with mothers that had also been challenged with KLH were on average highly neophobic and this was especially true for sons. Maternal immune challenge may alter the deposition of other egg components, besides antibodies, with subsequent effects on offspring phenotype. For example, elevated yolk testosterone levels have been demonstrated to increase the latency to feed in the presence of a novel object in male zebra finches, but not females (Tobler and Sandell, 2007).
Although very few studies have examined the potential for neonatal or maternal immune challenges to impact adult behavior and the mechanisms underlying this process are poorly understood, a number of studies have addressed the potential for neonatal exposure to a stressor or manipulation of glucocorticoid levels to impact adult behavior in both rodent and avian systems (e.g., Bangasser and Shors, 2010; Fumagalli et al., 2007; Schoech et al., 2011). In zebra finches, experimental elevation of corticosterone levels during the post-hatching period (days 12–28) resulted in greater HPA reactivity of these birds when subjected to a standardized capture and restraint protocol at 60 days post-hatch (Spencer et al., 2009). In a similar study in which corticosterone levels were experimentally elevated between days 7–18, treated zebra finches were demonstrated to exhibit reduced neophobic responses to novel objects when tested at the ages of 27–35 days and 50–60 days (Spencer and Verhulst, 2007). This response was also shown to be sex-specific such that only male behavior was significantly impacted by developmental elevation of corticosterone levels (Spencer and Verhulst, 2007). Challenge with LPS is known to activate the HPA axis as well as the immune response (Karrow, 2006). Thus, some of the effects observed here of developmental immune challenge on adult behavior might be the result of elevation of corticosterone levels during development. However, low doses of KLH are not thought to activate the HPA axis, at least in rodents (Stenzel-Poore et al., 1993).
Although offspring treatment was demonstrated to impact adult learning and behavior, it is unclear whether challenge on day 5 or day 28 or the combination of these two induced the effects observed on adult behavior. A recent study of mallards demonstrated that immunization during development affected adult behavior (Butler et al., 2012). These authors immunized ducklings at different developmental stages and were able to determine that challenges during the middle period of growth (following the completion of somatic growth) and during the late growth period (during the molt into nuptial plumage) impacted adult behavior, but challenge earlier, during maximum somatic growth did not impact behavior (Butler et al., 2012). The day 5 challenge of zebra finches would correspond to the early growth period of ducklings and the day 28 challenge of zebra finches would correspond to the middle growth period of ducklings. On day 5, nestlings were beginning rapid somatic growth and on day 28, juveniles had completed most somatic growth, but had not yet attained sexually dimorphic plumage (Zann, 1996). Thus, if the mechanisms underlying effects of offspring immune challenge on adult behavior are similar in mallards and zebra finches, this would suggest the secondary immunization was most important in impacting neophobia. However, this remains to be tested directly. Rodent studies have demonstrated that infection during the early postnatal period induces memory impairments later in life and induces altered glial activation and cytokine expression, but infection later in development does not induce behavioral or physiological effects (Bilbo et al. 2006). This sensitive period is thought to be a result of developmental changes in glial morphology and function (Bilbo and Schwarz, 2009).
As predicted, offspring immune challenge reduced offspring growth rates. In particular, control offspring had higher rates of body mass gain between the primary and secondary immunizations than immunized offspring. Contrary to our predictions, KLH challenged offspring had even lower growth rates than LPS challenged offspring. We predicted that LPS challenged offspring would have the slowest growth rates due to the high usage costs associated with the inflammatory response stimulated by LPS (Klasing and Leshchinsky, 1999). However, the developmental costs of a specific immune response such as that induced by KLH may be high enough to reduce growth rates as well (Klasing and Leshchinsky, 1999). There were also sex-dependent effects of maternal and offspring treatments on offspring growth rates suggesting that the effects of immune system activation may be different for males and females. We predicted that maternal antibodies might block some of the growth-suppressive effects of offspring immune challenge as was documented previously in precocial Japanese quail (Grindstaff, 2008). However, offspring challenged with the same antigen as mothers (maternal) had equivalent or even lower growth rates than offspring challenged with a different antigen than mothers (novel). In altricial birds, maternal antibodies may be catabolized more quickly than in precocial birds (Addison et al., 2009; Pihlaja et al., 2006), which would provide both a shorter period of pathogen protection and a shorter period of protection from the growth-suppressive effects of immune system activation. Additionally, immune challenged offspring may prioritize investment in other life history traits at the expense of investment in growth.
All cross-fostered young within a brood were assigned to the same treatment group as the foster female to minimize within nest differences in offspring behavior after immune challenge that might have induced treatment-dependent begging hierarchies (Romano et al., 2011). However, with this design the potential effect of foster female treatment on offspring growth and behavior cannot be separated from the effect of offspring treatment. A complete, reciprocal cross-fostering design would have been ideal, but logistically impractical due to the large number of potential combinations of maternal and offspring treatments (27 for this design). Nonetheless, foster female treatment was administered before egg laying and young were not cross-fostered until after hatch (day 0–day 3). Thus, approximately three weeks had elapsed between foster female treatment and cross-fostering and it seems unlikely that immunization with low doses of non-replicating antigens would have significantly impacted adult behavior over this time scale (Grindstaff et al., 2006; Koutsos and Klasing, 2001). The most parsimonious interpretation is that offspring growth and behavior were impacted by developmental immune challenge.
Conclusions
In conclusion, this study provides evidence that the developmental environment, specifically immune challenge, influences learning ability and behavior in adulthood in a sex-dependent manner. Furthermore, neophobia, is influenced both by maternal and offspring immune challenges. Additional work is required to determine the mechanistic basis for these effects, especially in non-mammalian systems and to determine the adaptive significance of these behavioral modifications.
Supplementary Material
Highlights.
Maternal and neonatal immune activation separately influence behavior.
We tested potential for maternal and neonatal immune challenges to interact.
Female and offspring zebra finches were immune challenged.
Maternal and neonatal immune challenges interacted to affect neophobia.
Sons challenged with LPS had reduced learning ability in adulthood.
Acknowledgments
We thank Staci Bilbo and Sabra Klein for the opportunity to contribute to this special issue and two anonymous reviewers for helpful comments. Matt Anderson, Kent Andersson, Courtney Cupps, Sara Friedemann, Lisa Hughes, Amanda Neujahr, Alecia Pippin, Arielle Shanahan, and Matt Waselik provided invaluable assistance with zebra finch care. Anna Forsman and Britt Heidinger provided helpful comments on a previous version of the manuscript. Funding for the project was provided by NIH grant 1R15HD066378-01. SNC was supported as an undergraduate researcher through the Oklahoma IDeA Network of Biomedical Research Excellence funded by NIH grant P20RR016478. VRH was supported as an undergraduate researcher through the NSF REU program at Oklahoma State University funded by NSF grant SMA1063091.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addison B, Klasing KC, Robinson WD, Austin SH, Ricklefs RE. Ecological and life-history factors influencing the evolution of maternal antibody allocation: A phylogenetic comparison. Proc R Soc Lond, Ser B Biol Sci. 2009;276:3979–3987. doi: 10.1098/rspb.2009.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. Critical brain circuits at the intersection between stress and learning. Neurosci Biobehav Rev. 2010;34:1223–1233. doi: 10.1016/j.neubiorev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:1–14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogert NJ, Giraldeau LA, Lefebvre L. Song complexity correlates with learning ability in zebra finch males. Anim Behav. 2008;76:1735–1741. [Google Scholar]
- Butler MW, Toomey MB, McGraw KJ, Rowe M. Ontogenetic immune challenges shape adult personality in mallard ducks. Proc R Soc Lond, Ser B Biol Sci. 2012 doi: 10.1098/rspb.2011.0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison F, Magor KE, Kaspers B. The avian mucosal immune system. In: Davison F, Kaspers B, Schat KA, editors. Avian immunology. Elsevier; New York: 2008. pp. 107–127. [Google Scholar]
- de Kloet ER, Grootendorst J, Karssen AA, Oitzl MS. Gene × environment interaction and cognitive performance: Animal studies on the role of corticosterone. Neurobiol Learn Mem. 2002;78:570–577. doi: 10.1006/nlme.2002.4079. [DOI] [PubMed] [Google Scholar]
- Dixon FJ, Jacot-Guillarmod H, McConahey PJ. The antibody responses of rabbits and rats to hemocyanin. J Immunol. 1966;97:350–355. [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL. Maternal antibodies reduce costs of an immune response during development. J Exp Biol. 2008;211:654–660. doi: 10.1242/jeb.012344. [DOI] [PubMed] [Google Scholar]
- Grindstaff JL, Brodie ED, Ketterson ED. Immune function across generations: Integrating mechanism and evolutionary process in maternal antibody transmission. Proc R Soc Lond, Ser B Biol Sci. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson JÅ, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: Maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc R Soc Lond, Ser B Biol Sci. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre EM, Galic MA, Mouihate A, Noorbakhsh F, Pittman QJ. Neonatal inflammation produces selective behavioural deficits and alters n-methyl-d-aspartate receptor subunit mRNA in the adult rat brain. Eur J Neurosci. 2008;27:644–653. doi: 10.1111/j.1460-9568.2008.06031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behav Ecol Sociobiol. 1999;45:167–175. [Google Scholar]
- Heller ED, Leitner G, Drabkin N, Melamed D. Passive immunization of chicks against Escherichia coli. Avian Pathol. 1990;19:345–354. doi: 10.1080/03079459008418685. [DOI] [PubMed] [Google Scholar]
- Holveck MJ, Riebel K. Low-quality females prefer low-quality males when choosing a mate. Proc R Soc Lond, Ser B Biol Sci. 2010;277:153–160. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurincic-Winkler C, Klippel KF, Beuth J, Markl J. Value of keyhole limpet hemocyanin in prevention of superficial bladder-cancer recurrence. Onkologie. 1995;18:41–50. [Google Scholar]
- Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: Lessons learned from the model inflammagen, lipopolysaccharide. Brain Behav Immun. 2006;20:144–158. doi: 10.1016/j.bbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kentner AC, McLeod SA, Field EF, Pittman QJ. Sex-dependent effects of neonatal inflammation on adult inflammatory markers and behavior. Endocrinol. 2010;151:2689–2699. doi: 10.1210/en.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitraki E, Kremmyda O, Youlatos D, Alexis M, Kittas C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Stress: Current neuroendocrine and genetic approaches. 2004:323–327. doi: 10.1196/annals.1296.039. [DOI] [PubMed] [Google Scholar]
- Klasing KC, Leshchinsky TV. Functions, costs, and benefits of the immune system during development and growth. In: Adams NJ, Slotow RH, editors. 22nd International Ornithological Congress; BirdLife South Africa, Durban, South Africa. 1999. pp. 2817–2835. [Google Scholar]
- Kohman RA, Tarr AJ, Sparkman NL, Bogale TMH, Boehm GW. Neonatal endotoxin exposure impairs avoidance learning and attenuates endotoxin-induced sickness behavior and central IL-1 beta gene transcription in adulthood. Behav Brain Res. 2008;194:25–31. doi: 10.1016/j.bbr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Kortet R, Hedrick AV, Vainikka A. Parasitism, predation and the evolution of animal personalities. Ecol Lett. 2010;13:1449–1458. doi: 10.1111/j.1461-0248.2010.01536.x. [DOI] [PubMed] [Google Scholar]
- Koutsos EA, Klasing KC. The acute phase response in Japanese quail (Coturnix coturnix japonica) Comp Biochem Physiol. 2001;128:255–263. doi: 10.1016/s1532-0456(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la Konrad Lorenz? Scand J Immunol. 1999;50:348–354. doi: 10.1046/j.1365-3083.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. SAS Institute Inc; Cary, NC: 2006. [Google Scholar]
- Martins TLF, Roberts ML, Giblin I, Huxham R, Evans MR. Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Horm Behav. 2007;52:445–453. doi: 10.1016/j.yhbeh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Sonoda Y, Isobe N, Yoshimura Y. Effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokines and the subsequent recruitment of immunocompetent cells in the oviduct of laying and molting hens. Poult Sci. 2011;90:2332–2341. doi: 10.3382/ps.2011-01596. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA, Peters S. Brain development, song learning and mate choice in birds: A review and experimental test of the “Nutritional stress hypothesis”. J Comp Physiolog A Neuroethol Sens Neural Behav Physiolog. 2002;188:1003–1014. doi: 10.1007/s00359-002-0361-3. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley NT, Turner M, Hahn TP, Wingfield JC. Hormonal, behavioral, and thermoregulatory responses to bacterial lipopolysaccharide in captive and free-living white-crowned sparrows (Zonotrichia leucophrys gambelii) Horm Behav. 2006;49:15–29. doi: 10.1016/j.yhbeh.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Pihlaja M, Siitari H, Alatalo RV. Maternal antibodies in a wild altricial bird: Effects on offspring immunity, growth and survival. J Anim Ecol. 2006;75:1154–1164. doi: 10.1111/j.1365-2656.2006.01136.x. [DOI] [PubMed] [Google Scholar]
- Romano A, Rubolini D, Caprioli M, Boncoraglio G, Ambrosini R, Saino N. Sex-related effects of an immune challenge on growth and begging behavior of barn swallow nestlings. Plos One. 2011;6:e22805. doi: 10.1371/journal.pone.0022805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielzeth H, Forstmeier W. Conclusions beyond support: Overconfident estimates in mixed models. Behav Ecol. 2009;20:416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Sol D. The cognitive-buffer hypothesis for the evolution of large brains. In: Dukas R, Ratcliffe JM, editors. Cognitive ecology II. The University of Chicago Press; Chicago: 2009. pp. 111–134. [Google Scholar]
- Spencer KA, Evans NP, Monaghan P. Postnatal stress in birds: A novel model of glucocorticoid programming of the hypothalamic-pituitary-adrenal axis. Endocrinol. 2009;150:1931–1934. doi: 10.1210/en.2008-1471. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Verhulst S. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata) Horm Behav. 2007;51:273–280. doi: 10.1016/j.yhbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Wimpenny JH, Buchanan KL, Lovell PG, Goldsmith AR, Catchpole CK. Developmental stress affects the attractiveness of male song and female choice in the zebra finch (Taeniopygia guttata) Behav Ecol Sociobiol. 2005;58:423–428. [Google Scholar]
- Stenzel-Poore M, Vale WW, Rivier C. Relationship between antigen-induced immune stimulation and activation of the hypothalamic-pituitary-adrenal axis in the rat. Endocrinol. 1993;132:1313–1318. doi: 10.1210/endo.132.3.8382598. [DOI] [PubMed] [Google Scholar]
- Tobler M, Sandell MI. Yolk testosterone modulates persistence of neophobic responses in adult zebra finches, Taeniopygia guttata. Horm Behav. 2007;52:640–645. doi: 10.1016/j.yhbeh.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Wynne O, Horvat JC, Osei-Kumah A, Smith R, Hansbro PM, Clifton VL, Hodgson DM. Early life infection alters adult BALB/c hippocampal gene expression in a sex specific manner. Stress: Int J Biol Stress. 2011;14:247–261. doi: 10.3109/10253890.2010.532576. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: A synthesis of field and laboratory studies. Oxford University Press; New York: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.