Abstract
Objectives
Late perimenopause and early postmenopause confer an increased risk of depression in the population, yet bipolar disorder mood course during these times remains unclear.
Methods
Clinic visits in 519 premenopausal, 116 perimenopausal including 13 women transitioning from perimenopause to postmenopause, and 133 postmenopausal women with bipolar disorder who received naturalistic treatment in the multisite STEP-BD study over 19.8±15.5 months were analyzed for mood state. History of postpartum and perimenstrual mood exacerbation and current hormone therapy were evaluated as potential mood predictors.
Results
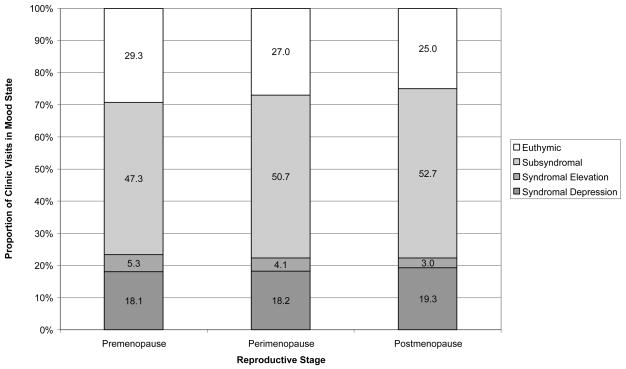
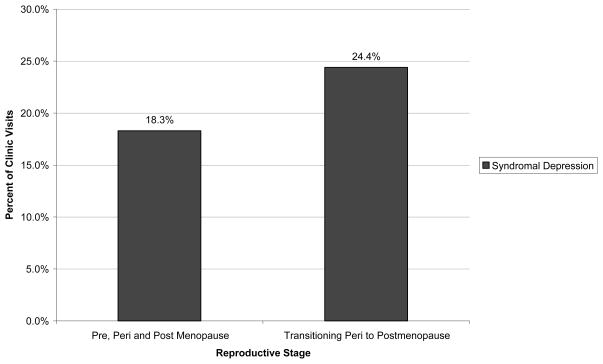
A progression in female reproductive stage (premenopause, perimenopause, and postmenopausae) was significantly associated with percent of visits decreasing in euthymia (29.3%, 27.0%, 25.0%, respectively, p<0.05) decreasing in syndromal mood elevation (5.3%, 4.1%, and 3.0%, respectively, p<0.001), and increasing in subsyndromal symptoms (47.3%, 50.7%, and 52.7%, respectively, p = 0.05). Thirteen women transitioning from peri- to postmenopause had a significantly greater proportion of visits in syndromal depression (24.4%, p<0.0005) compared to premenopausal, perimenopausal and postmenopausal women, while depression in the latter three groups (18.1%, 18.1%, and 19.3%, respectively) did not differ. Perimenstrual and/or postpartum mood exacerbation, or hormone therapy did not significantly alter depression during perimenopause.
Conclusions
A progression in female reproductive stages was associated with bipolar illness exacerbation. A small number of women transitioning from perimenopause to postmenopause had significantly greater depression than other female reproductive groups. Euthymia and mood elevation decreased with progressing female reproductive stage. Menstrual cycle or postpartum mood exacerbation, or current hormone therapy use, was not associated with perimenopausal depression. Future studies, which include hormonal assessments, are needed to confirm these preliminary findings.
Keywords: Bipolar Disorder, Menopause, Depression, Mood Disorders, Women
INTRODUCTION
Women with bipolar disorder are at risk of severe mood episodes, particularly depression, during the postpartum (1, 2), a time of decline in reproductive hormones. Overall women with bipolar disorder differ from men by bearing a greater depressive burden with more frequent depressive episodes (3, 4), rapid cycling (4, 5), and mixed mania (6, 7). Yet little is known about the mood episode course in bipolar disorder during the menopausal transition, another time of marked gonadal hormone fluctuation and decline.
While most women in the population do not experience a depressive episode during the menopausal transition, psychological symptoms are frequent and consist of irritability, tearfulness, anxiety, depression, emotional lability, low energy, low motivation, poor concentration and interrupted sleep (8–10). These symptoms may overlap or potentially contribute to a mood episode and an expanding literature is reporting an increased risk of major (and minor) depression in women with or without a history of depression during the late perimenopause or early postmenopause (11–14). The likelihood of depressed mood during the menopausal transition has been reported to be approximately 30% to 300% greater compared to premenopause (15).
Neurobiological changes during the menopausal transition indicate the importance of evaluating the relationship between reproductive hormonal fluctuation and mood during this time period. Sex steroid receptors are located in the cognitive and mood processing regions of the brain, including the amygdala, hippocampus, cingulate cortex, locus ceruleus, midbrain raphe, and central grey matter (16). Estrogens, progestins, and androgens affect a wide rangeof neuromodulator processes, including the neuromodulators serotoninand norepinephrine, implicated in the development of depression (17) Estradiol stimulates synaptogenesis, and leads to a significant increase in serotonin 5-HT2A binding sights, increase in serotonin synthesis, decrease in monoamine oxidase concentrations, and thus in sum, to a possible antidepressant-like effect (18).
Gonadal steroids such as estrogen and progesterone fluctuate greatly during the menopausal transition, especially in the early transition (change by at least seven days from regular cycle frequency) where estradiol levels can even rise above the normal menstrual cycle range. During the late menopausal transition (greater than 60 days between menstruations) gonadal sex steroids often have their greatest decline. After 365 days of not menstruating a woman is considered postmenopausal although estrogen and progesterone levels continue to vary, and from an endocrinological perspective the immediately post-menopausal period may not be distinguished from the long cycles of perimenopausal women (19, 20).
Prior studies of women with bipolar disorder during the menopausal transition have been small (21–23) or retrospective (11, 24). Most involved cross-sectional or retrospective assessment of mood (22, 24, 25). The literature in bipolar disorder suggests an increase in mood instability during the menopausal transition (21, 22, 24, 25), particularly an increase in depression (21, 25) or high rate of depression (23). However, to date no study has compared mood episodes in women undergoing the menopausal transition with premenopausal and postmenopausal women with bipolar disorder nor used longitudinal menstrual cycle data. We investigated whether women with bipolar disorder were at an increased risk of syndromal depression during the menopausal transition, particularly during the late perimenopause and early postmenopause compared to premenopause or postmenopause. We also examined whether a history of mood exacerbation associated with reproductive events predicted worse mood course during the perimenopause or if use of hormone therapy (HT) was associated with mood stability.
METHODS
Subjects
Prospectively collected systematic clinical data were analyzed for 268 women with a DSM-IV diagnosis of bipolar disorder type I, II, or NOS or schizoaffective disorder, bipolar type (26), who were then selected for reproductive stage of premenopausal, perimenopausal and postmenopausal as defined below in Subject Classification. Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) included 4108 female and male participants enrolled between September 1998 and September 2005 who were treated according to model practice procedures, which included published pharmacotherapy guidelines (26) at twelve academic U.S. sites (ClinicalTrials.gov identifier NCT00012558).
DSM-IV diagnoses were based on assessments using the STEP-BD Affective Disorders Evaluation (ADE) (26), which includes the mood disorders module of the Structured Clinical Interview for DSM-IV (SCID) (27) and independently confirmed using the Mini International Neuropsychiatric Interview (MINI) (28). Longitudinal observation of mood episodes was performed with the STEP-BD Clinical Monitoring Form (CMF) which yielded DSM-IV mood status for each visit and has been validated with robust correlations with formal mood symptom rating scales (29). All assessors were psychiatrists trained to use standardized criteria for mood and symptom ratings. Mood status at each visit was categorized as recovering/recovered (euthymic), roughening/continued subsyndromally symptomatic (subsyndromal depressive and or subsyndromal mood elevation), and the following based on DSM-IV criteria: syndromal depression (major depressive episode), or syndromal mood elevation (mania, hypomania, mixed mania). Mood status determination details are published previously (29).
Subject Classification
Premenopausal Women were defined as 28–38 years old at enrollment and having menstruation recorded every 60 days or fewer. Self-report of regular menstruation was considered evidence of non-pregnancy and intact uterus (no hysterectomy).
Perimenopausal Women were defined as women 42 to 60 years old who had 60 to 365 days between the first days of consecutive reported menstruations according to STRAW criteria for the late menopausal transition (30). All visits of women ages 42 to 60 years old who had record of menstruating between 60 and 365 days apart were included in the perimenopausal group.
Postmenopausal women were defined as over 42 years old and having a reported menstrual cycle after which 365 days passed without further menstruation (i.e. if at any time she had two menstrual cycles less than 365 days apart, she was considered perimenopausal). If over 60 years old women were considered postmenopausal even if no menstrual cycle data were recorded.
Transitioning Perimenopausal to Postmenopausal Women were defined as a subgroup of perimenopausal women whose menstrual cycle frequency changed from 60 to 365 days to greater than 365 days without menstruation during participation in STEP-BD
Procedure
The STEP-BD protocol was approved by the human subjects review panel of each site, and subjects provided verbal and written informed consent prior to participation. Baseline demographic, diagnostic, illness history, psychiatric comorbidity and reproductive data were obtained from the baseline STEP-BD ADE assessments. Longitudinal data regarding mood status as defined by DSM-IV, treatment received, and menstrual status at each visit were obtained from the STEP-BD CMF assessments performed at each clinical visit. STEP-BD patients received treatment in accordance with guidelines that exceeded rates previously reported in randomized controlled trials of guideline implementation (31).
Specific baseline variables used from the baseline ADE included age, sex, bipolar diagnosis, current comorbid psychiatric diagnoses including substance use and anxiety disorders, medication at intake (specifically, hormone therapy), age of first mood episode, rapid cycling status, history or perimenstrual mood exacerbation, history of postpartum mood exacerbation, menstrual and reproductive history. Variables used from the CMF assessments included current clinical mood status (syndromal depression, syndromal mood elevation, subsyndromal mood symptoms or euthymic), and self-reported date of first day of most recent menses.
Analysis
Proportions of clinic visits with syndromal depression, syndromal elevation and euthymia for women transitioning from late perimenopause to postmenopause were compared to those of premenopausal, non-transitioning perimenopausal and postmenopausal women using Chi square analysis. Pair-wise comparisons between individual reproductive groups for differences in proportions of clinic visits with syndromal depression were performed using a Z test of proportions.
A within-woman comparison of mood states in the perimenopause stage compared to postmenopause stage in the group of women transitioning from perimenopause to postmenopause was performed by t-test.
Analysis by clinic visit for an overall difference in proportion of clinic visits with differing mood states between premenopausal, perimenopausal and postmenopausal was performed by Chi square test.
Differences between premenopausal, perimenopausal and postmenopausal women in known covariates of mood severity were examined. Possible predictors of recurrence in bipolar disorder were assessed for presence of significant differences between premenopausal, perimenopausal and postmenopausal women including bipolar diagnostic subtype, rapid cycling status, age at onset (32), and current comorbid anxiety (33) and substance use (34) disorders.
Perimenstrual and Postpartum Mood Exacerbation
To examine whether the presence compared to the absence of a reported history of postpartum and/or perimenstrual mood exacerbation was associated with an increased proportion of clinic visits with syndromal depression or syndromal mood elevation in the menopausal transition women a within-woman analysis using a Z test of proportions.
Hormone Therapy
The proportion of clinic visits with syndromal depression or syndromal elevation in women with bipolar disorder taking and not taking hormonal therapy in both the perimenopausal and postmenopausal women were compared using a Z-test of proportions.
RESULTS
Study Sample
Among 4,108 participants who enrolled in STEP-BD and had an initial diagnostic assessment with the ADE, 1,740 had complete baseline data. Among 4,108 enrolled subjects 2,196 were women, 1,548 of whom had a recorded age (though not necessarily complete base line data). Total visit count for the 768 women who met inclusion criteria for one of the three reproductive groups was 9960.
The overall mean ± SD duration of longitudinal monitoring for all three reproductive groups was 19.8 ± 15.5 months, with an average frequency of 0.8 ± 0.6 visits a month.
Premenopausal Women
There were 640 women (8943 visits) age 28–38 years of whom 533 (6279 visits) had information on menses. Of the 533 women with menses information, 29.7% of clinic visits were missing menstrual cycle data and 519 women (5989 visits) met the inclusion criteria of menses within sixty days from consent.
Perimenopausal Women
There were 810 women (with 11803 visits) between 42 and 60 years old. Of these women, 68.8% of clinic visits were missing menstrual cycle data and 369 (4538 visits) had information on menses. From the women with information on menses, 116 women (2046 visits) meet the criteria of a record of menses within 60–365 days.
Postmenopausal Women
Postmenopausal women were between 42 and 83 years old on study entry. 43 (488 visits) women were under 60 years old and had visits at least 365 days after last menses. 98 women (1437 visits) were over the age of 60 with 99.5% missing menstrual data, presumably because the clinician assumed that they were well past their final menstrual period and did not continue to record a last menstrual cycle many years prior. A total of 133 postmenopausal women with 1924 visits were used for the postmenopausal analysis.
Transitioning Peri to Postmenopausal Women
Within the perimenopausal women, thirteen women met criteria for transitioning from perimenopause into postmenopause by progressing from 60 to 365 days between menstrual cycles to greater than 365 days from last menstrual period.
Sample Characteristics
The majority of the subjects were Caucasian and there was no significant difference between the three reproductive groups in bipolar subtype (I, II, or NOS) or rapid cycling status. Age of first mood episode was significantly different between the three reproductive groups, with premenopausal women having the youngest age of onset and postmenopausal women having the oldest. Also, rates of comorbid anxiety disorders significantly differed across reproductive groups with notably lower rates in postmenopausal women. There was no significant difference across the three groups with respect to comorbid alcohol or substance use disorders (Table 1). Pre-, peri-, and post-menopausal women had statistically similar rates of taking mood stabilizing (antipsychotic, antiepileptic or lithium) agents (92.7%, 100.0%, and 92.4%, respectively), antidepressants with mood stabilizing agents (64.1%, 75.9%, and 63.9%), and antidepressants without mood stabilizing agents (3.6%, 0.0%, 3.0%)
Table 1.
Subject Characteristics
| Premenopausal1 (n=519) | Perimenopausal2 (n=116) | Postmenopausal3 (n=133) | Premenopausal, Perimenopausal, and Postmenopausal Groups Combined (n=768) | P-value | |
|---|---|---|---|---|---|
|
|
|||||
| N (%) | N (%) | N (%) | N (%) | ||
| Number of Clinic Visits | 5989 | 2046 | 1925 | 9960 | |
| Race | |||||
| White or Caucasian | 460 (89) | 102 (88) | 119 (89) | 681 (89) | 0.0464 |
| Black or African American | 30 (6) | 13 (11) | 10 (8) | 53 (7) | |
| Other | 29 (5) | 1 (1) | 4 (3) | 34 (4) | |
| Bipolar Diagnosis | |||||
| Bipolar I | 317 (61) | 67 (58) | 87 (65) | 471 (61) | 0.7943 |
| Bipolar II | 166 (32) | 42 (36) | 37 (28) | 245 (32) | |
| Bipolar NOS | 31 (6) | 5 (4) | 8 (6) | 44 (6) | |
| Schizoaffective Disorder | 5 (1) | 2 (2) | 1 (1) | 8 (1) | |
| Rapid cycling | |||||
| Yes | 160 (31) | 39 (34) | 35 (26) | 234 (30) | 0.0596 |
| No | 152 (29) | 34 (29) | 57 (43) | 243 (32) | |
| Current Substance Abuse or Dependency | |||||
| Yes | 14 (3) | 3 (3) | 1 (1) | 18 (2) | 0.3219* |
| No | 505 (97) | 112 (97) | 131 (99) | 748 (98) | |
| Current Alcohol Abuse or Dependency | |||||
| Yes | 31 (6) | 7 (6) | 5 (4) | 43 (6) | 0.6051 |
| No | 486 (94) | 106 (91) | 126 (95) | 718 (93) | |
| Number of Previous Episodes | |||||
| 0 | 3 (0.6) | 2 (2) | 5 (4) | 10 (1) | 0.0002* |
| 1–2 | 20 (4) | 0 (0) | 5 (4) | 25 (3) | |
| 3–4 | 34 (7) | 5 (4) | 2 (2) | 41 (5) | |
| 5–6 | 160 (31) | 46 (39) | 61 (46) | 267 (35) | |
| 7 | 109 (21) | 21 (18) | 17 (13) | 147 (19) | |
| Anxiety Diagnosis | |||||
| Yes | 201 (39) | 44 (38) | 30 (23) | 275 (36) | 0.0021 |
| No | 318 (61) | 72 (62) | 103 (77) | 493 (64) | |
| HT Use | |||||
| Yes | 65 (13) | 20 (17) | 49 (37) | 134 (17) | <.0001 |
| No | 454 (87) | 96 (83) | 84 (63) | 634 (83) | |
|
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
P-value (ANOV A) | |
| Age at entry | 33.09 (3.21) 28–38 |
47.96 (3.80) 42–59 |
60.95 (8.68) 42–83 |
<.0001 | |
| Age at Onset of Depression | 16.40 (6.59) 3–37 |
19.32 (9.94) 5–46 |
25.88 (13.41) 4–61 |
18.45 (9.32) 3–61 |
<.0001 |
| Age at Onset of Mania | 20.32 (6.90) 2–36 |
24.98 (10.76) 5–45 |
30.08 (13.58) 6–67 |
22.72 (9.76) 2–67 |
<.0001 |
| Months in Clinic | 17.29 (14.23) 0–57.50 |
28.65 (17.02) 0–60.88 |
22.15 (15.73) 0–59.30 |
19.84 (15.49) 0–60.88 |
<.0001 |
| Average Number of Visits | 11.54 (11.78) 1–86 |
17.64 (13.39) 1–72 |
14.47 (12.74) 1–63 |
12.97 (12.39) 1–86 |
<.0001 |
| Average Number of Visits per Month | 0.86 (0.67) 0.04–4.35 |
0.72 (0.44) 0.11–2.61 |
0.78 (0.55) 0.12–4.68 |
0.82 (0.62) 0.04–4.68 |
0.0562 |
P-values for categorical variables from chi-square test unless asterisk is present. Asterisk designates p-value from likelihood ratio chi-square.
Reproductive characteristics varied across the three reproductive cohorts. All three groups reported similar age of menarche and number of miscarriages, however postmenopausal women had more births and fewer abortions than the younger two groups. While there was no significant difference between the three groups in the percentage of women who reported postpartum mood exacerbation, significantly fewer postmenopausal women compared to the younger cohorts reported a history of perimenstrual mood exacerbation (Table 2).
Table 2.
Reproductive History of Subjects
| Premenopausal1 (n=519) | Perimenopausal2 (n=116) | Postmenopausal3 (n=133) | Premenopausal, Perimenopausal, and Postmenopausal Groups Combined (n=768) | P-value | |
|---|---|---|---|---|---|
|
|
|||||
| N (%) | N (%) | N (%) | N (%) | ||
| History of Postpartum Exacerbation of Depression | |||||
| Yes | 116 (22) | 35 (30) | 42 (32) | 193 (25) | 0.5963 |
| No | 151 (29) | 47 (41) | 69 (52) | 267 (35) | |
| History of Perimenstrual Exacerbation of Depression | |||||
| Yes | 258 (50) | 53 (46) | 33 (25) | 344 (45) | <.0001 |
| No | 214 (41) | 49 (42) | 87 (65) | 350 (46) | |
| Number of Miscarriages | |||||
| 0 | 172 (33) | 45 (39) | 68 (51) | 285 (37) | 0.8754* |
| 1 | 42 (8) | 15 (13) | 12 (9) | 69 (9) | |
| 2 | 14 (3) | 5 (4) | 5 (4) | 24 (3) | |
| 3+ | 10 (2) | 3 (3) | 4 (3) | 17 (2) | |
| Number of Abortions | |||||
| 0 | 141 (27) | 41 (35) | 77 (58) | 259 (34) | 0.0001 |
| 1 | 62 (12) | 16 (14) | 8 (6) | 86 (11) | |
| 2 | 28 (5) | 5 (4) | 1 (0.8) | 34 (4) | |
| 3+ | 10 (2) | 5 (4) | 2 (2) | 17 (2) | |
| Number of Live Births | |||||
| 0 | 63 (12) | 12 (10) | 5 (4) | 80 (10) | <.0001 |
| 1 | 76 (15) | 17 (15) | 12 (9) | 105 (14) | |
| 2 | 72 (14) | 24 (21) | 40 (30) | 136 (18) | |
| 3+ | 38 (7) | 19 (16) | 37 (28) | 94 (12) | |
| Current Contraceptive Method | |||||
| None | 158 (30) | 53 (46) | 74 (56) | 285 (37) | <.0001 |
| Oral birth control | 80 (15) | 5 (4) | 4 (3) | 89 (12) | |
| Barrier | 92 (18) | 6 (5) | 2 (2) | 100 (13) | |
| Abstinence | 54 (10) | 8 (7) | 5 (4) | 67 (9) | |
| Other | 83 (16) | 30 (26) | 29 (22) | 142 (18) | |
|
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
P-value (ANOV A) | |
| Age at Menarche | 12.61 (1.68) 8–18 |
12.57 (1.71) 9–18 |
12.69 (1.75) 8–19 |
12.62 (1.70) 8–19 |
0.8771 |
| Number of Live Births | 1.42 (1.20) 0–7 |
1.83 (1.29) 0–6 |
2.41 (1.26) 0–6 |
1.72 (1.29) 0–7 |
<.0001 |
P-values for categorical variables from chi-square test unless asterisk is present. Asterisk designates p-value from likelihood ratio chi-square.
Overall Mood State Description by Reproductive Group
There was a significant overall difference in number of visits in different mood states (euthymic, subsyndromal, depressed or elevated) at clinic visits between the premenopausal, perimenopausal and postmenopausal women with bipolar disorder, χ2 (6, N = 9959) = 31.6, p <0.0001.
An ordinal logistic regression model revealed a gradient of worsening mood state from premenopause to perimenopausal (estimate = 0.0773; 95% CI: 0.9 to −0.1) to postmenopausal women (estimate = 0.1501 95% CI: −0.06 to 0.4). Thus, there was a significant increase in the proportion of visits in a subsyndromal state from premenopausal (47.3±32%) to perimenopausal (50.7±28%) to postmenopausal (52.7±32%) women F(2, N=768) 3.0, p=0.05. Euthymia and syndromal mood elevations decreased (both detailed below) and there was no significant difference between the proportion of visits in a depressed state between the three reproductive groups (Figure 1).
Figure 1.
Proportion of clinic visits symptomatic of mood by advancing reproductive stage (premenopause, perimenopause and postmenopause) in women with bipolar disorder. χ2 (6, N = 9960) = 31.6, p < 0.0001.
Women Transitioning from Perimenopause to Postmenopause
Thirteen women had a recorded menstrual cycle transition from late menopausal transition (perimenopause) to postmenopause. These women experienced a significantly greater proportion of clinic visits in a syndromal depression (24.4±18%) compared to premenopausal, non-transitioning perimenopausal or postmenopausal women χ2 (3, N = 9960) = 19.8, p <0.0002. However, while a significant difference in proportion of visits in euthymia in women transition from perimenopause to postmenopause (29±14%) on comparison of the remaining three reproductive groups was foundχ2 (3, N = 9960) = 9, p =0.046, there was no notable difference of transitioning peri to postmenopausal women on pair-wise comparisons with premenopause, non-transitioning perimenopausal and postmenopausal women in proportion of visits in a euthymic state. Likewise, no significant difference in proportion of visits in a mood elevation (3.2±0.5%) was found in a pair-wise comparison of the transitioning peri to post group compared to premenopausal, non-transitioning perimenopausal and postmenopausal women (Figure 2).
Figure 2.
Proportion of clinic visits in a syndromal depression of women with bipolar disorder transitioning from late perimenopause to postmenopause (n=13) compared to premenopausal (n=519), non-transitioning perimenopausal (n=103) and postmenopausal (n=133) women with bipolar disorder. χ2 (3, N = 9960) = 19.8, p <0.0002.
These thirteen women did not have a significant within woman change in the proportion of clinic visits in a syndromal depression t (24)=0.8, p=0.4, syndromal elevation t (24)= −0.34, p=0.7 or euthymia t(24)= −0.7, p=0.5, from late menopausal transition to early postmenopause stages.
Proportion of Visits with Syndromal Depression Across Reproductive Stages
There was no significant difference in the proportion of visits with syndromal depression among premenopausal, perimenopausal and postmenopausal women when analyzed by clinic visit,χ2 (2, N = 9960) = 1.6, p=0.4. Nor were any of the reproductive group pair-wise comparisons significantly different in the proportion of clinic visits depressed (Table 3).
Table 3.
Pair-wise Comparisons of Proportion of Clinic Visits in Depression, Mood Elevation or Euthymia by Reproductive Cohort
| Mood State | Proportion of Visits in Mood State (%) | Z-test for Proportion Value; P value | |
|---|---|---|---|
| Syndromal Depression | |||
| Reproductive age vs Perimenopause | 18.1 vs 18.1 | Z=−0.01. p=0.9 | |
| Reproductive age vs Postmenopause | 18.1 vs 19.3 | Z=1.1; p=0.2 | |
| Peri- vs Post-Menopause | 18.1 vs 19.3 | Z=0.9; p=0.3 | |
| Syndromal Elevation | |||
| Reproductive age vs Perimenopause | 5.3 vs 4.1 | Z=2.1; p=0.03* | |
| Reproductive age vs Postmenopause | 5.3 vs 3.0 | Z=4.1, p<0.001* | |
| Peri- vs Post-Menopause | 4.1 vs 3.0 | Z=1.8. p=0.06 | |
| Euthymia | |||
| Reproductive age vs Perimenopause | 29.2 vs 28.0 | Z=1.0, p=0.3 | |
| Reproductive age vs Postmenopause | 29.2 vs 26.0 | Z=2.7, p=0.007* | |
| Peri- vs Post-Menopause | 28.0 vs 26.0 | Z=1.4;p=0.2 |
P-values are from a z-test of two proportions
Proportion of Visits with Syndromal Mood Elevation across Reproductive Stages
There was a significant overall difference in the proportion of visits with syndromal mood elevation (manic, hypomanic or mixed episodes) among reproductive age (5.3%), perimenopausal (4.1%) and postmenopausal (3.0%) women with bipolar disorder when analyzed by visit,χ2 (2, N = 9960) = 19.5, p <0.0001.
In a pair-wise comparison, premenopausal women had a significantly greater proportion of clinic visits in mood elevation than either postmenopausal or perimenopausal while postmenopausal compared to perimenopausal women did not significantly differ in proportion of visits with syndromal mood elevation (Table 3).
Proportion of Visits in a Euthymic Mood across Reproductive Stages
There was a significant overall difference in the proportion of visits in a euthymic healthy mood among the premenopausal (29.2%), perimenopausal (27.0%) and postmenopausal (25.0%) women with bipolar disorder when analyzed by visit χ2(2, N=9960)=7.7, p=0.02.
In a pair-wise comparison, postmenopausal women had a significantly smaller proportion of clinic visits in euthymic mood than premenopausal women while there was no significant difference in the proportion of visits in a euthymic mood between premenopausal and perimenopausal or peri and postmenopausal women (Table 3).
History of Perimenstrual and or Postpartum Mood Exacerbation
Of perimenopausal women with bipolar disorder, 46% gave a history of perimenstrual mood exacerbation, 42% gave a history of no perimenstrual mood exacerbation and 12% had missing data. Women with versus without a history of perimenstrual mood exacerbation did not significantly differ in proportion of visits with syndromal depression (18% vs 19%) or with syndromal mood elevation (4% vs 4%) during the perimenopause.
Of perimenopausal women with bipolar disorder, 30% gave a history of postpartum mood exacerbation, 40% gave a history of no postpartum mood exacerbation and 30% had missing postpartum mood data. Women with versus without a history of postpartum mood exacerbation did not significantly differ in proportion of visits with syndromal depression (18% vs 14%) or with syndromal mood elevation (3% vs 4%) during the perimenopause.
Likewise, perimenopausal women with bipolar disorder who reported both perimenstrual and postpartum mood exacerbations (29%) did not significantly differ from those who reported no mood exacerbation at either time (24%) in the proportion of visits in a depression (18% vs 14%) or mood elevation (3% vs 3%) during the perimenopause.
Hormone Therapy Use
In the 28% of perimenopausal and postmenopausal women with bipolar disorder who used HT, 16% of their clinic visits were in a syndromal depression and 2% were in a syndromal mood elevation. These ratios did not significantly differ from those of the 72% of women not using HT, in whom 16% of visits were with syndromal depression and 4% were with syndromal mood elevation. Similarly, use of oral contraceptive pill by premenopausal women was not associated with a difference in proportion of visits elevated or depressed compared to premenopausal women not using an oral contraceptive.
Hormone Therapy use did not predict a greater proportion of visits in a depressed state by logistic regression χ2(2, N=768)=1.9, p=0.38.
DISCUSSION
Bipolar Illness Exacerbation with Progression Toward Late Female Reproductive Stages
We found a progression toward later female reproductive life (premenopausal, perimenopausal, and postmenopausal) associated with increasing overall mood disturbance (increasing percentage of visits with mood symptoms). This decreasing proportion of visits in a healthy euthymic state with advancing stage of female reproductive life is consistent with Kraepelin’s report that well intervals between episodes tend to become shorter with age in individuals with bipolar disorder (35), but is not consistent with Angst’s more recent report that longitudinal occurrence of syndromes remains more or less constant with advancing age (3). This decline in the proportion of visits in a euthymic state with reproductive age despite treatment may have been due to age-related episode accumulation yielding worsening illness due to sensitization (36) or kindling (37). However, this worsening of illness with age in women could also be related to progressing stage of female reproductive life, from premenopause to perimenopause and then to postmenopause. For example, in the unipolar depression literature within-woman change in menopausal status along with increased levels of follicle-stimulating hormone and luteinizing hormone, which are increased in postmenopausal women, have been found significantly associated with high Center for Epidemiologic Studies Depression Scale (CES-D) scores (more depressive symptoms) (38). In another examination of depression in relation to reproductive phase, higher testosterone levels but not estradiol, follicle-stimulating hormone, or dehydroepiandrosterone sulfate, were associated with higher CES-D scores during the menopausal transition (39)
Despite of the above finding for depression in the general population during the menopausal transition, we observed no significant difference in the proportion of clinic visits with syndromal depression experienced by premenopausal, perimenopausal or postmenopausal women with bipolar disorder. This is in contrast to the few small studies in women with bipolar disorder that report significant or notable worsening of depression during the perimenopause compared to younger reproductive years either within woman by retrospective report (25, 40) or in contrast to a longitudinal report in a concurrent cohort (23).
However, the thirteen women who transitioned from late perimenopause into postmenopause during study participation had a significantly greater proportion of visits in a syndromal depression than any of the original three reproductive groups. Within the thirteen women who transitioned from late perimenopause to postmenopause there was no significant difference in rates of depression from the former to latter reproductive phase. These results are in accordance with multiple prospective studies in the unipolar depression literature, where both the menopausal transition (11, 13, 14, 38, 41) and early postmenopause have been found to have an increased rate of depression (15, 38, 39). In women without bipolar disorder studies find that within the menopausal transition the late stage is associated with greater risk of depression than the early (41, 42). Our study was unable to compare the specific late and early menopause stages.
From an endocrinological perspective the immediate post-menopausal period may not be distinguished from the period of long cycles of perimenopause (19, 20). It is possible that during the transition into early postmenopause when gonadal hormones undergo marked fluctuation women with treated bipolar are at maximal vulnerability to syndromal depression. Alternatively, as found by Bromberger et al. in women with unipolar depression, higher testosterone levels may contribute to greater depressive symptoms (39).
Mood Elevation in Menopausal Transition
We also report that advancing stage of female reproductive life (premenopausal, perimenopausal, and postmenopausal) was associated with decreasing mood elevation, a finding consistent with a previous report of no increased risk of syndromal mood elevation during the perimenopause compared to the reproductive years (23). Another study reported the perimenopause as a time of increased mood cycling in bipolar disorder (22). Our study may be the first to report a decreased rate of syndromal mood elevation in the perimenopause and postmenopause compared to reproductive years.
Mood elevations tend to be easier to treat and prevent with medication than bipolar depression and perhaps the older groups found an effective treatment strategy for mood elevations. Alternatively, the reduction in gonadal hormones associated with youth and vitality may reduce the upward drive toward mania.
Potential Confounders
Although previous studies have found that alcohol or substance use disorders were associated with worse mood course in bipolar disorder (43–46), in our subjects, rates of current alcohol/substance use disorders were low and did not differ significantly between the three reproductive groups. Alcohol or substance us is an unlikely contributor to our observed mood worsening with progression toward later female reproductive life.
Comorbid anxiety disorders have also been associated with a worse mood course in bipolar disorder (4, 33, 47–49), but in the current study, anxiety disorders were more prevalent in the reproductive age group, followed by the perimenopausal group, followed in turn by the postmenopausal group, making anxiety an unlikely contributor to the increased mood symptoms observed with progression toward later stage of female reproductive life.
Earlier age of first mood episode has also been associated with a worse mood course in bipolar disorder (50), but in our study age of first mood episode was earliest in premenopausal women, intermediate in perimenopausal group, and latest in postmenopausal women, making age of onset unlikely to be a contributor to the greater mood symptomolgy associated with advancing stage of female reproductive life.
History of Mood Exacerbation with Reproductive Events
Reporting a history of mood exacerbation in association with the postpartum period or menstrual cycle or both did not predict syndromal depression or syndromal mood elevation in the perimenopause in women with treated bipolar disorder. Thus, women with bipolar disorder in this study did not carry a lifelong vulnerability to mood episodes associated with reproductive events.
Early retrospective descriptive studies report a significantly increased risk of ‘manic-depressive’ relapse during the two years before and after cessation of menstruation (2, 51) or in the ‘climacteric or post-climacteric age’ (41–60yo) (51) in women with bipolar disorder who had postpartum depressive episodes. In contrast, our findings are in agreement with more recent retrospective studies (40, 52). Nonetheless, a case series of five women with bipolar disorder reported increased risk of mood recurrence in the perimenopause in women with a history of postpartum psychosis (53). As our study did not select out women with a postpartum psychotic from non-psychotic mood exacerbation, it may be that only the group of women with bipolar disorder who experience severe mood episodes in relation to reproductive events are at increased risk of mood disturbance during the perimenopause. Also the lack of association between prior premenstrual and/or postpartum mood exacerbation and mood instability during the perimenopause in this study could be due to the limitations of retrospective assessment of premenopausal phenomena.
Mood Episodes and Hormone Therapy
Use of hormone therapy in perimenopausal and postmenopausal women with treated bipolar was not associated with lower proportion of visits with syndromal depression compared to non-users. In this study, it appears that in women treated for bipolar disorder, HT did not confer antidepressant-like effects, although HT could potentially improve a lower baseline mood of users up to the range of non-users. In a retrospective report of women with bipolar disorder, women who were not using hormone therapy were significantly more likely than those who were using HT to report worsening of symptoms during perimenopause (25). However, as previously reported by this author in a smaller study, women age 45–55 years who did and did not take HT had similar rates of syndromal depression (40). While analyzed together, HT formulations varied and it is possible that individual formulations may confer differential mood effects. In previous reports in women without bipolar disorder, specific HT formulations have shown distinctive influences on mood, such as antidepressant efficacy of transdermal estradiol in depressed perimenopausal women (54, 55).
Strengths and Limitations
Strengths of this study include its large sample size compared to previous studies in the field. Longitudinal assessment averaged approximately a year and a half per patient. The non-randomized, open, naturalistic treatment subjects received generally reflected that seen in optimized evidence-based clinical practice. Ours is the first study to assess menstrual status in conjunction with mood course in bipolar disorder, and took a continuum approach to female reproductive life – from premenopause to peri to postmenopause. Mood at each clinic visit was classified by rigorous DSM-IV criteria (although a less sensitive measure than other mood rating instruments). Characteristics that may influence mood course for the worse in bipolar disorder were assessed. For thirteen women we performed a within-individual comparison of mood state from perimenopause to postmenopause.
Limitations include missing data, such as menstrual cycle reporting. Thus, perimenopausal women may have been mistakenly classified as postmenopausal if a menstrual cycle occurred but was not reported. Proportion of clinic visits in a mood state does not necessarily translate into duration of a mood episode, as patients likely attend doctor visits more often during syndromal mood episode than when euthymic. Thus, while presence of mood episode was defined, the duration and severity of mood episodes were not accounted for in the analysis of the impact of reproductive stage upon mood. The absence of baseline and longitudinal endocrinological assessment, as well as systematic assessments of medical and psychosocial stressors represents additional limitations. As full DSM-IV major depression criteria were used, if there were an increase minor depressions, as found in unipolar depression (14), during the perimenopause they would not have been detected.
The average duration of the current study did not permit more than thirteen transitioning perimenopausal to postmenopause within-individual comparisons of mood across reproductive phases.
It could be that limited sample size and hence limited statistical power could have attenuated ability to demonstrate differences between the reproductive groups. Exclusion of women in the correct reproductive group age ranges was primarily due to missing menstrual cycle data and including the excluded women might have modified findings of this study.
In spite of these limitations, our study indicates that additional research is warranted to assess the finding that bipolar illness may worsen with progression toward later stage of female reproductive life. Such studies would be aided by methodological refinements such as prospectively tracking menstrual cycle and mood course in women with bipolar disorder through the early and late perimenopause and early postmenopause.
Acknowledgments
The project was supported by Award Number KL2RR031981 (WKM) from the National Center for Research Resources (NCRR). The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) project has been funded by NIMH under contract 01MH80001. We thank Bruce Barton and Aimee Kroll for statistical support.
Footnotes
Disclosures
TAK has received grant/research support AstraZeneca, Cephalon, Eli Lilly & Co., Pfizer, and Sunovion; has received lecture honoraria from AstraZeneca, royalties from the American Psychiatric Publishing, Inc., and consultation fees from Merck & Co., Inc.; and his spouse is an employee of Janssen Pharmaceutica Products, LP. AJR has received grant/research support from NIMH, Cyberonics, Takeda, and St. Jude Medical Center; and has served as a consultant to Dey Pharma, Eisai Medical, GlaxoSmithKline, Eli Lilly & Co., and Pfizer. WKM, JVJ, and SLC have no conflicts of interest to report.
References
- 1.Kendell RE, Chalmers JC, Platz C. Epidemiology of puerperal psychoses. Br J Psychiatry. 1987;150:662–73. doi: 10.1192/bjp.150.5.662. [DOI] [PubMed] [Google Scholar]
- 2.Braftos O, Haug J. Puerperal mental disorders in manic-depressive females. Acta Psychiatr Scand. 1966;42:285–94. doi: 10.1111/j.1600-0447.1966.tb01933.x. [DOI] [PubMed] [Google Scholar]
- 3.Angst J. The course of affective disorders. II. Typology of bipolar manic-depressive illness. Arch Psychiatr Nervenkr. 1978;226:65–73. doi: 10.1007/BF00344125. [DOI] [PubMed] [Google Scholar]
- 4.Altshuler LL, Kupka RW, Hellemann G, Frye MA, Sugar CA, McElroy SL, et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. 2010;167:708–15. doi: 10.1176/appi.ajp.2009.09010105. [DOI] [PubMed] [Google Scholar]
- 5.Dunner DL, Patrick V, Fieve RR. Rapid cycling manic depressive patients. Compr Psychiatry. 1977;18:561–6. doi: 10.1016/s0010-440x(97)90006-7. [DOI] [PubMed] [Google Scholar]
- 6.McElroy SL, Keck PE, Jr, Pope HG, Jr, Hudson JI, Faedda GL, Swann AC. Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry. 1992;149:1633–44. doi: 10.1176/ajp.149.12.1633. [DOI] [PubMed] [Google Scholar]
- 7.Arnold LM, McElroy SL, Keck PE., Jr The role of gender in mixed mania. Compr Psychiatry. 2000;41:83–7. doi: 10.1016/s0010-440x(00)90137-8. [DOI] [PubMed] [Google Scholar]
- 8.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4:214–20. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 9.Hunter MS. Emotional well-being, sexual behaviour and hormone replacement therapy. Maturitas. 1990;12:299–314. doi: 10.1016/0378-5122(90)90009-u. [DOI] [PubMed] [Google Scholar]
- 10.Greene JG. A factor analytic study of climacteric symptoms. J Psychosom Res. 1976;20:425–30. doi: 10.1016/0022-3999(76)90005-2. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 12.Bromberger JT. The menopausal transition increases the risk of depressive symptoms and depression diagnosis in women without a history of depression. Evid Based Ment Health. 2006;9:110. doi: 10.1136/ebmh.9.4.110. [DOI] [PubMed] [Google Scholar]
- 13.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–90. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161:2238–44. doi: 10.1176/appi.ajp.161.12.2238. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW. Associations of depression with the transition to menopause. Menopause. 2010;17:823–7. doi: 10.1097/gme.0b013e3181db9f8b. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48:S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 17.Janowsky H, Halbreich U, Rausch J. Association among ovarian hormones, other hormones, emotional disorders, and neurotransmitters. In: Jensvold M, Halbreich U, Hamilton J, editors. Psychopharmacology and Women: Sex, Gender, and Hormones. Washington DC: American Psychiatric Press; 1996. pp. 85–106. [Google Scholar]
- 18.Kendall DA, Stancel GM, Enna SJ. The influence of sex hormones on antidepressant-induced alterations in neurotransmitter receptor binding. J Neurosci. 1982;2:354–60. doi: 10.1523/JNEUROSCI.02-03-00354.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metcalf MG. The approach of menopause: a New Zealand study. N Z Med J. 1988;101:103–6. [PubMed] [Google Scholar]
- 20.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during and after the menopause: a longitudinal study. Clin Endocrinol (Oxf) 1982;17:489–94. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 21.Marsh WK, Templeton A, Ketter TA, Rasgon N. Clinical Course of Bipolar Disorder Through Perimenopause: Chart Review; Annual Meeting; Atlanta, GA: American Psychiatric Association; 2005. [Google Scholar]
- 22.Kukopulos A, Reginaldi D, Laddomada P, Floris G, Serra G, Tondo L. Course of the manic-depressive cycle and changes caused by treatment. Pharmakopsychiatr Neuropsychopharmakol. 1980;13:156–67. doi: 10.1055/s-2007-1019628. [DOI] [PubMed] [Google Scholar]
- 23.Marsh WK, Ketter TA, Rasgon NL. Increased depressive symptoms in menopausal age women with bipolar disorder: age and gender comparison. J Psychiatr Res. 2009;43:798–802. doi: 10.1016/j.jpsychires.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Blehar MC, DePaulo JR, Jr, Gershon ES, Reich T, Simpson SG, Nurnberger JI., Jr Women with bipolar disorder: findings from the NIMH Genetics Initiative sample. Psychopharmacol Bull. 1998;34:239–43. [PubMed] [Google Scholar]
- 25.Freeman MP, Smith KW, Freeman SA, McElroy SL, Kmetz GE, Wright R, et al. The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry. 2002;63:284–7. doi: 10.4088/jcp.v63n0403. [DOI] [PubMed] [Google Scholar]
- 26.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–42. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 27.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition Version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4–57. [PubMed] [Google Scholar]
- 29.Sachs GS, Guille C, McMurrich SL. A clinical monitoring form for mood disorders. Bipolar Disord. 2002;4:323–7. doi: 10.1034/j.1399-5618.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- 30.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Stages of Reproductive Aging Workshop (STRAW) J Womens Health Gend Based Med. 2001;10:843–8. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 31.Dennehy EB, Bauer MS, Perlis RH, Kogan JN, Sachs GS. Concordance with treatment guidelines for bipolar disorder: data from the systematic treatment enhancement program for bipolar disorder. Psychopharmacol Bull. 2007;40:72–84. [PubMed] [Google Scholar]
- 32.Schurhoff F, Bellivier F, Jouvent R, Mouren-Simeoni MC, Bouvard M, Allilaire JF, et al. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000;58:215–21. doi: 10.1016/s0165-0327(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 33.Simon NM, Otto MW, Wisniewski SR, Fossey M, Sagduyu K, Frank E, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2004;161:2222–9. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein BI, Velyvis VP, Parikh SV. The association between moderate alcohol use and illness severity in bipolar disorder: a preliminary report. J Clin Psychiatry. 2006;67:102–6. doi: 10.4088/jcp.v67n0114. [DOI] [PubMed] [Google Scholar]
- 35.Kraepelin E. Manic-Depressive Insanity and Paranoia. Edinburgh: E.S. Livingsone; 1921. [Google Scholar]
- 36.Kessing LV, Andersen PK, Mortensen PB. Predictors of recurrence in affective disorder. A case register study. J Affect Disord. 1998;49:101–8. doi: 10.1016/s0165-0327(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 37.Post RM, Weiss SR. Sensitization, kindling, and anticonvulsants in mania. J Clin Psychiatry. 1989;50(Suppl):23–30. discussion 45–7. [PubMed] [Google Scholar]
- 38.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of Hormones and Menopausal Status With Depressed Mood in Women With No History of Depression. Arch Gen Psychiatry. 2006;63:375–82. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 39.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh WK, Templeton A, Ketter TA, Rasgon NL. Increased frequency of depressive episodes during the menopausal transition in women with bipolar disorder: preliminary report. J Psychiatr Res. 2008;42:247–51. doi: 10.1016/j.jpsychires.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, Mitchell S. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15:223–32. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 42.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassidy F, Ahearn EP, Carroll BJ. Substance abuse in bipolar disorder. Bipolar Disord. 2001;3:181–8. [PubMed] [Google Scholar]
- 44.Jaffee WB, Griffin ML, Gallop R, Meade CS, Graff F, Bender RE, et al. Depression precipitated by alcohol use in patients with co-occurring bipolar and substance use disorders. J Clin Psychiatry. 2009;70:171–6. doi: 10.4088/jcp.08m04011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord. 2000;2:269–80. doi: 10.1034/j.1399-5618.2000.20308.x. [DOI] [PubMed] [Google Scholar]
- 46.Ostacher MJ, Perlis RH, Nierenberg AA, Calabrese J, Stange JP, Salloum I, et al. Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2010;167:289–97. doi: 10.1176/appi.ajp.2009.09020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller MB. Prevalence and impact of comorbid anxiety and bipolar disorder. J Clin Psychiatry. 2006;67 (Suppl 1):5–7. [PubMed] [Google Scholar]
- 48.Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–24. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 49.Gaudiano BA, Miller IW. Anxiety disorder comobidity in Bipolar I Disorder: relationship to depression severity and treatment outcome. Depress Anxiety. 2005;21:71–7. doi: 10.1002/da.20053. [DOI] [PubMed] [Google Scholar]
- 50.Perlis RH, Miyahara S, Marangell LB, Wisniewski SR, Ostacher M, DelBello MP, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–81. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 51.Kinkelin M. Verlauf und Prognose des Manisch-Depressiven Irreseins. Arch Neruol Psyciat. 1954;73:100–46. [PubMed] [Google Scholar]
- 52.Payne JL, Roy PS, Murphy-Eberenz K, Weismann MM, Swartz KL, McInnis MG, et al. Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord. 2007;99:221–9. doi: 10.1016/j.jad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Robertson Blackmore E, Craddock N, Walters J, Jones I. Is the perimenopause a time of increased risk of recurrence in women with a history of bipolar affective postpartum psychosis? A case series. Arch Womens Ment Health. 2008;11:75–8. doi: 10.1007/s00737-008-0215-2. [DOI] [PubMed] [Google Scholar]
- 54.Soares CN, Phillips SD. Hormones in the treatment of depression. Womens Health (Lond Engl) 2006;2:435–45. doi: 10.2217/17455057.2.3.435. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann N Y Acad Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]