Abstract
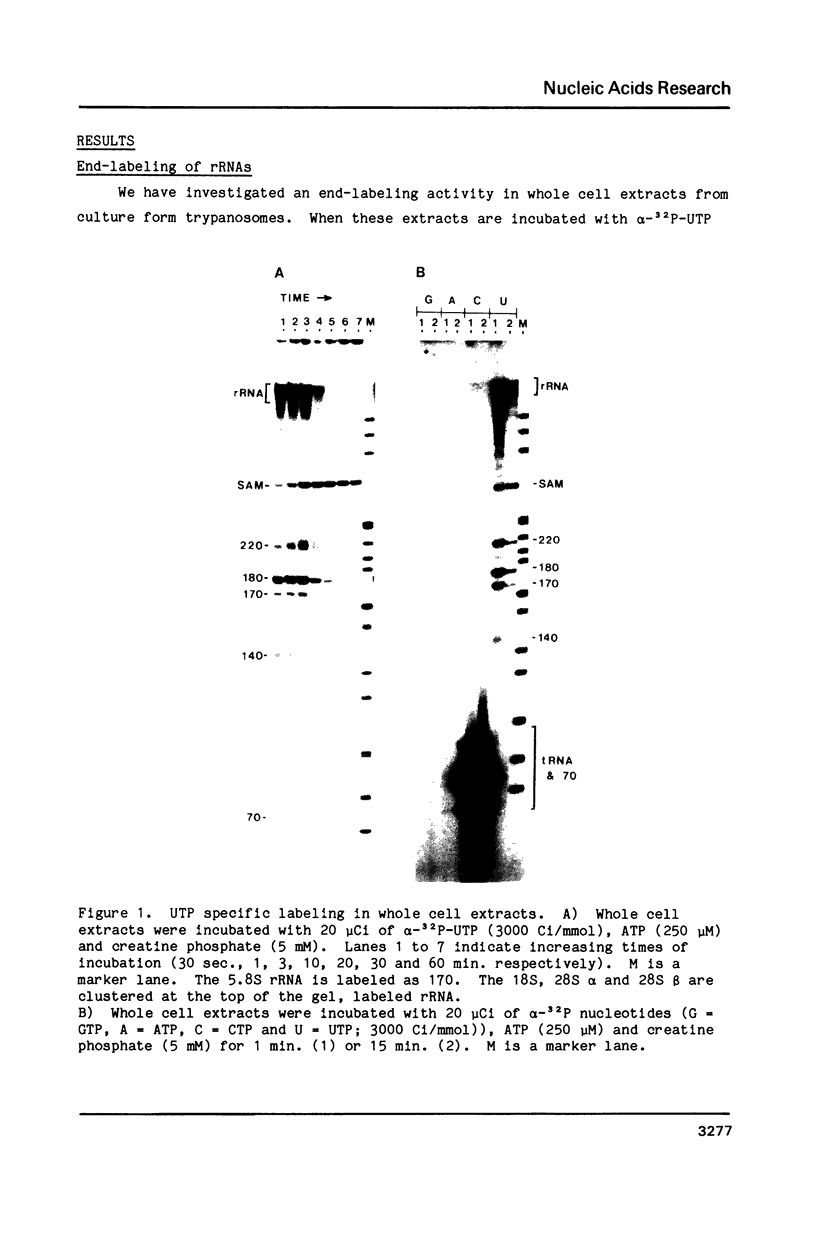
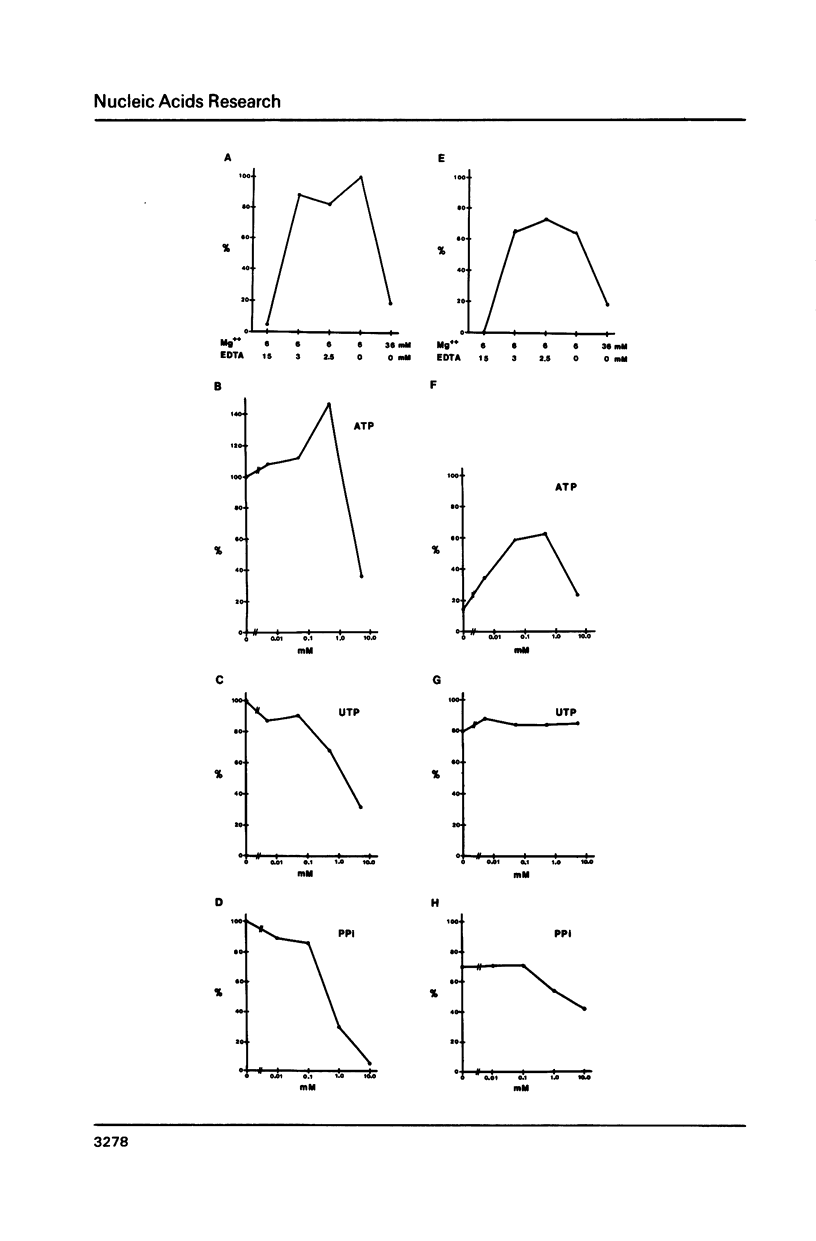
We have found two enzymatic activities in whole cell extracts from Trypanosoma brucei; an end-labeling reaction involving a single uridine at the 3' end of ribosomal RNAs (rRNAs) and an RNA ligase activity joining 5' monophosphates to 3' hydroxyl groups. The RNA ligase acts upon one of the small rRNAs (180 nucleotides) from the trypanosome ribosomal repeat unit, forming a circular RNA. The specific circularization of this small rRNA is probably dependent on the secondary structure of the molecule and is not detectable in vivo.
Full text
PDF



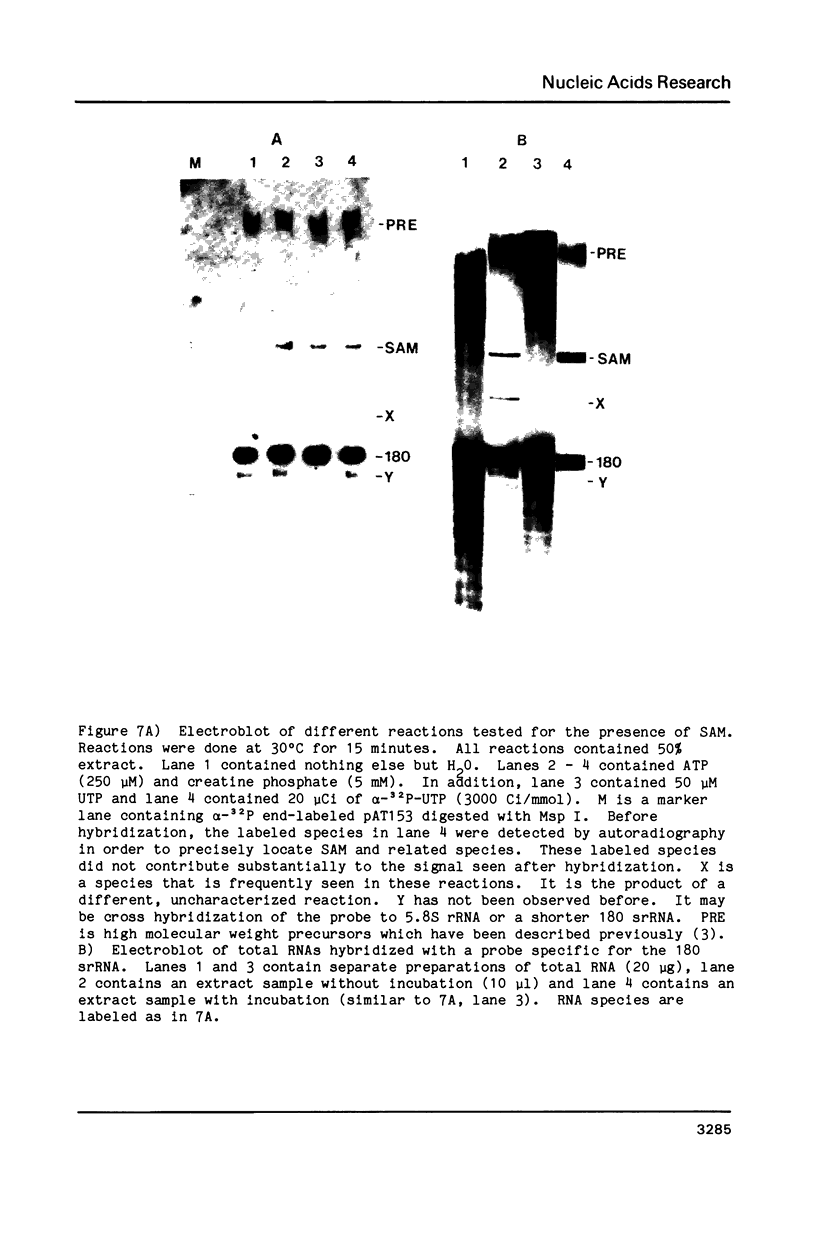





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Baltimore D. Purification of a terminal uridylyltransferase that acts as host factor in the in vitro poliovirus replicase reaction. Proc Natl Acad Sci U S A. 1986 Jan;83(2):221–225. doi: 10.1073/pnas.83.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. A poly(U) polymerase in tobacco leaves. Biochim Biophys Acta. 1975 Apr 2;383(4):351–358. doi: 10.1016/0005-2787(75)90304-4. [DOI] [PubMed] [Google Scholar]
- Bruce A. G., Uhlenbeck O. C. Reactions at the termini of tRNA with T4 RNA ligase. Nucleic Acids Res. 1978 Oct;5(10):3665–3677. doi: 10.1093/nar/5.10.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Lenardo M. J., Reddy L. V., Van der Ploeg L. H., Donelson J. E. The 5.8S ribosomal RNA gene of Trypanosoma brucei: structural and transcriptional studies. Nucleic Acids Res. 1985 May 24;13(10):3533–3549. doi: 10.1093/nar/13.10.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W., Konarska M., Gross H. J., Shatkin A. J. RNA 3'-terminal phosphate cyclase activity and RNA ligation in HeLa cell extract. Nucleic Acids Res. 1983 Mar 11;11(5):1405–1418. doi: 10.1093/nar/11.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev O. I., Nikolaev N., Hadjiolov A. A., Skryabin K. G., Zakharyev V. M., Bayev A. A. The structure of the yeast ribosomal RNA genes. 4. Complete sequence of the 25 S rRNA gene from Saccharomyces cerevisae. Nucleic Acids Res. 1981 Dec 21;9(24):6953–6958. doi: 10.1093/nar/9.24.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T. T., MacFarlane K. Comparison of endogenous and exogenous RNA primers of poly(U) polymerase in rat hepatic ribosomes. Biochem J. 1979 Mar 1;177(3):895–902. doi: 10.1042/bj1770895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Haruna I., Watanabe I., Mikoshiba K., Tsukada Y. Poly(U) polymerase in rat brain. Nature. 1975 Jul 24;256(5515):337–339. doi: 10.1038/256337a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G., Ogden R. C., Peebles C. L., Abelson J. Splicing of yeast tRNA precursors: structure of the reaction intermediates. Cell. 1979 Sep;18(1):37–45. doi: 10.1016/0092-8674(79)90351-9. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Gross H. J. RNA ligation via 2'-phosphomonoester, 3'5'-phosphodiester linkage: requirement of 2',3'-cyclic phosphate termini and involvement of a 5'-hydroxyl polynucleotide kinase. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1474–1478. doi: 10.1073/pnas.79.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., De Lange T., Borst P. Discontinuous synthesis of mRNA in trypanosomes. EMBO J. 1984 Oct;3(10):2387–2392. doi: 10.1002/j.1460-2075.1984.tb02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Leung M. F., Rawls W. E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979 Apr;30(1):98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. E., Piel N., McLaughlin L. W., Tromp M., Veeneman G. H., van der Marel G. A., van Boom J. H. Polymer supported DNA synthesis using hydroxybenzotriazole activated phosphotriester intermediates. Nucleic Acids Res. 1984 Nov 26;12(22):8639–8651. doi: 10.1093/nar/12.22.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Liu A. Y., Bernards A., Sloof P., Van der Bijl M. M., Schinkel A. H., Menke H. H., Borst P., Veeneman G. H., Tromp M. C. Activation of the genes for variant surface glycoproteins 117 and 118 in Trypanosoma brucei. J Mol Biol. 1983 Jun 5;166(4):537–556. doi: 10.1016/s0022-2836(83)80283-6. [DOI] [PubMed] [Google Scholar]
- Milchev G. I., Hadjiolov A. A. Association of poly(A) and poly(U) polymerases with cytoplasmic ribosomes. Eur J Biochem. 1978 Mar;84(1):113–121. doi: 10.1111/j.1432-1033.1978.tb12147.x. [DOI] [PubMed] [Google Scholar]
- Nishikura K., De Robertis E. M. RNA processing in microinjected Xenopus oocytes. Sequential addition of base modifications in the spliced transfer RNA. J Mol Biol. 1981 Jan 15;145(2):405–420. doi: 10.1016/0022-2836(81)90212-6. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polatnick J. Isolation of a foot-and-mouth disease polyuridylic acid polymerase and its inhibition by antibody. J Virol. 1980 Feb;33(2):774–779. doi: 10.1128/jvi.33.2.774-779.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Spencer D. F., Gray M. W. Primary structures of four novel small ribosomal RNAs from Crithidia fasciculata. Can J Biochem Cell Biol. 1983 Jan;61(1):38–45. doi: 10.1139/o83-006. [DOI] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Chain extension of ribonucleic acid by enzymes from rat liver cytoplasm. Biochem J. 1968 Oct;109(4):485–494. doi: 10.1042/bj1090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Dorssers L., Wernars K., Van Kammen A. Terminal uridylyl transferase of Vigna unguiculata: purification and characterization of an enzyme catalyzing the addition of a single UMP residue to the 3'-end of an RNA primer. Nucleic Acids Res. 1981 Jun 11;9(11):2433–2453. doi: 10.1093/nar/9.11.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]