Abstract
Nano-sized titanium dioxide (TiO2) is among the top five widely used nanomaterials for various applications. In this study, we determine the phototoxicity of TiO2 nanoparticles (nano-TiO2) with different molecular sizes and crystal forms (anatase and rutile) in human skin keratinocytes under UVA irradiation. Our results show that all nano-TiO2 particles caused phototoxicity, as determined by the MTS assay and by cell membrane damage measured by the lactate dehydrogenase (LDH) assay, both of which were UVA dose- and nano-TiO2 dose- dependent. The smaller the particle size of nano-TiO2 the higher the cell damage. The rutile form of nano-TiO2 showed less phototoxicity than anatase nano-TiO2. The level of photocytotoxicity and cell membrane damage is mainly dependent on the level of reactive oxygen species (ROS) production. Using polyunsaturated lipids in plasma membranes and human serum albumin as model targets, and employing electron spin resonance (ESR) oximetry and immuno-spin trapping as unique probing methods, we demonstrated that UVA irradiation of nano-TiO2 can induce significant cell damage, mediated by lipid and protein peroxidation. These overall results suggest that nano-TiO2 is phototoxic to human skin keratinocytes, and that this phototoxicity is mediated by ROS generated during UVA irradiation.
Keywords: TiO2 nanoparticles, Phototoxicity, Reactive oxygen species (ROS), Lipid peroxidation, Human HaCaT keratinocytes, ESR, Oximetry, Immuno-spin trapping
Introduction
Nanomaterials have been recently developed that show great potential for use in the biomedical, optical, and electronic fields. They are currently manufactured on a large scale to be used in thousands of consumer goods and industrial products. However, nanomaterials are potentially toxic due to their unique properties including extremely high surface-to-volume ratios, which can make them very reactive or catalytic. Furthermore, the tiny size of nanomaterials makes it possible for them to pass through cell membranes and other biological barriers. Therefore, investigation of cellular toxicity and phototoxicity of nanomaterials is critically needed.
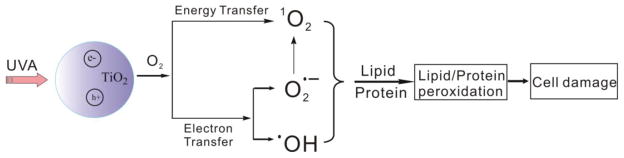
Nano-sized titanium dioxide (TiO2) is among the top five nanomaterials widely used in paints, plastics, cosmetics, personal care products, and as food additives and drug delivery agents (Kangwansupamonkon et al., 2009; Ray et al., 2009). The usefulness of TiO2 nanoparticles (nano-TiO2) is primarily linked to two of its functions: absorbing and deflecting ultraviolet (UV) radiation and semiconductor photocatalysis (Fujishima et al., 2000). Under UVA irradiation, electrons in the TiO2 valence band absorb the photon energy and jump to the conduction band, leaving valence band holes that extract electrons from water or hydroxyl ions and generate hydroxyl radicals (•OH). Formation of other reactive oxygen species (ROS), including superoxide (O2•−) and singlet oxygen (1O2), by different mechanisms has also been reported (Hirakawa and Hirano, 2006; Daimon and Nosaka, 2007). The photocatalytic properties make TiO2 nanoparticles successful candidates for degrading organic pollutants (Mura et al., 1999) or killing microorganisms (Kangwansupamonkon et al., 2009; Foster et al., 2011). On the other hand, potential risks associated with exposure to nano-TiO2 raise many concerns due to generation of ROS during UV irradiation; ROS are genotoxic or cytotoxic to organisms (Vevers and Jha, 2008; Ghosh et al., 2010; Sycheva et al., 2011).
Previous studies have suggested that nano-TiO2 that has not been irradiated with UV has limited cytotoxicity (Rehn et al., 2003). However, further reports have shown that TiO2 particles without illumination affected cell-matrix adhesion, which is not associated with ROS damage to cells (Fujita et al., 2009). Under UV irradiation, TiO2 nanoparticles exhibit toxic effects to both normal tissue and cancer cells, such as human dermal and lung cells (Liao et al., 2009), human keratinocyte cells (Shukla et al., 2011; Simon et al., 2011), rat lung alveolar macrophages (Afaq et al., 1998), intestinal cells (Koeneman et al., 2010) and HeLa cells (Cai et al., 1992). The human skin and eye are of particular concern as target sites for phototoxic damage because these tissues are constantly exposed to direct daily ambient radiation. It was reported that although micron-sized TiO2 cannot penetrate through the intact epidermal barrier (Kiss et al., 2008), topical application of TiO2 nanoparticles may induce dermal toxicity (Wu et al., 2009). The photoreactivity of TiO2 nanoparticles is largely dependent on their particle size, shape and crystal structure (Sharma, 2009; Liu et al., 2010). It was demonstrated that smaller nano-TiO2 particles tend to have higher phototoxicity, and anatase and amorphous forms of nano-TiO2 show higher cytotoxicity than its rutile form (Xue et al., 2010; Sanders et al., 2011).
In the present studies, we have evaluated the phototoxicity of nano-TiO2 with four different sizes (<25 nm, 31 nm, <100 nm, and 325 nm) and two crystal forms (anatase and rutile) towards human skin keratinocytes under UVA irradiation. We observe that their differences in phototoxicity are highly correlated with the production of ROS. By employing polyunsaturated lipids in plasma membrane and human serum albumin (HSA) as model targets, our unique electron spin resonance (ESR) oximetry and immuno-spin trapping techniques indicate that UVA photoirradiation of nano-TiO2 in biological systems can lead to significant cell damage.
Materials and methods
Chemicals
Nano-TiO2 (A25, <25 nm anatase, catalog # 637254; A325, 325 mesh anatase, catalog # 248576; R100, <100 nm rutile, catalog # 637262), HSA, xanthine, superoxide dismutase (SOD), diethylenetriaminepentaacetic acid (DTPA), 2,2,6,6-tetramethyl-4-piperidone (TEMP) and dimethyl sulfoxide (DMSO, cell-culture grade) were all purchased from Sigma Chemical Co. (St. Louis, MO). Titanium dioxide (P25, 31 nm anatase/rutile, catalog # Aeroxide® TiO2 P25) was from Degussa (Alpharetta, GA). Fetal bovine serum (FBS) was from Biofluids (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM), phosphate buffered saline (PBS, pH 7.4), trypsin-EDTA, penicillin, and streptomycin were purchased from Invitrogen Inc. (Grand Island, NY). The MTS assay kit (CellTiter 96® Aqueous) was purchased from Promega Co. (Madison, WI). 15N-labeled 4-oxo-2,2,6,6-tetramethylpiperidine-d16-1-oxyl (15N-PDT) was purchased from Cambridge Isotope Labs (Andover, MA). Xanthine oxidase (XOD) was from Roche Applied Science (Indianapolis, IN). The spin-trap 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO) was purchased from Applied Bioanalytical Labs (Sarasota, FL). Egg phosphatidylcholine was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). 5,5-dimethy-1-pyrroline N-oxide (DMPO) was purchased from Dojindo laboratories (Japan).
Preparation of TiO2 suspensions
The nano-TiO2 suspensions were prepared by first dispersing the nanoparticles in ultrapure water using a sonicator for 30 min to produce a 10 mg/ml stock nano-TiO2 suspension and then diluting according to the requirements of the experiments.
Characterization of nano-TiO2
X-ray diffraction (XRD) patterns were recorded on a Philips XPert PRO MPD X-ray diffractometer (Philips, Eindhoven, The Netherlands) operated at 35 kV and 45 mA with Cu-Kα radiation. Scanning Electron Microscope (SEM) images were taken on a Hitachi S4800 field scanning electron microscope (FESEM, Japan) operating at 10 kV. Samples were suspended in ethanol solution by sonication first. Approximately 10 μl of suspensions were spin-coated on a silicon wafer at 3000 rpm for 60 seconds. Dynamic light scattering (DLS) measurements of particle size were carried out using a light scattering Zetasizer Nano-S90 light scattering instrument (Malvern Instruments, Enigma Business Park, UK).
Cell culture and phototoxicity measurement
Human HaCaT keratinocytes, a transformed epidermal human cell line (Boukamp et al., 1988), were grown to ~95% confluence in 96-well plates at 37ºC in DMEM containing 10% FBS under 95% air/5% CO2. For phototoxicity tests, cells were exposed in the dark for 4 h at 37 ºC to different TiO2s (50 and100 μg/ml) in DMEM (FBS free). Control cells were treated with DMEM alone. After incubation the medium was removed and replaced by sterile PBS containing 10 mM glucose. Cells were then irradiated with UVA from four fluorescent PUVA lamps (Houvalite F20T12BL-HO; National Biological Co. Twinsburg, OH). The maximum emission of the UVA light lamps was determined to be between 320–390 nm. The light intensities at wavelengths below 315 nm (UVB light) and above 400 nm (visible light) are approximately two orders of magnitude lower than the maximum in the 320–390 nm spectral region (Zhao et al., 2010). The UVA exposure doses were 0, 2.5, 5.0, and 10 J/cm2 respectively, as measured with an YSI-Kettering Model 65A Radiometer (Yellow Springs Instrument Co., Yellow Springs, OH). After exposure the PBS/glucose solution was removed and replaced with DMEM containing 2% FBS and the cells were kept in the incubator overnight. Aliquots (10 μl) were removed and LDH release into the medium was assayed using the Cytotox 96 kit (Promega Corp., Madison, WI) according to the manufacturer’s directions. The remaining medium was removed and the cells were washed with PBS and replaced with 100 μl/well of PBS/glucose containing the MTS reagent (CellTiter 96 Aqueous Proliferation Assay; Promega Cop.). After incubation for 2 h at 37 ºC, the absorbance at 492 nm was recorded using a microplate reader (Spectrafluor Plus, Tecan US, RTP, NC).
ESR spectroscopy
Conventional ESR spectra were obtained with a Bruker EMX ESR Spectrometer (Billerica, MA). All spin trapping ESR measurements were carried out using the following settings for detection of the spin adduct between BMPO and O2•− (BMPO-OH): 10 mW microwave power, 100 G scan range and 1 G field modulation. All measurements were performed in replicates at ambient temperature. A light system consisting of a Xenon lamp coupled with a Schoeffel monochromator was used to generate UV light at 340 nm wavelength. All the samples for ESR measurements were freshly prepared in 100 mM phosphate buffer at pH 7.4. The buffer was treated with Chelex-100 resin for 24 h to remove traces of transition metal ions.
Spin label oximetry
ESR oximetry measurement is based on the bimolecular collision of molecular oxygen with a spin label which is a stable free radical. Collision of the spin label with O2 produces a spin exchange, resulting in shorter relaxation times and ESR signals with broader linewidths and decreasing peak height. Thus, oxygen consumption by such mechanisms as lipid peroxidation results in decreased line widths and increased peak height for the spin probe. The oximetry method determines the oxygen concentration by measuring the hyperfine structural changes at the low field line of the ESR spectrum using 15N-PDT as the spin label (Halpern et al., 1994; Swartz and Clarkson, 1998; Xia et al., 2007; Yin et al., 2008; Yin et al., 2009). During lipid peroxidation, the levels of dissolved O2 vary, with a time dependent decrease in linewidth and an increase in the peak intensity of the ESR signal indicating continuous O2 consumption. The value of oxygen concentration was obtained from a calibrated curve of the ESR linewidth versus the oxygen concentration.
Liposomes were prepared as previously described (Kusumi et al., 1986). Briefly, a suspension of 30 mg/ml egg PC liposomes and 0.1mM 15N-PDT with or without TiO2 was added to a quartz capillary tube. The lipid peroxidation was initiated by UV (340 nm) irradiation. The ESR spectra were then recorded at 4 min intervals for 16 min. Signals were obtained with a 0.05 G scanning width, 0.5 mW incident microwave power and with 100 kHz field modulation. All ESR spectra were recorded at the low field line of 15N-PDT and at ambient temperature.
ELISA and Western Blot
HSA protein samples were typically prepared by treating 600 μM HSA with 0.1 mg/ml TiO2, and 50 mM DMPO in 100 mM phosphate buffer (Chelex-treated with 25 μM DTPA) at pH 7.4 in a 6-well plate in the absence or presence of UVA irradiation (10 J/cm2). After treatment, samples were frozen at -80 ºC for further experiments.
The ELISA assay to measure DMPO nitrone adducts was performed essentially as described by Ehrenshaft and Mason (Ehrenshaft and Mason, 2006) with minor modifications. Two microgram aliquots of protein diluted in bicarbonate buffer, pH 9.4, were allowed to adsorb to the wells of a 96-well microtiter plate (Grenier Labortechnik) for 1.5 h. After washing once with wash buffer (1×PBS with 0.1% tween), the microtiter plate was incubated for 1 h in a 1:5000 dilution of rabbit polyclonal anti-DMPO antiserum, washed 4 times with wash buffer, incubated for 1 h more in a 1:5000 dilution of an alkaline phosphatase anti-rabbit secondary antibody, again washed 4 times with wash buffer, and finally washed once with 1×TBS, pH 9.6. After addition of CDP-Star (25 μM) (Roche Laboratories), the chemiluminescent product was detected using a microplate reader (Spectrafluor Plus, Tecan US, RTP, NC).
For Western Blot analysis, 10 μg of HSA protein from each reaction were electrophoresed under reducing conditions through a 4–12% BisTris NuPAGE acrylamide gel (Invitrogen) followed by electroblotting to a nitrocellulose membrane. Western Blot analysis was then performed as described (Ehrenshaft and Mason, 2006) except that DMPO-containing proteins were visualized using the Odyssey infrared imaging system (Licor).
Results
Characterization of nano-TiO2
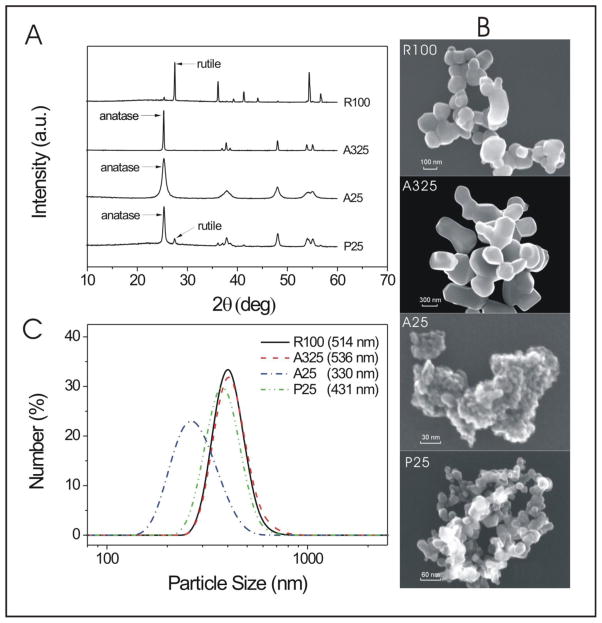
The XRD results shown in Fig. 1A reveal the crystal structure of nano-TiO2 samples, which are consistent with the crystallinity provided by the vendors. The crystalline composition of P25 was evaluated to be around 86% anatase and 14% rutile by the ratios of the integral areas of the three main diffraction peaks of the two phases (Zhao et al., 2008). SEM was used to visualize the particle size and shape of nano-TiO2. The SEM images of four nano-TiO2 samples are shown in Fig. 1B. The sizes are roughly consistent with the sizes claimed by the vendors. From the SEM images we also find that aggregates or big clusters are formed for all samples, especially for the smallest A25 sample. DLS measurements show the size and distribution of TiO2 aggregates in the aqueous medium, which are largely dependent on the concentration, dispersant, and primary particle size (Sanders et al., 2011). As shown in Fig. 1C, aggregates of the smallest A25 sample were between 150–500 nm and the other three were between roughly 250–700 nm at the lower concentration (50 μg/mL) tested in the phototoxicity experiments.
Figure 1. Characterization of Nano-TiO2 samples. (A) XRD patterns of various TiO2 samples;
(B) SEM images of various TiO2 samples; (C) DLS determination of particle size and distribution of 50 μg/ml nano-TiO2 samples in DMEM medium (FBS free).
Nano-TiO2-induced phototoxicity in human HaCaT keratinocytes
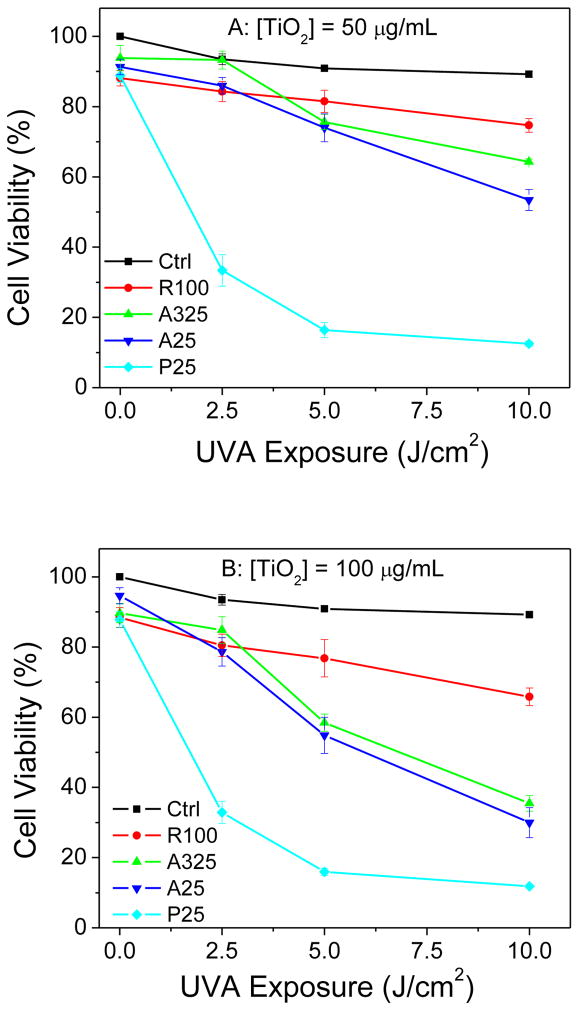
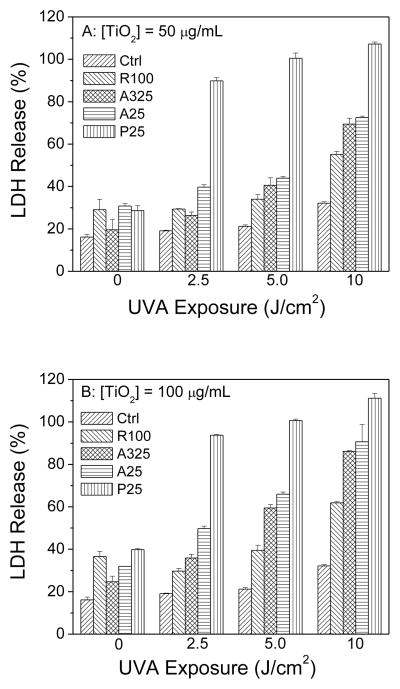
The phototoxicity of the four different sizes of nano-TiO2 towards HaCaT keratinocytes was determined using two different assays. The MTS assay measures the activity of (mainly mitochondrial) dehydrogenases, while cell membrane damage is measured by the release of cytosolic LDH. As measured by the MTS assay, no decrease in viability was observed when HaCaT cells were exposed to TiO2 samples or to UVA alone (Fig. 2A and 2B). However, in the presence of UVA radiation, two anatase TiO2 samples (A325 and A25) and P25 TiO2 caused UVA dose- and concentration-dependent damage to HaCaT cells. The rutile TiO2 sample (R100) showed much less phototoxicity towards HaCaT cells even at a high concentration of 100 μg/ml and 10 J/cm2 UVA exposure (Fig. 2B). The extent of photocytotoxicity to cells irradiated at 7.5 or 10 J/cm2 was in the following order: P25>A25>A325>R100. A similar result was seen with the LDH release assay (Fig. 3A and 3B).
Figure 2.
Effect of exposure to different TiO2 samples on the metabolic activity of HaCaT cells at concentrations of (A) 50 μg/ml and (B) 100 μg/ml as a function of UVA irradiation dosage, as measured by the MTS assay. Values are the means ± SE (n = 4).
Figure 3.
Effect of exposure to different TiO2 samples on the metabolic activity of HaCaT cells at concentrations of (A) 50 μg/ml and (B) 100 μg/ml as a function of UVA irradiation dosage, as measured by the LDH assay. Values are the means ± SE (n = 4).
ESR measurement of ROS generation
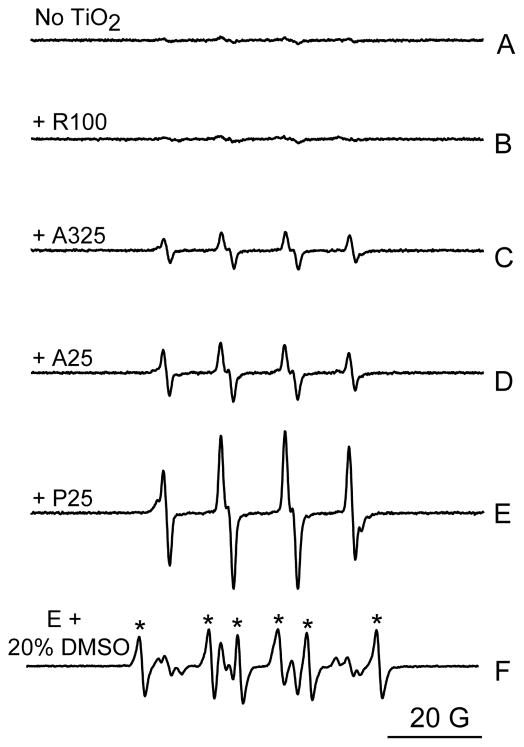
ESR spin trap spectroscopy was employed to determine whether or not nano-TiO2 under UVA light irradiation has the capability to generate ROS chemically. Generation of hydroxyl radicals was detected by the ESR spin trapping technique using BMPO. BMPO is a newly developed spin trap offering more advantages over the existing spin traps, such as greater stability and sensitivity (Zhao et al., 2001). The BMPO/•OH adduct has an ESR signal pattern similar to that of DMPO/•OH, which exhibits a characteristic set of four lines (1:2:2:1) in its ESR spectrum. In the absence of UVA irradiation, the ESR signal of the hydroxyl radical spin adduct was not detectable from the mixture of BMPO and nano-TiO2 (data not shown). No such ESR profile was generated when BMPO was irradiated under UVA light (340 nm) in the absence of nano-TiO2 (Fig. 4A) or in the presence of the rutile R100 TiO2 sample (Fig. 4B). However, UVA irradiation of BMPO in the presence of A325 nano-TiO2, A25 nano-TiO2, or P25 nano-TiO2 all produced the characteristic ESR profile (Fig. 4C, 4D, and 4E). As shown in Fig. 4, UVA irradiation of P25 nano-TiO2 resulted in the strongest signal of BMPO/•OH signal (Fig. 4E), followed by two anatase TiO2 samples, A325 nano-TiO2 and A25 nano-TiO2 (Fig. 4C and 4D). This signal was largely inhibited by the addition of 20% DMSO (Fig. 4F), further confirming the generation of OH radicals during the UVA irradiation.
Figure 4.
Generation of hydroxyl radical from photoirradiation of TiO2 samples under UVA light (340 nm). ESR spectra were recorded at room temperature 2 min after the UV light was turned on. Samples containing 25 mM BMPO and (A) without TiO2; (B) with 0.1 mg/ml R100; (C) 0.1 mg/ml A325; (D) 0.1 mg/ml A25; (E) 0.1 mg/ml P25 and (F) same as E, but with the addition of 20% DMSO. Stars (*) indicate the ESR signal of the BMPO/•CH3 adduct. Instrumental settings: microwave power, 10 mW; modulation frequency, 100 kHz; modulation amplitude, 1 G; scan range, 100 G.
We tested the generation of singlet oxygen by the ESR trapping technique using TEMP. It has been previously reported that 4-oxo-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPONE), a nitroxide radical detectable by EPR spectra, is generated from TEMP and singlet oxygen (Eq. 1) (Lion et al., 1976).
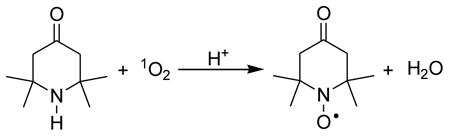 |
(1) |
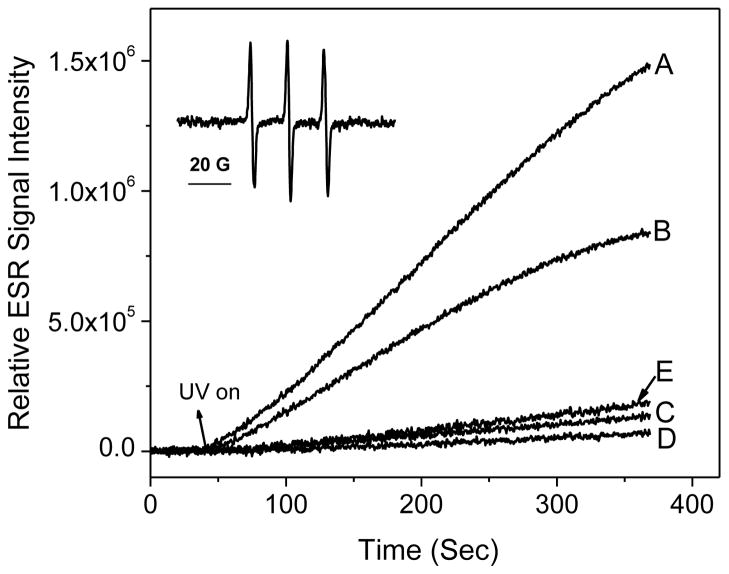
Irradiation of different nano-TiO2 samples (0.1 mg/ml) containing 20 mM TEMP resulted in ESR spectra of three lines with equal intensities (aN = 16.0 G), typical of nitroxide radicals (Fig. 5, inset). The hyperfine splitting constant and g factor of the photosensitized oxidation product of TEMP were identical to those of commercial TEMPONE. Fig. 5 shows the ESR intensity of the TEMPONE signal as a function of time during irradiation of different nano-TiO2 samples. The P25 nano-TiO2 solution (Fig. 5, curve A) showed the rate that increased the fastest, followed by the A25 nano-TiO2 sample (Fig. 5, curve B). The rates of production of singlet oxygen were much slower for A325 nano-TiO2 and R100 nano-TiO2 (Fig. 5, curves C and D). A control solution with TEMP only (data not shown) indicated no ESR signal increase. For P25 nano-TiO2, addition of a singlet oxygen quencher, NaN3, caused a substantial decrease in the rate of increase of the EPR signal (Fig. 5, curve E).
Figure 5.
Generation of singlet oxygen from photoirradiation of TiO2 samples under UVA light in a time- and crystal type-dependent manner. ESR spectra were recorded at room temperature. Samples containing 20 mM TEMP and (A) 0.1 mg/ml P25, (B) 0.1 mg/ml A25 (C) 0.1 mg/ml A325, (D) 0.1 mg/ml R100, and (E) 0.1 mg/ml P25 and 10 mM NaN3 were irradiated with UVA light at 340 nm. Inset: ESR signal of TEMPONE (aN = 16.0 G). The time scans of ESR signal intensity were obtained with a fixed field position (the peak position of the center line of the ESR spectrum), 15 mW microwave power and 1 G field modulation.
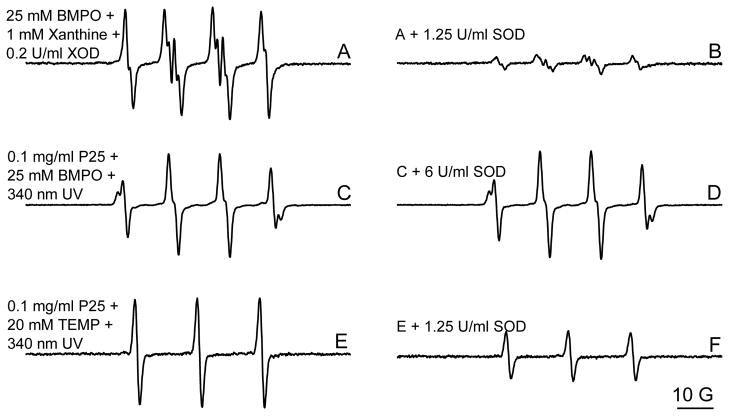
It has been reported that O2•− can be oxidized to 1O2 during TiO2 photocatalytic reactions (Lipovsky et al., 2012). Superoxide (O2•−) undergoes dismutation to produce H2O2, from which the •OH radical can also be formed through the Fenton reaction. To further elucidate the mechanisms of •OH and 1O2 generation, we studied the effect of SOD on the production of •OH and 1O2 by P25 nano-TiO2 during UVA irradiation. As a control study, addition of 1.25 U/ml SOD significantly inhibited the production of O2•− generated in a Xanthine system (Fig. 6A and 6B). However, no obvious effect was observed on •OH generation by P25 nano-TiO2 under UVA irradiation even at a high concentration of SOD (6 U/ml, Fig. 6C and 6D), indicating that nano-TiO2-generated •OH radicals were not evolved from O2• , at least for the most part. In contrast, the 1O2 generation was markedly reduced in the presence of 1.25 U/ml SOD (Fig. 6E and 6F), confirming that O2•− may be a main pathway in UVA-induced 1O2 generation by nano-TiO2 (Lipovsky et al., 2012). It is noteworthy that although O2•− was formed from UVA irradiation of nano-TiO2 and was attributed to the formation of 1O2, no obvious O2•− production was detected in our ESR study using BMPO as a trapping agent (Fig. 4), which is actually consistent with a previous report (Konaka et al., 2001). Its short lifetime under our experimental conditions warrants further investigation.
Figure 6.
Effect of SOD on the generation of hydroxyl radicals and singlet oxygen by P25 during UVA irradiation. The same conditions were used as shown in Fig. 4 and Fig. 5.
ESR oximetry measurement of lipid peroxidation
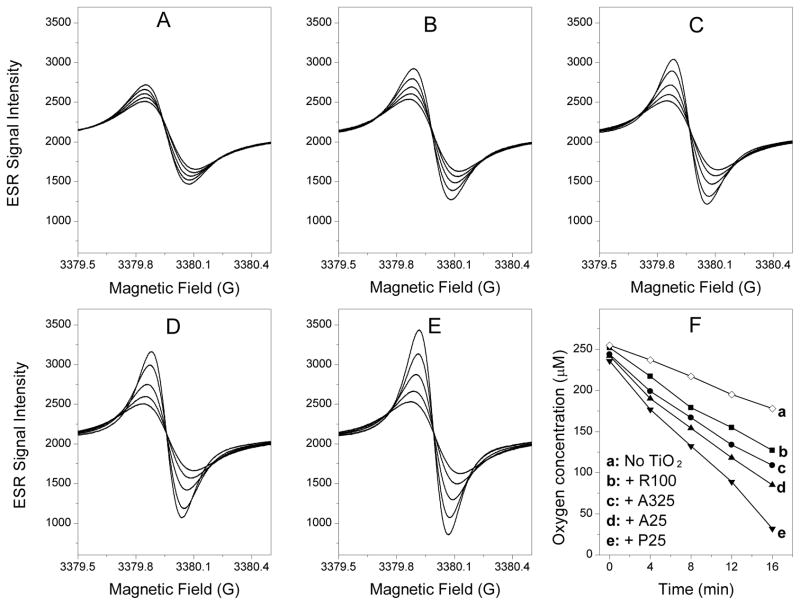
It is well known that ROS can produce a time-dependent peroxidation of the polyunsaturated lipids in plasma membrane. In this study, lipid peroxidation produced by UVA irradiation of different nano-TiO2 was assessed using ESR oximetry recorded at 4-min intervals for 16 min. The consumption of oxygen accompanying lipid peroxidation was measured as a time-dependent narrowing of the ESR signal for the spin probe 15N-PDT. As shown in Fig. 7, narrowing of the ESR signal was necessarily accompanied by an increase in the peak height of the ESR signal within the scan range. The results of lipid peroxidation produced by different nano-TiO2 samples (R100, A325, A25, and P25) after UVA irradiation are shown in Fig. 7B–7E, respectively. The final ESR signal intensities of R100 nano-TiO2 (Fig. 7B), A325 nano-TiO2 (Fig. 7C), A25 nano-TiO2 (Fig. 7D), and P25 nano-TiO2 (Fig. 7E) were approximately 7.5, 11.7, 16.2, and 26.3% higher than in the control (Fig. 7A). The progressive increases in peak-to-peak signal intensity (and accompanying progressive narrowing of line width) in each panel were due to time-dependent oxygen consumption resulting from lipid peroxidation. Fig. 6F presents the data as a decrease in oxygen concentration, reflecting the variations in the slope (oxygen concentration versus time) with different nano-TiO2, which decreases in the order of P25 > A25 > A325 > R100.
Figure 7.
Effect of TiO2 samples on lipid peroxidation in liposomes. Oxygen consumption was measured in a closed chamber using liposome suspensions and the spin label 15N-PDT. The liposome sample contained 30 mg/ml Egg PC and 0.1 mμ 15N-PDT spin label mixed with (A) no TiO2; (B) 0.03 mg/ml of R100; (C) 0.03 mg/ml of A325; (D) 0.03 mg/ml of A25 and (E) 0.03 mg/ml of P25. Lipid peroxidation was initiated by UV (340 nm) irradiation. The ESR spectra were recorded with the low field line of the 15N-PDT spin label every 4 min after the sample was sealed in a quartz capillary tube. The spectra were obtained with 0.5 mW incident microwave power and with 0.05 G field modulation at ambient temperature. The progressive increases in peak-to-peak signal intensity (and accompanying progressive narrowing of the linewidth) in each panel are due to time-dependent oxygen consumption resulting from lipid peroxidation, as shown in panel F. The enhancement effects of different TiO2 nanoparticles on lipid peroxidation may be seen as bigger changes in the peak-to-peak signal intensities seen in panels B, C, D and E compared to panel A.
Immuno-spin trapping measurement of protein radicals
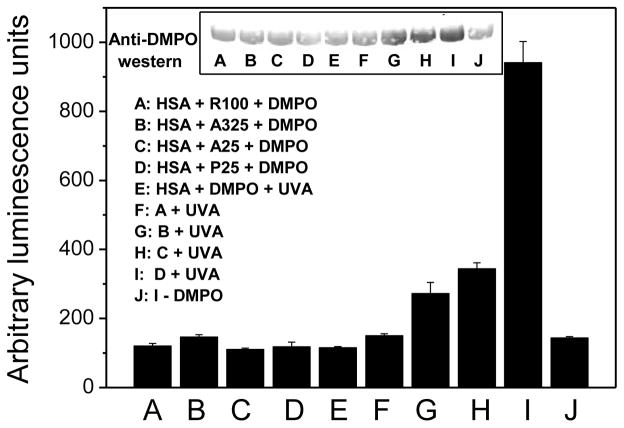
To further characterize the ability of ROS to oxidize target proteins, the most abundant plasma protein (human serum albumin, HSA) was incubated with DMPO and different nano-TiO2 samples with and without UVA irradiation. The reaction products were analyzed by ELISA and Western blotting using an anti-DMPO polyclonal antibody. Immunochemical detection of HSA-DMPO nitrone adducts was performed according to our previous studies (Ranguelova et al., 2010). As measured by both ELISA and Western blotting, HSA with DMPO exposed to different nano-TiO2 samples or UVA alone produced nitrone adducts at background levels (Fig. 8A–8E). While in the presence of UVA irradiation, P25 nano-TiO2 resulted in a significant increase in HSA-DMPO-derived nitrone adducts (Fig. 8I). The other three nano-TiO2 samples caused modest increases in adduct formation (Fig. 8F–8H). The order of the extent of nitrone adducts is the same as for photocytotoxicity, hydroxyl radical, singlet oxygen, and lipid peroxidation measurements.
Figure 8.
Effect of TiO2 samples and UVA irradiation on the formation of DMPO-HAS-derived radical nitrone adducts. Relative ELISA quantification and Western Blot analysis (inset).
Discussion
TiO2 is regarded as an inert and non-toxic substance by many regulatory bodies; e.g., the FDA approved TiO2 as a food color additive in 1996, and the Material Safety Data Sheets (MSDS) indicate that there are no exposure hazards to the health of occupational workers and public health. However, there have been several studies that have shown that TiO2 is a potential carcinogen and photocatalyst in biological tissues (Burnett and Wang, 2011). The cytotoxicity and/or phototoxicity of nano-TiO2 is still debated. Also, there is uncertainty about whether the properties of nano-TiO2 differ with its particular form and size. Human skin and ocular tissues are most affected by ambient radiation. In vitro studies have shown that when irradiated, the smallest form of TiO2, anatase (<25 nm), is phototoxic to human retinal cells (Sanders et al., 2011). UV-induced production of ROS and the resultant oxidative stress exposure plays an important role in photocarcinogenesis caused by UV irradiation. ROS are believed to be involved in many inflammatory skin disorders, skin cancer formation, and skin aging (He et al., 2005). Thus we report here a study of the photocytotoxicity and phototoxicity of nano-TiO2 towards human HaCaT keratinocytes.
Our results show that all nano-TiO2 tested in this paper caused UVA and nano-TiO2 dose-dependent phototoxicity in HaCaT cells. As expected, smaller particles resulted in higher phototoxicity than larger particles. Rutile nano-TiO2 showed less phototoxicity than anatase nano-TiO2. Moreover, small mixed anatase/rutile nano-TiO2 (P25) showed the highest phototoxicity, which is consistent with previous reported results (Hurum et al., 2003). The cellular toxicity of nano-TiO2 mainly depends on its ROS generation under UVA irradiation. Using ESR and spin trapping technique, a “gold standard” and state-of-the-art tool for detecting and quantifying ROS, we confirmed the generation of hydroxyl radicals and singlet oxygen. ROS generation by these four nano-TiO2 particles is consistent with the phototoxicity studies, further confirming our hypothesis that ROS production was probably involved in the phototoxic mechanism.
In addition to measurement of ROS generation with ESR, we explored two other assays to further characterize the phototoxicity of nano-TiO2. ROS are known to produce a time-dependent peroxidation of the polyunsaturated lipids in plasma membrane (Yin et al., 2008; Yin et al., 2009) as well as peroxidation of lipids and proteins inside the cells (Girotti, 1998). To mimic oxidation reactions with substrates in biological systems, we used polyunsaturated lipids in plasma membranes and human serum albumin as model targets for ROS. Using innovative ESR oximetry and immune-spin trapping techniques, we easily detected ROS-induced peroxidation, which was found to be in agreement with the ESR-detected ROS production. These results indicate that both methods are useful for qualitative screening of the phototoxicity of nano-TiO2.
Based on the results reported in this study, we propose the mechanism shown in Fig. 9. UVA irradiation results in electron-hole pairs in nano-TiO2. The photoexcited nano-TiO2 serves as the initiating species to transfer energy to molecular oxygen and generate singlet oxygen (1O2) or to extract electrons from water or hydroxyl ions to generate hydroxyl radicals (•OH). Our experimental results also indicate that part of the 1O2 formation proceeds via a superoxide-dependent mechanism, whereas the OH formation is not via superoxide (Fig. 6). It is well established that in the presence of a lipid or protein, the generated ROS can initiate lipid and/or protein peroxidation. Both ROS and lipid peroxidation are associated with aging-related diseases, including cancer (Aust et al., 1993; Xia et al., 2007).
Figure 9.
Proposed mechanism of TiO2 nanoparticle-induced free radicals and lipid/protein peroxidation leading to cell damage.
In conclusion, the studies presented here give an overall in vitro assessment of the phototoxicity by various nano-TiO2 particles towards HaCaT cells under UVA irradiation. This phototoxic damage was apparently mediated by production of ROS by photo-activated nano-TiO2. According to our results ROS are generated by UVA irradiation of nano-TiO2 with anatase and/or mixed anatase/rutile forms. Moreover, the phototoxicity of nano-TiO2 was less with larger particle size and surface areas, indicating that coarse particles of nano-TiO2 may consistently produce less ROS.
Highlights.
We evaluate the phototoxicity of nano-TiO2 with different sizes and crystal forms.
The smaller the particle size of nano-TiO2 the higher the cell damage.
The rutile form of nano-TiO2 showed less phototoxicity than anatase nano-TiO2.
ESR oximetry and immuno-spin trapping techniques confirm UVA-induced cell damage.
Phototoxicity is mediated by ROS generated during UVA irradiation of nano-TiO2.
Acknowledgments
This article is not an official US Food and Drug Administration (FDA) guidance or policy statement. No official support or endorsement by the US FDA is intended or should be inferred. This work was supported by a regulatory science grant under the FY11 FDA Nanotechnology CORES Program (JJ Yin) and the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors are indebted to Dr. Jianxun Xu for his help in SEM image analysis, Dr. Zhanjun Gu, Yeteng Zhong for their help in XRD analysis, and Dr. Ann Motten, NIEHS, for critical reading of the manuscript.
Abbreviations
- 15N-PDT
4-Oxo-2,2,6,6-tetramethylpiperidine-d16-1-oxyl
- BMPO
5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide
- DLS
dynamic light scattering
- DMEM
Dulbecco’s modified Eagle’s medium
- DMPO
5,5-dimethy-1-pyrroline N-oxide
- DMSO
dimethyl sulfoxide
- ESR
electron spin resonance
- FBS
fetal bovine serum
- DTPA
diethylenetriaminepentaacetic acid
- HSA
human serum albumin
- LDH
lactate dehydrogenase
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
- SOD
superoxide dismutase
- TEMP
2,2,6,6-tetramethyl-4-piperidone
- UVA
ultraviolet A (315-400 nm)
- XOD
xanthine oxidase
- XRD
X-ray diffraction
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afaq F, Abidi P, Matin R, Rahman Q. Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide. J Appl Toxicol. 1998;18:307–312. doi: 10.1002/(sici)1099-1263(1998090)18:5<307::aid-jat508>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Aust SD, Chignell CF, Bray TM, Kalyanaraman B, Mason RP. Free radicals in toxicology. Toxicol Appl Pharmacol. 1993;120:168–178. doi: 10.1006/taap.1993.1100. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photo. 2011;27:58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- Cai R, Kubota Y, Shuin T, Sakai H, Hashimoto K, Fujishima A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992;52:2346–2348. [PubMed] [Google Scholar]
- Daimon T, Nosaka Y. Formation and behavior of singlet molecular oxygen in TiO2 photocatalysis studied by detection of near-infrared phosphorescence. J Phys Chem C. 2007;111:4420–4424. [Google Scholar]
- Ehrenshaft M, Mason RP. Protein radical formation on thyroid peroxidase during turnover as detected by immuno-spin trapping. Free Radical Bio Med. 2006;41:422–430. doi: 10.1016/j.freeradbiomed.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biot. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photoch Photobio C. 2000;1:1–21. [Google Scholar]
- Fujita K, Horie M, Kato H, Endoh S, Suzuki M, Nakamura A, Miyauchi A, Yamamoto K, Kinugasa S, Nishio K, Yoshida Y, Iwahashi H, Nakanishi J. Effects of ultrafine TiO(2) particles on gene expression profile in human keratinocytes without illumination: Involvement of extracellular matrix and cell adhesion. Toxicol Lett. 2009;191:109–117. doi: 10.1016/j.toxlet.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81 :1253–1262. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Halpern HJ, Yu C, Peric M, Barth E, Grdina DJ, Teicher BA. Oxymetry deep in tissues with low-frequency electron paramagnetic resonance. P Nat Acad Sci USA. 1994;91:13047–13051. doi: 10.1073/pnas.91.26.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Huang JL, Block ML, Hong JS, Chignell CF. Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. J Invest Dermatol. 2005;125:560–566. doi: 10.1111/j.0022-202X.2005.23851.x. [DOI] [PubMed] [Google Scholar]
- Hirakawa K, Hirano T. Singlet oxygen generation photocatalyzed by TiO2 particles and its contribution to biomolecule damage. Chem Lett. 2006;35:832–833. [Google Scholar]
- Hurum DC, Agrios AG, Gray KA, Rajh T, Thurnauer MC. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J Phys Chem B. 2003;107:4545–4549. [Google Scholar]
- Kangwansupamonkon W, Lauruengtana V, Surassmo S, Ruktanonchai U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomedicine : NBM. 2009;5:240–249. doi: 10.1016/j.nano.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Kiss B, Biro T, Czifra G, Toth BI, Kertesz Z, Szikszai Z, Kiss AZ, Juhasz I, Zouboulis CC, Hunyadi J. Investigation of micronized titanium dioxide penetration in human skin xenografts and its effect on cellular functions of human skin-derived cells. Exp Dermatol. 2008;17:659–667. doi: 10.1111/j.1600-0625.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- Koeneman BA, Zhang Y, Westerhoff P, Chen YS, Crittenden JC, Capco DG. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol Toxicol. 2010;26:225–238. doi: 10.1007/s10565-009-9132-z. [DOI] [PubMed] [Google Scholar]
- Konaka R, Kasahara E, Dunlap WC, Yamamoto Y, Chien KC, Inoue M. Ultraviolet irradiation of titanium dioxide in aqueous dispersion generates singlet oxygen. Redox Rep. 2001;6:319–325. doi: 10.1179/135100001101536463. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Subczynski WK, Pasenkiewicz-Gierula M, Hyde JS, Merkle H. Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochim Biophys Acta. 1986;854:307–317. doi: 10.1016/0005-2736(86)90124-0. [DOI] [PubMed] [Google Scholar]
- Liao CM, Chiang YH, Chio CP. Assessing the airborne titanium dioxide nanoparticle-related exposure hazard at workplace. J Hazard Mater. 2009;162:57–65. doi: 10.1016/j.jhazmat.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Lion Y, Delmelle M, van de Vorst A. New method of detecting singlet oxygen production. Nature. 1976;263:442–443. doi: 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- Lipovsky A, Levitski L, Tzitrinovich Z, Gedanken A, Lubart R. The different behavior of rutile and anatase nanoparticles in forming oxy radicals upon illumination with visible light: an EPR study. Photochem Photobiol. 2012;88:14–20. doi: 10.1111/j.1751-1097.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Yin LH, Pu YP, Li YH, Zhang XQ, Liang GY, Li XB, Zhang JA, Li YF, Zhang XY. The Immune Toxicity of Titanium Dioxide on Primary Pulmonary Alveolar Macrophages Relies on their Surface Area and Crystal Structure. J Nanosci Nanotechno. 2010;10:8491–8499. doi: 10.1166/jnn.2010.2685. [DOI] [PubMed] [Google Scholar]
- Mura GM, Ganadu ML, Lubinu G, Maida V. Photodegradation of organic waste coupling hydrogenase and titanium dioxide. Ann NY Acad Sci. 1999;879:267–275. doi: 10.1111/j.1749-6632.1999.tb10430.x. [DOI] [PubMed] [Google Scholar]
- Ranguelova K, Bonini MG, Mason RP. (Bi)sulfite oxidation by copper, zinc-superoxide dismutase: Sulfite-derived, radical-initiated protein radical formation. Environ Health Persp. 2010;118:970–975. doi: 10.1289/ehp.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Heal C Environ Carcinog Ecotoxicol Rev. 2009;27 :1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn B, Seiler F, Rehn S, Bruch J, Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol Appl Pharm. 2003;189:84–95. doi: 10.1016/s0041-008x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Sanders K, Degn LL, Mundy WR, Zucker RM, Dreher K, Zhao B, Roberts JE, Boyes WK. In Vitro Phototoxicity and Hazard Identification of Nano-scale Titanium Dioxide. Toxicol Appl Pharmacol. 2011;258:226–236. doi: 10.1016/j.taap.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Sharma VK. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment-A Review. J Environ Sci Heal A. 2009;44:1485–1495. doi: 10.1080/10934520903263231. [DOI] [PubMed] [Google Scholar]
- Shukla RK, Kumar A, Pandey AK, Singh SS, Dhawan A. Titanium Dioxide Nanoparticles Induce Oxidative Stress-Mediated Apoptosis in Human Keratinocyte Cells. J Biomed Nanotechnol. 2011;7:100–101. doi: 10.1166/jbn.2011.1221. [DOI] [PubMed] [Google Scholar]
- Simon M, Barberet P, Delville MH, Moretto P, Seznec H. Titanium dioxide nanoparticles induced intracellular calcium homeostasis modification in primary human keratinocytes. Towards an in vitro explanation of titanium dioxide nanoparticles toxicity. Nanotoxicology. 2011;5:125–139. doi: 10.3109/17435390.2010.502979. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- Sycheva LP, Zhurkov VS, Iurchenko VV, Daugel-Dauge NO, Kovalenko MA, Krivtsova EK, Durnev AD. Investigation of genotoxic and cytotoxic effects of micro- and nanosized titanium dioxide in six organs of mice in vivo. Mutat Res. 2011;726:8–14. doi: 10.1016/j.mrgentox.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Vevers WF, Jha AN. Genotoxic and cytotoxic potential of titanium dioxide (TiO2) nanoparticles on fish cells in vitro. Ecotoxicology. 2008;17:410–420. doi: 10.1007/s10646-008-0226-9. [DOI] [PubMed] [Google Scholar]
- Wu J, Liu W, Xue C, Zhou S, Lan F, Bi L, Xu H, Yang X, Zeng FD. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett. 2009;191:1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Xia Q, Yin JJ, Fu PP, Boudreau MD. Photo-irradiation of Aloe vera by UVA--formation of free radicals, singlet oxygen, superoxide, and induction of lipid peroxidation. Toxicol Lett. 2007;168:165–175. doi: 10.1016/j.toxlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Xue C, Wu J, Lan F, Liu W, Yang X, Zeng F, Xu H. Nano titanium dioxide induces the generation of ROS and potential damage in HaCaT cells under UVA irradiation. J Nanosci Nanotechnol. 2010;10:8500–8507. doi: 10.1166/jnn.2010.2682. [DOI] [PubMed] [Google Scholar]
- Yin JJ, Lao F, Fu PP, Wamer WG, Zhao Y, Wang PC, Qiu Y, Sun B, Xing G, Dong J, Liang XJ, Chen C. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials. 2009;30:611–621. doi: 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JJ, Lao F, Meng J, Fu PP, Zhao Y, Xing G, Gao X, Sun B, Wang PC, Chen C, Liang XJ. Inhibition of tumor growth by endohedral metallofullerenol nanoparticles optimized as reactive oxygen species scavenger. Mol Pharmacol. 2008;74:1132–1140. doi: 10.1124/mol.108.048348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Chignell CF, Rammal M, Smith F, Hamilton MG, Andley UP, Roberts JE. Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol. 2010;86:798–805. doi: 10.1111/j.1751-1097.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Zhang H, Karoui H, Kalyanaraman B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: a relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radical Bio Med. 2001;31:599–606. doi: 10.1016/s0891-5849(01)00619-0. [DOI] [PubMed] [Google Scholar]
- Zhao L, Han M, Lian H. Photocatalytic activity of TiO2 films with mixed anatase and rutile structures prepared by pulsed laser deposition. Thin Solid Films. 2008;516:3394–3398. [Google Scholar]