Abstract
Aims
To evaluate the pathobiologic effects of long-term treatment with nicotine of A/J mice susceptible to tobacco-induced lung carcinogenesis.
Main methods
Experimental group of mice received subcutaneous injections of the LD50 dose of (−)nicotine hydrogen tartrate of 3 mg/kg/day, 5 days per week for 24 months, and control group received the vehicle phosphate-buffered saline.
Key findings
Nicotine treated mice, 78.6%, but none of control of mice, developed neoplasms originating from uterus or skeletal muscle. Examination of the uterine neoplasms revealed leiomyosarcomas, composed of whorled bundles of smooth-muscle like cells with large and hyperchromatic nuclei. Sections of the thigh neoplasms revealed densely cellular tumors composed of plump spindle cells, with occasional formation of ‘strap’ cells, containing distorted striations. Both neoplasms were positive for desmin staining. A solitary pulmonary adenoma with papillary architecture also occurred in one nicotine treated mouse. Experimental mice also developed transient balding starting as small patches of alopecia that progressed to distinct circumscribed areas of complete hair loss or large areas of diffuse hair loss.
Significance
We demonstrate for the first time that chronic nicotine treatment can induce the development of muscle sarcomas as well as transient hair loss. These findings may help explain the association of childhood rhabdomyosarcoma with parental smoking and earlier onset of balding in smokers. It remains to be determined whether the pathobiologic effects of nicotine result from its receptor mediated action and/or its tissue metabolites cotinine and N'-nitrosonornicotine, or toxic effects of reactive oxygen species activated due to possible intracellular accumulation of nicotine.
Keywords: A/J mice, nicotine, leiomyosarcoma, rhabdomyosarcoma, alopecia
Introduction
Although consumption of tobacco products can lead to several types of cancer, the direct contribution of nicotine itself is unknown, and the safety of long-term usage of nicotine replacement products remains to be elucidated. Nicotine is metabolized in the liver by cytochrome P450 enzymes yielding its major metabolite cotinine. N'-nitrosonornicotine (NNN) is formed from nicotine by nitrosation in human organism (Stepanov et al., 2009). While NNN is believed to play an important role in the induction of tobacco-related cancers and classified by the International Agency for Research on Cancer (IRAC) as carcinogenic to humans, there is no direct evidence that nicotine and cotinine are oncogenic per se. The carcinogenic properties of pure nicotine have not been evaluated by the IARC, and it has not been assigned to an official carcinogen group.
The purpose of this pilot study was to evaluate the pathobiologic effects of long-term, high dose nicotine treatment of A/J mice susceptible to tobacco product-induced lung carcinogenesis. This mouse strain is widely used for assessing the carcinogenic potential of nitrosamines, such as NNN (Hecht, 1998). A/J mice carry the pulmonary adenoma susceptibility (Pas1) locus, a major locus affecting predisposition to lung cancer in mice (Manenti et al., 1997), and produce the EcoRI-generated 0.55 kb K-ras fragment associated with high susceptibility to lung tumor development (Malkinson, 1992).
The obtained results demonstrated for the first time that A/J mice chronically treated with nicotine can develop soft tissue sarcomas and reversible hair loss. These findings open novel avenues for studying tobacco-related morbidity and toxicity of nicotine replacement products.
Materials and Methods
Treatment of mice with nicotine
Female strain A/J mice, 6–8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed in polypropylene boxes with ad libitum access to food and water, and conventional bedding material. (−)-Nicotine hydrogen tartrate salt was obtained from Sigma-Aldrich Co. (St. Louis, MO; catalog number N-5260). The mice were randomized into two groups: experimental group (n = 15) and control group (n = 5). Experimental group mice received nicotine in phosphate buffered saline (PBS) by subcutaneous injection of 50 µl in the upper back of 3 mg/kg/day, 5 days per week for 24 months, and control group received the vehicle PBS.
Tumor analysis
Mice were euthanized at the first visible signs of tumor development, so that the tumor size never exceeded 1.5 cm in diameter, thus avoiding an excessive tumor burden. All internal organs of sacrificed animals underwent a thorough gross examination. The tumor masses were excised, embedded in paraffin, cut, stained by hematoxylin-and-eosin and subjected to microscopic examination. For desmin staining, we used paraffin embedded 7 µm thick sections, goat anti-desmin (Y-20) antibody from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and FITC-conjugated anti-goat secondary antibody (Vector Labs, Burlingame, CA). Sections were counterstained with DAPI.
In a search for lung tumors, the lungs were manually expanded to inspiratory volume by intratracheal instillation of Tellyesniczky’s fluid and incubated for at least 24 h. This fixative makes even small tumors stand out in a better contrast (Shimkin and Stoner, 1975). The tumors are localized beneath the pleura, and there is no advantage to examining serial lung sections. This procedure gives an accurate total number of all macroscopically discernible tumors in a mouse lung (Witschi, 2003).
Results
A/J mice treated with nicotine developed muscle sarcomas
During the 24 months period, 11 of 15 A/J treated with nicotine, but none of the control animals, developed grossly apparent tumor masses originating from the uterus (Fig. 1A; n = 3) or skeletal muscle (Fig. 1C; n = 8). Three experimental mice did not develop any tumors and one experimental mouse died from nicotine toxicity prior to completion of the studies. Thus, 78.6% of A/J mice that survived nicotine toxicity developed tumors.
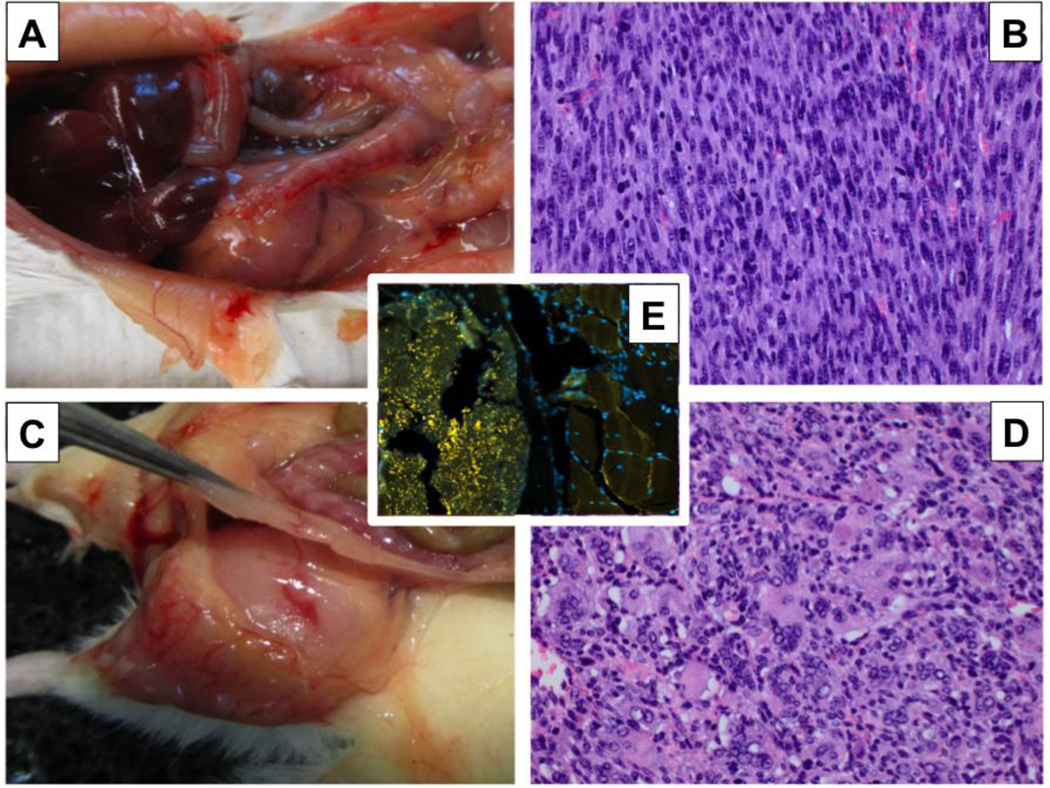
Figure 1. Nicotine-induced muscle sarcomas in A/J mice.
A, B: Uterine leiomyosarcoma. C, D: Rhabdomyosarcoma of the quadriceps muscle. E. Immunofluorescent analysis of rhabdomyosarcoma with anti-desmin antibody showing intense punctate cytoplasmic staining in the neoplastic cells (at left) and diffuse subsarcolemmal staining in the adjacent normal striated muscle (at right), serving as an internal positive control.
Light microscopic examination of H&E-stained uterine masses revealed spindle cell neoplasms composed of whorled bundles of pleomorphic smooth-muscle like cells with enlarged, hyperchromatic nuclei (Fig. 1B). More than 10 mitotic figures per 10 high power fields and areas of necrosis were present.
Sections of the quadriceps masses revealed a densely cellular neoplasm composed of plump spindle cells, with occasional formation of ‘strap’ cells, containing distorted striations (Fig. 1D). Scattered multinucleated giant cells and many mitotic figures were seen. Many areas of confluent tumor necrosis were present, with palisading tumor cells around their periphery.
Both neoplasms were positive for desmin staining (Fig. 1E). Immunofluorescent analysis showed intense punctate cytoplasmic staining in the neoplastic cells and diffuse subsarcolemmal staining in the adjacent normal striated muscle.
Based on these clinicopathological features, the uterine tumors were diagnosed as leiomyosarcoma, and those in quadriceps—as rhabdomyosarcoma.
A/J mice treated with nicotine developed transient hair loss
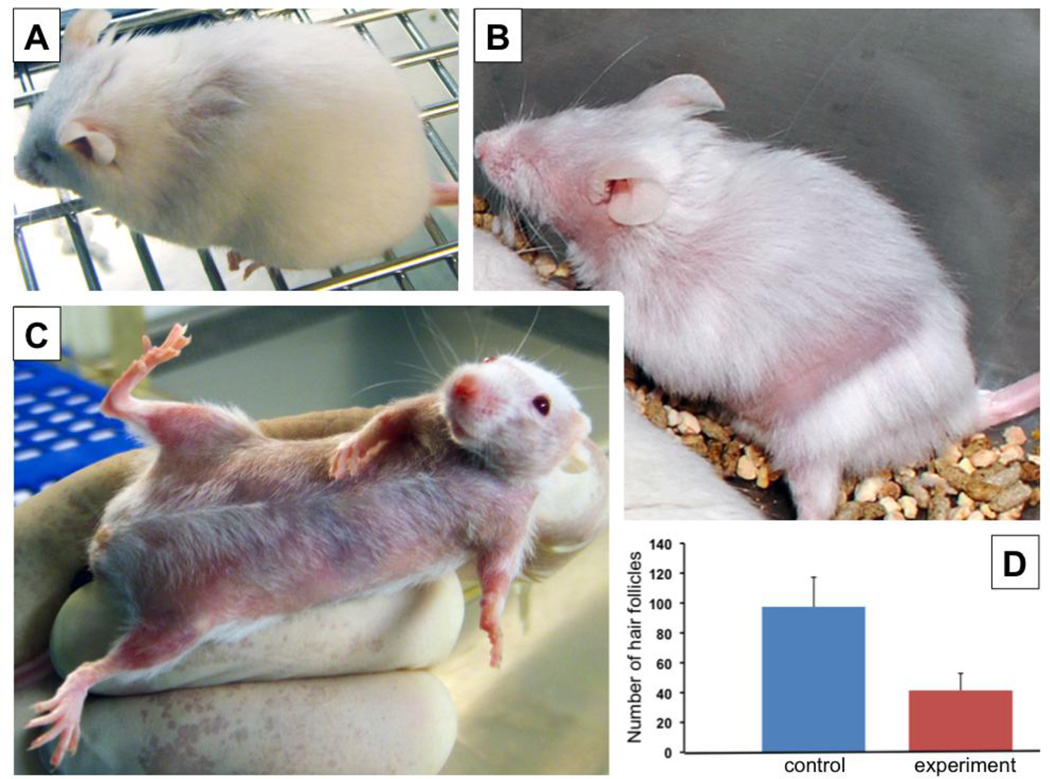
Experimental, but not control, A/J mice started to lose their hair after 8–9 months of treatment with nicotine. Any anatomical area of mouse skin could be affected. The balding started as small patches of alopecia (Fig. 2A) that progressed into distinct circumscribed areas of complete hair loss (Fig. 2B) or large areas of diffuse hair loss (Fig. 2C). The number of hair follicles in the areas of diffuse alopecia decreased by approximately 30% on average (Fig. 2D).
Figure 2. Hair loss in A/J mice.
Patchy (A, B) and diffuse (C) areas of alopecia observed at 10 months of treatment with nicotine. D: The representative differences of hair follicle numbers in the areas of diffuse alopecia, compared to the same cutaneous region of control mouse. Results were computed in 10 microscopic fields of horizontal sections of trunkal skin at magnification ×4, and presented as mean ± SD per 1 mm2 ; p = 0.003.
The hair loss spontaneously reversed within 7–8 months, so that by the end of additional 15–16 months of nicotine treatment all animals re-grew their hair.
Discussion
We demonstrated for the first time that chronic treatment of A/J mice with an LD50 dose of nicotine cause carcinogenic transformation of both smooth and striated muscles as well as transient hair loss. These findings help explain previous reports of association of childhood rhabdomyosarcoma with parental smoking (Grufferman et al., 1982) and earlier onset of hair loss in smokers (reviewed in (Ortiz and Grando, 2012)). Our results, therefore, should add nicotine to the list of potential carcinogens present in tobacco products and raise concern about the safety of long term usage of nicotine replacement products.
One of the key trends is creating novel smokeless tobacco products is to use alternative technologies of nicotine delivery. Some of new smokeless tobacco products also deliver higher than usual doses of nicotine. For example, each 8-ounce bottle of NICLite® contains 4 mg of nicotine. Therefore, the daily dose of nicotine received by experimental A/J mice approximates that consumed by a nicotine addict. Indeed, there are certain limitations of the rapid kinetics of daily nicotine injection compared the slower kinetics of nicotine administration by minipump or water. Although experimental A/J mice received the entire daily dose of nicotine via a single subcutaneous injection, we did not observe any behavioral abnormalities or changes in skin appearance at the injection sites.
Although there is no data published to date that nioctine can cause lung cancer per se (Catassi et al., 2008), it is well accepted that it exhibits tumor promoting effects, facilitating survival and proliferation of transformed cells (Minna, 2003, Paleari et al., 2008, Song et al., 2008). The pathobiologic effects of nicotine observed in A/J mice might result from its receptor-mediated and/or non-receptor actions, as well as from its endogenously produced metabolites cotinine and NNN. It is well known that by activating cellular nicotinic acetylcholine receptors (nAChRs) nicotine can foster cancer by acting as tumor promoter that facilitates the outgrowth of cells with genetic damage (epigenetic effect) (Ortiz and Grando, 2012). Binding of nicotine to nAChRs modulates the expression of a diverse set of genes that may be broadly categorized into four groups: transcription factors, protein processing factors, RNA-binding proteins, and plasma membrane-associated proteins (Dunckley and Lukas, 2003). In addition to activation of nAChRs, nicotine can render a non-receptor action elicited due to its transport across the cell membrane directly into cell without receptor-mediated interactions (Nair et al., 1997). Therefore, the mutagenic effect of nicotine was most likely mediated by reactive oxygen species activated due to the possible intracellular accumulation of nicotine (Fukada et al., 2002) that can cause DNA damage (Argentin and Cicchetti, 2004).
While nicotine did not cause lung cancer in A/J mice, chronic tobacco smoke exposure did (Witschi, 2003). The mechanism of tumorigenic action of tobacco smoke in lungs of A/J mice apparently involves both gas and particulate phase carcinogens (Witschi et al., 1997). The contribution of endogenously produced NNN to nicotine carcinogenicity is unlikely, because among the experimental A/J, only one animal developed three small pulmonary neoplasms. The largest was 3 mm in greatest dimension, and all had a pushing border, and lacked significant pleomorphism, necrosis, or mitotic figures, consistent with a benign pulmonary adenoma. No malignancy—a reliable marker of the carcinogenic action of NNN—was seen. It has been documented that A/J mice exposed to NNN have 80 to 100% lung tumor incidence (Amin et al., 1996). The absence of malignant lung tumors in experimental A/J mice is in keeping with a previous report that rats treated for 2 years with daily inhalation of nicotine did not develop microscopic or macroscopic lung tumors (Waldum et al., 1996). The rodent lung, therefore, may not be a preferable target for nicotine carcinogenicity. Hence, an increased incidence of lung cancers in tobacco users may involve additional mechanisms, such as genetic polymorphisms of nAChRs causing an aberrant downstream signaling (Chikova and Grando, 2011).
Our findings of muscle sarcomas and balding in mice chronically treated with nicotine were quite surprising, though not totally unexpected. In a case-control study of childhood rhabdomyosarcoma, a significant relative risk was associated with fathers' cigarette smoking (Grufferman et al., 1982). On the other hand, clinical observations of premature baldness in smokers are supported by experimental studies showing that C57BL/6 mice exposed to cigarette smoke for a prolonged period of time develop areas of alopecia and grey hair (D'Agostini et al., 2007). Since smoking leads to DNA damage of the hair follicle (Adams et al., 1997), the two separate pathologies related to nicotine/smoking —malignancy and alopecia—have DNA damage as a common pathophysiologic mechanism. Alternatively or additionally, nicotine might cause alopecia by accelerating senescence of hair follicles through chronic stimulation of the nAChR subtypes coupled to terminal differentiation and programmed cell death of epidermal keratinocytes (Nguyen et al., 2001)
In conclusion, we recognize that our findings pose more questions than provide answers, and hope that an in-depth analysis of the observed phenomena in future studies will ultimately resolve the issue about the potential carcinogenic activities of nicotine replacement products.
Acknowledgements
This work was supported by the NIH grants ES017009 and DE14173, and a grant from Lung Cancer Research Foundation to S.A.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interests to declare.
References
- Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: Reversibility with oral L-arginine but not vitamin C. J Am Coll Cardiol. 1997;29:491–497. doi: 10.1016/s0735-1097(96)00537-2. [DOI] [PubMed] [Google Scholar]
- Amin S, Desai D, Hecht SS, Hoffmann D. Synthesis of tobacco-specific N-nitrosamines and their metabolites and results of related bioassays. Crit Rev Toxicol. 1996;26:139–147. doi: 10.3109/10408449609017927. [DOI] [PubMed] [Google Scholar]
- Argentin G, Cicchetti R. Genotoxic and antiapoptotic effect of nicotine on human gingival fibroblasts. Toxicol Sci. 2004;79:75–81. doi: 10.1093/toxsci/kfh061. [DOI] [PubMed] [Google Scholar]
- Catassi A, Servent D, Paleari L, Cesario A, Russo P. Multiple roles of nicotine on cell proliferation and inhibition of apoptosis: implications on lung carcinogenesis. Mutat Res. 2008;659:221–231. doi: 10.1016/j.mrrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Chikova A, Grando SA. Naturally occurring variants of human α9 nicotinic receptor differentially affect bronchial cell proliferation and transformation. PLoS One. 2011;6:e27978. doi: 10.1371/journal.pone.0027978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostini F, Fiallo P, Pennisi TM, De Flora S. Chemoprevention of smoke-induced alopecia in mice by oral administration of L-cystine and vitamin B6. J Dermatol Sci. 2007;46:189–198. doi: 10.1016/j.jdermsci.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Dunckley T, Lukas RJ. Nicotine modulates the expression of a diverse set of genes in the neuronal SH-SY5Y cell line. J Biol Chem. 2003;278:15633–15640. doi: 10.1074/jbc.M210389200. [DOI] [PubMed] [Google Scholar]
- Fukada A, Saito H, Inui K. Transport mechanisms of nicotine across the human intestinal epithelial cell line Caco-2. J Pharmacol Exp Ther. 2002;302:532–538. doi: 10.1124/jpet.102.034629. [DOI] [PubMed] [Google Scholar]
- Grufferman S, Wang HH, DeLong ER, Kimm SY, Delzell ES, Falletta JM. Environmental factors in the etiology of rhabdomyosarcoma in childhood. J Natl Cancer Inst. 1982;68:107–113. [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Malkinson AM. Primary lung tumors in mice: an experimentally manipulable model of human adenocarcinoma. Cancer Res. 1992;52:2670s–2676s. [PubMed] [Google Scholar]
- Manenti G, Gariboldi M, Fiorino A, Zanesi N, Pierotti MA, Dragani TA. Genetic mapping of lung cancer modifier loci specifically affecting tumor initiation and progression. Cancer Res. 1997;57:4164–4166. [PubMed] [Google Scholar]
- Minna JD. Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer. J Clin Invest. 2003;111:31–33. doi: 10.1172/JCI17492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MK, Chetty DJ, Ho H, Chien YW. Biomembrane permeation of nicotine: mechanistic studies with porcine mucosae and skin. J Pharm Sci. 1997;86:257–262. doi: 10.1021/js960095w. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Hall LL, Zia S, Arredondo J, Chernyavsky AI, et al. Programmed cell death of keratinocytes culminates in apoptotic secretion of a humectant upon secretagogue action of acetylcholine. J Cell Sci. 2001;114:1189–1204. doi: 10.1242/jcs.114.6.1189. [DOI] [PubMed] [Google Scholar]
- Ortiz A, Grando SA. Smoking and the skin. Int J Dermatol. 2012;51:250–262. doi: 10.1111/j.1365-4632.2011.05205.x. [DOI] [PubMed] [Google Scholar]
- Paleari L, Grozio A, Cesario A, Russo P. The cholinergic system and cancer. Semin Cancer Biol. 2008;18:211–217. doi: 10.1016/j.semcancer.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Shimkin MB, Stoner GD. Lung tumors in mice: application to carcinogenesis bioassay. Adv Cancer Res. 1975;21:1–58. doi: 10.1016/s0065-230x(08)60970-7. [DOI] [PubMed] [Google Scholar]
- Song P, Sekhon HS, Fu XW, Maier M, Jia Y, Duan J, et al. Activated cholinergic signaling provides a target in squamous cell lung carcinoma. Cancer Res. 2008;68:4693–4700. doi: 10.1158/0008-5472.CAN-08-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Carmella SG, Han S, Pinto A, Strasser AA, Lerman C, et al. Evidence for endogenous formation of N'-nitrosonornicotine in some long-term nicotine patch users. Nicotine Tob Res. 2009;11:99–105. doi: 10.1093/ntr/ntn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldum HL, Nilsen OG, Nilsen T, Rorvik H, Syversen V, Sanvik AK, et al. Long-term effects of inhaled nicotine. Life Sci. 1996;58:1339–1346. doi: 10.1016/0024-3205(96)00100-2. [DOI] [PubMed] [Google Scholar]
- Witschi H. Induction of lung cancer by passive smoking in an animal model system. Methods Mol Med. 2003;74:441–455. doi: 10.1385/1-59259-323-2:441. [DOI] [PubMed] [Google Scholar]
- Witschi H, Espiritu I, Maronpot RR, Pinkerton KE, Jones AD. The carcinogenic potential of the gas phase of environmental tobacco smoke. Carcinogenesis. 1997;18:2035–2042. doi: 10.1093/carcin/18.11.2035. [DOI] [PubMed] [Google Scholar]