Abstract
Pictures of emotional facial expressions or natural scenes are often used as cues in emotion research. We examined the extent to which these different stimuli engage emotion and attention, and whether the presence of social anxiety symptoms influences responding to facial cues. Sixty participants reporting high or low social anxiety viewed pictures of angry, neutral, and happy faces, as well as violent, neutral, and erotic scenes, while skin conductance and event-related potentials were recorded. Acoustic startle probes were presented throughout picture viewing, and blink magnitude, probe P3 and reaction time to the startle probe also were measured. Results indicated that viewing emotional scenes prompted strong reactions in autonomic, central, and reflex measures, whereas pictures of faces were generally weak elicitors of measurable emotional response. However, higher social anxiety was associated with modest electrodermal changes when viewing angry faces and mild startle potentiation when viewing either angry or smiling faces, compared to neutral. Taken together, pictures of facial expressions do not strongly engage fundamental affective reactions, but these cues appeared to be effective in distinguishing between high and low social anxiety participants, supporting their use in anxiety research.
Introduction
The study of emotion relies on the use of cues or contexts that reliably engage the bodily reactions that collectively have defined emotion for the psychophysiologist for over a century. Among the visual cues often used to investigate emotion are pictures of facial expressions (Ekman & Friesen, 1976; Hansen & Hansen, 1998; Gur et al., 2002; Lundqvist, Flykt, & Öhman, 1998; Matsumoto & Ekman, 1989; Tottenham et al., 2009) and pictures of pleasant and unpleasant natural scenes (Lang, Bradley, & Cuthbert, 2008). Previous studies, however, report small or null effects of emotional expression on autonomic measures of emotional reactivity (e.g., Alpers, Adolph & Pauli, 2011; Surcinelli & Codispoti, 2007). In fact, faces are more often utilized as affective cues in behavioral paradigms (e.g., dot-probe, Mogg & Bradley, 2002; Perez-Edgar et al., 2010; visual search, Frischen, Eastwood, & Smilek, 2008; Juth, Lundqvist, Karlsson, & Öhman, 2005) or neuroscience investigations (e.g., Eimer & Holmes, 2007; Portois & Vuilleumier, 2006) in which emotional expressions can affect reaction time, ERPs or BOLD activity.
One possibility is that pictures of emotional expressions can influence attention and other cognitive processes, without necessarily strongly activating the autonomic and somatic reactions indicative of emotional engagement. Thus, in the current study, we measure emotional and attentional indices of processing for faces and scenes. In particular, we measure three different reactions to a startling acoustic probe presented during picture viewing: the blink reflex, the P3 component elicited by the startle probe, and speeded reaction time. Central questions concern whether emotional faces prompt affective modulation of the blink reflex, and whether concurrent measures of attention, including reaction time and the event-related potential (ERP) to the startle probe, will show evidence of heightened processing (or avoidance) of emotional expressions, particularly for participants reporting high social anxiety.
The blink component of the startle reflex is reliably potentiated when viewing aversive compared to neutral scenes, and is attenuated for pleasant scenes (e.g., Lang & Bradley, 2010); thus it is a useful measure of emotional reactivity during picture viewing (Vrana, Spence, & Lang, 1988). Several previous studies have examined startle reflex modulation during affective face viewing, with some studies reporting modest startle potentiation when participants view unpleasant facial expressions (Anokhin & Golosheykin, 2010; Dunning, Auriemmo, Castille, & Hajcak, 2010; Grillon & Charney, 2011; Hess, Sabourin, & Kleck, 2007; Springer, Rosas, McGetrick, & Bowers, 2007).
A number of paradigmatic specifications have been noted, however. For example, Anokhin & Golosheykin (2010) reported startle potentiation for angry and fearful faces that was only significant for female participants. In contrast, Springer et al. (2007) reported potentiated startle when participants viewed angry, but not fear, faces, regardless of gender. Hess et al. (2007) also reported startle potentiation specifically prompted by angry faces, but only for pictures depicting male expressors. Using angry faces only, Dunning et al. (2010) manipulated picture arousal by systematically morphing faces to display varying levels of intensity, and only found potentiation for angry faces rated as maximally intense. Studying fear faces specifically, Grillon and Charney (2011) reported startle potentation when viewing fearful expressions, but only when participants were also under threat of shock (Grillon & Charney, 2011). Moreover, at least two studies have reported that startle magnitude is enhanced when participants view either happy or angry expressions compared to neutral faces (Alpers et al., 2011; Garner, Clarke, Graystone, & Baldwin, 2011)—a different pattern of reflex modulation than observed when viewing arousing pleasant and unpleasant scenes. In the current study, we intermixed pictures depicting pictures depicting happy, angry, and neutral facial expressions with pictures of emotional and neutral natural scenes, which allowed a direct comparison of startle reflex modulation across picture type.
Assuming that startle potentiation will not be strong for facial expressions, however, our next question concerned possible differences in attention allocation to pictures of facial expressions. Previous studies in our laboratory and others (Cuthbert, Schupp, Bradley, McManis, & Lang, 1998; Schupp, Cuthbert, Bradley, Birbaumer, & Lang, 1997; Bradley, Cuthbert, & Lang, 1999; Smith, Hillman, & Duley, 2005) have clearly indicated that the amplitude of the P3 component elicited to a startling probe presented during picture viewing serves as an accurate index of resource allocation: When people view emotional pictures, the probe P3 is attenuated throughout the viewing interval, suggesting fewer resources are available for processing the secondary probe when attention is captured by the affective foreground cue. Moreover, reaction time to startle probes is similarly affected, particularly shortly after picture onset, with slower reactions for emotional, compared to neutral, pictures (Bradley et al., 1999). In the current study, we measured both ERPs and speeded reaction time to startle probes presented when viewing faces or scenes, to gauge heightened attention allocation even in the absence of evidence of strong emotional engagement.
Moreover, previous research suggests that socially anxious individuals may particularly exhibit enhanced attentional and emotional processing when viewing emotional faces, and information processing theories of social phobia propose that symptoms may be maintained, in part, by a face-specific attentional bias (e.g., Heinrichs & Hoffman, 2001). For example, social phobia patients, compared to controls, have shown increased selective attention to angry faces in a variety of behavioral task paradigms (e.g., Mogg & Bradley, 2002; Juth, Lundqvist, Karlsson, & Öhman, 2005; Putman, Hermans, & van Honk, 2004). High social anxiety has also been associated with faster visual orienting to emotional faces (happy or angry) in eye-tracking research (e.g., Garner, Mogg, & Bradley, 2006; Wieser, Pauli, Weyers, Alpers, & Mühlberger, 2009), and with enhanced ongoing visual attention to emotional faces in ERP paradigms (McTeague, Shumen, Wieser, Lang, & Keil, 2011; Mühlberger et al., 2009). Thus, an additional aim of the current study is to assess the extent to which reports of heightened social anxiety are associated with greater affective reactivity to emotional faces.
The Current Study
The present research examines affective and attentional engagement when participants view intermixed sets of 1) standardized happy, neutral, and angry facial expressions, and 2) pictures of emotional and neutral natural scenes. Face cues were selected from the Karolinska Directed Emotional Faces set (KDEF; Lundqvist et al., 1998). These images are more naturalistic than exaggerated or posed collections (i.e., Ekman & Friesen, 1976; Tottenham et al., 2009) and have been controlled for perceptual variables that might impact ERP measures.
Emotional scenes were selected from the International Affective Picture System (Lang et al., 2008) and included erotic and violent pictures. It is well-known that the visual scenes that strongly elicit responses in autonomic and somatic systems indicative of emotion are those that are rated as highly arousing (Balaban & Taussig, 1994; Bernat, Patrick, Benning & Tellegen, 2006; Bradley, Codispoti, Cuthbert, & Lang, 2001; Codispoti, Bradley, & Lang, 2001; De Ceserai & Codispoti, 2010; Lang, Greenwald, Bradley, & Hamm, 1993). In a systematic investigation of the effects of rated arousal and semantic content on psychophysiological reactivity, we found that pictures of erotica and violence most reliably engage emotional reactions, whereas contents rated lower in arousal, such as babies, pollution, and illness are more similar to neutral in their affective profile (Bradley et al., 2001). In the current study, therefore, we included pictures of erotica and violence as high arousal anchors for evaluating reactivity to facial expressions, but expected these scenes to generate strong emotional reactions compared to facial expressions, based on previous findings that emotional faces are rated relatively low in arousal and do not strongly elicit emotional reactions (Alpers et al. 2011; Lang et al., 2008; Surcinelli & Codispoti, 2007). Of major interest was whether pictures of facial expressions would differ from a set of neutral natural scenes also included in the stimulus set.
In addition to three responses to the startle probe, including speeded reaction time, the reflexive startle blink, and the amplitude of the P3 component of the ERP, the measurement array included continuous recording of skin conductance and picture-related ERPs. This array provides a profile of emotional and attentional engagement across the viewing interval: initial attention capture is reflected by slowed RT to startle probes; patterns of appetitive and defensive response mobilization are distinguished by startle reflex attenuation and potentiation, respectively; and probe P3 amplitude indexes continued attention capture across the viewing interval. Skin conductance tracks sympathetic activity associated with response mobilization, and the amplitude of the late positive potential provides a brain-based index that a cue is “significant”—activating appetitive or defensive motivational systems (Bradley, 2009).
Method
Participants and Screening Measures
Sixty (30 female) participants from University of Florida introductory psychology classes received course credit for participation. Participants were selected based on their total score on the Liebowitz Social Anxiety Scale—Self Report Version (LSAS-SR; Fresco et al., 2001), which was administered in an initial online prescreening session. The LSAS-SR is a 24-item questionnaire assessing dimensional severity of social anxiety symptoms. A total score of 60 has been established as the cutoff for significant symptoms of generalized social phobia (Rytwinksi et al., 2009), so to ensure recruitment of high- and low-anxiety participants, those with pre-screening LSAS total scores of 60 or greater, or lower than 40, were invited to participate.
The LSAS was re-administered at the laboratory session, and participants were assigned to either a high or low socially anxious group according to a median split on LSAS total score. The high anxiety group (n=30, 21 male) reported an average total score of 67.8 (s.d.=19.1) and the low anxiety group (n=30, 11 male) reported an average total score of 23.7 (s.d.=9.0).
Materials and Design
Participants viewed 108 affective pictures representing pleasant, neutral, and unpleasant content, half depicting standard emotional facial expressions (54 pictures), and half depicting natural scenes (54 pictures). Emotional facial expressions were selected from the KDEF set (Lundqvist et al., 1998) and natural scenes from the IAPS (Lang et al., 2008). Specific contents portrayed scenes of human violence, neutral people in everyday situations, and erotic couples, and male and female facial expressions of anger, neutrality, and happiness. Mean (s.d.) normative arousal ratings for erotic, neutral, and violent scenes were 6.1(0.5), 3.4(0.5), and 6.3(0.5) (Lang et al., 2008), and for happy, neutral, and angry faces were 3.6 (0.3), 2.5(0.3), and 3.8(0.6) (Goeloeven et al., 2008).
Apparatus and Physiological Response Measurement
A PC running VPM (Cook, 2002) and a PC running Presentation (Neurobehavioral Systems, Albany, CA) controlled data acquisition and stimulus events, respectively. Pictures were displayed in color on an LCD monitor 1m from the participant, subtending approximately 38.5 degrees horizontally and 22.3 degrees vertically. Each trial began with a 3-second baseline in which a fixation cross appeared on the center of the screen, followed by a 3 s presentation of a picture, and an inter-trial interval (ITI) that varied from 5 to 10 s in duration. Acoustic startle stimuli (50 ms, 98 dB) were presented over headphones during every picture either 250 ms, 750 ms, or 2500 ms after picture onset. Startle probes were also presented in one-sixth of the ITIs, occurring 5 s after picture offset. Three different picture orders were used across participants, and counterbalanced so that each picture was probed at each latency, and no more than 3 pictures of the same hedonic content were presented in a row.
The eyeblink component of the startle response was measured via 4mm Ag-AgCl electrodes over the left orbicularis oculi muscle (Fridlund & Cacioppo, 1986). The raw orbicularis EMG signal was sampled at 1000 Hz, amplified by 30,000, filtered from 28–500 Hz, and integrated (20 ms time constant). Skin conductance was recorded from 8 mm electrodes filled with .5 M NaCl paste on the hypothenar eminence of the left palm. Raw skin conductance data was continuously sampled at 20 Hz.
Event-related potentials were sampled at 250 Hz using a 129-sensor dense array EEG system. Signals were bandpass filtered (0.1–100 Hz) with a vertex reference. Data were filtered off-line at 30 Hz and transformed to an average reference. For analysis of the probe P300, stimulus-locked epochs were extracted from 100 ms before to 600 ms after startle probe onset and were baseline-corrected (100 ms pre-startle onset). For analyses of the late-positive potential (LPP) elicited by picture onset, epochs time-locked to picture onset were also extracted and baseline corrected (100 ms before to 800 ms after picture onset). Reaction time was recorded in milliseconds via a serial-port button press device communicating with the VPM computer.
Procedure
Participants were comfortably seated in a sound-attenuated room, where they initially completed the LSAS-SR before the researcher applied sensors and headphones. Participants were instructed that pictures would be displayed on the screen, and that they should look at each picture for the duration of its presentation. They were informed that brief noises would be intermittently presented over the headphones and that they should quickly press the provided button each time a noise was detected.
Data reduction
Primary experimental conditions were devised according to cue type—either face or scene—and further by hedonic content, either pleasant (happy face/erotic scene), neutral (neutral face/neutral scene), or unpleasant (angry face/violence scene). Additional specifications within measures are outlined below.
For startle magnitude, eyeblink data were reduced off-line using a peak-scoring algorithm implemented in VPM (Cook, 2002) that determines the onset latency and amplitude of ach blink (Balaban, Losito, Simons, & Graham, 1986). Trials with clear artifacts were rejected and trials with no responses scored as zero magnitude blinks. Average blink magnitude was calculated for probes presented during the ITI, and blinks were then converted to T-scores using the mean and standard deviation of the ITI distribution. Blink magnitude was computed over the two later startle probe times (750ms, 2500ms) separately for each cue type (face/scene) and hedonic content (pleasant/neutral/unpleasant) for each participant.
Skin conductance data were converted into microSiemens and averaged into half-second bins. These values were computed as change scores relative to the 1 second prior to picture onset, and the maximum skin conductance change between 1 and 6 s after picture onset was determined.
Probe P3 amplitude was calculated as the mean positivity over 24 centro-parietal sensors in a window from 260–340ms after probe onset, relative to the 100 ms pre-startle baseline. Average P3 amplitude was calculated across the two later (750, 2500ms) startle probe times for each cue type and hedonic content. Because P3 reduction is associated with greater attention to the picture, for statistical tests the data were multiplied by −1 so that greater P3 reduction was characterized by a more positive value. The LPP elicited by each picture was measured as the average baseline-corrected cortical positivity 400–700 ms after picture onset, over 36 centro-parietal sensors. Average LPP amplitude was computed for each cue type and hedonic content.
For RT data, a log transformation normalized the distribution of raw RT values. Average RT was computed for each startle probe time (250, 750, 2500ms), cue type, and hedonic content.
Data analysis
Data were analyzed using SPSS (Version 17.0). A multivariate repeated-measures ANOVA was conducted for skin conductance, LPP, and probe P300 measures, as these are similarly modulated by rated stimulus arousal. A 3 (Measure: skin conductance, LPP, probe P300) × 2(Cue: face, scene) × 3(Content: pleasant, neutral, unpleasant) × 2(Anxiety: low, high) design was used. Attention capture across time, measured by RT, was assessed using a 3(Time: 250, 750, 2500ms) × 2(Cue) × 3(Content) × 2(Anxiety) mixed design ANOVA. To assess defensive and appetitive engagement, startle magnitude was analyzed using a 2(Cue) × 3(Content) × 2(Anxiety) mixed design ANOVA. Greenhouse-Geisser corrections were used where appropriate.
Results
Skin conductance, probe P3, LPP
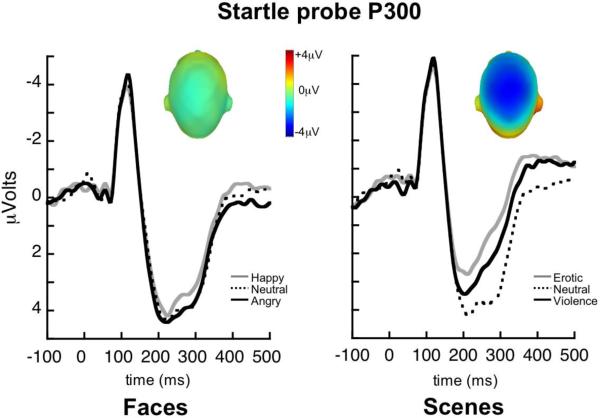
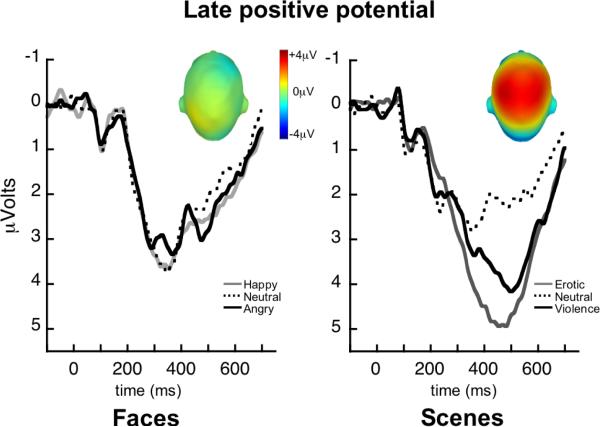
Viewing affective naturalistic scenes was significantly more evocative than viewing pictures of emotional facial expressions, as confirmed by the overall MANOVA1 and a significant three-way interaction, Measure × Cue × Content: F(4,55)=7.5, p<0.001, η2p=0.35. Follow-up analyses for each measure indicated a significant effect of hedonic content only for scenes, as illustrated in Figure 1. Viewing either erotic or violent pictures, compared to neutral scenes, was associated with enhanced skin conductance, Content: F(2,118)=6.9, p<0.01, η2p=0.10 (see Figure 1), increased LPP amplitude, Content: F(2,118)=23.7, p<0.001, η2p=0.29 (see Figure 2), and reduced probe P3 amplitude, Content: F(2,118)=44.4, p<0.001, η2p=0.43 (see Figure 3; all erotic v. neutral and violence v. neutral comparisons p<0.05). In contrast, neither autonomic nor electrocortical measures were reliably modulated by facial expression content (univariate Content effects for faces within each measure: Fs[2,118]<2.6, n.s.). Means are reported in Table 1.
Figure 1.
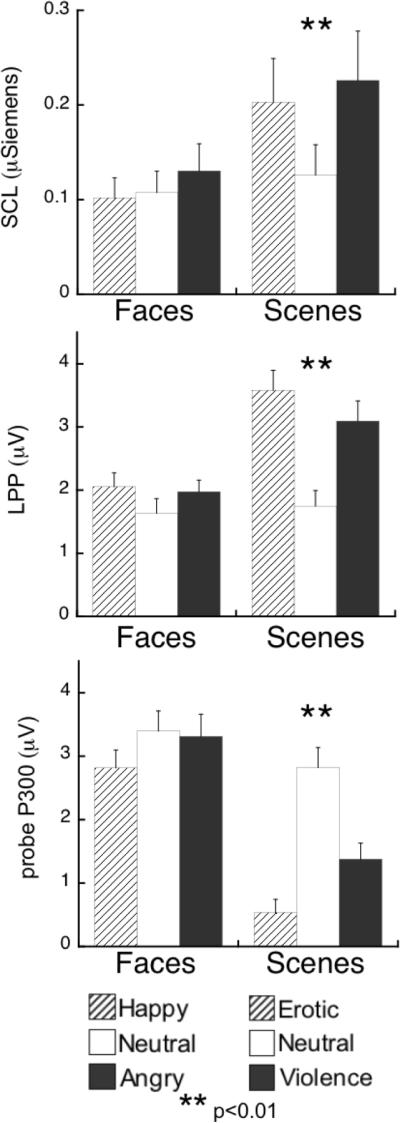
Average electrodermal and electrocortical reactivity during happy, neutral, and angry facial expressions, and erotic, neutral, and violent scenes. Top panel: maximum change in skin conductance level (SCL). Middle panel: scalp positivity elicited by picture onset, over centroparietal sensors in the late positive potential window (LPP; 400–700ms). Bottom panel: scalp positivity elicited by startle probes, over centro-parietal sensors in the probe P300 window (260–340ms). Error bars represent standard error of the mean.
Figure 2.
Grand average ERP waveforms (24 centro-parietal sensors) elicited by acoustic startle probes that were presented when participants viewed happy, neutral, and angry facial expressions, and erotic, neutral, and violent scenes. Insets depict scalp topography (top view) of the difference between emotional (pleasant and unpleasant) and neutral picture processing for each cue type, in the probe P300 window (240–360ms).
Figure 3.
Grand average ERP waveforms (32 centro-parietal sensors) elicited by picture onset when participants viewed happy, neutral, and angry facial expressions, and erotic, neutral, and violent scenes. Insets depict the scalp topography (top view) of the difference between emotional (pleasant and unpleasant) and neutral picture processing for each cue type, in the late positive potential window (400–700ms).
Table 1.
Mean physiological reactivity for faces and scenes, by social anxiety group.
| Low social anxiety (n=30) | High social anxiety (n=30) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pleasant | Neutral | Unpleasant | Pleasant | Neutral | Unpleasant | |
| Skin conductance (uSiemens) | ||||||
| Faces | 0.1(0.03) | 0.1(0.03) | 0.1(0.03) | 0.1(0.04) | 0.1(0.03) | 0.2(0.04) |
| Scenes | 0.2(0.03) | 0.1(0.03) | 0.2(0.05) | 0.2(0.09) | 0.1(0.06) | 0.3(0.1) |
| Late positive potential (uVolts) | ||||||
| Faces | 2.3(0.3) | 1.7(0.3) | 2.1(0.5) | 1.8(0.3) | 1.6(0.4) | 1.9(0.2) |
| Scenes | 3.8(0.5) | 2.0(0.3) | 3.4(0.5) | 3.3(0.4) | 1.5(0.4) | 2.8(0.4) |
| Probe P300 (uVolts) | ||||||
| Faces | 3.5(0.3) | 3.4(0.3) | 3.3(0.4) | 2.1(0.4) | 3.4(0.5) | 3.4(0.6) |
| Scenes | 0.7(0.3) | 3.2(0.4) | 1.3(0.2) | 0.4(0.3) | 2.4(0.5) | 1.5(0.5) |
| Startle magnitude (T-score) | ||||||
| Faces | 48.1(0.8) | 47.6(1.1) | 47.5(0.9) | 48.8(0.7) | 46.8(0.7) | 48.7(0.8) |
| Scenes | 46.1(0.9) | 49.3(0.9) | 50.0(1.2) | 46.8(1.0) | 48.3(0.8) | 50.4(0.8) |
Note. Mean (s.d.) activity within each psychphysiological measure when high and low socially anxious participants viewed Happy (Pleasant), Neutral, and Angry (Unpleasant) facial expressions, and Pleasant, Neutral, and Unpleasant scenes.
Probe Reaction Time
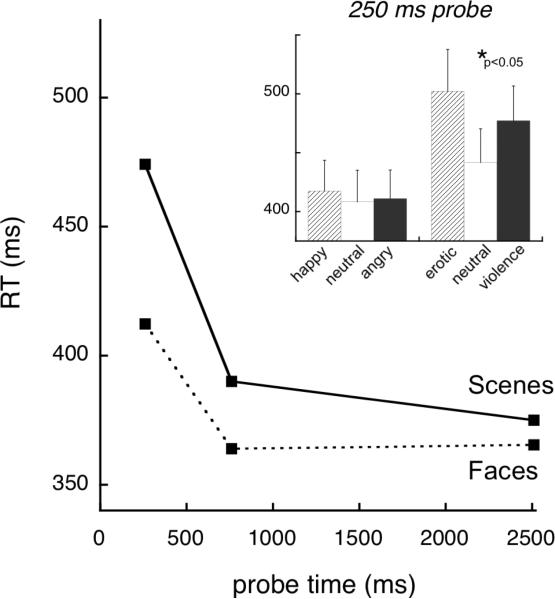
As illustrated in Figure 4, probe RT was slower when viewing scenes compared to facial expressions, Cue: F(1,58)=45.0, p<0.001, η2p=0.44, and this difference was greatest 250 ms after picture onset when attention capture was strongest, Time × Cue: F(2,116)=16.2, p<0.001, η2p=0.22. Furthermore, initial attention capture (250ms) was enhanced for emotional compared to neutral contents (Content × Time: F(4,232)=6.7, p<0.001, η2p=0.10). Attention allocation to the picture then waned as viewing persisted, Time: F(2,116)=58.4, p<0.001, η2p=0.50 (all comparisons p<0.001). Importantly, a Cue × Content interaction, F(2,116)=6.0, p<0.01, η2p=0.09, indicated that probe RT was significantly slower when participants viewed emotional naturalistic scenes compared to neutral scenes (Content within scenes: F[2,57]=7.5, p<0.01, η2p=0.21), but that RT did not vary as a function of emotional expression for face cues (Content within faces, F[2,57]<1). Means are reported in Table 2.
Figure 4.
Reaction time to acoustic startle probes that were presented 250 ms, 750 ms, or 2500 ms into the picture-viewing period, averaged separately for facial expression and naturalistic scene stimuli. Inset illustrates mean RT at the early (250ms) probe time when participants viewed happy, neutral, and angry faces, and erotic, neutral, and violent scenes. Error bars represent standard error of the mean.
Table 2.
Mean reaction time across picture viewing for faces and scenes, by social anxiety group.
| Low social anxiety (n=30) | High social anxiety (n=30) | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Pleasant | Neutral | Unpleasant | Pleasant | Neutral | Unpleasant | |
| 250 ms probe time | ||||||
| Faces | 421(35) | 412(35) | 408(31) | 414(38) | 404(40) | 414(36) |
| Scenes | 486(39) | 424(33) | 477(40) | 519(58) | 460(46) | 478(43) |
| 750 ms probe time | ||||||
| Faces | 365(29) | 371(31) | 388(31) | 352(33) | 340(33) | 367(35) |
| Scenes | 387(31) | 390(34) | 381(31) | 416(40) | 381(33) | 386(34) |
| 2500 ms probe time | ||||||
| Faces | 356(26) | 373(28) | 365(29) | 368(39) | 365(35) | 366(31) |
| Scenes | 374(30) | 386(31) | 373(27) | 377(28) | 380(34) | 362(29) |
Note. Mean (s.d.) reaction time (in milliseconds) for instructed button presses to acoustic startle probes that were presented 250ms, 750ms, and 2500ms after the onset of a picture stimulus. Data are shown separately for high and low socially anxious participants when viewing Happy (Pleasant), Neutral, and Angry (Unpleasant) facial expressions, and Pleasant, Neutral, and Unpleasant scenes.
Startle Magnitude
Although a main effect of hedonic content indicated that startle reflex magnitude was greater, on average, when viewing unpleasant pictures compared to neutral and pleasant content, F(2,116)=8.8, p<0.001, η2p=0.13 (unp v. neu, pl contrasts ps<0.004), this effect was importantly qualified by a significant interaction between Cue and Content, F(2,116)=15.8, p<0.001, η2p=0.22. As expected, startle reflexes were strongly potentiated during violent compared to neutral scenes (violent v. neutral: p<0.01) and were markedly attenuated during erotic scenes compared to neutral (erotic v. neutral: p<0.01, Content within scenes: F[2,116]=18.5, p<0.001, η2p=0.22). A different pattern of modulation emerged for face stimuli, however. Blinks were somewhat larger when participants viewed either happy or angry faces, compared to neutral faces, (quadratic contrast: F[1,59]=3.9, p<0.05) and this effect was less reliable than that for scenes, Content (faces): F[2,116]=3.1, p=0.05, η2p=0.05.
Affective Faces versus Neutral Scenes
Reactions prompted by emotional face cues were similar in magnitude to reactions elicited by neutral scenes. Skin conductance, LPP amplitude, probe P3 amplitude and startle magnitude did not significantly differ between emotional expressions and neutral scenes, all Fs<1.3, n.s. Follow-up t-tests confirmed that this contrast (emotional face v. neutral scene) was not significantly different from zero, ts(59)<1.2, n.s., for any measure, with the exception of probe RT, in which reaction time was significantly slower when participants viewed neutral scenes compared to emotional faces, F(1,59)=14.0, p<0.001, η2p=0.19, Measure × Content: F(4,56)=3.1, p<0.05, η2p=0.18.
Social Anxiety
A marginal Anxiety × Measure × Cue × Content interaction, F(4,55)=1.9, p=0.06, η2p=0.12 suggested that individual differences in social anxiety affected reactivity when viewing pictures of emotional expressions. Participants reporting low social anxiety showed no significant modulation of skin conductance, probe P3 amplitude, or LPP amplitude when viewing emotional facial expressions (all Content effects: Fs[2,57]<1.5, n.s.; see Table 1 for means). On the other hand, participants reporting high social anxiety showed enhanced skin conductance activity when viewing angry faces compared to neutral or happy expressions (quadratic contrast: F[1,29]=6.7, p<0.02, η2p=0.19; see Table 1). Both high and low socially anxious participants showed similar skin conductance reactions when viewing emotional scenes, all Anxiety effects: F(2,57)>2.5, p<0.05. Neither the LPP or probe reaction times varied significantly between anxiety groups (all Fs<2.9, n.s.), although high socially anxious participants showed a slightly smaller probe P3 amplitude when viewing happy, compared to neutral or angry faces, quadratic F(1,29)=8.8, p<0.05, η2p=0.17.
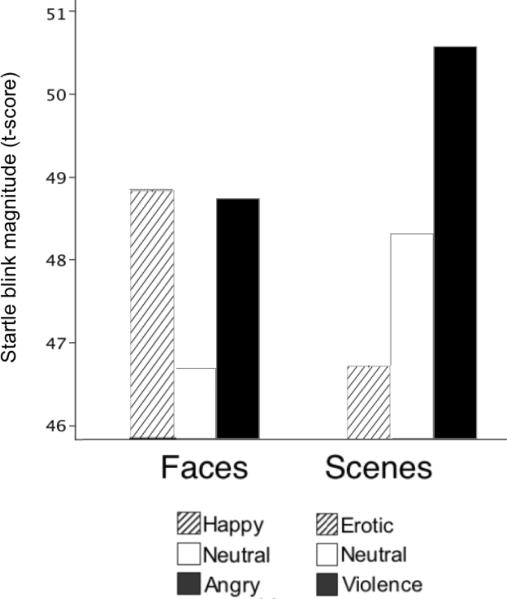
For startle modulation, although the Anxiety × Cue × Content interaction was not significant (F<1) planned analyses based on previous data (Garner et al., 2011) indicated that it was only the participants reporting high social anxiety who showed a significant difference in blink magnitude as a function of facial expression when viewing angry (p<0.01) or happy (p<0.01) expressions, compared to neutral faces, F(2,58)=5.6, p<0.01, η2p=0.17 (see Figure 5). For the low social anxiety group, startle magnitude did not significantly differ when viewing emotional and neutral faces (Content: F[2,58]<1, n.s.).
Figure 5.
Startle reflex magnitude (t-score) for participants reporting high social anxiety, when viewing happy, neutral, or angry facial expressions, and erotic, neutral, or violence scenes. T-scores were calculated in reference to the average startle magnitude during the inter-trial interval (ITI=50).
Discussion
For the psychophysiologist, emotion is induced in the laboratory when measurable changes in a variety of autonomic and somatic measures are elicited that signal affective engagement. Replicating previous studies (e.g., Alpers et al., 2011; Surcinelli & Codispoti, 2007), the current data indicate that psychophysiological reactions consistent with affective engagement are not readily apparent when participants view pictures of people with happy or angry facial expressions. Although participants in the current study were selected based on reported social anxiety (low or high), overall findings were similar to these previous studies with more normative, unselected samples. Indeed, for the sample as a whole, reactivity prompted by emotional faces did not generally differ from that elicited by neutral natural scenes. Happy and angry faces did not prompt changes in either skin conductance or the amplitude of the centroparietal late positive potential that differed from that measured when viewing either neutral faces or neutral scenes. These data are consistent with previous neuroimaging findings that the strength and extent of visual cortex activation is not different when viewing angry faces, compared to either neutral faces or neutral scenes, whereas emotionally arousing scenes are associated with heightened neural activity (Bradley et al., 2003).
Additional measures of attention capture were also not generally influenced by whether a facial expression was neutral, happy or angry. Neither the speed of reaction time nor the amplitude of the P3 component elicited by a startle probe showed modulation by facial expression. Studies reporting significant effects of emotional expression on reaction time indices of attention have often used paradigms such as visual search, in which the detection of an angry face among other faces is more rapid (e.g., Juth et al., 2005; Öhman et al., 2001), or the dot-probe task, in which RT is faster when a probe appears in the same spatial location as a fearful or angry expression (e.g., Mogg & Bradley, 2002). Both paradigms seem to be tuned to differences in spatial attention allocation, whereas secondary RT is typically interpreted as a more general index of resource allocation (e.g., Desimone & Duncan, 1995). Nonetheless, a recent meta-analysis of 172 studies utilizing dot-probe, Stroop, and other attentional paradigms concludes that non-anxious individuals do not show reliable evidence of heightened attention to threatening faces (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), which is also consistent with the lack of overall differences in this study.
Facial expressions from other stimulus sets—specifically the NimStim set—have been reported as eliciting ratings of arousal that are somewhat higher than those elicited by faces in the Karolinska set used here (Adolph & Alpers, 2010). The differences in rated arousal, though statistically significant, are quite small (e.g., a difference of 0.3 to 0.7 on a 9-point scale), making it difficult to predict that facial images from a different set would prompt reliably stronger motivational engagement. Nonetheless, future investigations exploring reactions to other sets of facial expressions are certainly warranted.
Social Anxiety and Facial Expressions
Alpers et al. (2011) reported that the magnitude of the startle blink was modestly larger when participants viewed either happy or angry, compared to neutral facial expressions. This pattern is different from the reflex modulation for emotional scenes, in which the blink is reduced when viewing pleasant arousing pictures. In the current study, however, this pattern of modulation for happy and angry faces was uniquely found for participants reporting high social anxiety: low anxious participants showed no difference in blink magnitude as a function of emotional expression.
Alpers et al. (2011) interpreted larger blink reflexes when viewing either happy or angry expressions as reflecting general arousal. Because the current study intermixed scenes and faces (avoiding differences due to startle habituation, as well as potential context effects), it can be seen (Figure 4) that blinks elicited when viewing happy and angry expressions for high socially anxious participants were similar in magnitude to those elicited when viewing neutral scenes (i.e., not potentiated), whereas blinks elicited when viewing neutral faces were attenuated. This suggests the alternative possibility that smaller blinks for neutral faces may reflect increased attention allocation. Blink inhibition by attention has been demonstrated in a number of different cognitive tasks (Filion, Dawson, & Schell, 1993; Graham, 1975), and this interpretation is consistent with Vrana & Gross (2004), who found modest cardiac deceleration (another psychophsyiolgoical index of attention and orienting; Graham & Clifton, 1966; Bradley, 2009) when people viewed pictures of neutral, compared to emotional, facial expressions. Vrana & Gross (2004) suggest that neutral faces draw enhanced attention because these expressions are emotionally and semantically ambiguous. This interpretation remains tentative, however, as other measures of attention (probe RT and P3) did not show the same effects for socially anxious individuals, remaining a relevant issue for further study.
Neuroimaging studies have reported that social anxiety patients, compared to healthy participants, show greater amygdala activation when viewing affective faces (e.g., Campbell et al., 2007; Yoon et al, 2007), which is often taken as an index of defensive mobilization. Typically, one might expect associated response outputs (e.g., blink potentiation, electrodermal reactivity, heightened LPP) to accompany amygdala activation, and yet there was little evidence of heightened reactivity in the sample as a whole. Nonetheless, some modest differences in reactivity were obtained for participants reporting high social anxiety. Participants selected on the basis of high self-reported social anxiety showed enhanced skin conductance when viewing angry facial expressions, whereas reactivity in the low-anxious group did not differ as a function of emotional expression. This finding suggests that even relatively mild expressions of threat may prompt sympathetic reactivity associated with avoidance and escape in participants reporting high social anxiety, and is supported by previous studies showing enhanced processing of angry faces in social phobia patients (e.g., Gamble & Rapee, 2010), and increased electrodermal responding when social anxiety patients imagine aversive social scenes (McTeague, Lange, Laplante, Cuthbert, Strauss, & Bradley, 2009).
Although modest, differences in reactivity obtained for participants reporting high social anxiety suggest that pictures of facial expressions might be utilized to examine emotional reactivity in socially anxious and non-anxious participants, even in a non-clinical sample. As Lissek and colleagues (Lissek, Pine, & Grillon, 2006) have suggested, individual differences in emotional reactivity might be more apparent for affective cues that are less potent for the healthy individual or more specific to the individual's personal fear, and therefore better suited for observing different patterns of affective reactivity between anxiety patients and healthy controls. Highly arousing natural scenes—which depict evocative survival-relevant information—strongly engaged both high and low anxious participants in the current study, a finding consistent with previous data that social phobia patients do not differ from controls when imagining standard scenes of survival threat (Cuthbert, Lang, Strauss, Drobes, Patrick, & Bradley, 2003; McTeague et al., 2009).
Affective engagement by natural scenes
Viewing erotic and violent scenes reliably prompted a physiological profile reflecting marked activation of the brain's motivational circuitry, replicating many previous studies (e.g., Amrhein, Mühlberger, Pauli & Wiedemann, 2004; Bradley et al., 2001; Cuthbert et al., 1998; DeCeserai & Codispoti, 2010; Lang & Bradley, 2010) Lang et al., 1993): Startle magnitude was reliably potentiated when viewing violent scenes and attenuated during erotic scenes, reflecting reliable activation of the fundamental motivational systems of defense and appetite, and modulation of both probe P3 amplitude and reaction time to the startling probe were consistent with heightened attention to emotional, compared to neutral, pictures. Skin conductance and the amplitude of the late positive potential were both heightened for emotional scenes. Together these findings affirm that arousing natural scenes induce strong emotional engagement and provide an upper anchor from which to assess and compare the extent to which other types of cues are emotionally evocative.
Conclusions
Pictures of emotional scenes and emotional facial expressions are both used extensively in the study of emotion. In the current study, a comprehensive psychophysiological assessment when participants viewed emotional faces (Lundqvist et al., 1998) or scenes (Lang et al., 2008) indicates that pictures of emotional expressions show little evidence of measurable emotional reactivity or heightened attention, consistent with the general findings of previous studies (Alpers et al., 2011; Surcinelli & Codispoti, 2007). Nevertheless, the data also suggest that pictures of facial expressions might be effective in distinguishing between low and high socially anxious individuals, for whom face cues may be more salient.
Highlights
We examine activation of motivational systems when participants view emotional facial expressions and affective scenes
We compare these responses between groups reporting either higher or lower social anxiety
Emotional reflex responses are robustly activated during processing of affective scenes, whereas reflexes are not strongly activated during processing of affective faces.
When viewing angry or happy faces, individuals reporting high social anxiety show stronger affective reactions compared to individuals reporting low social anxiety
Acknowledgments
This research was supported in part by NIMH grants P50-MH072850 and MH082702 to Peter J. Lang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1The overall multivariate ANOVA included significant main effects of Cue, F(1,58)=79.8, p<0.001, η2p=0.58, hedonic Content, F(2,57)=41.0, p<0.001, η2p=0.59, and Measure, F(2,57)=121, p<0.001, η2p=0.81 as well as a significant interactions of Measure × Cue, F(2,57)=37.4, p<0.001, η2p=0.58, Measure × Content, F(2,55)=20.4, p<0.001, η2p=0.35, and Cue × Content: F(2,57)=15.4, p<0.001, η2p=0.35.
References
- Adolph D, Alpers GW. Valence and arousal: A comparison of two sets of emotional facial expressions. The American Journal of Psychology. 2010;123(2):209–219. doi: 10.5406/amerjpsyc.123.2.0209. [DOI] [PubMed] [Google Scholar]
- Alpers GW, Adolph D, Pauli P. Emotional scenes and facial expressions elicit different psychophysiological responses. International Journal of Psychophysiology. 2011;80:173–181. doi: 10.1016/j.ijpsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Amrhein C, Mühlberger A, Pauli P, Wiedemann G. Modulation of event-related brain potentials during affective picture processing: a complement to startle reflex and skin conductance response? International Journal of Psychophysiology. 2004;54(3):231–40. doi: 10.1016/j.ijpsycho.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S. Startle modulation by affective faces. Biological Psychology. 2010;83(1):37–40. doi: 10.1016/j.biopsycho.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban M, Losito B, Simons RF, Graham FK. Off-line latency and amplitude scoring of the human reflex eye blink with Fortran IV. Psychophysiology. 1986;23(5):612. [Google Scholar]
- Balaban MT, Taussig HN. Salience of fear/threat in the affective modulation of the human startle blink. Biological Psychology. 1994;38(2–3):117–131. doi: 10.1016/0301-0511(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43(1):93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46(1):1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43(5):486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell A, Boehmelt A, editors. Startle Modification: Implications for Neuroscience, Cognitive Science and Clinical Science. Cambridge; Stanford, CA: 1999. pp. 157–183. [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Campbell DW, Sareen J, Paulus MP, Goldin PR, Stein MB, Reiss JP. Time-varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry. 2007;62(5):455–463. doi: 10.1016/j.biopsych.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Bradley MM, Lang PJ. Affective reactions to briefly presented pictures. Psychophysiology. 2001;38(3):474–478. [PubMed] [Google Scholar]
- Cook EW., III . VPM reference manual. Author; Birmingham, AL: 2002. [Google Scholar]
- Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: Activation and emotion. Psychophysiology. 1996;33:103–111. doi: 10.1111/j.1469-8986.1996.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40(30):402–22. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, McManis MH, Lang PJ. Probing affective pictures: Attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- De Cesarei A, Codispoti M. Effects of picture size reduction and blurring on emotional engagement. PLoS One. 2010;5(10):e13399. doi: 10.1371/journal.pone.0013399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Reviews of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dunning J, Auriemmo A, Castille C, Hajcak G. In the face of anger: Startle modulation to graded facial expressions. Psychophysiology. 2010;47:874–878. doi: 10.1111/j.1469-8986.2010.01007.x. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45(1):15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. The facial action coding system. Consulting Psychologist's Press; Palo Alto, CA: 1976. [Google Scholar]
- Filion DL, Dawson ME, Schell AM. Modification of the acoustic startle-reflex eyeblink: A tool for investigating early and late attentional processes. Biological Psychology. 1993;35:185–200. doi: 10.1016/0301-0511(93)90001-o. [DOI] [PubMed] [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. The Liebowitz Social Anxiety Scale: A comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31(6):1025–35. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Gamble A, Rapee R. The time-course of attention to emotional faces in social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(1):39–44. doi: 10.1016/j.jbtep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Garner M, Clarke G, Graystone H, Baldwin DS. Defensive startle response to emotional social cues in social anxiety. Psychiatry Research. 2011;186(1):150–2. doi: 10.1016/j.psychres.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115(4):760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Goeloeven E, De Raedt R, Leyman L, Verschuere B. The Karonlinska directed emotional faces: A validation study. Cognition & Emotion. 2008;22(6):1094–1118. [Google Scholar]
- Graham FK. Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12(3):238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65(5):305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Grillon C, Charney DR. In the face of fear: Anxiety sensitizes defensive responses to fearful faces. Psychophysiology. 2011 doi: 10.1111/j.1469-8986.2011.01268.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. Journal of Neuroscience Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Hansen C, Hansen R. Finding the face in the crowd: An anger superiority effect. Journal of Personality and Social Psychology. 1998;54:917–924. doi: 10.1037//0022-3514.54.6.917. [DOI] [PubMed] [Google Scholar]
- Heinrichs N, Hofmann SG. Information processing in social phobia: a critical review. Clinical Psychology Reviews. 2001;21(5):751–770. doi: 10.1016/s0272-7358(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Juth P, Lundqvist D, Karlsson A, Öhman A. Looking for foes and friends: perceptual and emotional factors when finding a face in the crowd. Emotion. 2005;5(4):379–395. doi: 10.1037/1528-3542.5.4.379. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M. Emotion and the motivational brain. Biological Psychology. 2010;84(3):437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. University of Florida; Gainesville, FL: 2008. Technical Report A-8. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–70. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska directed emotional faces—KDEF. Department of Clinical Neuroscience, Psychology section, Karolinksa Institutet; Stockholm, Sweden: 1998. [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization comorbidity and physiological reactivity. Biological Psychiatry. 2009;65(5):374–82. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Shumen JR, Wieser MJ, Lang PJ, Keil A. Social vision: sustained perceptual enhancement of affective facial cues in social anxiety. Neuroimage. 2011;54(2):1615–24. doi: 10.1016/j.neuroimage.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behavior Research and therapy. 2002;40(12):1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wieser M, Herrmann M, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2009;116(6):735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80(3):381–396. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Bar-Haim Y, McDermott JM, Gorodetsky E, Hodgkinson CA, Goldman D, et al. Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biological Psychology. 2010;83(3):269–271. doi: 10.1016/j.biopsycho.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portois G, Vuilleumier P. Dynamics of emotional effects on spatial attention in the human visual cortex. Progress in Brain Research. 2006;156:67–91. doi: 10.1016/S0079-6123(06)56004-2. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans E, van Honk J. Emotional Stroop performance for masked angry faces: It's BAS, not BIS. Emotion. 2004;4(3):305–311. doi: 10.1037/1528-3542.4.3.305. [DOI] [PubMed] [Google Scholar]
- Rytwinksi NK, Fresco DM, Heimberg RG, Coles ME, Liebowitz MR, Cissell S, et al. Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety. 2009;26(1):34–38. doi: 10.1002/da.20503. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Smith DP, Hillman CH, Duley AR. Influences of age on emotional reactivity during picture processing. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60(1):49–56. doi: 10.1093/geronb/60.1.p49. [DOI] [PubMed] [Google Scholar]
- Springer US, Rosas A, McGetrick J, Bowers D. Differences in startle reactivity during the perception of angry and fearful faces. Emotion. 2007;7(3):516–525. doi: 10.1037/1528-3542.7.3.516. [DOI] [PubMed] [Google Scholar]
- Surcinelli P, Codispoti M. Autonomic changes during the perception of emotional facial expressions. Psychophysiology. 2007;44:S1, S108. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Gross D. Reactions to facial expressions: effects of social context and speech anxiety on responses to neutral, anger, and joy expressions. Biological Psychology. 2004;66(1):63–78. doi: 10.1016/j.biopsycho.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–91. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wieser M, Pauli P, Weyers P, Alpers G, Mühlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: An eye-tracking study. Journal of Neural Transmission. 2009;116(6):717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- Yoon K, Fitzgerald D, Angstadt M, McCarron R, Phan K. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: A 4-Tesla functional MRI study. Psychiatry Research: Neuroimaging. 2007;154(1):93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]