Abstract
Barrier-protective agonists induce association of focal adhesions (FA) and adherens junctions (AJ) in endothelial cells. Here we identified specific domains of FA protein paxillin interacting with AJ protein and examined regulation of paxillin domain interactions with β-catenin by Rac GTPase. Co-expression of paxillin LD-1,2; LD-3,4; LIM-1,2; and LIM-3,4 domains with β-catenin showed exclusive interaction of LIM-1,2 and LIM-3,4 with β-catenin, which was enhanced by agonist-induced Rac activation or expression of activated Rac mutant. These results demonstrate a novel function of paxillin LIM domains in targeting β-catenin in a Rac-dependent manner, which may play a role in Rac-dependent control of FA-AJ interactions and monolayer integrity.
Keywords: Focal adhesions, adherens junctions, protein interactions, Rac GTPase
1. INTRODUCTION
Focal adhesions (FA) and adherens junctions (AJ) are critical structural elements in the maintenance of monolayer integrity [1]. In addition, specific proteins from FA, AJ and tight junction adhesive complexes may interact with each other [2–4]. Paxillin is a multi-domain adapter FA protein that functions as a molecular scaffold for protein recruitment to FA and thereby facilitates protein networking and efficient signal transmission [5,6]. The N-terminal domain of paxillin contains several copies of the so called LD motif, which function as binding sites for other FA-associated proteins. The C-terminal half of paxillin contains four LIM domains, also involved in protein-protein interactions. The LIM-3 domain targets paxillin to focal adhesions, while the LIM-2 domain plays a minor role in this process (see [7] for review). These LIM domains engage binding partners to direct and/or tether paxillin to focal adhesions, but the complete list of LIM-domain binding partners remains to be identified. Through the multiple SH2- and SH3-binding domains, LIM, and LD motifs, paxillin interacts with the signaling proteins focal adhesion kinase (FAK), p60Src-kinase, Crk, Csk, Pyk2, ILK, and the structural focal adhesion-associated proteins vinculin, actopaxin, and tubulin [5,8]. In addition to the classical paxillin binding partners, new candidate molecules have been recently reported, and the sites of paxillin interaction with these proteins remain to be defined.
We have previously reported that in pulmonary endothelium oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (OxPAPC) induced paxillin peripheral translocation and co-localization with the AJ protein β-catenin [9], which was linked to endothelial barrier enhancement. This study used a molecular dissection approach to identify paxillin domains involved in interaction with β-catenin and examined their potential role in OxPAPC-induced endothelial signaling.
2. MATERIAL AND METHODS
An expanded Materials and Methods section is available in the online data supplement.
2.1. Reagents and cell culture
Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Paxillin, β-catenin, Rac1, and GIT2 antibodies were purchased from BD Transduction Laboratories (San Diego, CA); GST, p-cofilin, and p-Vav2 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant EGF was from R&D Systems (Minneapolis, MN). HEK293T cells (ATTC, Washington, DC) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (Allendale, NJ), cultured in cell growth medium (EGM-2, Lonza) containing 10% fetal bovine serum (FBS) and used at passages 5–8.
2.2. Plasmid construction
Human paxillin from HPAEC was cloned, and paxillin truncated mutants encompassing LD-1,2; LD-3,4; LIM-1,2; and LIM-3,4 domains were generated, as described in the Online Supplementary Data.
2.3. GST pull-down assays and immunoprecipitation
Control or EGF-stimulated HEK293T cells transfected with GST-tagged full length or paxillin domians, or 6-His-tagged β-catenin were subjected to pulldown assays, as described in the Online Supplementary Data. Co-immunoprecipitation of β-catenin – paxillin assays have been described previously [9].
2.4. Rac activation assay
Activation of Rac1 GTPase was monitored by accumulation of a GTP-bound Rac1 pool in stimulated cells using a Rac in vitro pulldown assay with PAK-PBD beads, as described elsewhere [10].
2.5
Measurements of transendothelial electrical resistance across confluent endothelial cell monolayer were performed using the electrical cell-substrate impedance sensing system ECIS-1600 (Applied Biophysics, Troy, NY) as described elsewhere [10].
2.6. Statistical analysis
Results are expressed as mean ± SD of three to six independent experiments. Experimental samples were compared to controls by unpaired Student’s t-test. For multiple-group comparisons, a one-way analysis of variance (ANOVA) and Tukey’s post hoc multiple-comparison test were used. P<0.05 was considered statistically significant.
3. RESULTS
3.1. Paxillin β-catenin interaction is mediated by LIM domains of paxillin
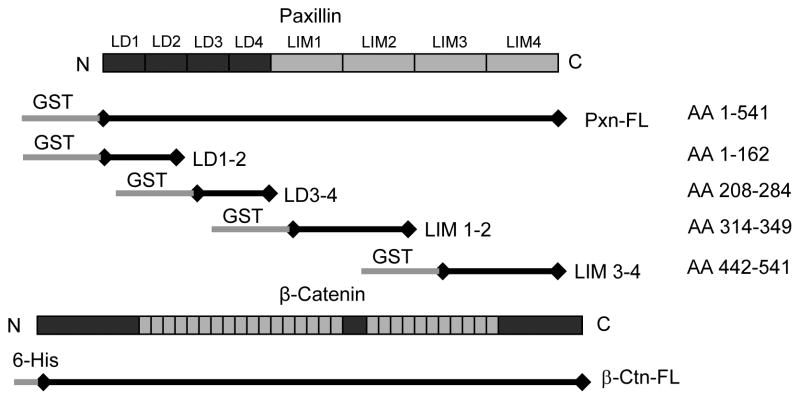
In order to identify paxillin regions interacting with β-catenin, we cloned human paxillin from human pulmonary endothelial cells and generated paxillin truncated mutants encompassing LD-1,2; LD-3,4; LIM-1,2; and LIM-3,4 domains (Supplementary Data, Figure S1). Schematic paxillin domain structure (see [7] for review) and diagrams of GST-fused paxillin constructs used in this study are presented in Figure 1.
Figure 1. Expression of paxillin and its truncated domains in HEK293T.
Schematic presentation paxillin and β-catenin constructs used in this study.
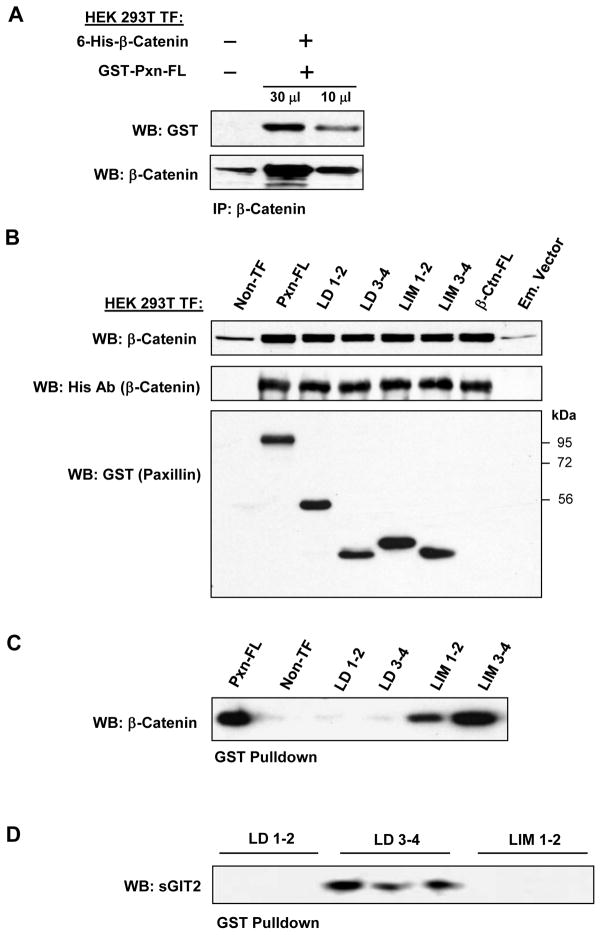
Paxillin - β-catenin interactions were detected in HEK293T cells ectopically expressing paxillin and β-catenin in co-immunoprecipitation assays under non-denaturing conditions (Figure 2A). The content of paxillin in β-catenin immunoprecipitates was determined using GST antibody. Non-transfected cells served as a negative control.
Figure 2. Determination of paxillin domains involved in interaction with β-catenin.
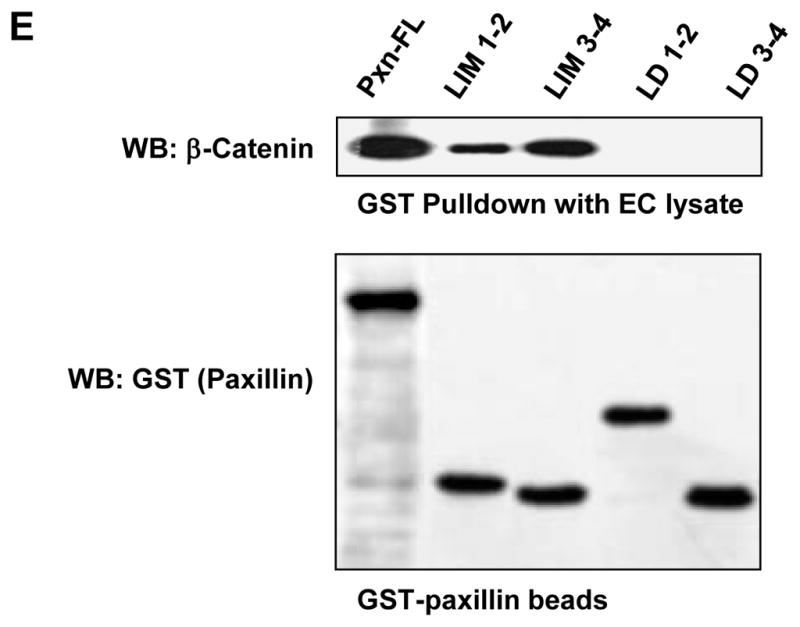
A: HEK293T cells were transected with GST-tagged paxillin or its domains and 6-His-tagged β-catenin constructs. After 48 hrs of transfection, immunoprecipitation of β-catenin was performed under non-denaturing conditions using β-catenin antibody. The content of recombinant paxillin in the immunoprecipitates was determined using GST antibodies. B – D: HEK293T cells were co-transected with paxillin and β-catenin constructs. Control transfections were performed with empty vectors. Expression of GST-tagged full length paxillin and its truncated domains was determined by western blot analysis with GST antibody. Expression of 6-His-tagged β-catenin was confirmed by western blot analysis with His antibody or β-catenin antibody (B). GST-tagged full length paxillin and truncated domains were immobilized on GSH-beads. Total cell lysates from HEK293T overexpressing 6-His-tagged β-catenin were added to the beads, and bound proteins were analyzed by western blot. Content of β-catenin (C) and short form of GIT2 (D) bound to GSH-beads was detected with corresponding antibodies. E: HPAEC lysates were loaded onto GSH-beads conjugated with full length GST-paxillin or corresponding paxillin domains expressed in bacteria. A pulldown assay was performed, and the amount of β-catenin bound to GST-tagged bacterial proteins was determined by western blot with β-catenin antibody. Equal loading of recombinant paxillin beads used for pulldown assays with HPAEC lysates was determined by western blot with GST antibody.
In the next experiments, GST-tagged full length paxillin or its domains were overexpressed in HEK293T (Figure 2B) followed by immobilization on GSH beads. Pulldown of β-catenin was performed by loading of HEK293T lysates with overexpressed 6-His-tagged β-catenin onto GSH-paxillin beads. We observed strong interactions of β-catenin with full length paxillin, LIM-1,2, and LIM-3,4 domains, while no β-catenin interaction was detected with paxillin mutants containing LD-1,2 and LD-3,4 domains (Figure 2C). To ensure that paxillin domains in these pulldown experiments maintained their protein binding capacities, we tested the well-characterized interaction between paxillin and its FA partner, short form GIT2, using a panel of paxillin deletion mutants. In agreement with previous studies showing interaction of GIT2 with the LD-4 domain of paxillin [11], the paxillin mutant containing LD-3,4 domains interacted with sGIT2, whereas no interaction with other domains was found (Figure 2D).
In agreement with the experiments in HEK293T cells, GST pulldown assays revealed a strong interaction of endogenous β-catenin from human pulmonary EC lysates with bacterially expressed recombinant full length paxillin and its LIM-1,2 and LIM-3,4 domains. Of note, no interaction between β-catenin and paxillin LD domains was detected (Figure 2E).
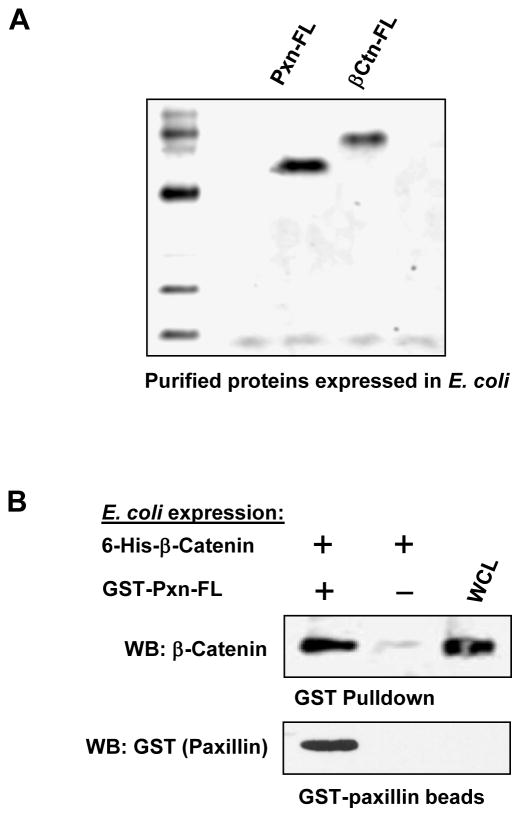
3.2. Bacterially expressed purified paxillin and β-catenin interact in pulldown assay
Because paxillin may interact with any of the large number of proteins within the focal adhesion multi-protein complex in mammalian cells, it is conceivable that the interaction with β-catenin observed in our co-immunoprecipitation and GST pull-down experiments performed in the HEK293T cell system may be indirect. To rule out this possibility, we separately expressed 6-His-tagged full-length β-catenin and GST-fused full length paxillin (Figure 3A) in a bacterial system (BL21-AI E.coli), and performed a GST pulldown assay of purified proteins. In agreement with experiments performed in transfected HEK293T cells, paxillin and β-catenin expressed in E. coli efficiently interacted with each other, thus supporting a direct mechanism of paxillin-β-catenin interaction (Figure 3B).
Figure 3. Interaction between paxillin and β-catenin expressed in E. coli.
A: Full length paxillin and β-catenin were expressed in E. coli (strain BL21-AI). Fusion proteins were purified using GSH-sepharose and Ni-column, respectively, and analyzed by SDS-PAGE followed by Coomassie staining. B: 6-His-tagged β-catenin and full length GST-tagged paxillin expressed in the bacterial system were tested in a GST-pulldown assay. GST conjugated to the beads alone served as negative control. After washing steps, beads were treated with SDS sample buffer, and bound paxillin and β-catenin were determined by western blot with corresponding antibody.
3.3. Rac regulation of β-catenin interactions with paxillin domains and control of Rac signaling by LIM domains
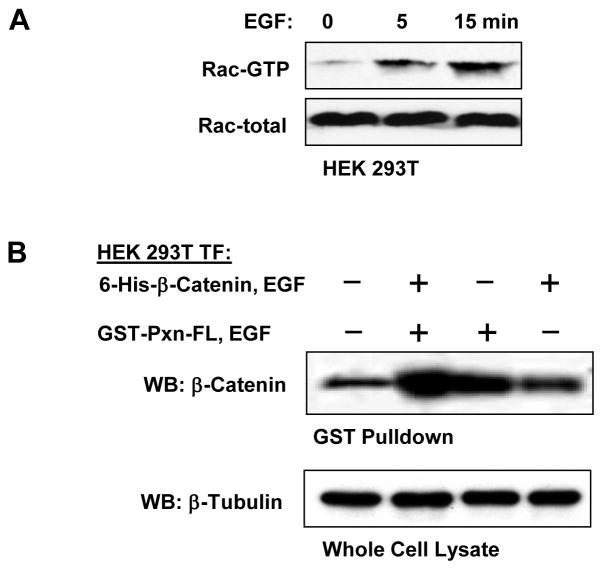
The following studies examined whether Rac GTPase activation stimulates interactions between ectopically expressed β-catenin and paxillin. Our test experiments showed that epithelial growth factor (EGF) causes pronounced Rac activation in HEK293T (Figure 4A). Cells were transfected separately with full length paxillin or B-catenin constructs and treated with EGF before lysis. Cell lysates from paxillin or β-catenin transfected cells were combined, and GST-paxillin was pulled down with GSH-beads. EGF stimulation of either full length paxillin or β-catenin expressing cells increased paxillin-β-catenin interaction, as compared to non-stimulated lysates. However, maximal binding was observed when both paxillin-expressing and β-catenin-expressing cells were stimulated with EGF simultaneously (Figure 4B).
Figure 4. Interaction between paxillin and β-catenin is Rac1 dependent.
A: HEK293T were stimulated with EGF (500 ng/ml) for the indicated periods of time. Rac activation was evaluated by Rac-GTP pulldown assay. Upper panel depicts the levels of activated GTP-bound Rac. The total Rac content in whole cell lysates is shown on the lower panel. B: HEK293T were transfected with full length GST-paxillin or 6-His-β-catenin followed by stimulation with vehicle or EGF (500 ng/ml, 15 min). Cells lysates were combined, and GST pulldown was performed as described in Materials and Methods. Content of paxillin-bound β-catenin was determined by western blot with β-catenin antibody. Equal protein loading was confirmed by detection of the housekeeping protein β-tubulin in whole cell lysates.
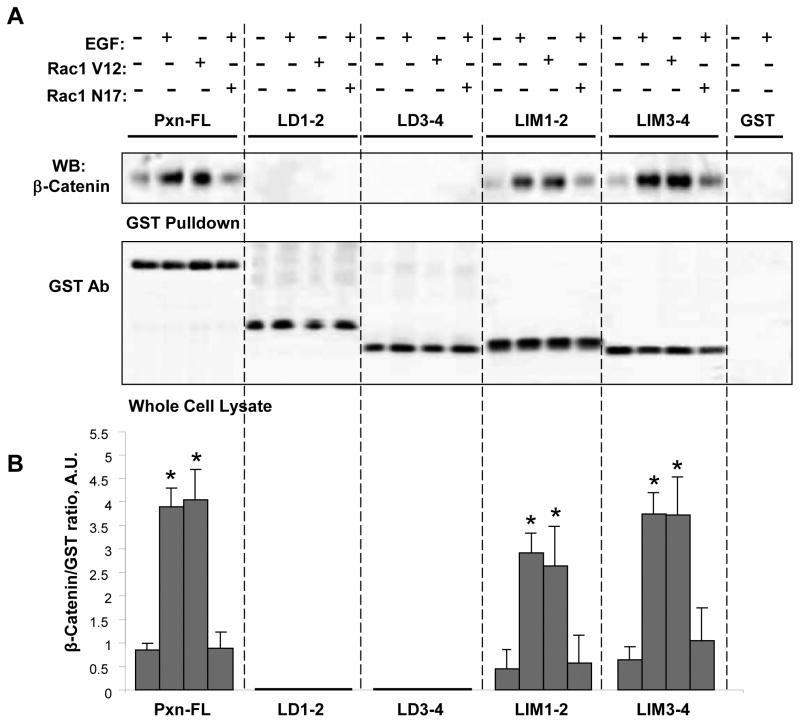
Next, we investigated the role of Rac in the regulation of interactions between paxillin domains and β-catenin. HEK293T cells were co-transfected with full length paxillin or its domains and dominant negative or constitutively active Rac mutants (Rac1-N17 and Rac1-V12, respectively). GST proteins were immobilized on GSH beads and used for GST pulldown assays with cell lysates overexpressing β-catenin. In consistence with results obtained in non-stimulated cells, neither Rac activation using EGF treatment, nor expression of activated Rac caused interactions between β-catenin and paxillin LD domains (Figure 5A). In contrast, agonist-induced Rac activation or expression of Rac1-V12 mutant dramatically increased interactions between β-catenin and full length paxillin or its LIM domains. In contrast, molecular inhibition of Rac activity by overexpression of dominant negative Rac significantly suppressed EGF-induced β-catenin interactions with full length paxillin and its LIM domains. These experiments demonstrate the Rac-dependent mechanism of increased paxillin-β-catenin interactions. Quantitative analysis of pulldown results is presented in Figure 5B.
Figure 5. Interaction between paxillin and β-catenin is Rac dependent.
A: HEK293T were co-transfected with full length GST-tagged paxillin or its domains, HA-tagged dominant negative (Rac1-N17) or constitutively active (Rac1-V12) Rac mutants, and full length His-tagged β-catenin. After 48 hrs of transfection, the cells were stimulated with vehicle or EGF (500 ng/ml, 15 min) and lysed. Pulldown assays on GSH beads were performed as described in Materials and Methods. The content of β-catenin bound to GST-paxillin or its domains was determined by western blot analysis with corresponding antibody (upper panel). Expression levels of GST-tagged paxillin mutants were detected in whole cell lysates using GST antibody (lower panel). B: Quantification of pulldown experiments. Amount of β-catenin bound to GSH beads with paxillin or its domains was determined by quantitative densitometry of western blot membranes and normalized to the amount of immobilized paxillin and its domains on the beads. Results of densitometry are expressed as β-catenin/GST ratio and shown as mean ± SD, * p<0.05 vs. non-stimulated control; n=4.
3.4. Functional significance of paxillin LIM-3,4 domains in endothelial cells
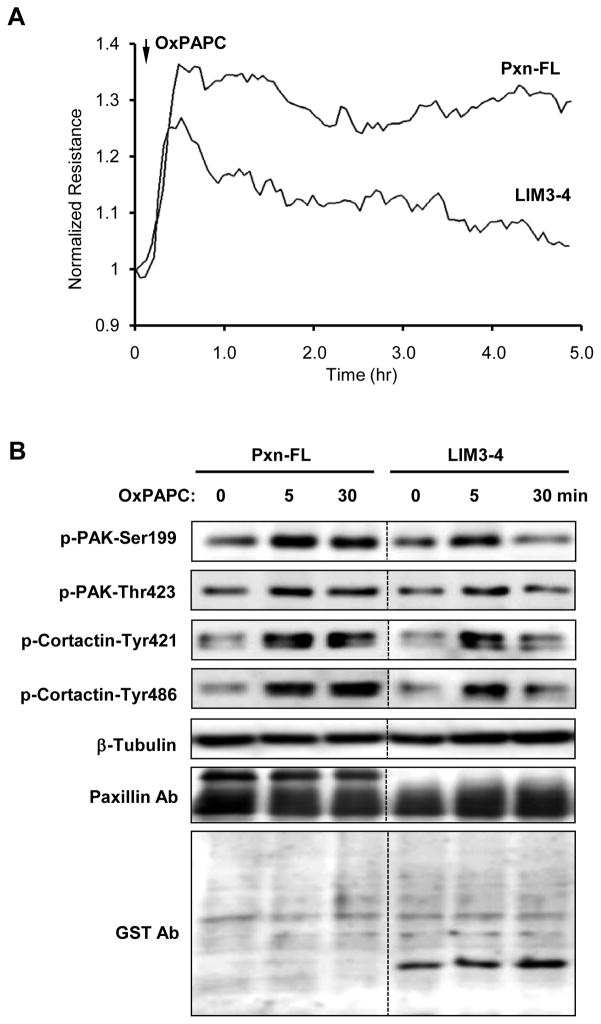
The functional significance of paxillin LIM-3,4 domains was further tested in human pulmonary artery endothelial cells (HPAEC), which were transiently transfected with full length GFP-tagged paxillin or a truncated paxillin mutant containing LIM-3,4 domains. OxPAPC-induced elevation of transendothelial electrical resistance (TER) reflecting HPAEC barrier enhancement was monitored over time. Similarly to non-transfected cells [Birukov, 2004 #911], OxPAPC stimulation of cells expressing full length paxillin caused a rapid and sustained TER increase. Ectopic expression of LIM-3,4 did not significantly affect the acute phase of OxPAPC-induced barrier response, but suppressed the prolonged phase of barrier enhancement (Figure 6A).
Figure 6. Effects of paxillin LIM-3,4 domain expression on OxPAPC-induced endothelial barrier enhancement and Rac pathway activation in pulmonary endothelial cells.
HPAEC were transfected with full length GST-tagged paxillin or its LIM-3,4 domain. After 48 hrs of transfection, the cells were stimulated with vehicle or OxPAPC (15 mg/ml). A: Changes in transendothelial electrical resistance were monitored over time. B: Site-specific phosphorylation of PAK and cortactin was detected by Western blot. Equal protein loading was confirmed by determination of β-tubulin and paxillin content in total cell lysates. Expression level of GFP-tagged full length paxillin was detected in whole cell lysates using paxillin monoclonal antibody antibody (clone 349), GST- LIM-3,4 was detected using GST antibody.
We next evaluated the effects of LIM-3,4 expression on OxPAPC-induced signaling. Similarly to non-transfected cells [13], OxPAPC treatment of HPAEC transfected with full length paxillin induced sustained phosphorylation/activation of the Rac downstream target, PAK1 and also caused phosphorylation of the regulator of actin remodeling, cortactin (Figure 6A). Ectopic expression of the paxillin LIM-3,4 domain did not affect OxPAPC-induced PAK1 and cortactin phosphorylation at early time points (5 min), but caused significant reduction at the later (30 min) time points. Collectively, these results suggest negative effects of paxillin LIM-3,4 domain expression on OxPAPC-induced signaling and EC barrier enhancement.
4. DISCUSSION
This study demonstrates for the first time a key role of LIM domains in paxillin interaction with β-catenin. Pulldown assays with bacterially expressed proteins further confirmed the direct nature of these interactions. We found that both paxillin fragments, each containing two LIM domains (LIM-1,2 and LIM-3,4, respectively), interacted with β-catenin. Because LIM domains exhibit a high degree of homology, we speculate that β-catenin may potentially interact with any LIM domain depending on its availability in particular conditions. However, other potential binding regions, such as short linker regions connecting LIM domains cannot be completely excluded. It was noted that paxillin binding to other protein partners (actopaxin, FAK) may also involve more than one domain [14,15].
Several complementary approaches used in this study, including EGF-induced activation of endogenous Rac, ectopic expression of constitutively active Rac mutant, and Rac inhibition by expression of dominant negative Rac mutant strongly suggest direct involvement of Rac in the regulation of paxillin - β-catenin interactions via paxillin LIM domains. We also do not exclude Rac-induced activation of tyrosine phosphorylation as an additional step to enhancement of β-catenin interactions with paxillin. Published studies noted that paxillin phosphorylation at serine and threonine residues may influence paxillin conformation and thereby allosterically affect its ability to interact with specific binding partners [7].
Our previous report described a positive feedback mechanism of OxPAPC-induced Rac activation via the PAK1 – paxillin – βPIX protein complex [13]. This mechanism contributes to the maintenance of local Rac activity at the cell periphery which promotes enhancement of cortical cytoskeleton and cell-cell junctions leading to strengthening of the endothelial barrier. The expression of paxillin LIM-3,4 domains in this study attenuated OxPAPC-induced Rac signaling and abrogated the sustained phase of EC barrier enhancement.
We speculate that in non-transfected cells, OxPAPC-induced Rac activation promotes interaction of β-catenin with paxillin via LIM domains and therefore targets paxillin to adherence junctions. In turn, over-expression of LIM domains uncouples Rac-induced association of β-catenin with endogenous full-length paxillin, as well as abrogates positive feedback regulation of Rac activity, which involves paxillin phosphorylation at Serine-273 [13,16]. Altogether, these events may lead to the formation of less stable cell-cell interactions and compromised EC barrier enhancement. We also do not exclude other LD-dependent signaling mechanisms which can affect OxPAPC-mediated barrier enhancement. Finally, overexpression of LIM domains may also displace endogenous paxillin from the focal adhesions and further compromise interactions between adherens junctions and focal adhesions upon OxPAPC stimulation. These potentially important mechanisms await further investigation.
5. CONCLUSIONS
This study identified LIM domains as domains mediating paxillin interaction with β-catenin. A direct mechanism of β-catenin – paxillin interactions has been confirmed in experiments with recombinant proteins expressed in E. coli. However, Rac-dependent enhancement of β-catenin - paxillin complex formation has been observed, which suggests an allosteric mechanism of regulation, and which possibly involves other paxillin domains. These results suggest that perturbations in paxillin LIM domain interactions with other protein partners may affect cell signaling and cytoskeletal dynamics, the key features of cell motility and endothelial permeability. Future characterization of functional activities of paxillin LIM domains may reveal novel mechanisms regulating Rac signaling and endothelial permeability in physiological and pathological settings.
Supplementary Material
Highlights.
Paxillin directly interacts with adherens junction protein β-catenin
This interaction occurs via LIM-1,2 and LIM-3,4 paxillin domains
Paxillin - β-catenin interaction is controlled by Rac1 GTPase
Acknowledgments
Supported by National Heart, Lung, and Blood Institutes grants HL87823, HL76259, and HL58064 for KGB; HL89257 and HL107920 for AAB
The authors acknowledge Yufeng Tian and Nicolene Sarich for superior technical assistance in western blot experiments and permeability studies. The authors wish to thank Katherine Higginbotham for proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 2.Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226:2052–62. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takai Y, Ikeda W, Ogita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol. 2008;24:309–42. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- 4.Geny B, Grassart A, Manich M, Chicanne G, Payrastre B, Sauvonnet N, Popoff MR. Rac1 inactivation by lethal toxin from Clostridium sordellii modifies focal adhesions upstream of actin depolymerization. Cell Microbiol. 12:217–32. doi: 10.1111/j.1462-5822.2009.01392.x. [DOI] [PubMed] [Google Scholar]
- 5.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2:E231–6. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 6.Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593–9. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–39. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 8.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–61. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin - {beta}-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007;293:L199–211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- 10.Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–20. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamieson JS, Tumbarello DA, Halle M, Brown MC, Tremblay ML, Turner CE. Paxillin is essential for PTP-PEST-dependent regulation of cell spreading and motility: a role for paxillin kinase linker. J Cell Sci. 2005;118:5835–47. doi: 10.1242/jcs.02693. [DOI] [PubMed] [Google Scholar]
- 12.Birukov KG, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 13.Birukova AA, Alekseeva E, Cokic I, Turner CE, Birukov KG. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2008;295:L593–602. doi: 10.1152/ajplung.90257.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151:1435–48. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–40. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 16.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–99. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.