Abstract
We investigated the in-vivo cartilage contact biomechanics of the tibiofemoral joint in patients after reconstruction of a ruptured anterior cruciate ligament (ACL). A dual fluoroscopic and MR imaging technique was used to investigate the cartilage contact biomechanics of the tibiofemoral joint during in-vivo weight-bearing flexion of the knee in eight patients six months following clinically successful reconstruction of an acute isolated ACL rupture. The location of tibiofemoral cartilage contact, size of the contact area, cartilage thickness at the contact area, and magnitude of the cartilage contact deformation of the ACL-reconstructed knees were compared with those previously measured in intact (contralateral) knees and ACL-deficient knees of the same subjects. Contact biomechanics of the tibiofemoral cartilage after ACL reconstruction were similar to those measured in intact knees. However, at lower flexion, the abnormal posterior and lateral shift of cartilage contact location to smaller regions of thinner tibial cartilage that has been described in ACL-deficient knees persisted in ACL-reconstructed knees, resulting in an increase of the magnitude of cartilage contact deformation at those flexion angles. Reconstruction of the ACL restored some of the in vivo cartilage contact biomechanics of the tibiofemoral joint to normal. Clinically, recovering anterior knee stability might be insufficient to prevent postoperative cartilage degeneration due to lack of restoration of in vivo cartilage contact biomechanics.
Keywords: anterior cruciate ligament (ACL), cartilage deformation, fluoroscopy, ACL reconstruction, postoperative osteoarthritis (OA)
INTRODUCTION
Rupture of the ACL is a common acute injury that is associated with an increased risk for developing osteoarthritis (OA) in the affected knee 1,2. Each year ~200,000 patients opt for ACL reconstruction in the United States3, drawn by the excellent postoperative stability, health-related quality of life, and the ability to return to sports4,5. However, no long-term difference in OA prevalence has been detected between patients that opted for conservative treatment and those that opted for surgery6,7.
Since current ACL reconstruction techniques can restore the main mechanical function of the ACL (i.e., control of AP translation of the tibiofemoral joint) on clinical evaluation, other pathogenic processes such as the release of inflammatory cytokines in the synovial fluid8 or occult cartilage abnormalities9,10 that are already present following ACL injury have been implicated in post-reconstruction cartilage degeneration. However, the contribution of mechanics should not be ruled out in the long-term postoperative OA pathogenesis. Studies that examined knee motion under functional loading conditions with advanced imaging modalities all showed that abnormal translations and rotations of the joint persist following ACL reconstruction in spite of clinically satisfactory anterior stability11–13. These studies have greatly enhanced our insight into the efficacy of contemporary reconstruction techniques to reproduce normal in vivo knee kinematics. More importantly, they triggered the quest for surgical techniques that more closely restore normal kinematics11–13, since even minimal alterations are believed to affect cartilage contact,14 which in turn has been hypothesized to contribute to the initiation of OA15. However, no methodology is available with the necessary accuracy to directly quantify the extent of the biomechanical cartilage alteration in response to such persistent minimal alterations in knee kinematics after ACL reconstruction.
In a recent study with a dual fluoroscopic and MRI technique, we indirectly found that –during a quasi-static lunge activity – ACL deficiency shifted the articular contact location to smaller regions of thinner cartilage, and increased the cartilage contact deformation, providing insight in the possible underlying biomechanical dimension of cartilage degeneration14. Our current objective was to re-analyze the cartilage contact biomechanics in our study sample of 8 patients with an acute isolated ACL rupture14, now 6 mos following clinically successful ACL reconstruction. We hypothesized that reconstruction is unable to correct the abnormal contact location and size, the cartilage thickness at the contact area, and magnitude of cartilage contact deformation caused by rupture of the ACL during in vivo weight-bearing flexion from 0° to 90°. The healthy contralateral knees were considered as a normal group.
METHODS
Eight patients (5 men, 3 women, from 19 to 38 yrs old) that were recruited for our previous study were included in the present study14. Injury to other ligaments, noticeable cartilage lesions or meniscal damage on MRI at 4.5 ± 3 mos after injury and during arthroscopic examination, and injury to the underlying bone were reasons for exclusion. The patients were active on a minimal to moderate athletic level before injury based on the IKDC demographic form, and had a primary complaint of persistent instability that limited their athletic participation or work and prompted the decision to undergo ACL reconstruction. Each patient signed a consent form that had been approved by our Institutional Review Board. All the patients were scheduled for ACL reconstruction surgery within 1 wk after their preoperative study.
ACL reconstruction technique
The patients underwent arthroscopic ACL reconstruction at 4.5 ± 3 mos after injury. All surgeries were done by one surgeon. A diagnostic arthroscopy was performed before graft placement. Reconstruction was performed with a central 10-mm bone-patellar tendon-bone (BPTB) autograft. A 10-mm tibial tunnel was drilled using a 55° guide (Linvatec-Conmed, Largo, FL) centered 7 mm anterior to the PCL on the downslope of the medial tibial spine. A 10-mm femoral tunnel was drilled using a 6-mm femoral offset guide (Arthrex, Naples, FL) centered at the 10:30 position for right knees (1:30 for left). The graft was passed in retrograde fashion, and the femoral and tibial bone blocks were secured with titanium interference screws (Guardsman, Linvatec-Conmed). The femoral screw length was 25 mm and was placed with the knee in maximal flexion. The tibial screw length was 30 mm. The graft was fully tensioned with the knee in full extension. Screw diameter was determined based on graft-tunnel fit. Examination confirmed that there was no notch impingement, and cycling of the knee revealed < 2 mm of graft motion. The anterior laxity of the reconstructed knee as measured with the KT-1000 arthrometer was similar to that of the intact contralateral knee.
Imaging procedure14,16
6 mos post-operatively, the ACL-reconstructed knee of each patient was simultaneously imaged using 2 orthogonally placed fluoroscopes (OEC 9800; GE Healthcare, Salt Lake City, UT) as the patient performed a single-leg quasi-static lunge at 0, 15, 30, 60, and 90° of flexion – similar to the lunge activity performed by the patient prior to the reconstruction14. At each angle, the patient was asked to pause for 5 secs while simultaneous fluoroscopic images were taken. Throughout the experiment, the leg being tested supported the patient’s body weight, while the other leg provided stability. Next, the images were imported into solid modeling software (Rhinoceros; Robert McNeel and Assoc, Seattle, WA) and placed in the orthogonal planes based on the position of the fluoroscopes during imaging of the patient.
The 3D surface mesh models of the tibia, fibula, femur, and articulating cartilage of the ACL-deficient knees constructed based on 3T MR images pre-operatively were used for the present study: an MR scanner (Siemens, Malvern, PA) equipped with a surface coil and 3D double-echo water excitation sequence (field of view 16 × 16 × 12 cm) acquired sagittal plane images that were then imported into solid modeling software to construct the bone and cartilage surfaces of the knee14. The 3D MRI-based model was imported into the same software that contained the postoperative fluoroscopic images, viewed from the 2 orthogonal directions corresponding to the orthogonal fluoroscopic setup, and independently manipulated in 6DOF inside the software until the projections of the model matched the outlines of the fluoroscopic images, so that the model reproduced the in vivo position of the ACL-reconstructed knee. This analysis technique has an error of <0.1 mm and a repeatability of <0.3° in measuring the position and orientation of matched bones, respectively17.
Cartilage thickness was calculated by finding the smallest Euclidian distance connecting a vertex of the articular surface to the cartilage-bone interface of the 3D surface models. The size of the contact area was determined by computing the area of tibial cartilage that intersected the femoral cartilage14. The accuracy and repeatability of cartilage thickness measurement using MRI-based models of the knee joint has been validated to be 0.04 ± 0.01 mm 14. Cartilage contact deformation was then defined for each vertex of the articular surface mesh as the amount of cartilage surface intersection (Fig. 1) divided by the sum of the tibial and femoral cartilage surface thicknesses 14. Previous validation studies showed an accuracy of 4% and 14% when this technique was used to measure the cartilage contact deformation18 and cartilage contact area16, respectively. In this study, cartilage contact deformation and its location were defined as the magnitude and location of peak cartilage deformation, referenced to Cartesian coordinate systems on the tibial plateaus14. The origin of each coordinate system was located at the center of a circle that was fit to the posterior edge of each tibial compartment. The AP and mediolateral axes split each plateau into quadrants. In the AP direction, a location anterior to the mediolateral axis was considered positive. In the mediolateral direction, a location lateral to the AP axis was considered positive.
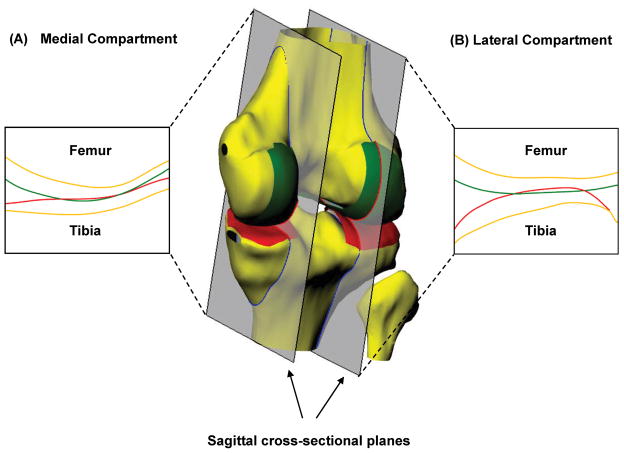
Figure 1.
A 3D MRI-based knee model to illustrate the intersection of tibiofemoral cartilage surfaces in the medial (A) and lateral (B) compartments in response to weight bearing. Reproduced, with permission, from Hosseini A, et al. Osteoarthritis Cartilage. 2010; 18: 909–16.
Statistical Analysis
A two-way repeated-measures ANOVA and Newman-Keuls post hoc test were used to determine if differences between group-means of cartilage contact biomechanics existed measured in the 8 subjects at the 5 flexion angles. For each knee compartment (medial and lateral), the dependent variables were location, contact area, thickness, and cartilage contact deformation. The independent variables were the state of the knee (healthy contralateral before ACL reconstruction, ACL-deficient14, and ACL-reconstructed) and the flexion angle. P values < 0.05 were considered significant. Analyses were performed with Statistica software (Statisticac©, StatSoft Inc., Tulsa, OK).
RESULTS
Location of cartilage contact
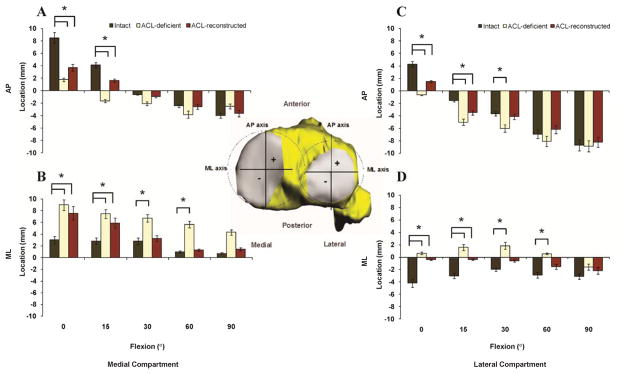
ACL reconstruction did not restore the combined lateral and posterior shift of the location of peak cartilage contact deformation on the tibial plateaus that was measured in ACL-deficient knees to normal levels at lower flexion angles. At 0° in the medial (lateral) compartment, contact in ACL-reconstructed knees remained 4.8±2.7mm (2.8±2.7 mm) posterior and 4.5±3.5 mm (3.8±2.7 mm) lateral (P < 0.0001) from the location of contact in intact knees (Fig. 2). Between 30 and 90°, no significant differences were found between the cartilage contact location in either mediolateral or AP direction between the intact and ACL-reconstructed knees (P > 0.05).
Figure 2.
Location of cartilage contact on the medial tibial plateau in the AP (A) and mediolateral (ML) (B) directions, and on the lateral tibial plateau in the AP (C) and ML (D) directions in intact, ACL-deficient knees14, and ACL-reconstructed knees.
Size of contact area
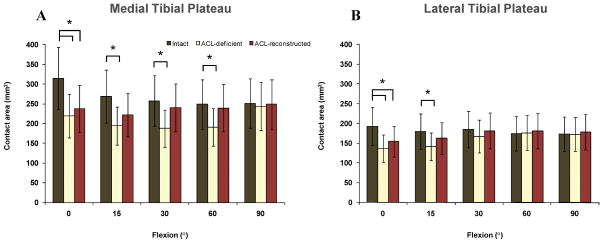
The cartilage contact area after ACL reconstruction remained significantly smaller than the normal cartilage contact area at 0° [314±114 mm2 in intact knees, 220±69 mm2 in ACL-deficient knees, and 237±98 mm2 inACL-reconstructed knees in the medial compartment, P = 0.008; 193±75 mm2 inintact knees, 137±64 mm2 in ACL-deficient knees, and 155±77 mm2 in ACL-reconstructed knees in the lateral compartment, P = 0.005]. The cartilage contact areas between 15 and 90° were not significantly different (although smaller) compared to those of normal group at these flexion angles (P > 0.05) (Fig. 3).
Figure 3.
Cartilage contact area on the medial (A) and lateral (B) tibial plateaus in intact, ACL-deficient knees14, and ACL-reconstructed knees.
Cartilage thickness at the contact area
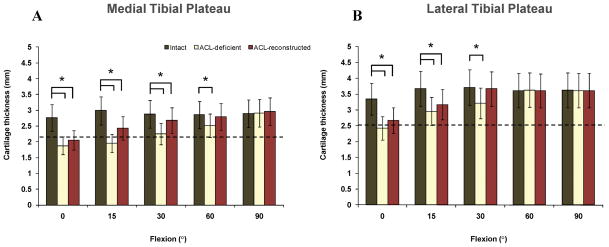
Maximum cartilage contact deformation remained at areas where cartilage thickness was an average of 0.5±0.2 mm thinner than the normal condition between 0 (P = 0.004) and 30° (P = 0.04) on the medial compartment and an average of 0.6±0.1 mm thinner than the normal condition at 0 (P = 0.041) and 15° (P = 0.036) on the lateral compartment (Fig. 4).
Figure 4.
Cartilage thickness in regions of contact on the medial (A) and lateral (B) tibial plateaus in intact, ACL-deficient knees14, and ACL-reconstructed knees. Broken horizontal line indicates the total average cartilage thickness.
Magnitude of cartilage contact deformation
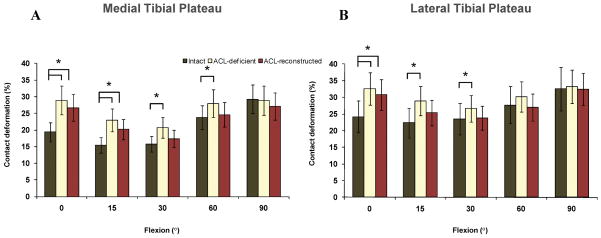
In the medial compartment, significant differences in the magnitude of cartilage contact deformation persisted between healthy contralateral knees and ACL-reconstructed knees at 0 (P < 0.0001) and 15° (P = 0.038) (Fig. 5). The maximum increase in cartilage deformation occurred at 0°, where a deformation of 19±4% was found in intact knees, 29±9% in ACL-deficient knees, and 27±3% in ACL-reconstructed knees (P < 0.0001) (a 42% increase from the deformation in the intact knee). Similarly, at 0° cartilage contact deformation in the lateral compartment of ACL-reconstructed knees was increased 29% compared to the value measured in intact knees (24±9% intact knee, 33±6% ACL-deficient knee, 31±3% ACL-reconstructed knee, P = 0.006) (Fig. 5). In Figure 6, the cartilage contact areas of intact, ACL-deficient and ACL-reconstructed knees of a typical patient are shown at full extension (Fig. 6A) and 90° of flexion (Fig. 6B).
Figure 5.
Peak cartilage contact deformation on the medial (A) and lateral (B) tibial plateaus in intact, ACL-deficient knees14, and ACL-reconstructed knees.
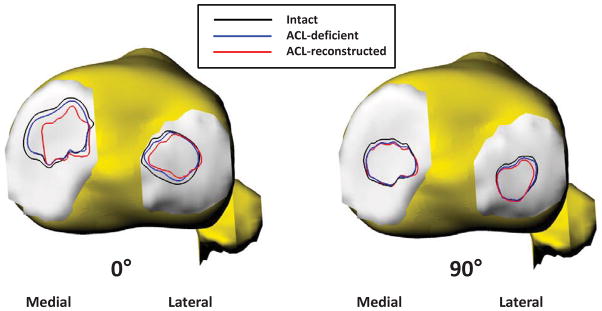
Figure 6.
Comparison of the cartilage contact areas of intact, ACL-deficient, and ACL-reconstructed knees of a typical patient at full extension (A) and 90° of knee flexion (B).
DISCUSSION
We used a dual fluoroscopic and MR imaging technique to investigate the cartilage contact biomechanics indirectly in 8 patients after ACL reconstruction. We found that the in vivo tibiofemoral cartilage contact biomechanics after clinically successful ACL reconstruction were not restored to those measured in intact contralateral knees at lower flexion angles in a lunge activity (when the ACL is primarily functioning). In general, around 0 and 15° of flexion, the abnormal posterior and lateral shift of cartilage contact location to smaller regions of thinner cartilage seen in ACL-deficient knees persisted in the ACL-reconstructed knees, resulting in a persistent increase of cartilage contact deformation at those flexion angles.
Many clinical studies have demonstrated that AP stability can be restored after ACL reconstruction19,20. However, when the forces in the graft were measured in cadaver knees, the graft forces were dramatically larger compared to those of the intact ACL21,22. Such abnormal force vectors could explain the altered knee kinematics that have been observed both in vitro23,24 and in vivo11,12,25–27 after ACL reconstruction. Although the absolute changes in tibiofemoral kinematics vary widely from study to study, a general trend of persistent increased anterior tibial translation combined with rotational changes in knee kinematics were observed in ACL-reconstructed knees during weight-bearing28–30. These changes in tibiofemoral kinematics after ACL reconstruction are hypothesized to lead to changes in the cartilage contact characteristics ultimately wearing down the articular cartilage31. In the present study, we found that cartilage contact was located posteriorly and laterally on the tibial plateaus at 0 and 15° of flexion, despite a clinically successful normalization of anterior knee stability. Analogous to our observations in ACL deficiency14, a shift in cartilage contact resulted in a considerable change in cartilage loading distribution within the knee joint despite surgical reconstruction of the ACL.
In a recent study, two different ACL reconstruction methods (anteroproximal versus anatomic graft placement) were compared to quantify the effect of femoral graft placement on the ability of ACL reconstruction in restoring normal knee kinematics32. During a quasi-static lunge activity, the knees with anatomic graft placement more closely matched the contralateral knee kinematics (AP and mediolateral tibial translations and internal tibial rotation). In the current study, only a transtibial reconstruction technique was used. Therefore, no solid conclusion could be made about the effect of surgical techniques and femoral graft placement on restoration of tibiofemoral cartilage contact biomechanics.
We acknowledge several limitations to this study. Similar to the study of cartilage contact biomechanics of ACL-deficient knees14, no ground reaction forces were measured to document whether global knee joint loading for the ACL-reconstructed knees were replicated with the intact and the ACL-deficient knees. We acquired data from only one functional activity, a single leg lunge, using a goniometer to measure the flexion angle. However, knee joint kinematics and consequently cartilage contact deformation are activity-dependent33,34 so the present findings cannot be extrapolated to other knee movements. For example, with biplane radiography, Deneweth et al. found that single-legged hopping – similar to the demanding jumping and cutting movements of high-level competitive sports – elicited significant differences in all examined degrees of freedom except adduction of the tibia relative to the femur35, whereas downhill running only caused differences in tibial adduction and external rotation30. If feasible, the incorporation of cartilage morphology into such high-speed motion analysis might further quantify the impact of persistent kinematic changes following ACL reconstruction during movements that are relevant to the this patient population. We did not include meniscus–cartilage contact. However, the fluoroscopic images reflected the in vivo structure, including menisci, so they influenced the kinematics and the relative position of the cartilage surfaces.
We cannot discern whether the enduring deformation seen in the ACL-reconstructed knees will eventually cause healthy cartilage to breakdown or whether the increased deformation was the result of cartilage damage that was already present at the time of injury and surgery, remaining more compliant throughout the postoperative follow-up. In addition, longitudinal degenerative changes in response to altered joint loading have been well-demonstrated in animal models where a transection of the ACL reliably triggers cartilage degeneration36,37. However, an analogous study that tracks the longitudinal effect of instability-induced cartilage deformation on the integrity of undamaged cartilage tissue at baseline has not been performed in patients.
The same 3D surface mesh models previously constructed of the ACL-deficient knees were used for the corresponding ACL-reconstructed knees at the 6-mo follow-up visit to reduce variability in model construction. Although in general significant changes are not expected in cartilage within such a time frame, there might be changes from the acute stage right after injury to this sub-acute stage38. Another predicament has been the absence of valid markers that correlate with and precede structural and biomechanical cartilage changes. Patients with discernible cartilage and meniscal lesions on 3T MRI at 4.5 ± 3 mos of injury were excluded from the study. However, conventional MRI sequences such as the 3D double-echo water excitation that was used in this study are not particularly sensitive to cartilage and meniscal lesions: cartilage damage is often only visible multiple years after the initiation of OA on conventional MR images9,10,39. Such delay in the identification of underlying bone marrow or cartilage abnormalities in the knees of some ACL deficient patients9 has historically impeded recruitment to only patients with truly isolated ruptures of the ACL. Therefore, rather than relying solely on clinical MR imaging, the final evaluation of cartilage and meniscal damage in our study occurred during arthroscopic examination at the time of ACL reconstruction. We measured the kinematics of healthy contralateral knees pre-operatively. However, kinematics might have changed after ACL reconstruction. It would be interesting to study and compare the kinematics of contralateral knees pre-and post-operatively.
Progress toward a more comprehensive insight in the development of OA in ACL-reconstructed knees has been made by OA biomarker imaging assessment, such as T1ρ, delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC), and sodium MR9,40,41. In a recent study, Li et al. evaluated cartilage matrix changes with T1ρ and T2 relaxation time quantification in 12 patients with acute ACL injuries and 1 yr following reconstruction39. The elevated T1ρ values found at baseline in the posterolateral region of the tibial cartilage of ACL-deficient knees, which were related to underlying bone marrow lesions at the time of injury, decreased closer to normal values at 1 yr, whereas the initially normal T1ρ values in the medial tibiofemoral compartment significantly increased in the weightbearing subcompartments. Interestingly, in the present study, a relatively greater persistent increase in cartilage deformation was measured in the medial tibiofemoral compartment as compared with the lateral compartment both at the time of injury and at 6-mo follow-up. Those changes in T1ρ values would indicate changes in the cartilage matrix, which would agree with the changes in cartilage deformation seen in this study.
We believe our results provide insight into the changes in in vivo tibiofemoral cartilage contact deformation following ACL reconstruction and identify important directions for future research. Clinically successful ACL reconstruction restored some of the in vivo cartilage contact biomechanics of the tibiofemoral joint to normal, but at lower flexion angles an increased magnitude of cartilage contact deformation persisted.
Acknowledgments
The authors gratefully acknowledge the support of NIH (R01 AR055612, F32 AR056451) and thank Bijoy Thomas, Louis DeFrate, and Jeffrey Bingham for their technical assistance.
References
- 1.Roos H, Adalberth T, et al. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 2.Oiestad BE, Engebretsen L, et al. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37:1434–1443. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 3.Brophy RH, Wright RW, et al. Cost analysis of converting from single-bundle to double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37:683–687. doi: 10.1177/0363546508328121. [DOI] [PubMed] [Google Scholar]
- 4.Lewis PB, et al. Systematic review of single-bundle ACL reconstruction outcomes: a baseline assessment for consideration of double-bundle techniques. AJSM. 2008;36:2028–2036. doi: 10.1177/0363546508322892. [DOI] [PubMed] [Google Scholar]
- 5.Barenius B, Nordlander M, et al. Quality of life and clinical outcome after ACL reconstruction using patellar tendon graft or quadrupled semitendinosus graft: an 8-year follow-up of a randomized controlled trial. Am J Sports Med. 2010;38:1533–1541. doi: 10.1177/0363546510369549. [DOI] [PubMed] [Google Scholar]
- 6.Daniel DM, Stone ML, et al. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 7.Lohmander LS, Englund PM, et al. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 8.Cameron M, Buchgraber A, et al. The natural history of the aACL-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 9.Bolbos RI, Ma CB, et al. In vivo T1rho quantitative assessment of knee cartilage after ACL injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43:782–788. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Molina G, Guermazi A, et al. Central bone marrow lesions in symptomatic knee OA and their relationship to ACL tears and cartilage loss. Arthritis Rheum. 2008;58:130–136. doi: 10.1002/art.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashman S, Collon D, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 12.Papannagari R, Gill TJ, et al. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34:2006–2012. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 13.Ristanis S, Stergiou N, et al. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:1323–1329. doi: 10.1016/j.arthro.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 14.Van de Velde SK, Bingham JT, et al. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60:3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriacchi TP, Mundermann A, et al. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 16.Bingham JT, Papannagari R, et al. In vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology (Oxford) 2008;47:1622–1627. doi: 10.1093/rheumatology/ken345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defrate LE, Papannagari R, et al. The 6 degrees of freedom kinematics of the knee after ACL deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34:1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 18.Wan L, de Asla RJ, et al. In vivo cartilage contact deformation of human ankle joints under full body weight. Journal of orthopaedic research. 2008;26:1081–1089. doi: 10.1002/jor.20593. [DOI] [PubMed] [Google Scholar]
- 19.Fox JA, Nedeff DD, et al. Anterior cruciate ligament reconstruction with patellar autograft tendon. Clin Orthop Relat Res. 2002:53–63. doi: 10.1097/00003086-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Howe JG, Johnson RJ, et al. Anterior cruciate ligament reconstruction using quadriceps patellar tendon graft. Part I. Long-term followup. Am J Sports Med. 1991;19:447–457. doi: 10.1177/036354659101900505. [DOI] [PubMed] [Google Scholar]
- 21.Hoher J, Kanamori A, et al. The position of the tibia during graft fixation affects knee kinematics and graft forces for ACL reconstruction. Am J Sports Med. 2001;29:771–776. doi: 10.1177/03635465010290061601. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Papannagari R, et al. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 2006;77:267–274. doi: 10.1080/17453670610046019. [DOI] [PubMed] [Google Scholar]
- 23.Shoemaker SC, Adams D, et al. Quadriceps/anterior cruciate graft interaction. An in vitro study of joint kinematics and anterior cruciate ligament graft tension. Clin Orthop. 1993;294:379–390. [PubMed] [Google Scholar]
- 24.Yoo J, Papannagari R, et al. The Effect of ACL Reconstruction On Knee Joint Kinematics Under Simulated Muscle Loads. Am J Sports Med. 2005;33:240–246. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 25.Georgoulis AD, Papadonikolakis A, et al. Three-dimensional tibiofemoral kinematics of the ACL-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 26.Nordt WE, 3rd, Lotfi P, et al. The in vivo assessment of tibial motion in the transverse plane in anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27:611–616. doi: 10.1177/03635465990270051101. [DOI] [PubMed] [Google Scholar]
- 27.Brandsson S, Karlsson J, et al. Kinematics and laxity of the knee joint after ACL reconstruction: pre- and postoperative radiostereometric studies. Am J Sports Med. 2002;30:361–367. doi: 10.1177/03635465020300031001. [DOI] [PubMed] [Google Scholar]
- 28.Georgoulis AD, Ristanis S, et al. Tibial rotation is not restored after ACL reconstruction with a hamstring graft. Clin Orthop Relat Res. 2007;454:89–94. doi: 10.1097/BLO.0b013e31802b4a0a. [DOI] [PubMed] [Google Scholar]
- 29.Ferretti A, Monaco E, et al. Double-bundle anterior cruciate ligament reconstruction: a comprehensive kinematic study using navigation. Am J Sports Med. 2009;37:1548–1553. doi: 10.1177/0363546509339021. [DOI] [PubMed] [Google Scholar]
- 30.Tashman S, Kolowich P, et al. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 31.Stergiou N, Ristanis S, et al. Tibial rotation in ACL-deficient and ACL-reconstructed knees: a theoretical proposition for the development of osteoarthritis. Sports Med. 2007;37:601–613. doi: 10.2165/00007256-200737070-00004. [DOI] [PubMed] [Google Scholar]
- 32.Abebe ES, Utturkar GM, et al. The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. Journal of biomechanics. 2011;44:924–929. doi: 10.1016/j.jbiomech.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozanek M, Hosseini A, et al. Tibiofemoral kinematics and condylar motion during the stance phase of gait. J Biomech. 2009;42:1877–1884. doi: 10.1016/j.jbiomech.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu F, Kozanek M, et al. In vivo tibiofemoral cartilage deformation during the stance phase of gait. J Biomech. 2009;43:658–665. doi: 10.1016/j.jbiomech.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deneweth JM, Bey MJ, et al. Tibiofemoral joint kinematics of the ACL-reconstructed knee during a single-legged hop landing. Am J Sports Med. 2010;38:1820–1828. doi: 10.1177/0363546510365531. [DOI] [PubMed] [Google Scholar]
- 36.Brandt KD. Transection of the anterior cruciate ligament in the dog: a model of osteoarthritis. Semin Arthritis Rheum. 1991;21:22–32. doi: 10.1016/0049-0172(91)90037-z. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Burton-Wurster N, et al. Spontaneous and experimental osteoarthritis in dog: similarities and differences in proteoglycan levels. Journal of orthopaedic research. 2003;21:730–737. doi: 10.1016/S0736-0266(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 38.Frobell RB, Le Graverand MP, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. OAC. 2009;17:161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Kuo D, et al. Cartilage in Anterior Cruciate Ligament-Reconstructed Knees: MR Imaging T1{rho} and T2--Initial Experience with 1-year Follow-up. Radiology. 2010;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samosky JT, et al. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res. 2005;23:93–101. doi: 10.1016/j.orthres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Taylor C, et al. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Img. 2009;27:779–784. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]