Abstract
Objective
The purpose of this study was to evaluate the ability of multiple wavelength pulse CO-oximetry (SpCO) to screen for environmental tobacco smoke (ETS) exposure in children.
Background
Exposure to ETS is associated with an increased risk of perioperative respiratory complications in children. It is often difficult to obtain an accurate history for ETS exposure, so a preoperative screening tool is desirable. Carbon monoxide is a measurable product of tobacco combustion. Multiple wavelength pulse CO-oximetry is a recently developed point-of-care monitor.
Methods
Following IRB approval and parental consent, 220 children aged 1–16 years having outpatient surgical procedures were enrolled. SpCO was measured preoperatively 3 times with the Radical-7 Rainbow SET CO-oximeter (Masimo, Irvine, CA). Immediately following induction of anesthesia, a blood sample for laboratory measurement of carboxyhemoglobin (COHb) and serum cotinine was obtained. Regression analysis determined the correlation of SpCO with serum cotinine values. Receiver operator characteristic (ROC) curves analyzed the discriminating ability of SpCO or COHb to predict ETS exposure based on cotinine cutoff values known to be present in children exposed to ETS. Agreement of SpCO and COHb values was assessed using Bland-Altman plots.
Results
SpCO did not correlate with cotinine (R2=0.005). Both SpCO and COHb had poor discriminating ability for ETS exposure (area under the ROC curve = 0.606 and 0.562, respectively). SpCO values had poor agreement with COHb values.
Conclusions
The point-of-care multiple wavelength pulse CO-oximeter does not appear to be a useful preoperative screening tool for ETS exposure in children.
Keywords: Child, tobacco smoke, carbon monoxide, carboxyhemoglobin, oximetry
Introduction
Exposure to environmental tobacco smoke (ETS) is associated with an increased risk of perianesthetic respiratory complications in children, including laryngospasm, severe coughing, breath holding, oxygen desaturation, and increased oxygen requirement (1,2,3). One study demonstrated that 42% of children exposed to ETS experienced adverse respiratory events in the perianesthetic period (2), and another observed a ten times greater incidence of laryngospasm during emergence from anesthesia in children exposed to ETS (1).
Preoperative identification of children exposed to ETS could enhance patient safety and clinical decision making. Choice of anesthetic drugs and airway management could be affected if a greater risk of respiratory complications were identified preoperatively. Informed consent could be made more precise, and outcomes potentially improved with such foreknowledge.
Since parental history of exposure to ETS can be inaccurate and unreliable (4), a quantitative point-of-care preoperative screening tool for ETS in children is desirable. Cotinine levels in blood, urine, or saliva correlate well with ETS exposure and have been used in several pediatric studies to quantitate ETS exposure (2,3,5,6), but measurement of this metabolite of nicotine cannot be performed quickly. Carbon monoxide (CO), a product of combustion of tobacco smoke, correlates with number of cigarettes smoked in smokers (7) and can be measured by CO-oximetry as blood carboxyhemoglobin (COHb). This test is rapid but requires a blood sample, so is not easily applied as a preoperative screening test in children. Exhaled (end-tidal) CO measurement is too difficult for young children to perform (8).
Pulse oximetry technology (near infrared spectroscopy) can be applied to the measurement of COHb, and a new monitor has been developed for this purpose. This device has been shown to be accurate when compared to blood COHb levels in adults (9) and to be clinically useful as a screening tool for CO toxicity (10). As a screening tool, this device offers benefits of being noninvasive, simple, and immediate. Carboxyhemoglobin, as measured by this device, is recorded as SpCO. The primary objective of this study was to evaluate SpCO measured by multiple wavelength pulse CO-oximetry as a screening tool for exposure to ETS in preanesthetic children. The secondary objective was to evaluate the accuracy and repeatability of SpCO measurements made by the device in this population.
Methods
Following approval from the hospital’s Institutional Review Board, parental consent and child assent when appropriate, 220 children, ages 1 through 16 years, were enrolled in the study. Children who were to be admitted into same day surgery and scheduled for general anesthesia were eligible for enrollment. Exclusion criteria were diagnoses of hemolytic disorders, chronic liver disease or chronic lung disease.
A Radical-7 Rainbow SET Pulse CO-oximeter (software version 7.5.0.3, Masimo, Irvine, CA, USA) was used to determine SpCO. The CO-oximeters used in this study were calibrated on a monthly basis per manufacturer’s guidelines and a Masimo Clinical Specialist trained the research assistants who collected SpCO values. Based on subject weight, a reusable finger sensor (model DCI-dc3 for subjects ≥ 40 kg, model DCIP-dc3 for subjects ≤ 39.9 kg) and a black plastic light shield was placed upon the subject’s second or third digit on either hand while in the preoperative holding area. SpCO and oxygen saturation (SpO2) were recorded after a 15-second stable period had passed. Subjects were instructed to keep their hands still during the data collection phase to reduce the possibility of interference. Blood pressure cuffs were not placed on the subjects’ arms during data collection. This process was carried out three times by removing and replacing the sensor each time, and the mean of these three SpCO values was calculated and labeled as “preoperative SpCO”. Additionally, the subject’s parent or guardian was given a one-page questionnaire designed to determine exposure to ETS and other sources of CO in the household (8) (Appendix).
Inhalational induction of anesthesia with sevoflurane, nitrous oxide and oxygen (total flow >5 L/min) took place in the operating room 5–30 minutes later. When consciousness was lost, a peripheral intravenous catheter was placed and a 2mL blood sample was obtained for laboratory measurement of COHb and serum cotinine. The time interval between onset of anesthetic induction and blood sampling was <5 minutes. Neither insertion of an artificial airway nor controlled ventilation was performed prior to blood sampling. Whole blood COHb levels were assessed with a CO-oximeter measurement module (Rapid Lab C1265, Siemens Healthcare Diagnostic, Deerfield, IL, USA). Serum cotinine values were quantitated with an Agilent 1100 Series HPLC system interfaced with a Sciex API5000 tandem mass spectrometer (Agilent 1100 series, Agilent Technologies, Palo Alto, CA, USA) and controlled by Analyst Software (version 1.4.1). A fourth SpCO measurement (“simultaneous SpCO”) was obtained by the method described above at the time of the blood draw in 111 subjects.
Statistical Analysis
Sample size calculation: We assumed 0.75 sensitivity and 0.8 specificity to be criteria for minimal acceptable validity of a device to distinguish ETS exposure, while 0.9 sensitivity and 0.9 specificity approach the ideal. Therefore, (sensitivity =0.75 and specificity =0.8) is the null hypothesis, and (sensitivity =0.9 and specificity =0.9) is a value of the alternative hypothesis (sensitivity >0.75 and specificity >0.8). Desiring a power of 0.8 to detect a statistically significant difference when using alpha =0.05, 203 subjects are required.
Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). To test the primary objective, regression analysis determined the correlation of SpCO with serum cotinine values, and receiver operator characteristic (ROC) curves were used to analyze the ability of SpCO or COHb to predict ETS exposure based on serum cotinine cutoff values known to be present in children exposed to ETS (0.9 ng/mL for children aged <12 yr, 0.6 ng/mL for children aged ≥12 yr) (6). Values from ETS exposed and non-exposed subjects were compared using one way analysis of variance.
To test the accuracy of the device, Bland-Altman plots were used to compare simultaneous SpCO values to the standard, COHb, with calculation of bias (mean of the difference of values), precision (1 SD of the difference), and limits of agreement (bias ± 1.96 SD). The association between SpCO and COHb was further investigated by linear regression analysis. Repeatability of the device was determined by calculation of repeatability coefficients (1.96 SD of the difference) from Bland-Altman plots and intra-class correlation coefficient (ICC), which was estimated with a mixed effects model using the three successive preoperative SpCO and SpO2 values. 95% confidence intervals (CI) for ICC and repeatability coefficient were estimated using 2000 bootstrap samples. Bland-Altman plots were also used to assess bias, precision, and limits of agreement of the device compared to COHb in children weighing <25 kg and >25 kg. Mean preoperative SpCO values were compared to simultaneous SpCO values by paired t-test.
Results
Screening for ETS
Sixteen subjects were withdrawn from the study due to incomplete data or patient choice, resulting in 204 evaluable subjects ranging in age from 1.9 to 16.9 (median 6.9) years. Using the cotinine criteria above, ETS status was found to be positive for 23 subjects and negative in 181 subjects. Subject characteristics and results are displayed in Table 1.
Table 1.
Results in ETS exposed and non-exposed subjects
| N | Age (yr) | Weight (kg) | Preoperative SpCO (%) |
COHb (%) | Cotinine (ng/mL) |
|
|---|---|---|---|---|---|---|
| All subjects | 204 | 6.9 (1.9–16.9) | 24 (11–92) | 4.9 ± 3.6 | 0.76 ± 0.28 | 2.73 ± 0.65 |
| ETS (+) | 23 | 8.4 (3.0–16.2) | 29 (12–81) | 3.3 ± 1.8* | 0.84 ± 0.30 | 4.83 ± 7.17* |
| ETS (−) | 181 | 6.8 (1.9–16.9) | 23 (11–92) | 5.1 ± 3.7 | 0.75 ± 0.26 | 0.14 ± 0.13 |
Data are presented as median (range) for age and weight and as mean ± SD for other values.
Significantly different from the ETS (−) group (P<0.05).
Poor correlation was observed between preoperative SpCO values and cotinine values (Figure 1). Neither SpCO nor COHb was a significant factor for predicting positive ETS in logistic regression analysis. ROC analysis demonstrated that SpCO has poor discriminating ability to predict ETS exposure (Figure 2) with C-statistics of 0.61 (95% CI 0.49–0.72). Similarly, ROC analysis indicated that laboratory measurements of COHb have poor discriminating ability to predict ETS exposure (Figure 3) with C-statistics of 0.56 (95% CI 0.43–0.70). Sensitivity and specificity of the questionnaire for ETS using the same cotinine criteria were 43% (95% CI 23.2 – 65.5%) and 73% (95% CI 65.9% –79.4) respectively.
Figure 1.

Figure 2.

Figure 3.

Repeatability and Accuracy of device
The pooled repeatability coefficient for the three preoperative SpCO values was 5.02% (95% CI 4.10–5.16) SpCO. The pooled repeatability coefficient of the three successive SpO2 values measured by the same device was 2.56% (95% CI 2.11–2.59%) SpO2. The ICC for the three preoperative SpCO values was 0.78 (95% CI 0.77 – 0.85).
Results of Bland-Altman analyses of SpCO values vs COHb values are presented in Table 2. Simultaneously measured SpCO values vs COHb demonstrated a proportionally sloped bias of 4.55%, precision of 3.53%, and limits of agreement of −2.37 to 11.47% (Figure 4). Linear regression analysis demonstrated poor correlation of COHb and simultaneous SpCO (Figure 5).
Table 2.
Bland-Altman analyses for SpCO vs COHb
| Comparison vs COHb |
N | Bias (mean of diff) |
Precision (1 SD) |
Limits of Agreement (±1.96 SD) |
|---|---|---|---|---|
| Simultaneous SpCO | 111 | 4.55 | 3.53 | −2.37 to 11.47 |
| Subjects <25 kg | 61 | 5.76 | 3.12 | −0.36 to 11.88 |
| Subjects >25 kg | 50 | 3.06 | 3.47 | −3.74 to 9.86 |
| Preoperative SpCO | 203 | 4.24 | 3.63 | −2.87 to 11.35 |
Figure 4.

Figure 5.
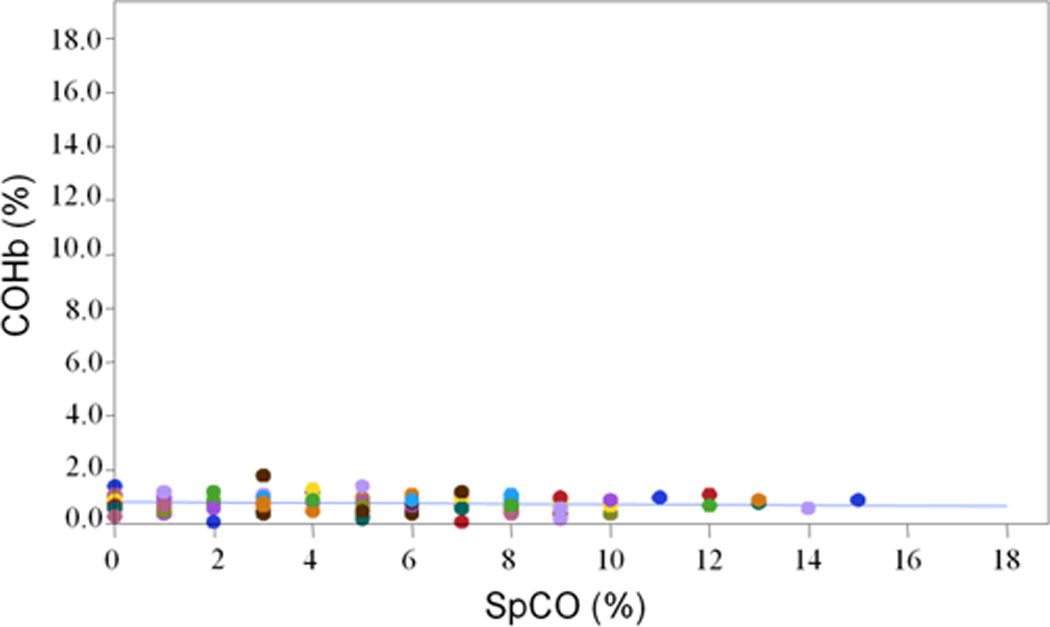
The preoperative SpCO value was 4.94 ± 3.58 (mean ± SD), which did not differ significantly from the simultaneous SpCO value of 5.25 ± 3.50 (P=0.251). Bland-Altman results were slightly better in subjects weighing >25 kg than in smaller subjects (Table 2).
Discussion
The Masimo Radical-7, Rainbow SET Pulse CO-oximetry device did not prove to be a useful screening tool for ETS exposure in preanesthetic children. SpCO did not correlate with cotinine values and had poor discriminating ability to identify children exposed to ETS. Laboratory blood COHb values also were unable to discriminate ETS exposure in our study, an observation consistent with a prior study that demonstrated no significant increase in COHb levels in healthy adult subjects following ETS exposure (11).
Since this is the first study to associate SpCO with serum cotinine values, there is no additional evidence to support or contradict our findings. A previous study examined the association of SpCO with ETS and other environmental sources of CO exposure in children (12). That study demonstrated wide confidence intervals and insignificant differences of SpCO values between exposed and non-exposed subjects, but such exposure was determined by parental questionnaire, rather than a quantitative measure. The poor sensitivity of the parental questionnaire to predict ETS exposure in our subjects illustrates the unreliability of self-reporting of cigarette smoking and is consistent with the experience of previous investigations (8,13).
The pulse CO-oximeter demonstrated precision consistent with its product specification of about 3% (14). Accuracy was unsatisfactory in our population of children with low, non-toxic levels of CO. There was a positive and sloped bias of SpCO compared to COHb. SpCO values measured by the device neither agreed nor correlated with blood COHb values determined in the laboratory, and sometimes we observed high SpCO values in children with low COHb levels. Although the ICC was acceptable, the repeatability coefficient and limits of agreement were too broad to be useful in this population and setting.
The pulse CO-oximeter was designed to identify patients with CO toxicity and seems to have better clinical applicability in that setting. A study of adult volunteers exposed to CO demonstrated acceptable bias (−1.2%) and precision (2.2%) when SpCO measurements were compared to laboratory COHb values (9). Similarly, SpCO measurements in adults with CO toxicity demonstrated bias of −2.5% and precision of 2.4% (15). Correlation between SpCO and COHb was strong in both studies. The device may perform better in the presence of high COHb values. In another study of adult subjects, SpCO bias was −1.1% in subjects with CO toxicity but −4.2% when low COHb values from non-toxic subjects were added (10).
On the other hand, the accuracy of the pulse CO-oximetry device in pediatric patients has been questioned. A study in an emergency department setting of children and adults with COHb values ranging from 0–38% demonstrated unacceptably wide limits of agreement of SpCO with COHb (−11.6% to 14.4%) (16). A recent study in children with sickle cell disease with fairly low COHb values (0.4–4.4%) observed better SpCO precision (2.1%) and limits of agreement (−4.1 to 4.2%) than we observed, but with a similarly proportionally sloped bias (17).
It is possible that the reusable sensor design is not suited to work well with the small fingers of children. We tried to avoid inaccurate SpCO measurements by using appropriately sized sensors according to weight guidelines set by the manufacturer, making sure that the child’s finger was positioned correctly in the sensor, and using black plastic shields to block the admittance of ambient light. Bias and limits of agreement, however, were somewhat better in subjects weighing >25 kg than in those <25 kg (Table 2), suggesting that the sensor does not function as well when used on smaller fingers. Nevertheless, these sensors demonstrated a satisfactory repeatability coefficient when measuring SpO2 in our subjects, suggesting that the SpCO software, rather than the sensor hardware design, may contribute more to the inaccuracy of SpCO measurement in our subjects.
It is possible that the anesthetic induction could have affected our subjects’ CO levels, since CO can be detected in the inspiratory limb of anesthesia breathing circuits when carbon dioxide absorbents degrade volatile anesthetics. However, this phenomenon is associated with low fresh gas flows and duration of exposure, and changes in COHb in children have been observed to be minor during a one hour exposure to volatile anesthetics (18). This was highly unlikely to affect our results due to the high fresh gas flows and short time interval between onset of anesthetic induction and the measurement of simultaneous SpCO and COHb in our study. Furthermore, since SpCO and COHb were measured simultaneously, both would have been affected equally.
We conclude that the Masimo Radical-7 Rainbow SET Pulse CO-oximeter does not provide acceptable accuracy for discriminating low levels of CO that are in the physiologic to ETS exposure range. A rapid, noninvasive, quantitative preoperative screening tool for ETS exposure in children, therefore, remains elusive.
Acknowledgement
This study was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The pulse CO-oximeters used in this study were purchased from Masimo by Children’s Hospital Colorado. The reusable finger sensors and SpCO software were loaned to the investigators by Masimo.
Appendix
QUESTIONNAIRE Subject #_______
Carbon Monoxide Screening in Preanesthetic Children
Thank you for agreeing to participate in this study. Your answers to this
questionnaire will not be identified by your or your child’s name.
1. Do you live in a: house____ apartment____ trailer____ cabin____
2. Do you live in a big city (for example, the Denver or Colorado Springs
metropolitan areas)? Yes____ No____
3. Do you usually: drive a car____ ride the bus or train____
4. In your home, do you have (check all that apply):
wood burning stove____ wood burning fireplace____ gas fireplace _____
gas stove or oven_____ space heater______ gas furnace________
Is your gas furnace over 30 years old? Yes____ No____ Don’t know________
5. Do any persons in the house smoke cigarettes? Yes____ No____
How many persons in the house smoke cigarettes? 1____ 2____ more____
How many cigarettes are smoked by everyone in the house per day?
Less than 1 pack____ 1–2 packs____ 2–4 packs____ more than 4 packs ____
6. Do any persons in the house smoke pipes or cigars? Yes____ No____
7. Does your child have asthma? Yes____ No____
8. Does you child have reactive airways disease? Yes____ No____
9. Has your child had bronchitis in the past month? Yes____ No____
10. Has your child had pneumonia in the past month? Yes____ No____
11. Has your child had a cold in the past month? Yes____ No____
12. Has your child had a cold in the past week? Yes____ No____
Footnotes
Conflict of interest: No Conflict of interest declared
References
- 1.Lakshmipathy N, Bokesch PM, Cowan DE, et al. Environmental tobacco smoke: a risk factor for pediatric laryngospasm. Anesth Analg. 1996;82:724–727. doi: 10.1097/00000539-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Skolnick ET, Vomvolakis MA, Buck KA, et al. Exposure to environmental tobacco smoke and the risk of adverse respiratory events in children receiving general anesthesia. Anesthesiology. 1998;88:1144–1153. doi: 10.1097/00000542-199805000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Drongowski RA, Lee D, Reynolds PI, et al. Increased respiratory symptoms following surgery in children exposed to environmental tobacco smoke. Pediatr Anesth. 2003;13:304–310. doi: 10.1046/j.1460-9592.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 4.Koop CE. Adverse anesthesia events in children exposed to environmental tobacco smoke. Anesthesiology. 1998;88:1141–1142. doi: 10.1097/00000542-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg RA, Haley NJ, Etzel RA, Loda FA. Measuring the exposure of infants to tobacco smoke: nicotine and cotinine in urine and saliva. N Engl J Med. 1984;310:1075–1078. doi: 10.1056/NEJM198404263101703. [DOI] [PubMed] [Google Scholar]
- 6.Pirkle JL, Flegal KM, Bernert JT, et al. Exposure of the US population to environmental tobacco smoke: the third national health and nutrition examination survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 7.Sarkar M, Liu J, Koval T, et al. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro Snus. Nicotine Tob Res. 2010;12:105–116. doi: 10.1093/ntr/ntp183. [DOI] [PubMed] [Google Scholar]
- 8.Dukellis D, Zuk J, Pan Z, et al. Exhaled carbon monoxide screening for environmental tobacco smoke exposure in preanesthetic children. Pediatr Anesth. 2009;19:848–853. doi: 10.1111/j.1460-9592.2009.03083.x. [DOI] [PubMed] [Google Scholar]
- 9.Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry. Anesthesiology. 2006;105:892–897. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Suner S, Partridge R, Sucov A, et al. Non-invasive pulse co-oximetry screening in the emergency department identifies occult carbon monoxide toxicity. J Emerg Med. 2008;34:441–450. doi: 10.1016/j.jemermed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Maniscalco M, Di Mauro V, Farinaro E, et al. Transient decrease of exhaled nitric oxide after acute exposure to passive smoke in healthy subjects. Arch Environ Health. 2002;57:437–440. doi: 10.1080/00039890209601434. [DOI] [PubMed] [Google Scholar]
- 12.Yee BE, Ahmed MI, Brugge D, et al. Second-hand smoking and carboxyhemoglobin levels in children: a prospective observational study. Pediatr Anesth. 2010;20:82–89. doi: 10.1111/j.1460-9592.2009.03192.x. [DOI] [PubMed] [Google Scholar]
- 13.Apseloff G, Ashton HM, Friedman H, Gerber N. The importance of measuring cotinine levels to identify smokers in clinical trials. Clin Pharmacol Ther. 1994;56:460–462. doi: 10.1038/clpt.1994.161. [DOI] [PubMed] [Google Scholar]
- 14. [accessed 7 February 2012]; www.masimo.com/spco/accuracy.htm.
- 15.Piatkowski A, Ulrich D, Grieb G, Pallua N. A new tool for the early diagnosis of carbon monoxide intoxication. Inhal Toxicol. 2009;21:1144–1147. doi: 10.3109/08958370902839754. [DOI] [PubMed] [Google Scholar]
- 16.Touger M, Birnbaum A, Wang J, et al. Performance of the RAD-57 pulse co-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56:382–388. doi: 10.1016/j.annemergmed.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Caboot JB, Jawad AF, McDonough JM, et al. Non-invasive measurements of carboxyhemoglobin and methemoglobin in children with sickle cell disease. Pediatr Pulmonol. 2012 doi: 10.1002/ppul.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy RJ, Nasr VG, Rivera O, et al. Detection of carbon monoxide during routine anesthetics in infants and children. Anesth Analg. 2010;110:747–753. doi: 10.1213/ANE.0b013e3181cc4b9f. [DOI] [PubMed] [Google Scholar]


