Abstract
During gait initiation (GI), consistency of foot placement while stepping is important in making successful transitions from a state of stable static posture to an unstable state of dynamic locomotion. In populations characterized by gait dysfunction and postural instability, such as persons with Parkinson’s disease (PD), the ability to generate a consistent stepping pattern during GI may be essential in the prevention of falls. However, little is known about GI variability in persons with PD as compared to their healthy elderly peers. Therefore, this study investigated spatiotemporal variability during the first two steps of GI in 46 persons with idiopathic PD and 49 healthy age-matched adults. Stepping characteristics, including the length, width, and time of the first two steps of GI as well as their coefficients of variation (CV) were compared between groups. Persons with PD initiated gait with significantly shorter steps (swing step length = .463 vs .537m, stance step length = .970 vs. 1.10m) and higher variability in step length (swing step CV = 8.82 vs. 5.45, stance step CV = 6.76 vs. 3.61). Persons with PD also showed significantly higher variability in the time of the swing step (swing step CV = 10.0 vs. 7.4). GI variability did not differ significantly between disease stages in persons with PD. Because greater variability in these measures during gait is related to an increased risk of falls, we propose that higher GI variability may play a considerable role in falls frequently observed during transitions from quiet standing in PD.
1. Introduction
Postural instability and gait dysfunction are unfortunate and common features of Parkinson’s disease (PD). Approximately 50% of individuals with PD fall during a three month follow up period [1] and people with PD have nine times greater risk of recurrent falls compared to healthy older adults [2]. Falls in PD are multifactorial with underlying disease-specific and balance- and mobility-related deficiencies contributing [3]. One mobility-related measure that has been shown to be predictive of future falls in older adults as well as in PD is increased gait variability [4,5,6]. Indeed, stride-to-stride variability, an indicator of rhythmicity and gait stability, is particularly abnormal in PD [7]. However, it remains unclear how changes in variability during discrete phases of gait, such as gait initiation (GI), may affect persons with PD.
While differences in gait variability are insightful, falling is more common to occur during transition movements where momentum must be generated or restrained and when the base of support is changing. Ashley, Tinetti, and colleagues [8,9] suggested that older persons more often fall when they are walking short distances and when the center of mass is displaced away from the base of support. Indeed, the potential for postural instability increases dramatically as the base of support changes from a 2-leg to a 1-leg stance during the initiation of walking [10]. Building upon the literature showing that increased gait variability contributes to fall risk, investigators have begun to evaluate step variability during GI. Several cross-sectional studies have reported that step length variability is significantly higher in populations with postural instability compared to age matched peers. Mbourou and colleagues reported that variability in the length of the first step in elderly fallers is more than twice that seen in elderly non-fallers [11]. Step length variability of the stepping limb during maximal-velocity GI was significantly greater in a small sample of individuals with PD compared to age-matched peers [12]. More recently, Wittwer and colleagues reported significantly increased temporal variability during GI in persons with Alzheimer’s disease who fall at three times the rate of healthy elderly adults [13]. The results from these studies provide early support that step variability during GI is a relevant marker of gait stability in older persons with and without neurodegenerative disease. Though continuous steady-state gait within-trial variability has been previously reported on in PD, to our knowledge, no study has assessed spatiotemporal variability bilaterally in a large sample of persons with PD while repetitively initiating gait at a comfortable, everyday walking speed across several discrete trials. This is surprising considering movement initiation and task switching involve basal ganglia circuitry [14], and thus variability during this time may provide deeper insight into fall risk in persons with PD than variability during steady state gait. Further, no study has evaluated the influence of disease severity on GI variability. Therefore, the aims of this study were 1) to investigate variability within spatiotemporal GI variables which have previously been shown to both be related to incidence of falls and also be different between controls and persons with PD during gait (such as length, width, and time of stepping) and 2) to determine the effect of disease severity on spatiotemporal GI variability. We hypothesize that persons with PD will have increased variability in the length, width, and time of their first steps bilaterally when compared to healthy older adults and that spatiotemporal GI variability will increase with disease severity.
2. Methods
2.1 Participants
Forty-six patients with idiopathic PD (age: 66.87 ± 9.11 y, mass: 81.39 ± 16.26 kg, height: 169.38 ± 8.34 cm) were recruited from the Movement Disorders Clinics at the University School of Medicine and Neurology offices in the surrounding area. The diagnosis of PD was confirmed by a movement disorders trained neurologist using the UK Brain Bank criteria and disease severity was quantified using the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS) and Hoehn and Yahr (H&Y) staging scale. All patients with PD were being treated with stable doses of antiparkinsonian medication and were tested in their best medicated state, as rated by each participant, approximately 1-1.5 hours following a dose of medication. Individuals reporting episodes of gait freezing were excluded. Forty-nine healthy older adults (age: 67.16 ± 7.81 y, mass: 77.42 ± 13.35 kg, height: 168.27 ± 8.11 cm) were age (±2 years) and gender-matched to the individuals with PD and were deemed free of any orthopedic or neurologic impairment after a general systems screening. The elderly controls were recruited from the university and surrounding community over the same general time period as the PD participants. All participants provided written informed consent before participating in the study as approved by the University Institutional Review Board.
2.2 Protocol
Gait initiation trials were performed along a 12-m walkway surrounded by an 8-camera optical motion capture system (120 Hz; Vicon Nexus, Lake Forrest, CA). Subjects were tested barefoot in form-fitting clothing. Three passive retroreflective markers were placed over the calcaneus, lateral malleolus, and second metatarsal head bilaterally. Participants began each trial standing quietly on the force platform in a relaxed position. Initial positioning of the feet was self-selected and was subsequently constrained for the remaining trials. Prior to data collection, participants were asked to start and stop walking several times within the collection space. If a participant started three consecutive trials with the same leg, it was deemed the preferred leg and served as the initial swing limb in the experimental trials. In response to a verbal cue of “ready”, the participants were asked to wait a moment and then initiate walking at their own discretion, continuing to walk for the length of the walkway.
2.3 Analysis
The swing (SW) limb was defined as the limb taking the first step during GI. Three to five GI trials were analyzed per person. The variation in the number of trials was due to the fact that some trials were excluded due to the participant stepping with the opposite swing limb or technical difficulties. Only trials which were performed with the initially self-selected swing limb were included in the analyses. Heel strikes and heel offs were manually labeled in Vicon software (Vicon Nexus, Lake Forrest, CA) based on marker velocity profiles. Length of the first SW step was defined as the displacement of the heel marker along the walking axis when initiating gait from the static pose to first heel strike. Width of the first SW step was defined as the mediolateral distance from the SW limb heel marker to the stance (ST) limb heel marker at the first SW heel strike. Time of the first SW step was defined as the time interval from the first heel off of the SW limb to the first heel strike. Length, width, and time of the first ST limb step were calculated similarly for the contralateral (ST) limb. Similar to previous literature, the coefficient of variation (CV = SD/mean * 100%) was used to describe within-subject variability of the length, width, and time of the first SW and ST steps [4,11,13].
One-way analyses of variance (ANOVAs) were used to compare mean length, width, and time of the first SW step and first ST step between the two groups. One-way analyses of covariance (ANCOVAs) were used to compare all CV values between groups after controlling for mean value (for instance, when analyzing SW step length CV, mean SW step length was included as a covariate). Pearson’s correlations were applied to analyze relationships between all CV measures and total UPDRS motor score, UPDRS sub item 25, and UPDRS sub item 29, individually. We highlight UPDRS 25 because impaired performance of rapidly alternating tasks has been related to falling in PD [3] and UPDRS 29 because this is the gait item of the UPDRS. An independent samples t-test was used to compare all CV values between the PD participants initially stepping with the more-affected limb and the PD participants initially stepping with the less-affected limb. All statistical analyses were performed in SPSS statistics version 17.0 (SPSS Inc., Chicago, IL) and statistical significance was set at α < 0.05.
3. Results
3.1 Mean Spatiotemporal GI Characteristics
Persons with PD took significantly shorter steps (SW step length: F = 17.131, p < .001; ST step length: F = 17.147, p < .001) than the healthy controls. There were no significant differences in the mean step width or step time measures between groups. Persons with PD also initiated gait with slower steps (SW step velocity: F = 13.293, p < .001; ST step velocity: F = 5.194, p = .025) than the healthy controls. Additionally, there were no significant differences in mean step length, width, or time measures between H&Y disease stages.
3.2 Variability in Length of First Swing and Stance Limb Steps
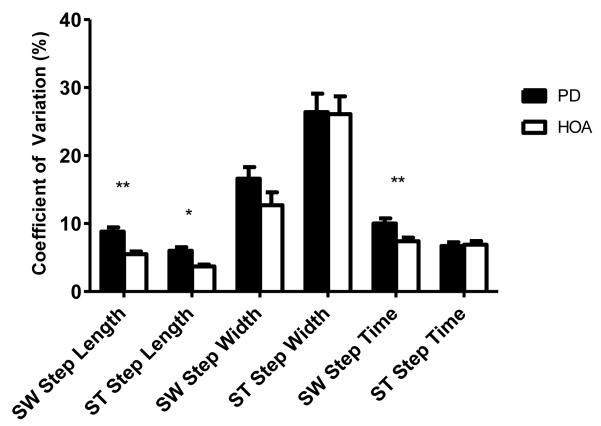
The CV of the length of the first SW step was significantly greater (F = 8.571, p = .004) in persons with PD when compared to their healthy elderly peers after controlling for mean SW step length. Additionally, the CV of the length of the first ST step was also significantly greater (F = 5.834, p = .018) in persons with PD when compared to their healthy elderly peers after controlling for mean ST step length.
3.3 Variability in Width of First Swing and Stance Limb Steps
The CVs of the width of the first SW and ST steps were not significantly different between the persons with PD and the control participants after controlling for mean SW step width and mean ST step width, respectively.
3.4 Variability in Time of First Swing and Stance Limb Steps
The CV of the time of the first SW step was significantly greater in persons with PD when compared to their healthy elderly peers (F = 7.558, p = .007) after controlling for mean SW step time. However, the CV of the time of the first ST step was not significantly different between the persons with PD and the control participants after controlling for mean ST step time.
3.5 Step Variability by Disease Severity
There were no significant correlations between any of the UPDRS measures of interest and any of our variability (Table 3). Additionally, there were no significant differences in any of the CV measures between the PD participants who stepped with the more-affected limb and those who stepped with the less-affected limb.
Table 3.
Pearson’s correlation coefficients (r) for CV of length, width, and time of first SW step and first ST step vs. UPDRS scores.
| Step Length CV | Step Width CV | Step Time CV | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| SW | ST | SW | ST | SW | ST | |
| Total Motor UPDRS | .164 | .155 | .050 | .100 | .061 | .058 |
| UPDRS 25 | .123 | −.157 | .086 | −.021 | .051 | −.189 |
| UPDRS 29 | −.079 | −.070 | .333 | .011 | −.034 | −.102 |
CV – Coefficient of Variation, UPDRS – Unified Parkinson’s Disease Rating Scale, SW – Swing Limb, ST – Stance Limb
No significant differences.
4. Discussion
Parkinsonian gait initiation is characterized by short, slow steps with a propensity for freezing episodes [15,16]. In this study, we have shown that persons with PD produce shortened step lengths and increased variability in the length and time of their first steps during GI. These findings extend previous literature reporting increased variability in gait initiation in several populations with postural instability [11,13] and increased step length variability during steady state gait in persons with PD. In PD, increased gait variability has been reported and has been associated with increased incidence of future falls. Taken together with our findings, it appears that increased gait variability may begin as early as initiation of the first step.
While the exact mechanism remains unknown, we postulate that alterations in cortical and subcortical control may contribute to the more variable GI performance. During gait initiation, anticipatory postural adjustments purposely uncouple movement of the center of pressure (COP) and center of mass (COM). Before any appreciable movement of the COM, the COP is shifted posterior and lateral towards the initial stepping limb (swing limb). Whereas the backward movements of the COP results from the bilateral deactivation of the triceps surae musculature coupled with activation of the tibialis anterior, the lateral COP movement is achieved by manipulation of hip abductor activity and stance limb knee flexion [17,18,19]. This pattern of muscular activity generates the initial momentum needed to begin walking while the COM remains within the base of support. The supplementary motor area (SMA) is thought to control the timing and planning of the anticipatory postural adjustments (APAs) that precede GI [20,21,22]. Indeed, Jacobs et al have recently used repetitive transcranial magnetic stimulation to demonstrate the role of the SMA in temporal control of APAs preceding GI [23]. Further, these investigators found that the temporal coordination of APAs is diminished in PD, which suggests that the SMA function may be impaired in this population. Moreover, altered cortical and basal ganglia influence on brain stem locomotor regions has also been recently implicated in walking disability in PD [24]. Specifically, corticopontine-cerebellar pathways appear to be involved in impaired stepping behavior [25]. Thus, we postulate that these disrupted connections together with altered motor cortical activity may be partially responsible for difficulty and increased variability in initiating gait in PD.
Peripheral changes in PD may also contribute to more variable GI performance. It has been reported that compared to neurologically healthy individuals, persons with PD produce reduced lower extremity force [26], greater co-contraction of muscles surrounding the ankle [27], and greater force production variability [28]. Additionally, muscle strength has been associated with impaired dynamic stability during GI in persons with PD [29]. Altogether, these muscular deficits may also contribute to the greater variability observed at GI in PD.
Hass and colleagues previously reported that persons with more advanced PD (Hoehn &Yahr stage ≥ 2.5) demonstrated greater impairment in dynamic stability during GI when compared to persons with less severe PD (Hoehn &Yahr stage <2.5) [30]. Our results do not support this trend as step length, width, and time variability did not correlate with the total UPDRS motor score nor UPDRS items 25 or 29. Limitations of our study included the narrow range of H&Y stage in the majority of our participants and relatively small number of trials per subject. As shown on Table 1, the bulk of our participants fell within a relatively limited range of H&Y stage (93% of participants within H&Y stages 2, 2.5, and 3). It is also possible that the UPDRS is not sensitive enough to detect subtle differences in GI variability in less-severe cases of PD. Finally, our results may be influenced by the relatively small number of GI trials performed by each participant. However, we did not find this to be the case as number of trials showed no significant effect as a covariate in our analyses.
Table 1.
Mean (SE) demographic characteristics of all participants.
| Group | N | Age (y) | Height (cm) | Mass (kg) | Disease Duration (mo) | H&Y | UPDRS Motor |
|---|---|---|---|---|---|---|---|
| PD | 46 | 66 ± 1 | 169.4 ± 1.2 | 81.4 ± 2.4 | 83 ± 9 | 2.6 ± .1 | 28± 1 |
| Control | 49 | 67 ± 1 | 168.3 ± 1.2 | 77.4± 1.9 | - | - | - |
H&Y - Hoehn and Yahr, UPDRS - Unified Parkinson’s Disease Rating Scale, SE – Standard Error
No significant differences.
For steady state walking, increased gait variability has frequently been associated with increased incidence of falls in several elderly populations, including PD [4,5,6]. Nakamura and colleagues propose that a stride length coefficient of variation of 7% puts the walker at a high risk of falling [6]. In the present experiment, the coefficient of variation of the first step length in the healthy older adults was below 5%. On the other hand, persons with PD surpassed the suggested 7% threshold, though the magnitudes of the CVs are less than those reported previously for elderly fallers (45%) [11] and for those with PD initiating gait as fast as possible (11%) [12]. While the finding that participants with PD exceeded the proposed fall-related variability threshold, further validation is needed to determine the thresholds sensitivity for predicting future falls during GI. Lastly, it appears that persons with PD should avoid initiating gait extremely quickly in order to minimize their GI variability and, potentially, minimize risk of falling during GI.
5. Conclusion
It is important to understand why falls occur during GI in persons with PD and to determine which GI parameters, if any, experience changes that may be useful in predicting these events. To our knowledge, this is the first study to investigate variability in step length, width, and time during self-paced GI in persons with PD. We have shown that persons with PD exhibit more variability in the length and time of stepping during GI when compared to their healthy elderly peers. Based on our work, we suggest that spatiotemporal GI variability is a clinically relevant GI disturbance in PD and that future investigations should prospectively evaluate the relationship between GI variability and fall risk.
Figure 1.
Coefficients of variation of the length, width, and time of the first swing (SW)- and stance(ST)-limb steps during gait initiation in persons with Parkinson’s disease (PD) and healthy older adults (HOA). * p-value <.05; ** p-value < .01
Table 2.
Mean (SE) and CV (SE) length, width, and time of first SW step and first ST step of PD and control participants.
| Group | N | Step Length | Step Width | Step Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| SW | ST | SW | ST | SW | ST | ||||||||
|
|
|||||||||||||
| Mean ± SE (m) |
CV ± SE (%) |
Mean ± SE (m) |
CV ± SE (%) |
Mean ± SE (m) |
CV ± SE (%) |
Mean ± SE (m) |
CV ± SE (%) |
Mean ± SE (m) |
CV ± SE (%) |
Mean ± SE (m) |
CV ± SE (%) |
||
| PD | 46 | .46±.01** | 8.8±.64** | .97±.02** | 6.0±.54* | .14±.01 | 16.6±1.7 | .11±.01 | 26.4±2.7 | .58±.01 | 10.0±.76** | .73±.01 | 6.7±.55 |
| Control | 49 | .54±.01 | 5.5±.39 | 1.1 ±.02 | 3.7±.27 | .15±.01 | 12.7±1.9 | .11±.01 | 26.1±2.6 | .57±.01 | 7.4±.54 | .77±.01 | 6.9±.52 |
SW – Swing Limb, ST – Stance Limb, SE –Standard Error
indicates significantly different from Control, p-value < .05
indicates significantly different from Control, p-value < .01
Acknowledgements
This work was supported in part by NIH grants R03HD054594, 1R21AG033284-01A2 and UF National Parkinson’s Foundation Center of Excellence
Footnotes
Conflict of interest statement The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pickering RM, Grimbergen YA, Rigney U, Ashburn A, Mazibrada G, Wood B, Gray P, Kerr G, Bloem BR. A meta-analysis of six prospective studies of falling in Parkinson’s disease. Mov Disord. 2007;22(13):1892–900. doi: 10.1002/mds.21598. [DOI] [PubMed] [Google Scholar]
- [2].Hong M, Perlmutter JS, Earhart GM. A kinematic and electromyographic analysis of turning in people with Parkinson disease. Neurorehabil Neural Repair. 2009;23(2):166–76. doi: 10.1177/1545968308320639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–24. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- [4].Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–6. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- [6].Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology. 1996;42(2):108–13. doi: 10.1159/000213780. [DOI] [PubMed] [Google Scholar]
- [7].Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19(2):026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ashley MJ, Gryfe CI, Amies A. A longitudinal study of falls in an elderly population II. Some circumstances of falling. Age Ageing. 1977;6(4):211–20. doi: 10.1093/ageing/6.4.211. [DOI] [PubMed] [Google Scholar]
- [9].Tinetti ME, Doucette JT, Claus EB. The contribution of predisposing and situational risk factors to serious fall injuries. J Am Geriatr Soc. 1995;43(11):1207–13. doi: 10.1111/j.1532-5415.1995.tb07395.x. [DOI] [PubMed] [Google Scholar]
- [10].Winter D, Ruder GD, MacKinnon CD. Control of balance of upper body during gait. In: Winters JM, Woo SL-Y, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. Springer-Verlag; New York: 1990. pp. 534–541. [Google Scholar]
- [11].Mbourou GA, Lajoie Y, Teasdale N. Step length variability at gait initiation in elderly fallers and non-fallers, and young adults. Gerontology. 2003;49(1):21–6. doi: 10.1159/000066506. [DOI] [PubMed] [Google Scholar]
- [12].Dibble LE, Nicholson DE, Shultz B, MacWilliams BA, Marcus RL, Moncur C. Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Posture. 2004;19(3):215–25. doi: 10.1016/S0966-6362(03)00065-1. [DOI] [PubMed] [Google Scholar]
- [13].Wittwer JE, Andrews PT, Webster KE, Menz HB. Timing variability during gait initiation is increased in people with Alzheimer’s disease compared to controls. Dement Geriatr Cogn Disord. 2008;26(3):277–83. doi: 10.1159/000160961. [DOI] [PubMed] [Google Scholar]
- [14].Johnson AM, Vernon PA, Almeida QJ, Grantier LL, Jog MS. A role of the basal ganglia in movement: the effect of precues on bi-directional movements in Parkinson’s disease. Motor Control. 2003;7(1):71–81. doi: 10.1123/mcj.7.1.71. [DOI] [PubMed] [Google Scholar]
- [15].Knutsson E. An analysis of Parkinsonian gait. Brain. 1972;95(3):475–86. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- [16].Fahn S. The freezing phenomenon in parkinsonism. Adv Neurol. 1995;67:53–63. [PubMed] [Google Scholar]
- [17].Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol. 1991;437:635–53. doi: 10.1113/jphysiol.1991.sp018616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brunt D, Lafferty MJ, Mckeon A, Goode B, Mulhausen C, Polk P. Invariant characteristics of gait initiation. Am J Phys Med Rehabil. 1991;70(4):206–12. doi: 10.1097/00002060-199108000-00009. [DOI] [PubMed] [Google Scholar]
- [19].Assaiante C, Woollacott M, Amblard B. Development of postural adjustment during gait initiation: kinematic and EMG analysis. J Mot Behav. 2000;32(3):211–26. doi: 10.1080/00222890009601373. [DOI] [PubMed] [Google Scholar]
- [20].Yazawa S, Shibasaki H, Ikeda A, Terada K, Nagamine T, Honda M. Cortical mechanism underlying externally cued gait initiation studied by contingent negative variation. Electroencephalogr Clin Neurophysiol. 1997;105(5):390–9. doi: 10.1016/s0924-980x(97)00034-9. [DOI] [PubMed] [Google Scholar]
- [21].Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–61. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- [22].Prentice SD, Drew T. Contributions of the reticulospinal system to the postural adjustments occurring during voluntary gait modifications. J Neurophysiol. 2001;85(2):679–98. doi: 10.1152/jn.2001.85.2.679. [DOI] [PubMed] [Google Scholar]
- [23].Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience. 2009;164(2):877–85. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, Henry-Lagrange C, Seigneuret E, Piallat B, Krack P, Le Bas JF, Benabid AL, Chabardès S, Pollak P. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133(Pt 1):205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- [25].Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ. Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait. Neuroreport. 2010;21(14):914–6. doi: 10.1097/WNR.0b013e32833ce5f1. [DOI] [PubMed] [Google Scholar]
- [26].Durmus B, Baysal O, Altinayar S, Altay Z, Ersoy Y, Ozcan C. Lower extremity isokinetic muscle strength in patients with Parkinson’s disease. J Clin Neurosci. 2010;17(7):893–6. doi: 10.1016/j.jocn.2009.11.014. [DOI] [PubMed] [Google Scholar]
- [27].Bishop M, Brunt D, Pathare N, Ko M, Marjama-Lyons J. Changes in distal muscle timing may contribute to slowness during sit to stand in Parkinsons disease. Clin Biomech (Bristol, Avon) 2005;20(1):112–7. doi: 10.1016/j.clinbiomech.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [28].Stelmach GE, Teasdale N, Phillips J, Worringham CJ. Force production characteristics in Parkinson’s disease. Exp Brain Res. 1989;76(1):165–72. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- [29].Nocera JR, Buckley T, Waddell D, Okun MS, Hass CJ. Knee extensor strength, dynamic stability, and functional ambulation: are they related in Parkinson’s disease? Arch Phys Med Rehabil. 2010;91(4):589–95. doi: 10.1016/j.apmr.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Arch Phys Med Rehabil. 2005;86(11):2172–6. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]