Abstract
Aim
Lifestyle modification, consisting of exercise and weight loss, delays the progression from prediabetes to type 2 diabetes (T2D). However, no study has determined the efficacy of exercise training on glucose metabolism in the different prediabetes subtypes.
Methods
Seventy-six older (65.1 ± 0.6yr) obese adults with impaired fasting glucose (IFG; n=12), impaired glucose tolerance (IGT; n=9), and combined glucose intolerance (IFG+IGT = CGI; n=22) were compared to normal glucose tolerant (NGT; n=15) and T2D (n=18) groups after 12 weeks of exercise training (60 min/d for 5d/wk at ~85% HRmax). An oral glucose tolerance test was used to assess glucose levels. Insulin sensitivity (euglycemic hyperinsulinemic clamp at 40 mU/m2·min−1), β-cell function (glucose stimulated insulin secretion corrected for insulin sensitivity), body composition (hydrostatic weighing/CT scan), and cardiovascular fitness (treadmill VO2max) were also assessed.
Results
Exercise training reduced weight and increased cardiovascular fitness (p < 0.05). Exercise training lowered fasting glucose levels in IFG, CGI and T2D (p < 0.05) and 2-hour glucose levels in IGT, CGI and T2D (p < 0.05). However, 2-hour glucose levels were not normalized in adults with CGI compared to IGT (p < 0.05). β-cell function improved similarly across groups (p < 0.05). Although not statistically significant, insulin sensitivity increased approximately 40% in IFG and IGT, but only 17% in CGI.
Conclusion
The magnitude of improvement in glucose metabolism after 12-weeks of exercise training is not uniform across the prediabetes subtypes. Given the high risk of progressing to T2D, adults with CGI may require more aggressive therapies to prevent diabetes.
Keywords: obesity, prediabetes, insulin resistance, beta-cell dysfunction, exercise
Introduction
Individuals with prediabetes have approximately a 30% chance of developing type 2 diabetes over a 10-year period [1-3]. Prediabetes is defined as impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or combined glucose intolerance (CGI) (i.e. IFG + IGT) [4]. Skeletal muscle insulin resistance, with concomitant β-cell dysfunction, characterizes individuals with IGT, whereas hepatic insulin resistance and first phase insulin secretion deficiency describes those with IFG [4]. Combinations of skeletal muscle and hepatic insulin resistance and β-cell dysfunction depict individuals with CGI [4]. Since approximately 40 million adults in the U.S. have prediabetes and are approximately 60 years of age, there is a great need to understand how to treat these various prediabetes subtypes [5].
Exercise, with concurrent weight loss, is recommended as a first-line therapy for improving abnormal glucose metabolism because it increases insulin sensitivity [6-8] and/or improves β-cell function [9-12]. Since IFG, IGT, and CGI are characterized by different pathophysiologic abnormalities in glucose metabolism [13], exercise and diet therapy may differentially lower diabetes risk [14,15]. Little is known, however, regarding the efficacy of lifestyle modification in individuals with different prediabetes subtypes [16,17]. Ramachandran et al. [16] demonstrated that lifestyle modification reduced the incidence of diabetes to the same degree in overweight middle-aged individuals with CGI and IGT. However, Jenkins and Hagberg, [17] showed that 6 months of exercise training lowered glucose levels to a greater extent in overweight middle-aged hyperglycemic individuals (i.e. IFG, IGT, or CGI) compared to normoglycemic controls (i.e. NGT). It was suggested that exercise lowers glucose levels more in adults with IGT than men and women with CGI [17], but this hypothesis has not been systematically investigated. Therefore, the purpose of this study was to determine the effect of exercise training on glucose levels, insulin sensitivity, and β-cell function in obese older adults. We hypothesized that exercise training with concurrent weight loss would improve glucose levels, increase insulin sensitivity, and lower insulin secretion more in individuals with IFG or IGT, than CGI.
Methods
Subjects
Older (65.1 ± 0.6 yr) obese men (n = 30) and women (n = 46) (see Table 1) were recruited from the Cleveland community as previously described [9,18,19]. All participants underwent a medical history and physical examination with blood work. Individuals were non-smoking, weight stable (<2 kg in previous 6 months), sedentary (activity less than <90 min/week), and free of chronic disease (i.e. hematological, renal, hepatic, cardiovascular) or medications (e.g. metformin and acarbose) known to affect glucose tolerance. Following an oral glucose tolerance test (OGTT), individuals were considered normal glucose tolerant (NGT: fasting glucose <100 mg/dl; 2-hour glucose <140 mg/dl), impaired fasting glucose (IFG: fasting glucose 100-125 mg/dl; 2-hour glucose <140 mg/dl), impaired glucose tolerant (IGT: fasting glucose <100 mg/dl; 2-hour glucose 140-199 mg/dl), combined glucose intolerant (CGI: fasting glucose 100-125 mg/dl; 2-hour glucose 140-199 mg/dl) or type 2 diabetic (T2D: fasting glucose >105 mg/dl; 2-hour glucose >200 mg/dl). All women were post-menopausal, and all subjects signed informed consent documents approved by our Institutional Review Board.
Table 1.
Body composition and cardiorespiratory fitness before and after exercise training.
| NGT | IFG | IGT | CGI | T2D | ANOVA | ||
|---|---|---|---|---|---|---|---|
| Pre vs. Post | |||||||
| Sex (n M/F) | 4M/11F | 8M/4F | 4M/5F | 7M/15F | 7M/11F | - | |
| Height (m) | 1.67±0.02 | 1.72 ±0.03 | 1.70±0.03 | 1.65 ±0.02 | 1.66 ±0.02 | 0.23 | |
| BMI (kg/m2) | Pre | 32.3 ± 1.2 | 33.8 ± 1.0 | 32.7 ± 1.1 | 35.6 ± 1.0 | 34.1 ± 1.3 | < 0.001 |
| Post | 30.3 ± 1.3 | 30.4 ± 1.0 | 30.2 ± 1.1 | 33.1 ± 1.0 | 32.8 ± 1.2 | ||
| WC (cm) | Pre | 102.2 ± 3.0 | 109.5 ±3.1 | 105.1 ±3.2 | 107.0 ±2.6 | 102.6 ±3.2 | < 0.001 |
| Post | 97.7±2.5 | 104.6 ±2.3 | 101.2 ±3.2 | 101.8 ±2.6 | 102.9 ±3.9 | ||
| Body Fat (%) | Pre | 42.1 ±2.0 | 41.0±2.1 | 42.6 ±3.7 | 43.2 ± 1.6 | 41.1 ± 1.3 | < 0.001 |
| Post | 39.8±2.8 | 36.2±2.4 | 42.9 ±2.1 | 41.7 ± 1.6 | 39.5 ± 1.8 | ||
| VAT (cm2)* | Pre | 137.4±23.9 | 139.9 ± 16.8 | 215.9 ±76.6 | 187.7 ± 19.1** | 139.9 ±23.7 | < 0.001 |
| Post | 90.5 ±21.2 | 86.6 ± 14.4 | 140.9 ±45.2 | 172.2 ± 19.9 | 109.0 ± 18.1 | ||
| SAT (cm2) | Pre | 421.3 ±43.2 | 443.7±41.0 | 456.2 ±61.8 | 449.5 ±30.8 | 379.6 ±38.5 | < 0.001 |
| Post | 354.8 ±47.3 | 365.7±44.7 | 410.5 ±72.4 | 373.8 ±26.8 | 330.1 ±31.4 | ||
| Fat-free mass (kg) | Pre | 52.3 ±3.3 | 54.4±4.3 | 57.3 ±3.9 | 55.5 ±2.5 | 54.7 ±3.1 | < 0.001 |
| Post | 50.4 ±3.1 | 52.9±4.1 | 53.5 ±4.1 | 50.2 ±3.2 | 50.6 ±4.3 | ||
| VO2 max (ml/kg- ffm/min) |
Pre | 39.2 ± 1.6 | 37.2±2.0 | 36.4 ± 1.8 | 37.0 ± 1.1 | 34.6 ± 1.0 | < 0.001 |
| Post | 43.7 ± 1.1 | 43.9 ± 3.5 | 41.7 ± 1.4 | 41.9 ± 1.3 | 39.5 ± 1.3 |
Data are mean ± standard error of mean. BMI was log-transformed for statistical analysis. No group x test interaction was observed.
Group effect (p < 0.05).
CGI compared to NGT and IFG (p ≤ 0.05).
Cardiovascular Fitness and Body Composition
Maximum oxygen consumption (VO2max) was determined using a continuous progressive exercise test on a treadmill. Weight was assessed on a digital platform in a hospital gown, and height was recorded on a stadiometer. Body fat and fat free-mass (FFM) was assessed by dual-x-ray absorptiometry (iDXA; Lunar Prodigy, Madison, WI) or hydrostatic weighing. Pre-and post body composition measurements were compared using the same assessment technique. Visceral and subcutaneous adipose tissue was assessed by computed tomography (Picker PQ6000 Scanner; Marconi/Picker, Highland Heights, OH) [20]. Waist circumference was measured in the standing position with a plastic tape measure approximately 2 cm above the umbilicus.
Metabolic Control Period
Metabolic testing was performed during a 3-day inpatient stay in the Clinical Research Unit. Subjects were provided isocaloric meals (resting metabolic rate x 1.2; 55% CHO, 30% fat, 15% protein). OGTT and insulin sensitivity measurements were made approximately 16-18 hours after the last exercise bout following the intervention.
OGTT
After a 10-12 hour overnight fast, a 75 gram glucose load was administered. Fasting and postprandial blood samples were obtained from an antecubital vein at 0, 30, 60, 90, and 120 minutes. Glucose and insulin area under the curve (AUC) during the OGTT were calculated using the trapezoidal rule.
Insulin Sensitivity
After a 10-12 hour overnight fast, subjects underwent a 120-minute euglycemic-hyperinsulinemic clamp. A constant infusion (40 mU/m2·min−1) of insulin was administered via catheters placed in an antecubital vein. Glucose (20%) was infused at a variable rate to maintain plasma glucose at 90 mg/dl. Arterialized plasma samples were collected from a hand warmed to approximately 60°C every 5 minutes for the analysis of glucose and every 15 minutes for analysis of insulin.
Exercise Training
Subjects participated in a fully-supervised aerobic treadmill-walking program 5 days/week at 60-65% of maximal heart rate (HRmax) for the first 4 weeks, and thereafter the intensity was increased and maintained at 80-85% HRmax. Subjects exercised for 50-60 minutes, with a 10-minute warm up and cool-down. To ensure proper consumption of macronutrients (i.e. ~55% carbohydrate, 30% fat and 15% protein), subjects met weekly with a registered dietitian.
Blood Analysis
Blood was collected and plasma was aliquoted to cryotubes for storage at −80°C until subsequent analysis. Plasma glucose was determined using a glucose oxidase assay (YSI 2300 STAT Plus, Yellow Springs, OH). Plasma insulin was measured by radioimmunoassay (Millipore, Billerica, MA).
Calculations
Insulin sensitivity (IS) was defined as the glucose disposal rate (GDR) per unit plasma insulin during the final 30 minutes of the clamp. Glucose stimulated insulin secretion was calculated as insulin (AUC) divided by glucose (AUC) levels during minutes 0-30 and 60-120 minutes of the OGTT (ΔI0-30/ΔG0-30 and ΔI60-120/ΔG60-120) to characterize first and second phase insulin secretion. β-cell function was defined as the reciprocal relationship between insulin secretion and insulin sensitivity, and was calculated as ΔI0-30/ΔG0-30 multiplied by IS. Homeostasis model assessment of insulin resistance (HOMA-IR) was also calculated as fasting glucose (mg/dl) x fasting insulin (μU/ml) divided by 405.
Statistical Analysis
Group means were compared using the R statistical software package (Version 2.4.0, The R foundation, Vienna, Austria, 2006). Non-normally distributed outcomes were log-transformed to minimize heterogeneity (see supplemental Table 1 for log-specific data). Age and height were assessed by one-way analysis of variance (ANOVA). All other outcomes were assessed using a two-way (group x test) repeated measures ANOVA. Sex was used as a covariate in our model, and it had no effect on any of the outcomes measured. Bonferroni’s post-hoc analysis was used to determine statistical differences between group means when there was a significant group or group x test interaction. Fischer’s exact test was used to examine prediabetes subtype prevalence (i.e. IGT yes or no). Pearson’s product moment correlation was used to examine associations between baseline glucose levels, the change in glucose levels as well as insulin sensitivity and β-cell function. Significance was accepted as α ≤ 0.05.
Results
Body composition and cardiovascular fitness
Groups were similar in age, despite NGT being slightly younger (60.5 ± 1.0 yr) than IFG (66.4 ± 1.4 yr), IGT (66.6 ± 1.0 yr), CGI (66.3 ± 1.1 yr) or T2D (64.8 ± 1.1 yr) (p < 0.05). Exercise training reduced body mass and total fat to a similar extent across groups (p < 0.001; Table 1). However, adults with CGI maintained more visceral fat after training than NGT and IGT (p ≤ 0.05). All groups increased VO2max by approximately 15% (p < 0.001; Table 1).
Glucose tolerance
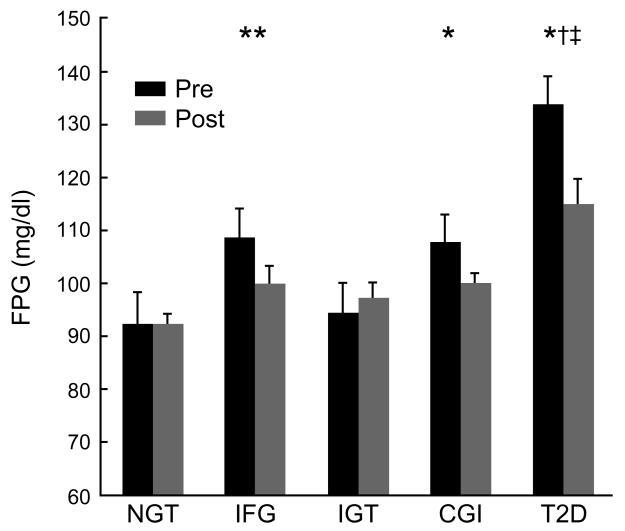
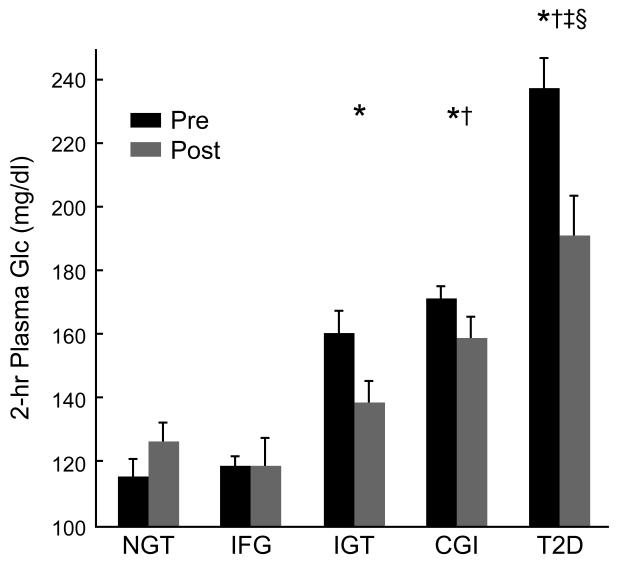
Fasting plasma glucose levels were higher in individuals with IFG, CGI and T2D compared to NGT and IGT (Figure 1a). Exercise training lowered fasting plasma glucose levels in individuals with IFG, CGI and T2D by 8-16% compared to NGT and IGT (effect of test: p < 0.001). Two-hour plasma glucose levels were higher in individuals with IGT, CGI and T2D compared to NGT (p < 0.05; Figure 1b). Exercise training lowered 2-hour glucose levels in individuals with IGT and T2D by approximately 25% (effect of time: p < 0.005), and 10% in those with CGI. After exercise training, 2-hour glucose levels were higher in individuals with CGI, but not IGT, compared to IFG (p = 0.05; Figure 1b). Approximately 86% of individuals with CGI maintained elevated 2-hour glucose levels (i.e. >140 mg/dl) compared to only 45% in adults with IGT after exercise training (p < 0.05). Fasting plasma insulin (effect of test; p < 0.005) and 2-hour plasma insulin levels (effect of test; p < 0.003) were lowered by approximately 35% after exercise training in all groups and there were no statistical differences across groups (Table 2).
Figure 1a.
Effects of exercise training on fasting glucose levels. Data are mean ± standard error of the mean. Fasting glucose was log-transformed for statistical analysis. Effect of time (p < 0.001). Group x time interaction (p < 0.0006). *CGI pre vs. NGT pre (p = 0.09). **IFG pre vs. NGT pre (p < 0.05). †T2D pre vs. IFG, IGT, and CGI pre (p < 0.05). ‡T2D post vs. NGT, IGT, and CGI post (p < 0.05).
Figure 1b.
Effects of exercise training on 2-hour glucose levels. Data are mean ± standard error of the mean. Effect of time (p < 0.004). Group x time interaction (p < 0.002). *IGT, CGI, T2D pre vs. NGT pre (p < 0.05). †CGI and T2D post vs. IFG post (p = 0.05). ‡Compared to NGT post (p <0.05). §T2D pre vs. IFG, IGT, and CGI pre (p < 0.05).
Table 2.
Insulin sensitivity and insulin secretion before and after exercise training.
| NGT | IFG | IGT | CGI | T2D | ANOVA | ANOVA | ||
|---|---|---|---|---|---|---|---|---|
| Pre vs. Post | group x test | |||||||
| GDR/I | Pre | 0.05 ±0.00 | 0.04±0.01 | 0.05 ±0.01 | 0.04 ±0.00 | 0.03 ±0.00# | < 0.001 | < 0.05 |
| Post | 0.08 ± 0.01 | 0.07±0.01$ | 0.08±0.01‡ | 0.06 ±0.00 | 0.04 ±0.00 ^† | |||
| β-cell function | Pre | 0.021 ±0.003 | 0.015±0.003 | 0.016 ±0.002 | 0.016 ±0.002 | 0.008 ± 0.001 ^^†† | < 0.02 | 0.07 |
| Post | 0.022±0.002 | 0.021 ±0.002 | 0.017 ±0.002 | 0.017 ±0.002 | 0.012 ±0.002 | |||
| HOMA-IR | Pre | 2.9 ± 0.3 | 4.7 ± 0.8 | 3.3 ±0.5 | 4.5 ±0.5 | 6.3 ±0.7 | < 0.003 | 0.54 |
| Post | 2.7 ± 0.4 | 4.1 ±0.9 | 2.7 ±0.4 | 3.6 ±0.3 | 4.4 ±0.6 | |||
| Fasting Insulin | Pre | 13.0 ± 1.4 | 23.8 ±5.7 | 14.6 ±2.4 | 20.0 ±2.8 | 27.1 ±7.3 | < 0.0003 | 0.90 |
| Post | 12.3 ± 1.9 | 14.3 ±2.8 | 11.2 ± 1.7 | 14.0 ± 1.2 | 15.9 ± 1.7 | |||
| 2-hr Insulin | Pre | 76.3 ± 13.4 | 114.1 ±35.4 | 132.5 ±33.3 | 142.1 ±33.4 | 128.0±27.5 | < 0.003 | 0.53 |
| Post | 38.6 ± 11.9 | 53.1 ± 14.3 | 70.1 ± 17.5 | 92.8 ± 18.9 | 102.6 ± 18.1 | |||
| ΔI0-30/ΔG0-30 | Pre | 0.44 ± 0.09 | 0.58±0.17 | 0.40 ±0.08 | 0.40 ±0.06 | 0.32 ± 0.06 | < 0.007 | 0.49 |
| Post | 0.32 ± 0.05 | 0.32 ±0.05 | 0.29 ±0.18 | 0.34 ±0.05 | 0.29 ± 0.05 | |||
| ΔI60-120/ ΔG60-120 | Pre | 0.72 ± 0.1 | 0.62 ±0.1 | 0.83 ±0.2 | 0.72 ±0.1 | 0.56 ± 0.1 | < 0.001 | 0.26 |
| Post | 0.49 ± 0.1 | 0.41 ±0.1 | 0.51 ±0.1 | 0.51±0.1 | 0.48 ± 0.1 |
Data are mean ± standard error of the mean. GDR/I = insulin sensitivity (mg/kg-ffm/min/uU/ml). Insulin = uU/ml. β-cell function, HOMA-IR, fasting insulin, 2-hr insulin, and insulin secretion were log-transformed for statistical analysis.
Compared to baseline (p < 0.05).
Compared to NGT pre (p < 0.05).
T2D pre vs. NGT pre (p = 0.09).
Compared to IFG pre (p < 0.05).
Compared to IGT and CGI (p = 0.07).
T2D pre compared to NGT and IGT pre (p< 0.05). T2D pre compared to IFG pre (p = 0.11).
T2D post vs. NGT post (p < 0.05).
T2D post vs. IFG post (p < 0.05).
IFG post vs. IFG pre (p = 0.09).
IGT post to T2D post (p = 0.08).
Insulin sensitivity
As expected, prior to the intervention individuals with T2D were more insulin resistant than those with NGT, IFG, and IGT (p < 0.05). Relative to baseline, exercise training raised insulin sensitivity in all groups (effect of test p < 0.05; Table 2). However, although not statistically different, insulin sensitivity increased approximately 40% in IFG and IGT, but only 17% in CGI.
Insulin secretion and β-cell function
Insulin secretion was not different between groups at the start of the intervention (Table 2). After exercise training, insulin secretion was lower in response to an oral glucose stimulus across all groups (p < 0.001). β-cell function was lower in individuals with T2D compared to NGT and IFG at baseline (p < 0.05; Table 2). Exercise training increased β-cell function across the prediabetes subtypes (effect of test: p < 0.05).
Correlations
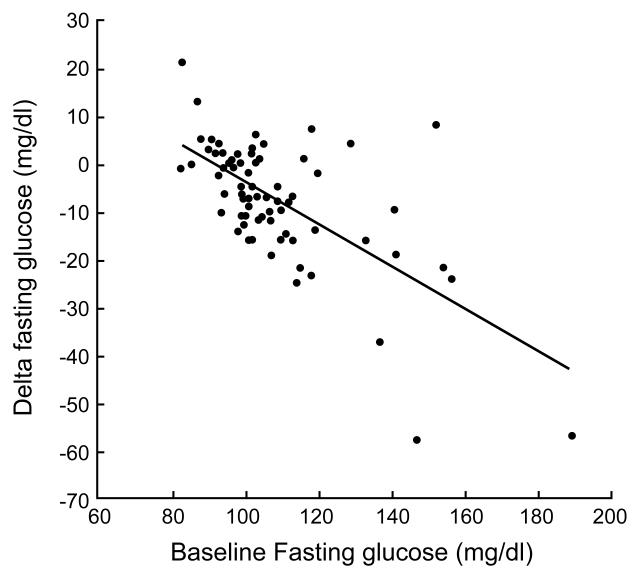
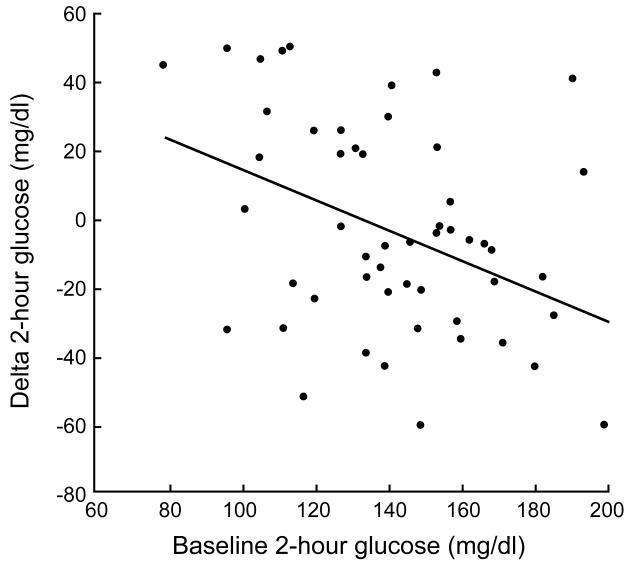
Baseline fasting glucose levels were significantly correlated with lower fasting glucose (r = −0.64; p < 0.05; Figure 2a) after exercise training. Baseline 2-hour glucose levels were also significantly associated with lower 2-hour glucose concentrations (r = −0.57; p < 0.05; Figure 2b) after the intervention. Increased β-cell function correlated with decreased 2-hour glucose levels (r = −0.29; p < 0.05) after exercise training.
Figure 2a.
Correlation between baseline fasting blood glucose levels and the change in fasting blood glucose levels.
Figure 2b.
Correlation between 2-hour blood glucose levels and the change in 2-hour blood glucose levels.
Discussion
Lifestyle interventions reduce the progression from prediabetes to type 2 diabetes [2,21,22], however, none of these previous studies examined the efficacy of exercise training on lowering diabetes risk in the prediabetes subtypes. Although lifestyle modification reduces the progression to diabetes similarly in adults with IGT or CGI [16], some clinical exercise studies have suggested otherwise. Malin et al. suggested that 12 weeks of aerobic and resistance exercise training tended to increase insulin sensitivity to a greater extent in obese middle-aged individuals with CGI compared to IGT [23]. Jenkins and Hagberg [17] showed that 6 months of aerobic exercise training lowered fasting and post-prandial glucose levels more in overweight middle-aged adults with hyperglycemia than normoglycemia. In our older obese subjects, 12 weeks of exercise training, with approximately 8% weight loss, reversed impaired fasting glucose levels in 70% of individuals with IFG and 55% of adults with CGI. In addition, we found that exercise training normalized 2-hour glucose levels in 55% of individuals with IGT, but only 14% of adults with CGI (between IGT and CGI: p < 0.05). This later finding is similar to the report by Jenkins and Hagberg [17] that aerobic exercise training reverses prediabetes prevalence most in individuals with IGT compared to those with CGI. It is unclear why CGI adults had an attenuated glucose tolerance response to exercise, but it is likely related to metabolic defects in both the periphery and splanchnic regions.
Gut glucose absorption may affect post-prandial glucose levels and influence insulin sensitivity and secretion [24]. Although exercise could have decreased glucose absorption to a greater extent in individuals with IGT compared to CGI, the effects of exercise on gut glucose absorption in individuals with different prediabetes subtypes is unknown. However, Bock et al. found that there were no difference in gut glucose appearance among individuals with IFG, IGT, and CGI [25], suggesting that glucose absorption is similar between the prediabetes subtypes, and an unlikely factor contributing to the glycemic response differences after exercise in individuals with CGI compared to IGT.
Elevated insulin sensitivity is known to lower post-prandial glucose levels, and the subtle differences across group means suggest that we observed an attenuated improvement in insulin sensitivity in individuals with CGI (~17%) compared to IGT (~40%). Although not statistically significant, the smaller rise in insulin sensitivity is worth considering. The attenuated rise in insulin sensitivity is not likely explained by weight loss or increased cardiovascular fitness because there were no differences between prediabetes subtypes. Small changes in fat-free mass (FFM) might have influenced skeletal muscle glucose uptake after training across the prediabetes groups. However, we scaled glucose uptake to FFM and still observed differences between prediabetes phenotypes. Independent of weight loss, reductions in visceral fat is strongly related to insulin resistance [20]. We found that individuals with CGI maintained more visceral fat compared to those with IGT after exercise training. Men generally have more visceral fat than women. Since there was a higher percentage of women in the CGI than IGT group, it is possible that exercise was less effective in decreasing visceral fat and this contributed to the preservation of glucose intolerance [20]. However, at baseline the visceral fat content in the CGI group was comparable to the IGT group, suggesting that it is unlikely that visceral fat mass alone explains the effects on 2-hour glucose levels. The maintenance of visceral fat in the CGI group could be a result of adipose insulin resistance. If insulin-stimulated suppression of lipolysis was impaired in adults with CGI then elevated free fatty acid levels during the OGTT may have attenuated glucose uptake [26,27] and/or elevated hepatic glucose output [18]. Future work measuring free fatty acid and glucose kinetics in the prediabetes subtypes after exercise training is needed to confirm this hypothesis.
Impaired glucose-stimulated insulin secretion may have also contributed to the glucose intolerance seen after exercise in individuals with CGI [28-30]. Recently, Bloem and Chang [11] reported that a 7-day exercise training intervention improved β-cell function measured by the intravenous glucose tolerance test in older obese adults with IGT. However, these same IGT adults had fasting glucose levels of approximately 101 mg/dl, suggesting that some were actually CGI. Therefore, the effect of exercise on each prediabetes subtype is unclear. We found that 12-weeks of exercise training reduced glucose stimulated plasma insulin secretion equally in individuals with IFG, IGT and CGI. Although this indicates that exercise favorably decreases insulin secretion in the prediabetes subtypes, changes in insulin sensitivity have reciprocal effects on insulin secretion [31]. Accordingly, we scaled insulin secretion to insulin resistance, and found that exercise training maintained/increased β-cell function. This finding is consistent with previous work in mild type 2 diabetics [9,10], and suggests that exercise interventions are able to increase β-cell function across the prediabetes subtypes. Improvement in β-cell function is clinically important because it was correlated with reductions in 2-hour glucose levels. Because glucose stimulated insulin secretion improved similarly between the prediabetes subtypes, the attenuated rise in insulin sensitivity in individuals with CGI compared to IGT explains the effects on 2-hour glucose levels. The exact mechanism by which fasting hyperglycemia blunts the reversal of IGT is unclear, but insulin resistance attenuates skeletal muscle expression of PGC-1α and AMPK after exercise [32-34]. Given that AMPK is important for GLUT4 translocation, an attenuated rise in AMPK after exercise in CGI adults may, in part, mitigate the rise in glucose uptake after exercise compared to IGT adults [32-34]. To gain mechanistic insight into glucose metabolism in the different prediabetes subtypes [35], future work should focus on cellular changes in skeletal muscle.
There are some limitations to the current study that warrant discussion. First, the nature of retrospective analysis may have increased inter-subject variability and minimized the ability to detect differences between groups. Second, the limited sample size in this study precludes our ability to perform subgroup analysis on the role of ethnicity in the different prediabetes subtypes. Third, the liver extracts approximately 50% of plasma insulin and weight loss alters hepatic insulin extraction [36]. Thus, our measures of insulin secretion may be less accurate than if we had used C-peptide to measure β-cell function. However, we have unpublished evidence that shows similar relations between glucose stimulated C-peptide and insulin levels after exercise (31). Fourth, this was not a dietary feeding study, so macronutrient intake may have fluctuated and influenced changes in insulin sensitivity or glucose tolerance. However, participants across all groups met with a dietitian for caloric counseling, received controlled mixed-meals prior to metabolic testing, and lost similar amounts of weight. Future work should determine if macronutrient composition differentially impacts glucose tolerance across the prediabetes phenotypes. Fifth, we did not use heart rate reserve (HRR) to prescribe exercise intensity as is suggested by the American College of Sports Medicine. As a result, exercise prescription based on HRR might produce different results than that used in the current study (i.e. HRmax). Lastly, because our NGT group was hyperinsulinemic and insulin resistant, the efficacy of exercise in the prediabetes subtypes may have been underestimated.
In conclusion, our findings support the role of exercise induced weight loss for improving glucose tolerance and reversing the progression towards type 2 diabetes. However, our exercise intervention did not reduce 2-hour glucose levels to a similar extent in individuals with IGT versus CGI, and this may be related to the magnitude of insulin resistance in these two groups. Together these findings suggest that each prediabetes subtype is a unique pathophysiological condition and future work should focus on understanding the effectiveness of exercise training on cardiometabolic risk in individuals with these different prediabetes subtypes.
Supplementary Material
Acknowledgments
S.K.M and J.P.K contributed to the study hypothesis. S.K.M was primarily responsible for data analysis, study organization, and statistical integrity. S.K.M and J.P.K. wrote the manuscript. We thank all those who contributed to data collection and organization. We also appreciate the dedicated effort of all the participants. The authors report no conflict of interest. This research was supported by RO1 AG12834 (to J.P.K.) and was supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1 RR-024989, Cleveland, Ohio.
Footnotes
Conflict of interest details: S.K.M and J.P.K contributed to the study hypothesis. S.K.M was primarily responsible for data analysis, study organization, and statistical integrity. S.K.M and J.P.K. wrote the manuscript.
Authorship details: no conflict of interests
References
- [1].Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med. 1988;319(23):1500–1506. doi: 10.1056/NEJM198812083192302. [DOI] [PubMed] [Google Scholar]
- [2].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Meigs J, Muller D, Nathan D, Blake D, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- [4].Abdul-Ghani M, DeFronzo R. Pathophysiology of prediabetes. Current Diabetes Report. 2009;9(3):193–199. doi: 10.1007/s11892-009-0032-7. [DOI] [PubMed] [Google Scholar]
- [5].CDC Diabetes Fact Sheet. 2011 [Google Scholar]
- [6].Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264(6 Pt 1):E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- [7].Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96(1):101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- [8].Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2009;64(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Solomon TPJ, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care. 2010;33(7):1561–1566. doi: 10.2337/dc09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dela F, von Linstow M, Mikines K, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287(5):E1024–E1031. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- [11].Bloem C, Chang A. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab. 2008;93(2):387–392. doi: 10.1210/jc.2007-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol. 1993;48(3):M84–M90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- [13].Abdul-Ghani M, Tripathy D, DeFronzo R. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- [14].Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, American Diabetes Association Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- [15].Faerch K, Borch Johnsen K, Holst JJ, Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52(9):1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- [16].Ramachandran A, Arun N, Shetty A, Snehalatha C. Efficacy of primary prevention interventions when fasting and postglucose dysglycemia coexist: analysis of the Indian Diabetes Prevention Programmes (IDPP-1 and IDPP-2) Diabetes Care. 2010;33(10):2164–2168. doi: 10.2337/dc09-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jenkins N, Hagberg J. Aerobic Training Effects on Glucose Tolerance in Prediabetic and Normoglycemic Humans. Med Sci Sports Exerc. 2011;43(12):2231–2240. doi: 10.1249/MSS.0b013e318223b5f9. [DOI] [PubMed] [Google Scholar]
- [18].Haus JM, Solomon TPJ, Marchetti CM, Edmison JM, Gonzlez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab. 2010;95(1):323–327. doi: 10.1210/jc.2009-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Solomon TPS, Haus JM, Kelly KR, Cook M, Filion J, Rocco M, Kashyap SR, Watanabe R, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr. 2010;92(6):1359–1368. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].O’Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol. 2006;100(5):1584–1589. doi: 10.1152/japplphysiol.01336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- [22].Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- [23].Malin S, Gerber R, Chipkin S, Braun B. Independent and Combined Effects of Exercise Training and Metformin on Insulin Sensitivity in Individuals With Prediabetes. Diabetes Care. 2011;35(1):131–136. doi: 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hucking K, Watanabe R, Stefanovski D, Bergman R. OGTT-derived measures of insulin sensitivity are confounded by factors other than insulin sensitivity itself. Obesity. 2008;16(8):1938–1945. doi: 10.1038/oby.2008.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes. 2006;55(12):3536–3549. doi: 10.2337/db06-0319. [DOI] [PubMed] [Google Scholar]
- [26].Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49(5):677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- [27].Solomon TPJ, Haus JM, Marchetti CM, Stanley W, Kirwan J. Effects of exercise training and diet on lipid kinetics during free fatty acid-induced insulin resistance in older obese humans with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2009;297(2):E552–E559. doi: 10.1152/ajpendo.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abdul Ghani M, Jenkinson C, Richardson D, Tripathy D, DeFronzo R. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55(5):1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- [29].Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC. Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes. 2000;49(6):975–980. doi: 10.2337/diabetes.49.6.975. [DOI] [PubMed] [Google Scholar]
- [30].Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo R, Abdul Ghani M. Impaired early-but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol. 2011;48(3):209–217. doi: 10.1007/s00592-011-0285-x. [DOI] [PubMed] [Google Scholar]
- [31].Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte D. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. J Nutr. 2001;131(2):354S–360S. doi: 10.1093/jn/131.2.354S. [DOI] [PubMed] [Google Scholar]
- [32].Layne A, Nasrallah S, South M, Howell MEA, McCurry M, Ramsey M, Stone M, Stuart C. Impaired muscle AMPK activation in the metabolic syndrome may attenuate improved insulin action after exercise training. J Clin Endocrinol Metab. 2011;96(6):1815–1826. doi: 10.1210/jc.2010-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino L. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294(3):E607–E614. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- [34].Heilbronn L, Gan S, Turner N, Campbell L, Chisholm D. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92(4):1467–1473. doi: 10.1210/jc.2006-2210. [DOI] [PubMed] [Google Scholar]
- [35].Faerch K, Vaag A. Metabolic inflexibility is a common feature of impaired fasting glycaemia and impaired glucose tolerance. Acta Diabetol. 2011;48(4):349–353. doi: 10.1007/s00592-010-0245-x. [DOI] [PubMed] [Google Scholar]
- [36].Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1988;66(5):979–986. doi: 10.1210/jcem-66-5-979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.